Uniaxial Negative Thermal Expansion and Mechanical Properties of a Zinc-Formate Framework
Abstract
:1. Introduction
2. Results and Discussion
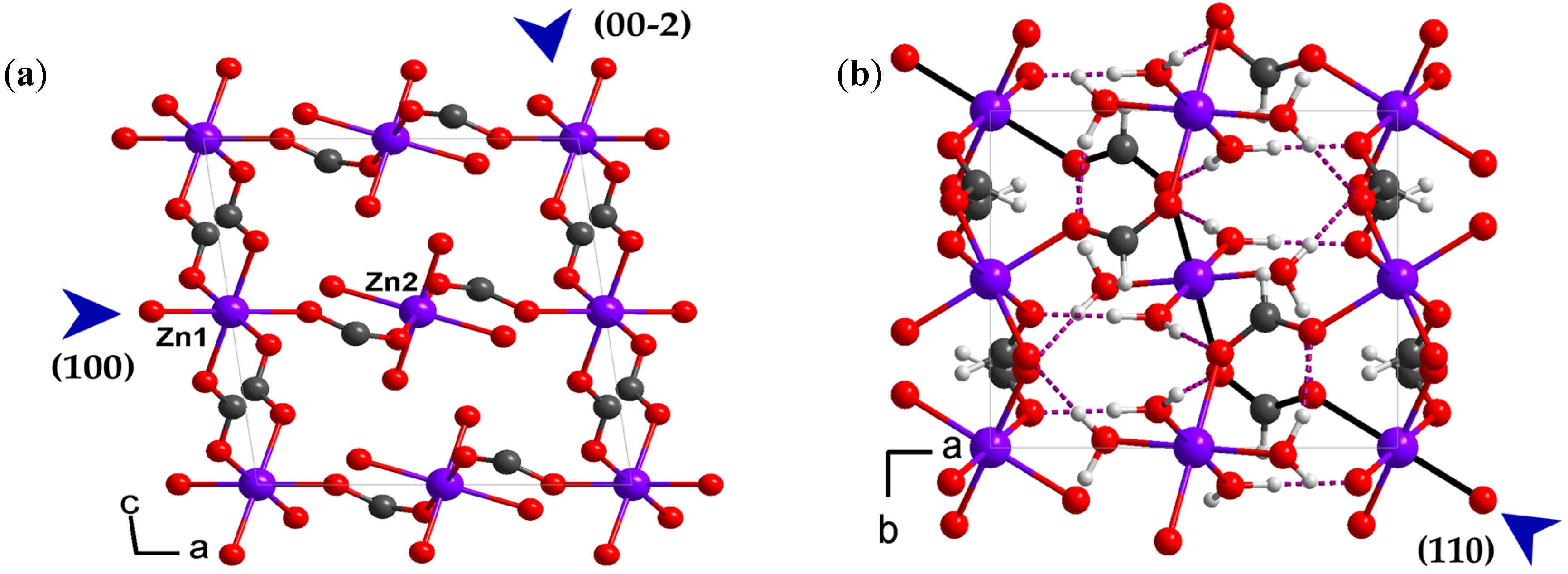
2.1. Crystal Structure Description
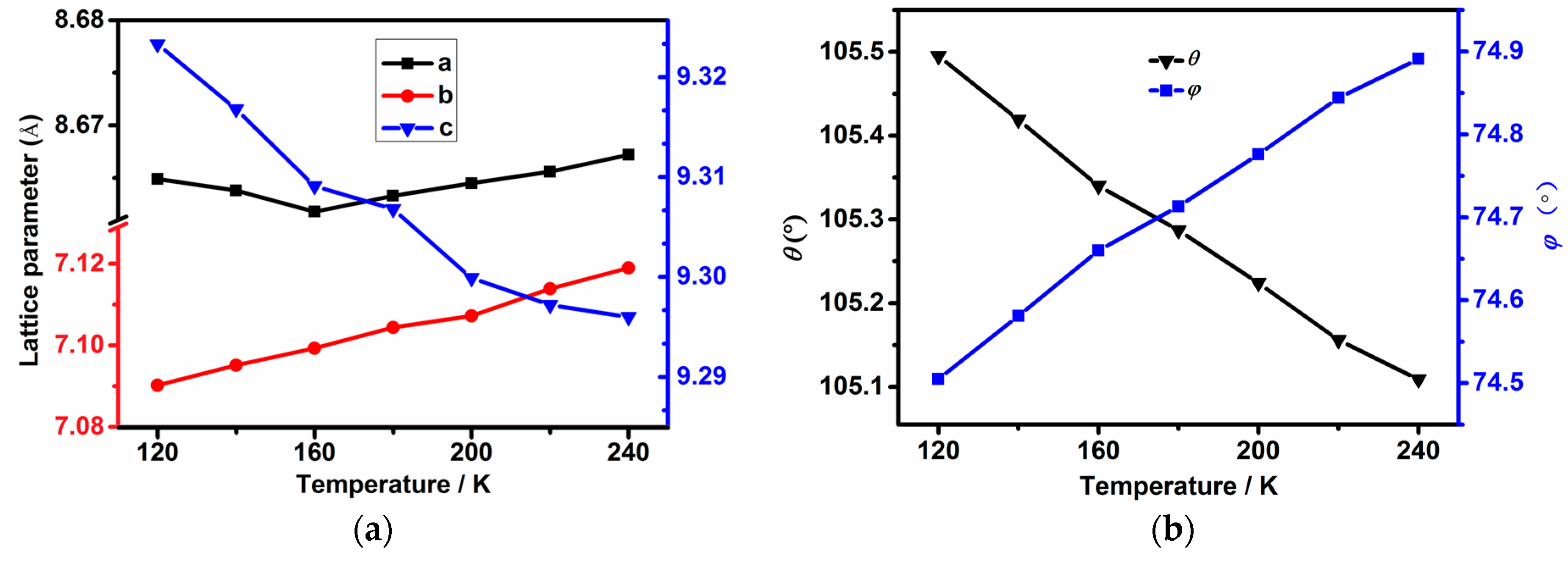
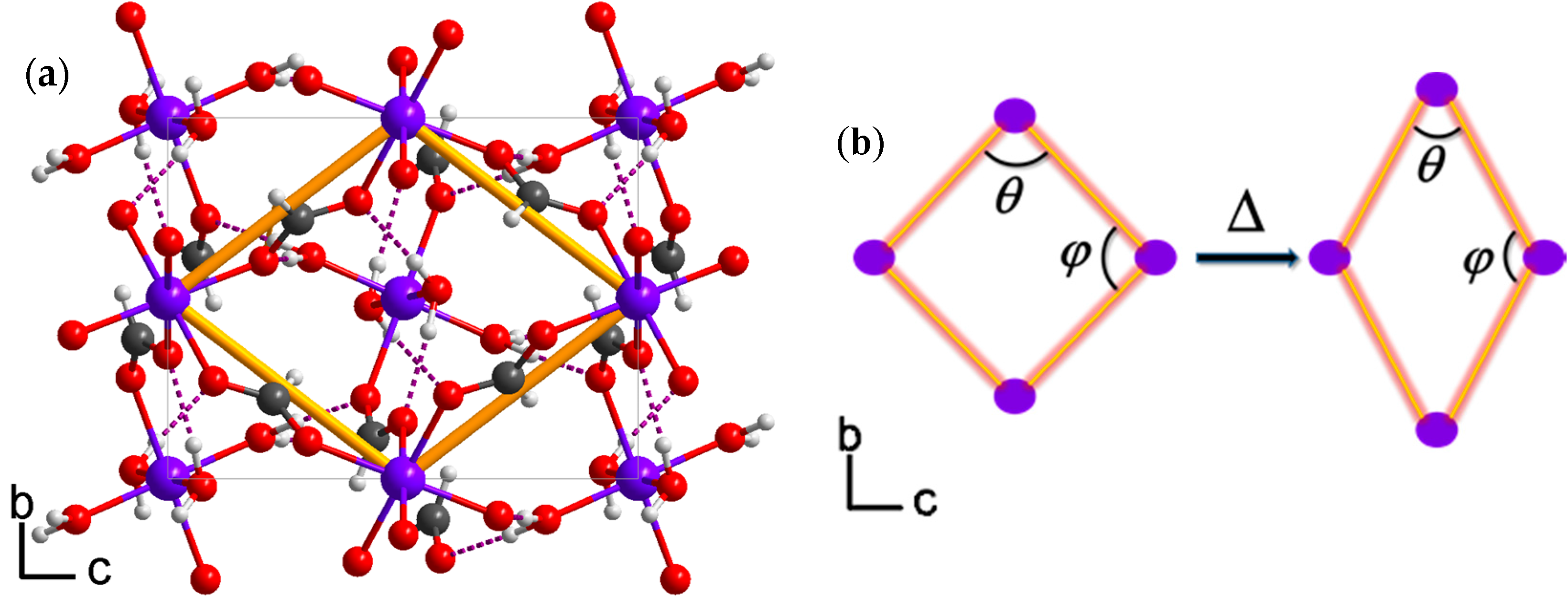
2.2. Thermal Expansion Study
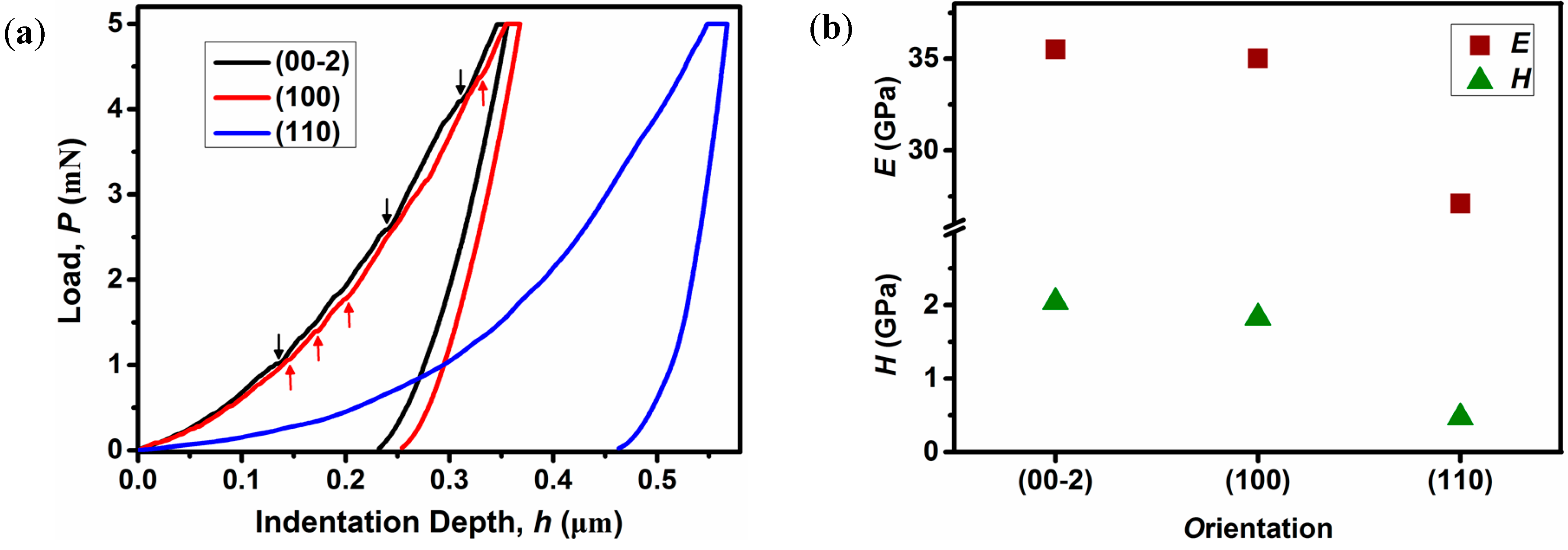
2.3. Mechanical Properties
3. Materials and Methods
3.1. Synthesis
3.2. Variable-Temperature Single Crystal X-ray Diffraction
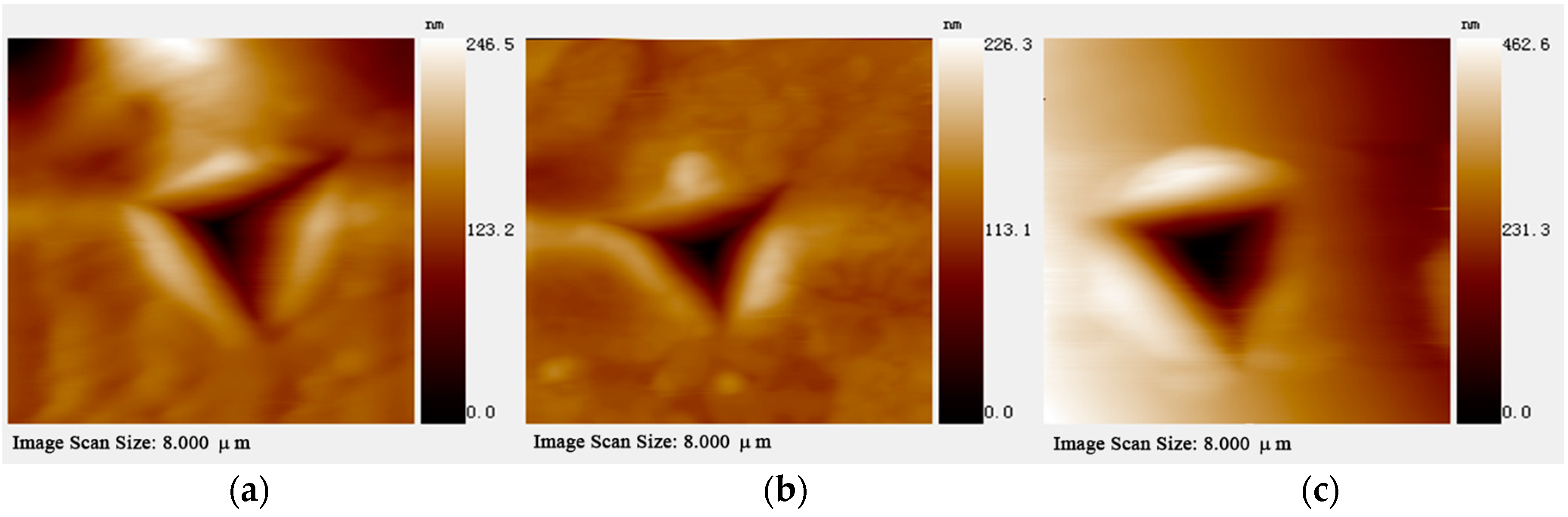
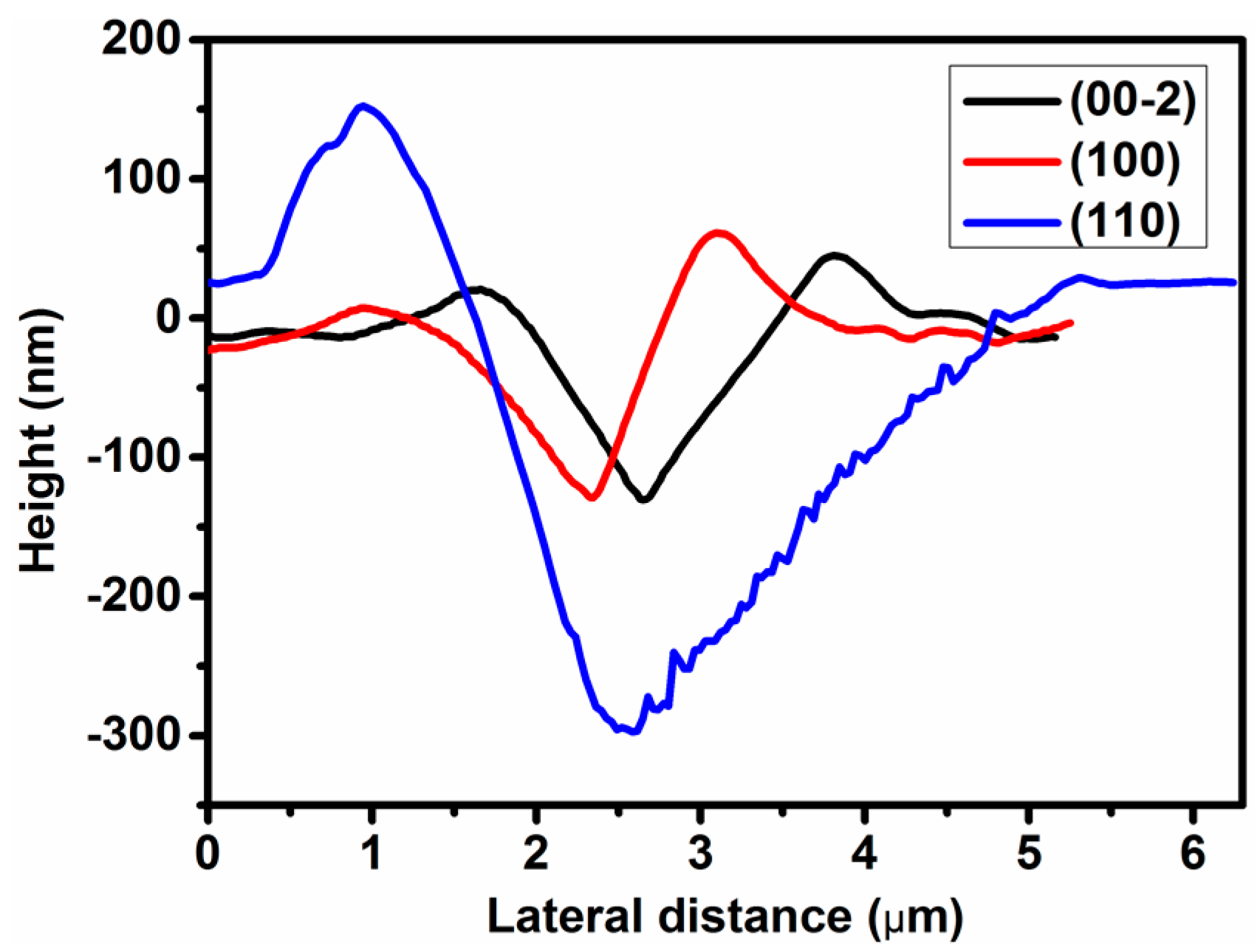
3.3. Nanoindentation Experiment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shang, R.; Chen, S.; Wang, Z.M.; Gao, S. Metal–Organic Frameworks: Functional Magnetic Materials with Formate. Encycl. Inorg. Bioinorg. Chem. 2014. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Inoue, K.; Fujiwara, H.; Otsuka, T.; Kobayashi, H.; Kurmoo, M. Occurrence of a rare 49•66 structural topology, chirality, and weak ferromagnetism in the [NH4][MII(HCOO)3](M = Mn, Co, Ni) frameworks. Inorg. Chem. 2007, 46, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.C.; Zhang, W.; Ma, X.M.; Chen, Y.H.; Zhang, L.; Cai, H.L.; Wang, Z.M.; Xiong, R.G.; Gao, S. Coexistence of magnetic and electric orderings in the metal–formate frameworks of [NH4][M(HCOO)3]. J. Am. Chem. Soc. 2011, 133, 14948–14951. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Di Sante, D.; Stroppa, A. Strain tuning of ferroelectric polarization in hybrid organic inorganic perovskite compounds. J. Phys. Chem. Lett. 2015, 6, 4553–4559. [Google Scholar] [CrossRef] [PubMed]
- Šimėnas, M.; Ciupa, A.; Mączka, M.; Völkel, G.; Pöppl, A.; Banys, J.R. EPR of Structural Phase Transition in Manganese-and Copper-Doped Formate Framework of [NH3(CH2)4NH3][Zn(HCOO)3]2. J. Phys. Chem. C 2016, 120, 19751–19758. [Google Scholar] [CrossRef]
- Wang, W.; Yan, L.Q.; Cong, J.Z.; Zhao, Y.L.; Wang, F.; Shen, S.P.; Zou, T.; Zhang, D.; Wang, Z.G.; Han, X.F.; et al. Magnetoelectric coupling in the paramagnetic state of a metal-organic framework. Sci. Rep. 2013, 3, 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Probert, M.R.; Kosa, M.; Bennett, T.D.; Thirumurugan, A.; Burwood, R.P.; Parinello, M.; Howard, J.A.K.; Cheetham, A.K. Negative linear compressibility of a metal–organic framework. J. Am. Chem. Soc. 2012, 134, 11940–11943. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Forse, A.C.; Sun, S.; Butler, K.T.; Kumagai, S.; Wu, Y.; Warren, M.R.; Walsh, A.; Grey, C.P.; Cheetham, A.K. Role of Amine–Cavity Interactions in Determining the Structure and Mechanical Properties of the Ferroelectric Hybrid Perovskite [NH3NH2]Zn(HCOO)3. Chem. Mater. 2016, 28, 312–317. [Google Scholar] [CrossRef]
- Kieslich, G.; Kumagai, S.; Forse, A.C.; Sun, S.; Henke, S.; Yamashita, M.; Grey, C.P.; Cheetham, A.K. Tuneable mechanical and dynamical properties in the ferroelectric perovskite solid solution [NH3NH2]1−x [NH3OH]xZn(HCOO)3. Chem. Sci. 2016, 7, 5108–5112. [Google Scholar] [CrossRef]
- Li, W.; Henke, S.; Cheetham, A.K. Research Update: Mechanical properties of metal-organic frameworks–Influence of structure and chemical bonding. APL Mater. 2014, 2, 123902. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Kojima, S. Brillouin scattering study of ferroelectric transition mechanism in multiferroic metal-organic frameworks of [NH4][Mn(HCOO)3] and [NH4][Zn(HCOO)3]. Appl. Phys. Lett. 2014, 104, 222903. [Google Scholar] [CrossRef]
- Maczka, M.; Ciupa, A.; Gagor, A.; Sieradzki, A.; Pikul, A.; Macalik, B.; Drozd, M. Perovskite metal formate framework of [NH2-CH(+)-NH2]Mn(HCOO)3]: Phase transition, magnetic, dielectric, and phonon properties. Inorg. Chem. 2014, 53, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Pato-Doldan, B.; Sanchez-Andujar, M.; Gomez-Aguirre, L.C.; Yanez-Vilar, S.; Lopez-Beceiro, J.; Gracia-Fernandez, C.; Haghighirad, A.A.; Ritter, F.; Castro-Garcia, S.; Senaris-Rodriguez, M.A. Near room temperature dielectric transition in the perovskite formate framework [(CH3)2NH2][Mg(HCOO)3]. Phys. Chem. Chem. Phys. 2012, 14, 8498–8501. [Google Scholar] [CrossRef] [PubMed]
- Collings, I.E.; Hill, J.A.; Cairns, A.B.; Cooper, R.I.; Thompson, A.L.; Parker, J.E.; Tang, C.C.; Goodwin, A.L. Compositional dependence of anomalous thermal expansion in perovskite-like ABX3 formates. Dalton Trans. 2016, 45, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Collings, I.E.; Tucker, M.G.; Keen, D.A.; Goodwin, A.L. Geometric switching of linear to area negative thermal expansion in uniaxial metal–organic frameworks. CrystEngComm 2014, 16, 3498–3506. [Google Scholar] [CrossRef]
- Maczka, M.; Pietraszko, A.; Macalik, B.; Hermanowicz, K. Structure, Phonon Properties, and Order–Disorder Transition in the Metal Formate Framework of [NH4][Mg(HCOO)3]. Inorg. Chem. 2014, 53, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Xu, G.C.; Wang, Z.M.; Gao, S. Phase Transitions, Prominent Dielectric Anomalies, and Negative Thermal Expansion in Three High Thermally Stable Ammonium Magnesium–Formate Frameworks. Chem. Eur. J. 2014, 20, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, X.; Feng, G.; Lin, Z.; Hu, B.; Li, W. Mechanical properties and negative thermal expansion of a dense rare earth formate framework. J. Solid State Chem. 2016, 233, 289–293. [Google Scholar] [CrossRef]
- Chen, S.; Shang, R.; Hu, K.L.; Wang, Z.M.; Gao, S. [NH2NH3][M(HCOO)3](M = Mn2+, Zn2+, Co2+ and Mg2+): Structural phase transitions, prominent dielectric anomalies and negative thermal expansion, and magnetic ordering. Inorg. Chem. Front. 2014, 1, 83–98. [Google Scholar] [CrossRef]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative thermal expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef]
- Phillips, A.E.; Goodwin, A.L.; Halder, G.J.; Southon, P.D.; Kepert, C.J. Nanoporosity and Exceptional Negative Thermal Expansion in Single-Network Cadmium Cyanide. Angew. Chem. Int. Ed. 2008, 47, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thirumurugan, A.; Barton, P.T.; Lin, Z.; Henke, S.; Yeung, H.H.M.; Wharmby, M.T.; Bithell, E.G.; Howard, C.J.; Cheetham, A.K. Mechanical tunability via hydrogen bonding in metal–organic frameworks with the perovskite architecture. J. Am. Chem. Soc. 2014, 136, 7801–7804. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, S.; André, V.; Martins, M.; Duarte, M.T. Zinc-Formate Metal–Organic Frameworks: Watch Out for Reactive Solvents. J. Chem. Crystallogr. 2015, 45, 178–188. [Google Scholar] [CrossRef]
- Jørgensen, M.R.V.; Cenedese, S.; Clausen, H.F.; Overgaard, J.; Chen, Y.S.; Gatti, C.; Iversen, B.B. Experimental and Theoretical Charge Densities of a Zinc-Containing Coordination Polymer, Zn(HCOO)2(H2O)2. Inorg. Chem. 2013, 52, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, D. Hydrogen bonding systems in metal (II) formate dihydrates, M (HCOO)2·2H2O (M = Mg, Mn, Co, Ni, Cu, and Zn). Double matrix infrared spectroscopy. J. Mol. Struct. 2006, 798, 141–148. [Google Scholar] [CrossRef]
- Barrera, G.D.; Bruno, J.A.O.; Barron, T.H.K.; Allan, N.L. Negative thermal expansion. J. Phys. Condens. Matter 2005, 17, R217–R252. [Google Scholar] [CrossRef]
- Ogborn, J.M.; Collings, I.E.; Moggach, S.A.; Thompson, A.L.; Goodwin, A.L. Supramolecular mechanics in a metal–organic framework. Chem. Sci. 2012, 3, 3011–3017. [Google Scholar] [CrossRef]
- DeVries, L.D.; Barron, P.M.; Hurley, E.P.; Hu, C.; Choe, W. “Nanoscale lattice fence” in a metal-organic framework: Interplay between hinged topology and highly anisotropic thermal response. J. Am. Chem. Soc. 2011, 133, 14848–14851. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mondal, A.; Kiran, M.S.R.N.; Ramamurty, U.; Reddy, C.M. The role of weak interactions in the phase transition and distinct mechanical behavior of two structurally similar caffeine co-crystal polymorphs studied by nanoindentation. Cryst. Growth Des. 2013, 13, 4435–4441. [Google Scholar] [CrossRef]
- Henke, S.; Li, W.; Cheetham, A.K. Guest-dependent mechanical anisotropy in pillared-layered soft porous crystals—A nanoindentation study. Chem. Sci. 2014, 5, 2392–2397. [Google Scholar] [CrossRef]
- Mishra, M.K.; Desiraju, G.R.; Ramamurty, U.; Bond, A.D. Studying microstructure in molecular crystals with nanoindentation: Intergrowth polymorphism in Felodipine. Angew. Chem. Int. Ed. 2014, 53, 13102–13105. [Google Scholar] [CrossRef]
- Li, W.; Kiran, M.S.R.N.; Manson, J.L.; Schlueter, J.A.; Thirumurugan, A.; Ramamurty, U.; Cheetham, A.K. Mechanical properties of a metal–organic framework containing hydrogen-bonded bifluoride linkers. Chem. Commun. 2013, 49, 4471–4473. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 2011, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2011, 19, 3–20. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
| A | M | α (MK−1) | T/K | Reference |
|---|---|---|---|---|
| CH3NH3+ | Mg2+ | αc = −20.0(5) | 100~300 | [14] |
| Mn2+ | αc = −49(2) | |||
| Fe2+ | αc = −25.1(1.2) | |||
| Co2+ | αc = −28.8(6) | |||
| Zn2+ | αc = −34.6(1.0) | |||
| Cd2+ | αc = −61(7) | |||
| C(NH2)3+ | Mn2+ | αc = −10.6(3) | ||
| Fe2+ | αc = −1.5(2) | |||
| Co2+ | αc = −6.7(2) | |||
| Zn2+ | αc = −5.3(6) | |||
| Cd2+ | αa = −16.8(9), αb = −16.8(9) | |||
| [(CH3)2NH3]+ | Cu2+ | αa = −14.3(1.0) | ||
| NH2NH3+ | Mg2+ | αa = −11, αb = −69~−89 | 290~400 | [19] |
| Mn2+ | αa = −96 | |||
| Co2+ | αa = −20, αb = −81~−100 | 290~405 | ||
| Zn2+ | αa = −108 | 290~375 | ||
| Er3+ | αb = −7.1(3) | 120~300 | [18] | |
| [NH3(CH2)4NH3]2+ | Mg2+ | αb = −648 | 390~410 | [17] |
| D-H⋯A | Lengths (Å) @120 K | @240 K | Angle (deg) @120 K | @240 K |
|---|---|---|---|---|
| O5 v-H3⋯O1 i | 2.769(2) | 2.770(2) | 169.3(3) | 165.0(3) |
| O5 v-H4⋯O3 iii | 2.726(3) | 2.746(3) | 156.6(4) | 150.4(4) |
| O6 vi-H5⋯O2 ii | 2.762(2) | 2.762(2) | 175.1(4) | 170.1(3) |
| O6 vi-H6⋯O4 iv | 2.721(3) | 2.727(3) | 174.1(4) | 162.7(3) |
| Metal-Formate Frameworks | Dc (g·cm−3) | Oriention | E (GPa) | H (GPa) | Reference |
|---|---|---|---|---|---|
| [NH4][Zn(HCOO)3] | 1.920 | (002) | 34.4(9) | - | [7] |
| (010) | 18.2(2) | - | |||
| [NH3NH2][Zn(HCOO)3] | 2.000 | (001) | 26.5 | 1.36 | [8] |
| (110) | 24.5 | 1.24 | |||
| [(CH2)3NH2][Mn(HCOO)3] | 1.735 | (010) | 12.6(3) | 0.66(3) | [22] |
| (101) | 11.7(3) | 0.59(3) | |||
| (10-1) | 11.5(4) | 0.58(3) | |||
| [C(NH2)3][Mn(HCOO)3] | 1.798 | (010) | 28.6(4) | 1.25(4) | |
| (101) | 24.5(5) | 1.18(4) | |||
| (10-1) | 23.5(6) | 1.11(5) | |||
| [NH2CHNH2][Er(HCOO)4] | 2.530 | (021) | 30.2(5) | 1.83(5) | [18] |
| (02-1) | 29.8(8) | 1.80(6) | |||
| Framework 1 | 2.215 | (00-2) | 35.5(6) | 2.04(8) | |
| (100) | 35.0(9) | 1.83(8) | |||
| (110) | 27.1(5) | 0.47(3) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wei, W.; Li, Y.; Wu, R.; Feng, G.; Li, W. Uniaxial Negative Thermal Expansion and Mechanical Properties of a Zinc-Formate Framework. Materials 2017, 10, 151. https://doi.org/10.3390/ma10020151
Gao H, Wei W, Li Y, Wu R, Feng G, Li W. Uniaxial Negative Thermal Expansion and Mechanical Properties of a Zinc-Formate Framework. Materials. 2017; 10(2):151. https://doi.org/10.3390/ma10020151
Chicago/Turabian StyleGao, Hongqiang, Wenjuan Wei, Yizhang Li, Rong Wu, Guoqiang Feng, and Wei Li. 2017. "Uniaxial Negative Thermal Expansion and Mechanical Properties of a Zinc-Formate Framework" Materials 10, no. 2: 151. https://doi.org/10.3390/ma10020151
APA StyleGao, H., Wei, W., Li, Y., Wu, R., Feng, G., & Li, W. (2017). Uniaxial Negative Thermal Expansion and Mechanical Properties of a Zinc-Formate Framework. Materials, 10(2), 151. https://doi.org/10.3390/ma10020151





