Ice as a Green-Structure-Directing Agent in the Synthesis of Macroporous MWCNTs and Chondroitin Sulphate Composites
Abstract
:1. Introduction
2. Results and Discussion
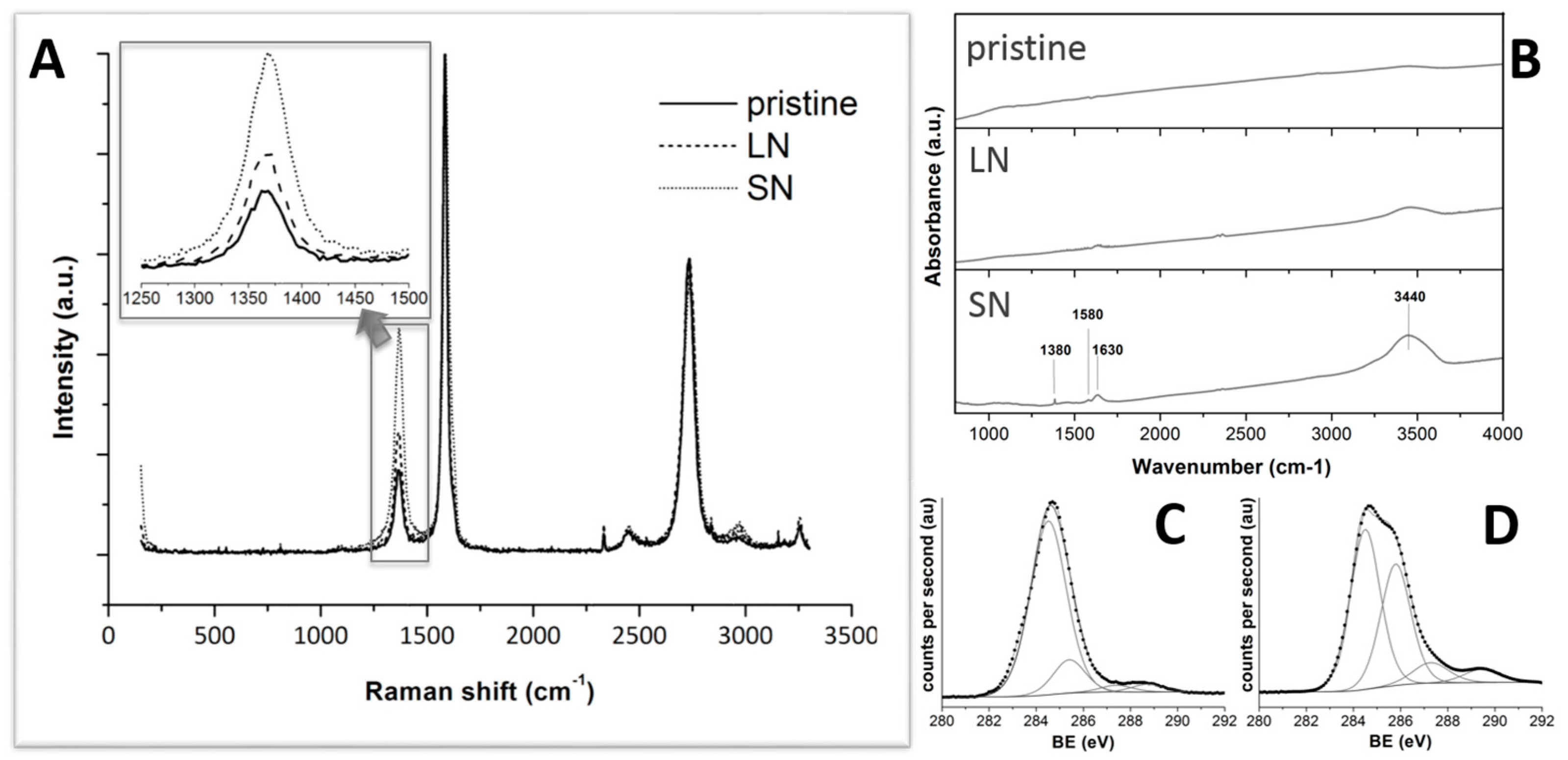
2.1. Characterization of Modified MWCNTs
2.2. Characterization of MWCNTs/CS Scaffolds
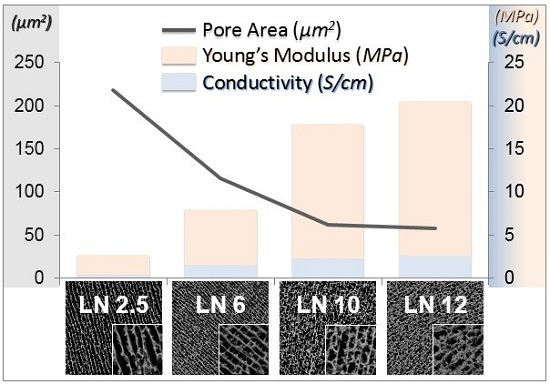
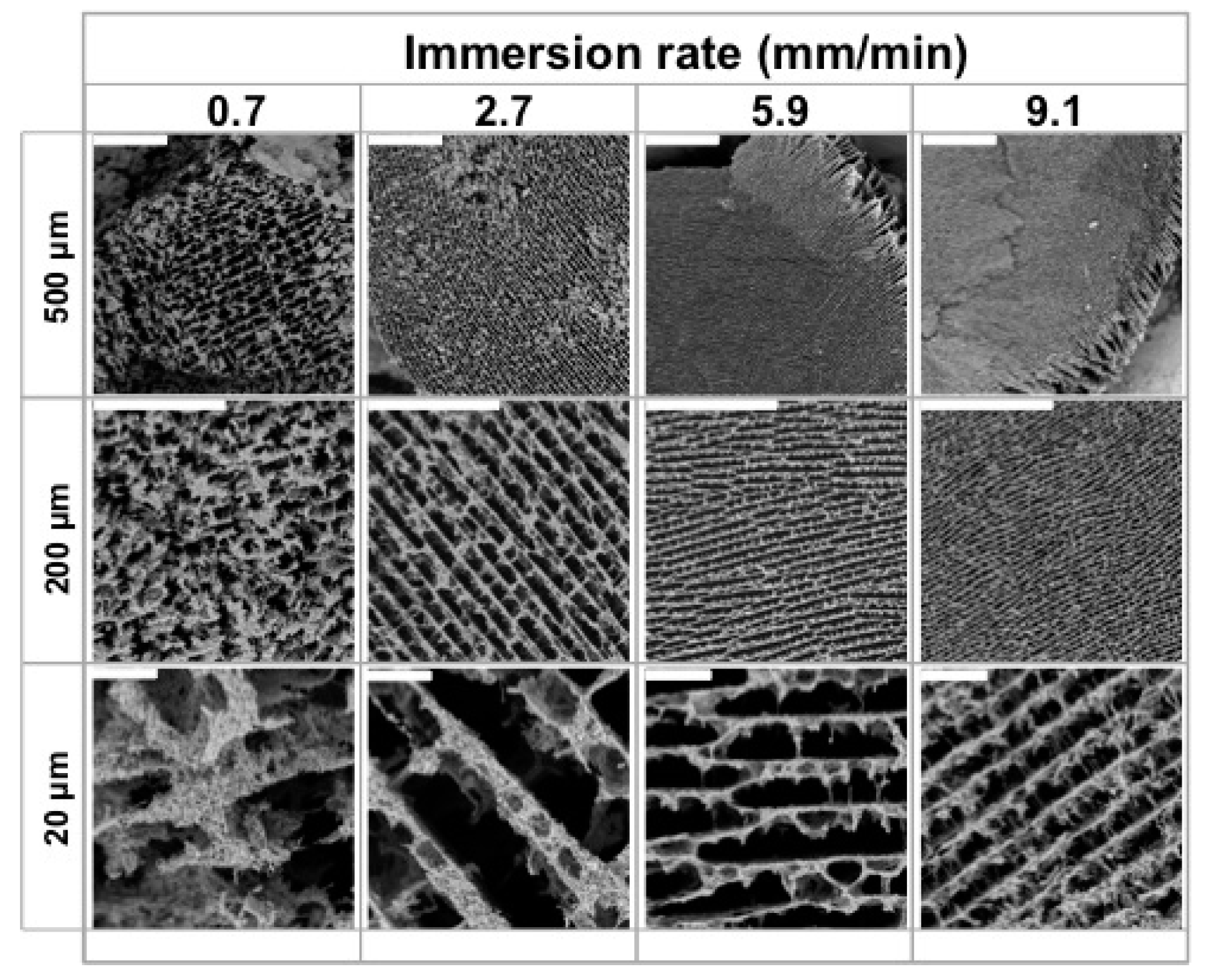
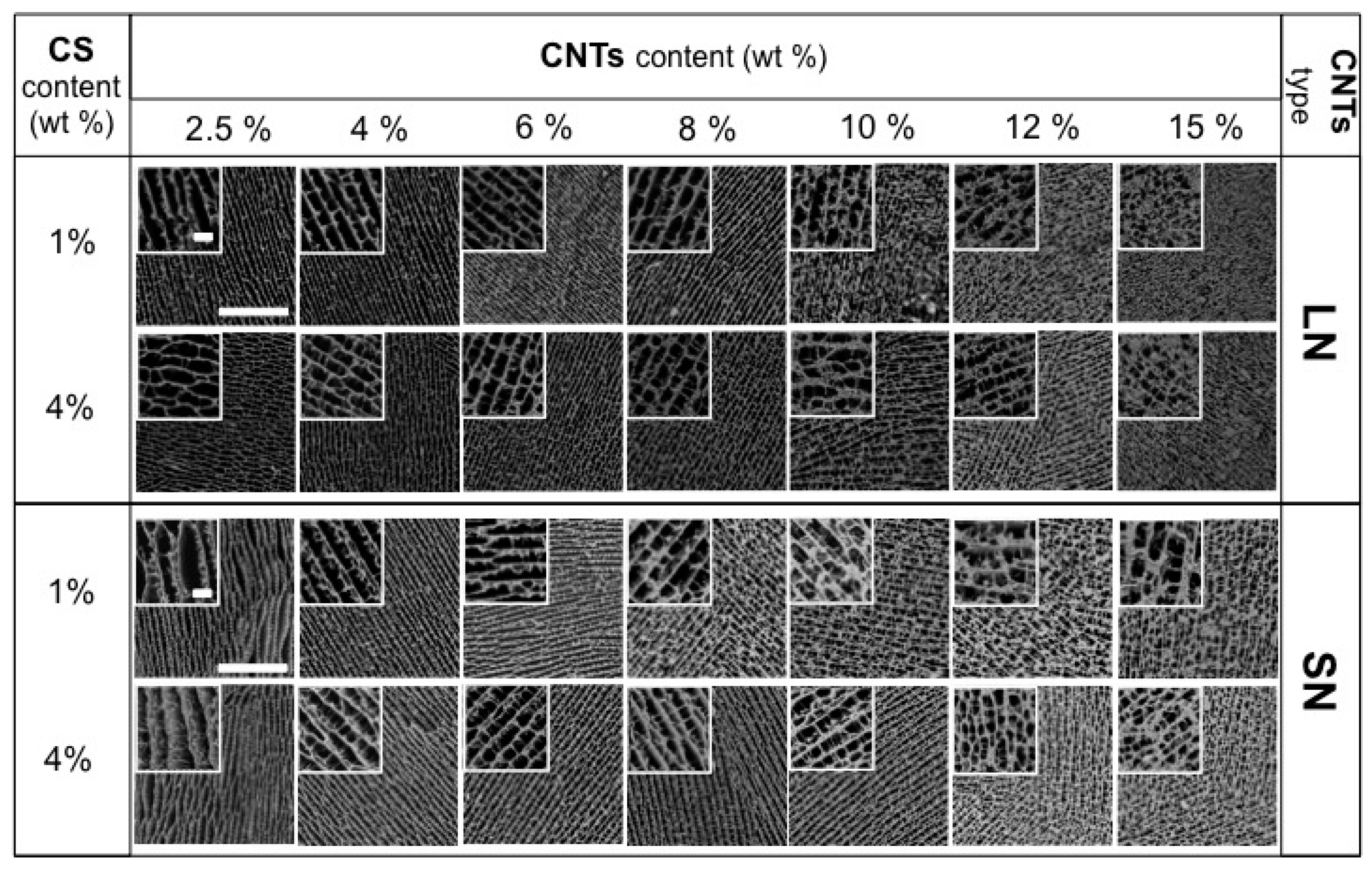
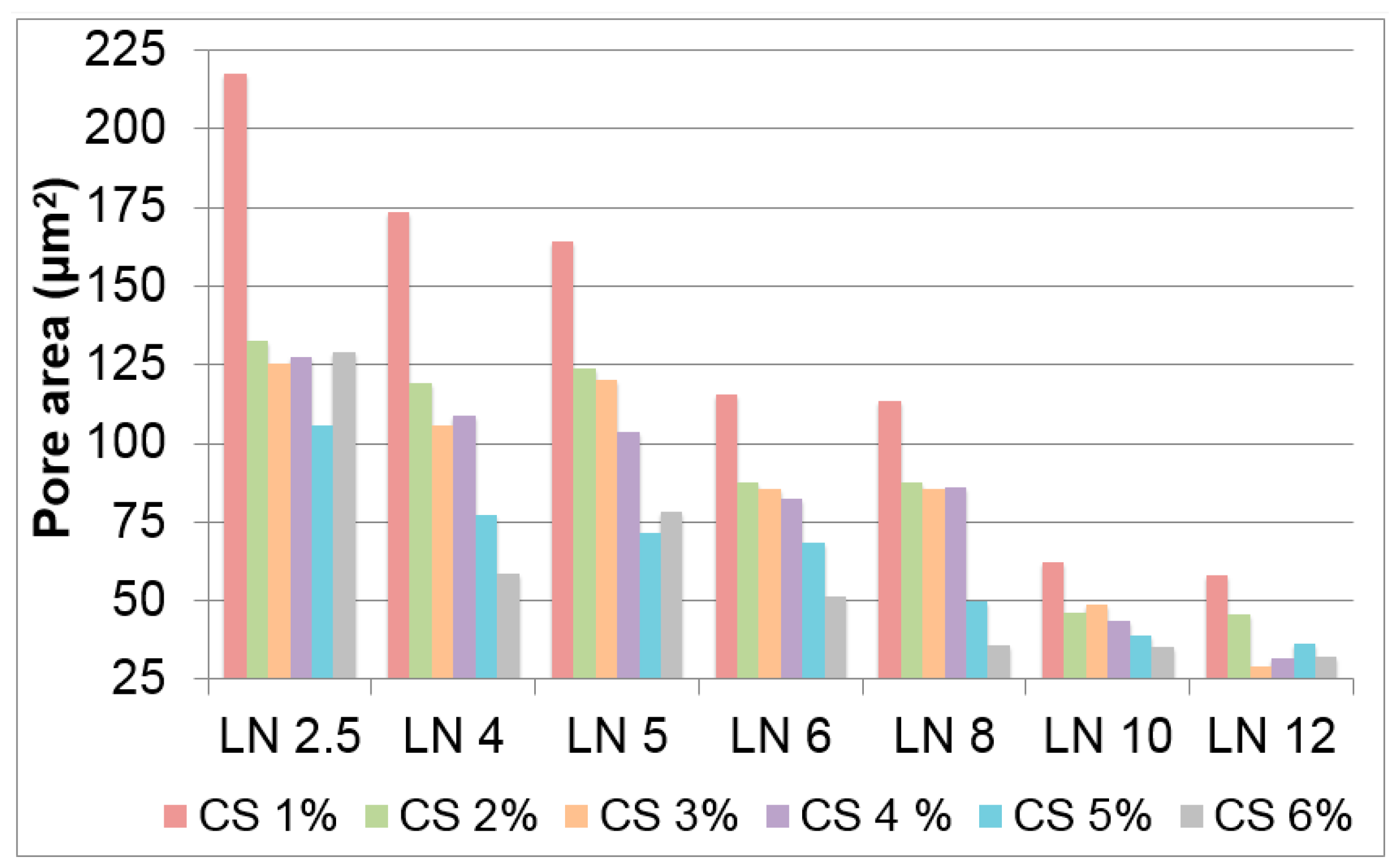
2.2.1. Scaffolds Morphology and Porosity
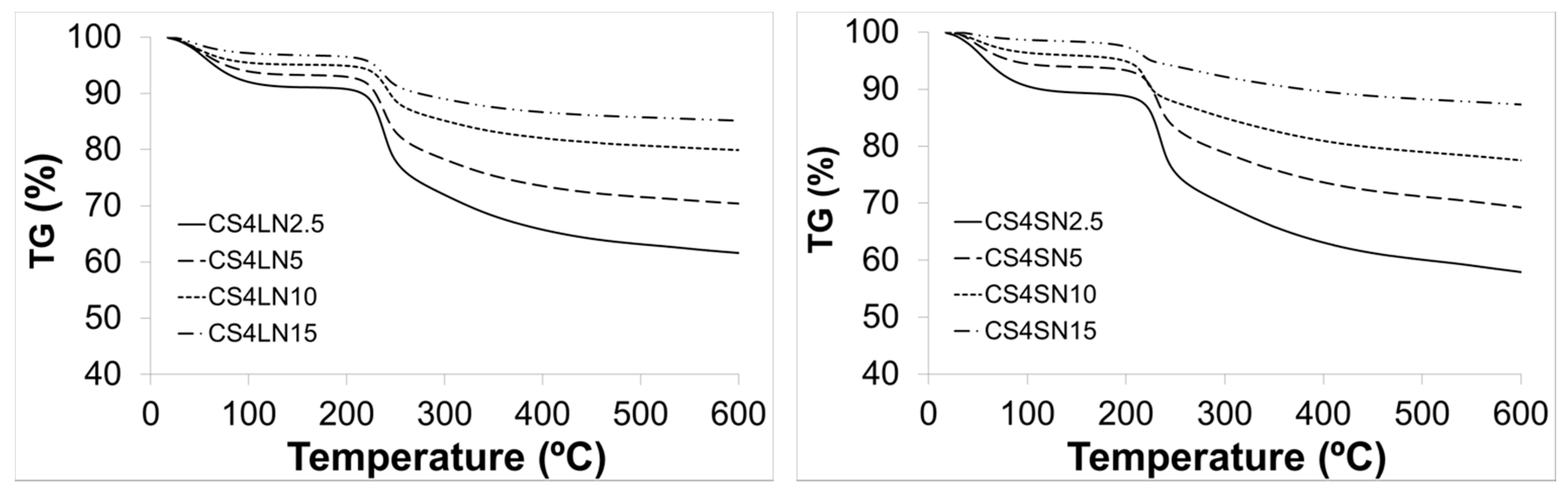
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Cross-Linking with Hexamethylene Diisocyanate (HMDI)
2.2.4. Water-Binding Capacity
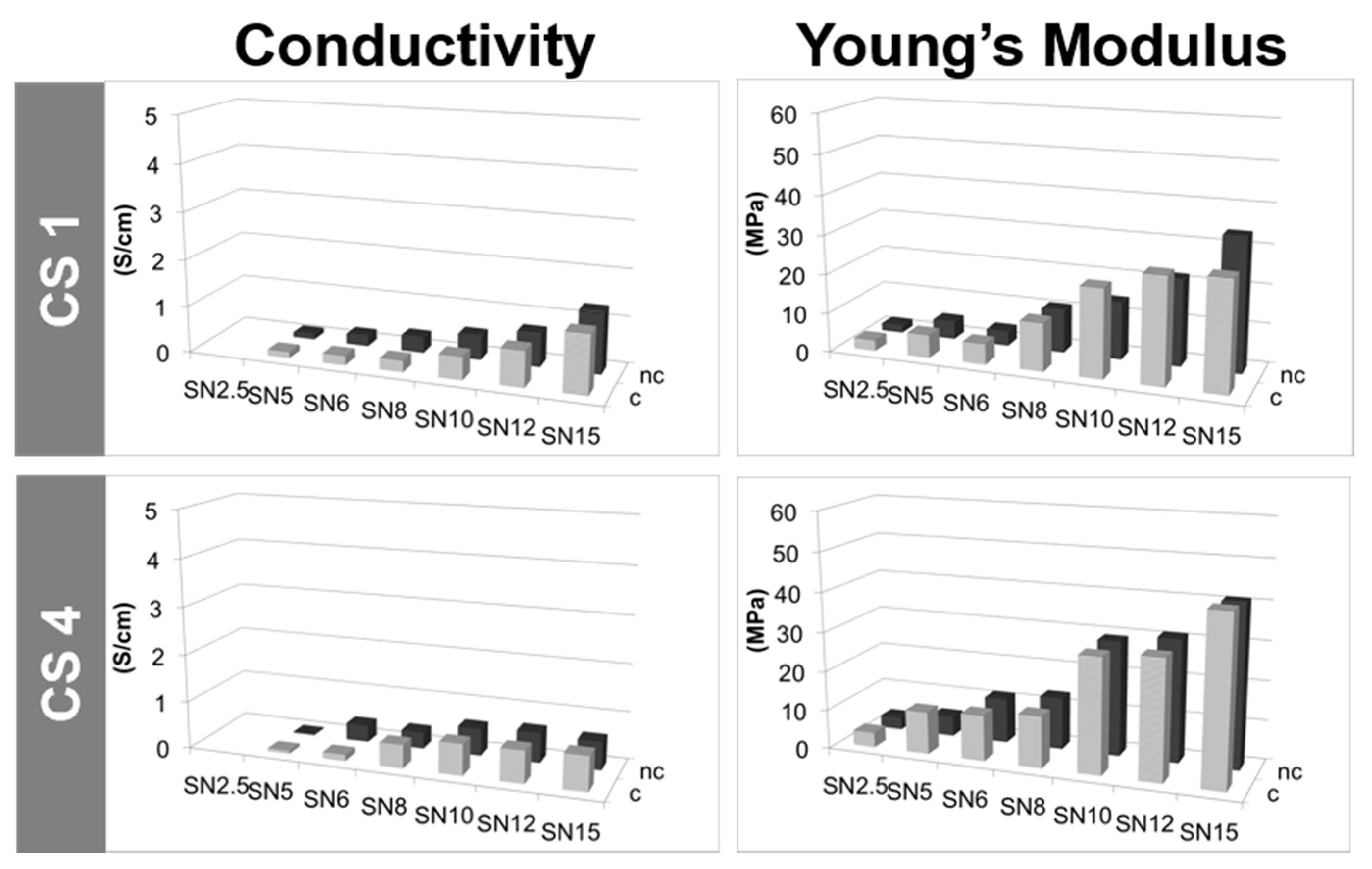
2.2.5. Conductivity Properties
2.2.6. Mechanical Properties
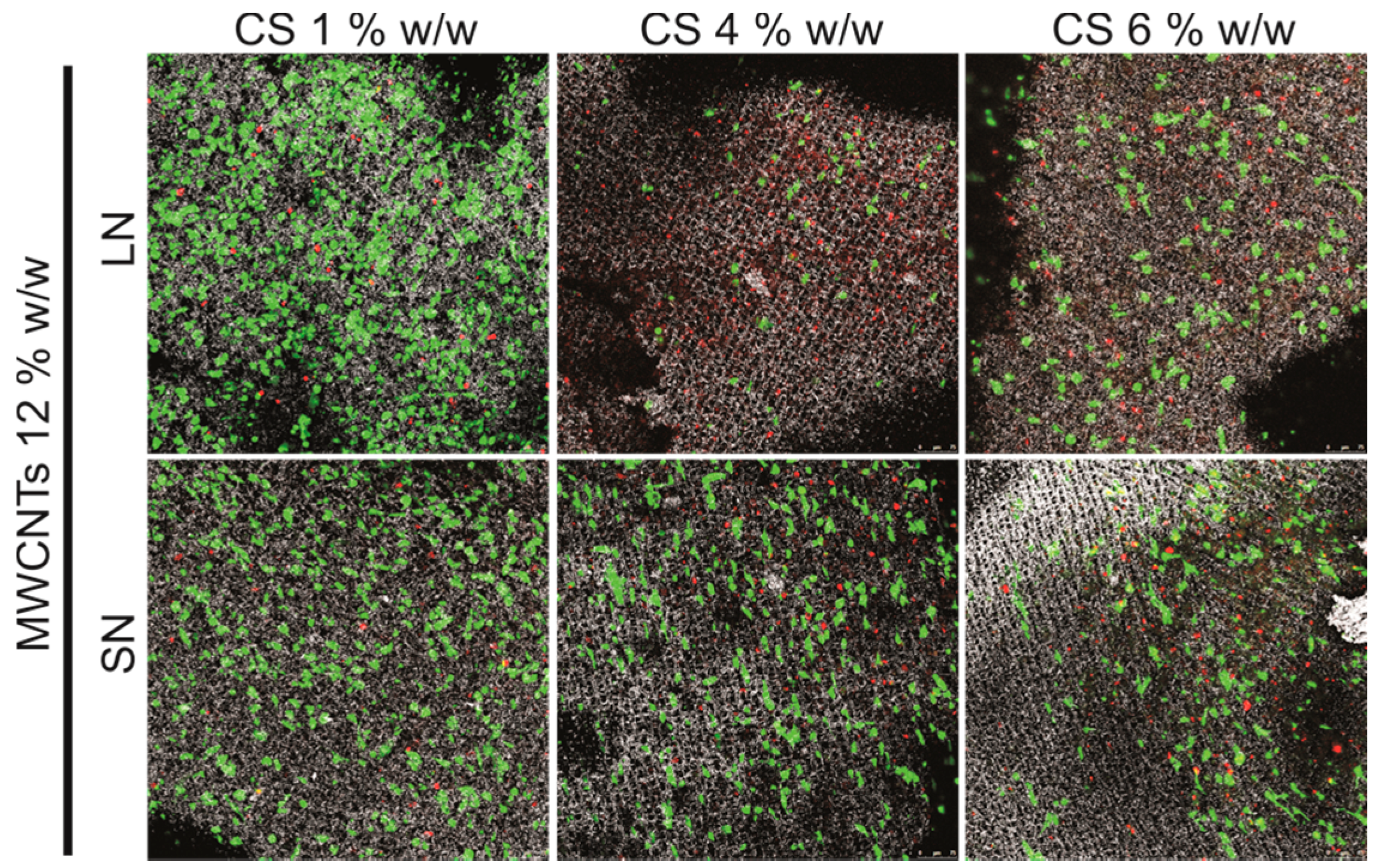
2.3. Biocompatibility Studies In Vitro
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of MWCNTs
3.3. Fabrication of MWCNT/CS Scaffolds
3.4. Morphological and Physico-Chemical Characteristics of MWCNT/CS Scaffolds
3.5. Biocompatibility Studies In Vitro
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Gutiérrez, M.C.; del Monte, F. Role of polymers in the design of 3D carbon nanotube-based scaffolds for biomedical applications. Prog. Polym. Sci. 2014, 39, 1448–1471. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Jobbágy, M.; Rapún, N.; Ferrer, M.L.; del Monte, F. A biocompatible bottom-up route for the preparation of hierarchical biohybrid materials. Adv. Mater. 2006, 18, 1137–1140. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Ice-templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation-induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Hortigüela, M.J.; Amarilla, J.M.; Jiménez, R.; Ferrer, M.L.; del Monte, F. Macroporous 3D architectures of self-assembled MWCNT surface decorated with Pt nanoparticles as anodes for a direct metanol fuel cell. J. Phys. Chem. C 2007, 111, 5557–5560. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; García-Carvajal, Z.Y.; Hortigüela, M.J.; Yuste, L.; Rojo, F.; Ferrer, M.L.; del Monte, F. Biocompaticle MWCNT scaffolds for immobilization and proliferation of E. coli. J. Mater. Chem. 2007, 17, 2992–2995. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gutiérrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, K.-H.; Roh, K.C.; Kim, K.B. Morphology control of three-dimensional carbon nanotube macrostructures fabricated using ice-templating method. J. Porous Mater. 2013, 20, 1289–1297. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. Understanding anisotropy and architecture in ice-templated biopolymer scaffolds. Mater. Sci. Eng. C 2014, 37, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Javadi, A.; Sabo, R.; Cai, Z.; Gong, S. Polyvinyl alcohol (PVA)-cellulose nanofibril (CNF)-multiwalled carbon nanotube (MWCNT) hybrid organic aerogels with superior mechanical properties. RCS Adv. 2013, 20, 1289–1297. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Wang, L.; Xu, S.; Hou, F. Effect of frozen conditions on dispersion morphologies of carbon nanotubes and electrical conductivity of carbon fiber/epoxy composites. Mater. Lett. 2014, 130, 180–183. [Google Scholar] [CrossRef]
- Nardecchia, S.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Modulating the cytocompatibility of tridimensional carbon nanotube-based scaffolds. J. Mater. Chem. B 2013, 1, 3064–3072. [Google Scholar] [CrossRef]
- Sweetman, L.J.; Moulton, S.E.; Wallace, G.G. Characterization of porous freeze conducting carbon nanotube-chitosan scaffolds. J. Mater. Chem. 2008, 18, 5417–5422. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; García-Carvajal, Z.Y.; Jobbágy, M.; Rubio, F.; Yuste, L.; Rojo, F.; Ferrer, M.L.; del Monte, F. Poly(vinyl alcohol) scaffolds with tailored morphologies for drug delivery and controlled release. Adv. Funct. Mater. 2007, 17, 3505–3513. [Google Scholar] [CrossRef]
- Nardecchia, S.; Serrano, M.C.; Gutiérrez, M.C.; Portolés, M.T.; Ferrer, M.L.; del Monte, F. Osteoconductive performance of carbon nanotube scaffolds homogeneously mineralized by flow-through electrodeposition. Adv. Funct. Mater. 2012, 22, 4411–4420. [Google Scholar] [CrossRef]
- Yan, L.Y.; Li, W.; Mesgari, S.; Leong, S.S.J.; Chen, Y.; Loo, L.S.; Mu, Y.; Chan-Park, M.B. Use of a chondroitin sulfate isomer as an effective and removable dispersant of single-walled carbon nanotunes. Small 2011, 7, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.; Linker, A.; Meyer, K. Chondroitin sulfates. Fed. Proc. 1958, 17, 1078–1086. [Google Scholar] [PubMed]
- Sechriest, V.F.; Miao, Y.J.; Niyibizi, C.; Westerhausen-Larson, A.; Matthew, H.W.; Evans, C.H.; Fu, F.H.; Suh, J.-K. GAG-augmented polysaccharide hydrogel: A novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 2000, 49, 534–541. [Google Scholar] [CrossRef]
- Van Susante, J.L.C.; Pieper, J.; Buma, P.; van Kuppevelt, T.H.; van Beuningen, H.; van der Kraan, P.M.; Veerkamp, J.H.; van den Berg, W.B. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 2001, 22, 2359–2369. [Google Scholar] [CrossRef]
- Gerber, I.; Oubenali, M.; Bacsa, R.; Durand, J.; Gonçalves, A.; Pereira, M.F.R.; Jolibois, F.; Perrin, L.; Poteau, R.; Serp, P. Theoretical and Experimental Studies on the Carbon-Nanotube Surface Oxidation by Nitric Acid: Interplay between Functionalization and Vacancy Enlargement. Chem. Eur. J. 2011, 17, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddon, R.C. Nitric acid purification of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.L.; Qing, Q.; Yang, Y.L.; Li, Q.W.; Liu, Z.F.; Guo, X.; Du, Z. Effect of Chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Pistone, A.; Ferlazzo, A.; Lanza, M.; Milone, C.; Iannazzo, D.; Piperno, A.; Piperopoulos, E.; Galvagno, S. Morphological modification of MWCNT functionalized with HNO3/H2SO4 mixtures. J. Nanosci. Nanotechnol. 2012, 12, 5054–5060. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rinzler, A.G.; Dai, H.G.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Jorio, A.; Brown, S.D.M.; Souza, A.G.; Dresselhaus, G.; Hafher, J.H.; Lieber, C.M.; Saito, R.; Dresselhaus, M.S. Diameter dependence of the Raman D-band in isolated single-wall carbon nanotubes. Phys. Rev. B 2001, 64, 041401–041404. [Google Scholar] [CrossRef]
- Kuan, H.-C.; Ma, C.-C.M.; Chang, W.-P.; Yuen, S.-M.; Wu, H.-H.; Lee, T.-M. Synthesis, thermal, mechanical and rheological properties of multiwall carbon nanotube/waterborne polyurethane nanocomposite. Compos. Sci. Technol. 2005, 65, 1703–1710. [Google Scholar] [CrossRef]
- Mawhinney, B.D.; Naumenko, V.; Kuznetsova, A.; Yates, J.T., Jr. Infrared spectral evidence for the etching of carbon nanotubes: Ozone oxidation at 298 K. J. Am. Chem. Soc. 2000, 122, 2383–2384. [Google Scholar] [CrossRef]
- Fanning, P.E.; Vannice, M.A. A drifts study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Gong, H.; Kim, S.-T.; Doo Lee, J.; Yim, S. Simple quantification of surface carboxylic acids on chemically oxidized multi-walled carbon nanotubes. Appl. Surf. Sci. 2013, 266, 219–224. [Google Scholar] [CrossRef]
- Porro, S.; Musso, S.; Vinante, M.; Vanzetti, L.; Anderle, M.; Trotta, F.; Tagliaferro, A. Purification of carbon nanotubes grown by termal CVD. Physica E 2007, 37, 58–61. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surface: A quantitative high-resolution xps and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16898–16878. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- He, F.-A.; Fan, J.-T. An effective approach for purifying, cutting, and functionalising of multi-walled carbon nanotubes. Mater. Sci. Technol. Ser. 2013, 29, 1423–1429. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Zemek, J.; Jiricek, P. Time dependent thermal treatment of oxidized MWCNTs studied by the electron and mass spectroscopy methods. Appl. Surf. Sci. 2012, 258, 7912–7917. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Aranaz, I.; Ferrer, M.L.; del Monte, F. Poly(vinyl alcohol) cryogels: Recent developments. In Macroporous Polymers: Production, Properties and Biotechnological/Biomedical Application, 1st ed.; Mattiasson, B., Kumar, A., Galeaev, I.Y., Eds.; CRC Press–Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 83–115. [Google Scholar]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.R.; Nishihara, H.; Tamon, H. Formation of monolithic silica gel microhoneycombs (SMHs) using pseudosteady state growth of microstructural ice crystals. Chem. Commun. 2004, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Shen, S.-S.; Lu, S.-C. Synthesis and characterization of chondroitin sulfate-methacrylate hydrogels. Carbohydr. Polym. 2003, 52, 389–396. [Google Scholar] [CrossRef]
- Van Luyn, M.J.A.; Van Wachem, P.B.; Olde Damink, L.H.H.; Dijkstra, P.J.; Feijen, J.; Nieuwenhuis, P. Relations between in vitro cytotoxicity and crosslinked dermal sheep collagens. J. Biomed. Mater. Res. 1992, 26, 1091–1110. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H. Chemistry and Technology of Isocyanates; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Chen, F.; Tian, M.; Zhang, D.; Wang, J.; Wang, Q.; Yu, X.; Zhang, X.; Wan, C. Preparation and characterization of oxidized alginate covalently cross-linked galactosylated chitosan scaffold for liver tissue engineering. Mater. Sci. Eng. C 2012, 32, 310–320. [Google Scholar] [CrossRef]
- Cael, J.J.; Isaac, D.H.; Blackwell, J.; Koenig, J.L.; Atkins, E.D.T.; Sheehan, J.K. Polarized infrared spectra of crystalline glycosaminoglycans. Carbohydr. Res. 1976, 50, 169–179. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Varum, K.M.; Smidsrod, O. Degradation of partially Nacetylated chitosans with hen egg white and human lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Thein, H.W.W.; Misra, R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Cooney, M.J. Conductive macroporous composite chitosan-carbon nanotube scaffolds. Langmuir 2008, 24, 7004–7010. [Google Scholar] [CrossRef] [PubMed]
- Saffar, K.P.-A.; Arshi, A.R.; JamilPour, N.; Najafi, A.R.; Rouhi, G.; Sudak, L. A cross-linking model for estimating Young’s modulus of artificial bone tissue grown on carbon nanotube scaffold. J. Biomed. Mater. Res. A 2010, 94, 594–602. [Google Scholar] [PubMed]
- Qin, Y.; Shi, J.; Wu, W.; Li, X.; Guo, Z.X.; Zhu, D. Coincise route to functionalized carbon nanotubes. J. Phys. Chem. B 2003, 107, 11899–12901. [Google Scholar] [CrossRef]
- Serrano, M.C.; Nardecchia, S.; García-Rama, C.; Ferrer, M.L.; Collazos-Castro, J.E.; del Monte, F.; Gutiérrez, M.C. Chondroitin sulphate-based 3D scaffolds containing MWCNTs for nervous tissue repair. Biomaterials 2014, 35, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, S.-Y. EDC/NHS-crosslinked type II collagen-chondroitin sulfate scaffold: Characterization and in vitro evaluation. J. Mater. Sci. Mater. Med. 2008, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Ganesh, N.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation and characterization of chitosan–gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2010, 80, 687–694. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Carriazo, D.; Tamayo, A.; Jiménez, R.; Picó, F.; Rojo, J.M.; Ferrer, M.L.; del Monte, F. Deep-eutectic-solvent-assisted synthesis of hierarchical carbón electrodes exhibiting capacitance retention at high current densities. Chem. Eur. J. 2011, 17, 10553–10537. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Gutiérrez, M.C.; Jiménez, R.; Ferrer, M.L.; del Monte, F. Synthesis of novel lidocaine-releasing poly(diol-co-citrate) elastomers by using deep eutectic solvents. Chem. Commun. 2012, 48, 579–581. [Google Scholar] [CrossRef] [PubMed]

| MWCNTs (%) x | CS1SNxH | CS4SNxH | CS1LNxH | CS4LNxH |
|---|---|---|---|---|
| 2.5 | 1633.8 | 983.4 | 1702.7 | 1049.4 |
| 4 | 1492.3 | 856.4 | 1594.8 | 920.4 |
| 6 | 1229.0 | 745.4 | 1379.5 | 800.9 |
| 8 | 990.6 | 697.8 | 1022.1 | 765.9 |
| 10 | 832.0 | 600.4 | 896.9 | 629.9 |
| 12 | 728.0 | 543.3 | 756.9 | 582.8 |
| 15 | 614.2 | 503.2 | 647.5 | 524.1 |
| 18 | 545.1 | --- | 512.2 | --- |
| 21 | 455.2 | --- | --- | --- |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardecchia, S.; Serrano, M.C.; García-Argüelles, S.; Maia Da Costa, M.E.H.; Ferrer, M.L.; Gutiérrez, M.C. Ice as a Green-Structure-Directing Agent in the Synthesis of Macroporous MWCNTs and Chondroitin Sulphate Composites. Materials 2017, 10, 355. https://doi.org/10.3390/ma10040355
Nardecchia S, Serrano MC, García-Argüelles S, Maia Da Costa MEH, Ferrer ML, Gutiérrez MC. Ice as a Green-Structure-Directing Agent in the Synthesis of Macroporous MWCNTs and Chondroitin Sulphate Composites. Materials. 2017; 10(4):355. https://doi.org/10.3390/ma10040355
Chicago/Turabian StyleNardecchia, Stefania, María Concepción Serrano, Sara García-Argüelles, Marcelo E. H. Maia Da Costa, María Luisa Ferrer, and María C. Gutiérrez. 2017. "Ice as a Green-Structure-Directing Agent in the Synthesis of Macroporous MWCNTs and Chondroitin Sulphate Composites" Materials 10, no. 4: 355. https://doi.org/10.3390/ma10040355
APA StyleNardecchia, S., Serrano, M. C., García-Argüelles, S., Maia Da Costa, M. E. H., Ferrer, M. L., & Gutiérrez, M. C. (2017). Ice as a Green-Structure-Directing Agent in the Synthesis of Macroporous MWCNTs and Chondroitin Sulphate Composites. Materials, 10(4), 355. https://doi.org/10.3390/ma10040355