Energy Donor Effect on the Sensing Performance for a Series of FRET-Based Two-Photon Fluorescent Hg2+ Probes
Abstract
:1. Introduction
2. Theoretical Methods and Computational Details
2.1. Theoretical Methods
2.2. Computational Details
3. Results and Discussion
3.1. Molecular Structure
3.2. One-Photon Absorption
3.3. Two-Photon Absorption
3.4. Fluorescent Emission
3.5. Responsive Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, Z.H.; Lin, Z.Z.; Chen, X.M.; Lai, Z.Z.; Huang, Z.Y. Carbon dots-based fluorescent probe for trace Hg2+ detection in water sample. Sens. Actuators B Chem. 2016, 222, 965–971. [Google Scholar] [CrossRef]
- Xuan, W.; Chen, C.; Cao, Y.; He, W.; Jiang, W.; Liu, K.; Wang, W. Rational design of a ratiometric fluorescent probe with a large emission shift for the facile detection of Hg2+. Chem. Commun. 2012, 48, 7292–7294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, X.; Lv, H.; Fu, H.; Yang, Y.; Huang, Z.; Han, A. A phenothiazine–rhodamine ratiometric fluorescent probe for Hg2+ based on FRET and ICT. Tetrahedron Lett. 2015, 56, 4293–4298. [Google Scholar] [CrossRef]
- Wang, M.; Wen, J.; Qin, Z.; Wang, H. A new coumarin–rhodamine FRET system as an efficient ratiometric fluorescent probe for Hg2+ in aqueous solution and in living cells. Dyes Pigm. 2015, 120, 208–212. [Google Scholar] [CrossRef]
- Sahana, S.; Mishra, G.; Sivakumar, S.; Bharadwaj, P.K. A 2-(2’-hydroxyphenyl)benzothiazole (HTB)—Quinoline conjugate: A highly specific fluorescent probe for Hg2+ based on ESIPT and its application in bioimaging. Dalton Trans. 2015, 44, 20139–20146. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Shinohara, Y.; Iwasaki, S.; Maeda, H. A coumarin-based fluorescent probe for Hg2+ and Ag2+ with an N’-acetylthioureido group as a fluorescence switch. Chem. Commun. 2011, 47, 5073–5075. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Sumiya, S.; Hirai, T. A coumarin-thiourea conjugate as a fluorescent probe for Hg(II) in aqueous media with a broad pH range 2–12. Org. Biomol. Chem. 2010, 8, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Zhang, Y.; Wang, H.; Kong, L.; Tian, Y.; Tao, X.; Bi, H.; Yang, J. Design of turn-on fluorescent probe for effective detection of Hg2+ by combination of AIEE-active fluorophore and binding site. Sens. Actuators B 2015, 221, 730–739. [Google Scholar] [CrossRef]
- Tang, Y.; Ding, Y.; Wu, T.; Lv, L.; Yan, Z. A turn-on fluorescent probe for Hg2+ detection by using gold nanoparticle-based hybrid microgels. Sens. Actuators B 2016, 228, 767–773. [Google Scholar] [CrossRef]
- Gong, Y.J.; Zhang, X.-B.; Chen, Z.; Yuan, Y.; Jin, Z.; Mei, L.; Zhang, J.; Tan, W.; Shen, G.L.; Yu, R.Q. An efficient rhodamine thiospirolactam-based fluorescent probe for detection of Hg2+ in aqueous samples. Analyst 2012, 137, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Zhang, X.B.; Wang, Q.Q.; Lv, Y.F.; Mao, G.J.; Luo, A.L.; Wu, Y.X.; Wu, Y.; Zhang, J.; Tan, W.H. Molecular engineering of a TBET-based two-photon fluorescent. J. Am. Chem. Soc. 2014, 136, 9838–9841. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.P.; Zhang, C.L.; He, W.J.; Yang, Z.H.; Gao, X.; Guo, Z.J. A highly sensitive ratiometric fluorescent probe for Cd2+ detection in aqueous solution and living cells. Chem. Commun. 2010, 46, 6138–6140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Yoon, J.; Spring, D.R. A selective and ratiometric Cu2+ fluorescent probe based on naphthalimide excimer-monomer switching. Chem. Commun. 2010, 46, 2563–2565. [Google Scholar] [CrossRef]
- Han, Z.X.; Zhang, X.B.; Li, Z.; Gong, Y.J.; Wu, X.Y.; Jin, Z.; He, C.M.; Jian, L.X.; Zhang, J.; Shen, G.L.; et al. Efficient fluorescence resonance energy transfer-based ratiometric fluorescent cellular imaging probe for Zn2+ using a rhodamine spirolactam as a trigger. Anal. Chem. 2010, 82, 3108–3113. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ren, A.M.; Guo, J.F.; Zou, L.Y.; Huang, S. Computational design of a two-photon excited FRET-based ratiometric fluorescent Cu2+ probe for living cell imaging. RSC Adv. 2015, 5, 98144–98153. [Google Scholar] [CrossRef]
- Fan, J.L.; Hu, M.M.; Zhan, P.; Peng, X.J. Energy transfer cassettes based on organic fluorophores: Construction and applications in ratiometric sensing. Chem. Soc. Rev. 2013, 42, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.J.; Zhang, X.B.; Zhang, C.C.; Luo, A.L.; Fu, T.; Tan, W.; Shen, G.L.; Yu, R.Q. Through bond energy transfer: A convenient and universal strategy toward efficient ratiometric fluorescent probe for bioimaging applications. Anal. Chem. 2012, 84, 10777–10784. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Mishra, S.; Mishra, S.K.; Suresh, E.; Mandal, A.K.; Shrivastav, A.; Das, A. Resonance energy transfer approach and a new ratiometric probe for Hg2+ in aqueous media and living organism. Org. Lett. 2009, 11, 2740–2743. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, X.; Zhao, Y.; Chen, M.; Liu, J.; Wang, P.; Guo, W. A naphthalimide–rhodamine ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer. Dyes Pigm. 2012, 92, 909–915. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Qian, X. A ratiometric fluorescent probe based on FRET for imaging Hg2+ ions in living cells. Angew. Chem. Int. Ed. 2008, 47, 8025–8029. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.Q.; Gao, X.; Chen, M.X.; Zheng, H.; Xu, J.G. A novel Hg2+ selective ratiometric fluorescent chemodosimeter based on an intramolecular FRET mechanism. J. Fluoresc. 2008, 18, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xue, L.; Zhu, D.J.; Li, G.P.; Jiang, H. Highly selective two-photon fluorescent probe for imaging of nitric oxide in living cells. Chin. Chem. Lett. 2014, 25, 19–23. [Google Scholar] [CrossRef]
- Sarkar, A.R.; Kang, D.E.; Kim, H.M.; Cho, B.R. Two-photon fluorescent probes for metal ions in live tissues. Inorg. Chem. 2014, 53, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.J.; Zhang, B.C.; Yu, H.Z.; Zhu, L.; Feng, Y.; Zhu, M.Z.; Guo, Q.X.; Meng, X.M. Two-photon fluorescent probes for biological Mg2+ detection based on 7-substituted coumarin. J. Org. Chem. 2015, 80, 4306–4312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhang, Q.Y.; Ding, H.J.; Song, X.N.; Wang, C.K. Responsive mechanism of 2-(20-hydroxyphenyl)benzoxazole-based two-photon fluorescent probes for zinc and hydroxide ions. Chin. Phys. B 2015, 24, 023301. [Google Scholar] [CrossRef]
- Jia, H.H.; Zhao, K.; Wu, X.L. Effects of torsional disorder and position isomerism on two-photonabsorption properties of polar chromophore dimers. Chem. Phys. Lett. 2014, 612, 151–156. [Google Scholar] [CrossRef]
- Gaussian 09. Available online: http://www.gaussian.com/ (accessed on 29 December 2016).
- Dalton2013. Available online: http://www.kjemi.uio.no/software/dalton/ (accessed on 29 December 2016).
- Atalay, Y.; Avcı, D.; Başoğlu, A. Linear and non-linear optical properties of some donor–acceptor oxadiazoles by ab initio Hartree-Fock calculations. Struct. Chem. 2008, 19, 239–246. [Google Scholar] [CrossRef]
- Myung Kim, H.; Rae Cho, B. Two-photon materials with large two-photon cross sections: Structure-property relationship. Chem. Commun. 2009, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Pons, Ò.; Luo, Y.; Ågren, H. Effects of conjugation length, electron donor and acceptor strengths on two-photon absorption cross sections of asymmetric zinc-porphyrin derivatives. J. Chem. Phys. 2006, 124, 094310. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Kim, D.Y.; Jeong, M.Y.; Kim, H.M.; Lee, Y.K.; Fang, X.; Jeon, S.-J.; Cho, B.R. Two-photon absorption properties of 2,6-bis(styryl)anthracene derivatives: Effects of donor–acceptor substituents and the π center. Chem. Eur. J. 2005, 11, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ren, A.M.; Wang, D.; Guo, J.F.; Feng, J.K.; Yu, X.Q. A theoretical investigation on two latest two-photon pH fluorescent probes. J. Photochem. Photobiol. A 2014, 293, 50–56. [Google Scholar] [CrossRef]
- Förster, T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann. Phys. 1948, 437, 55–75. (In German) [Google Scholar] [CrossRef]
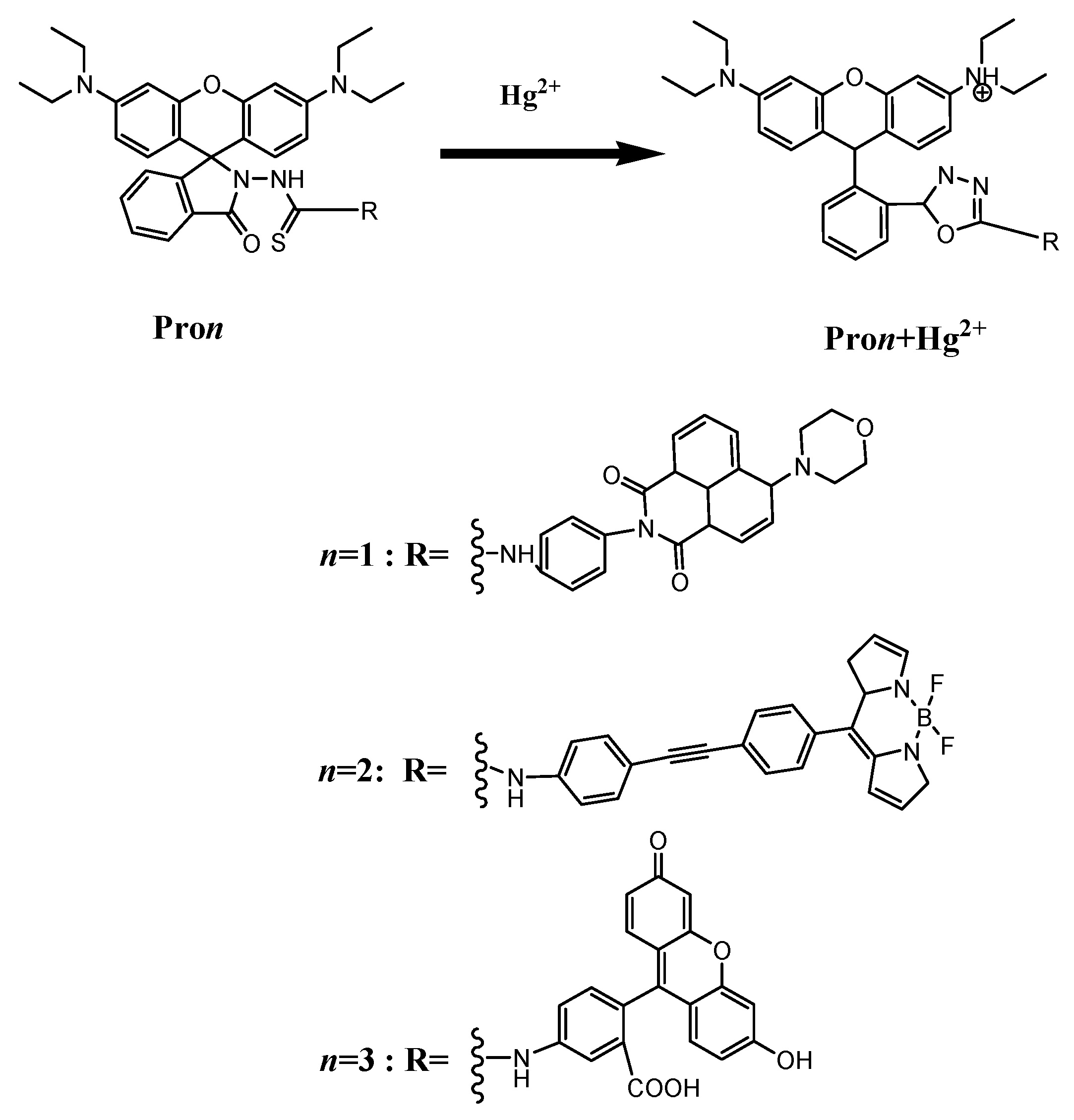
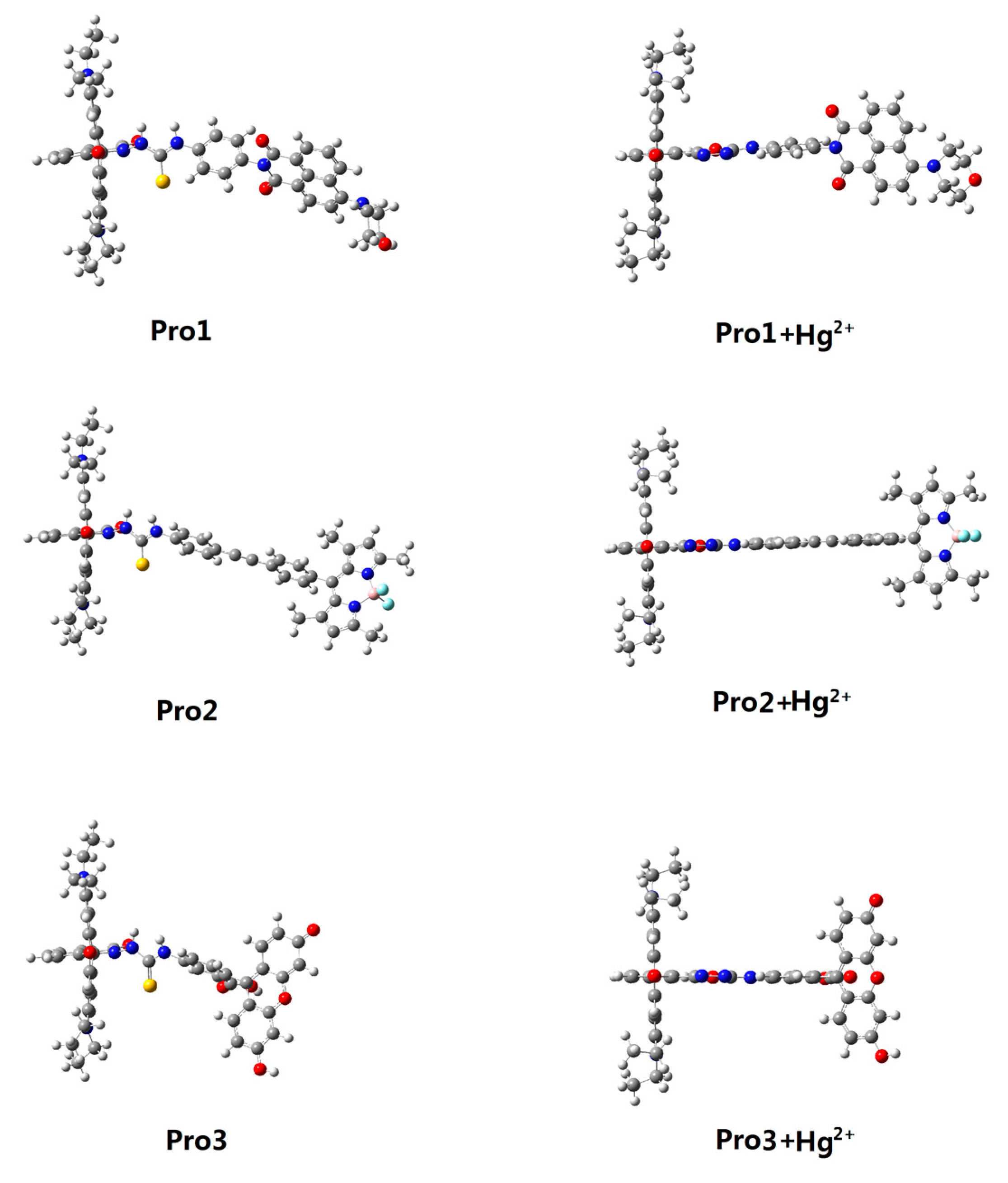
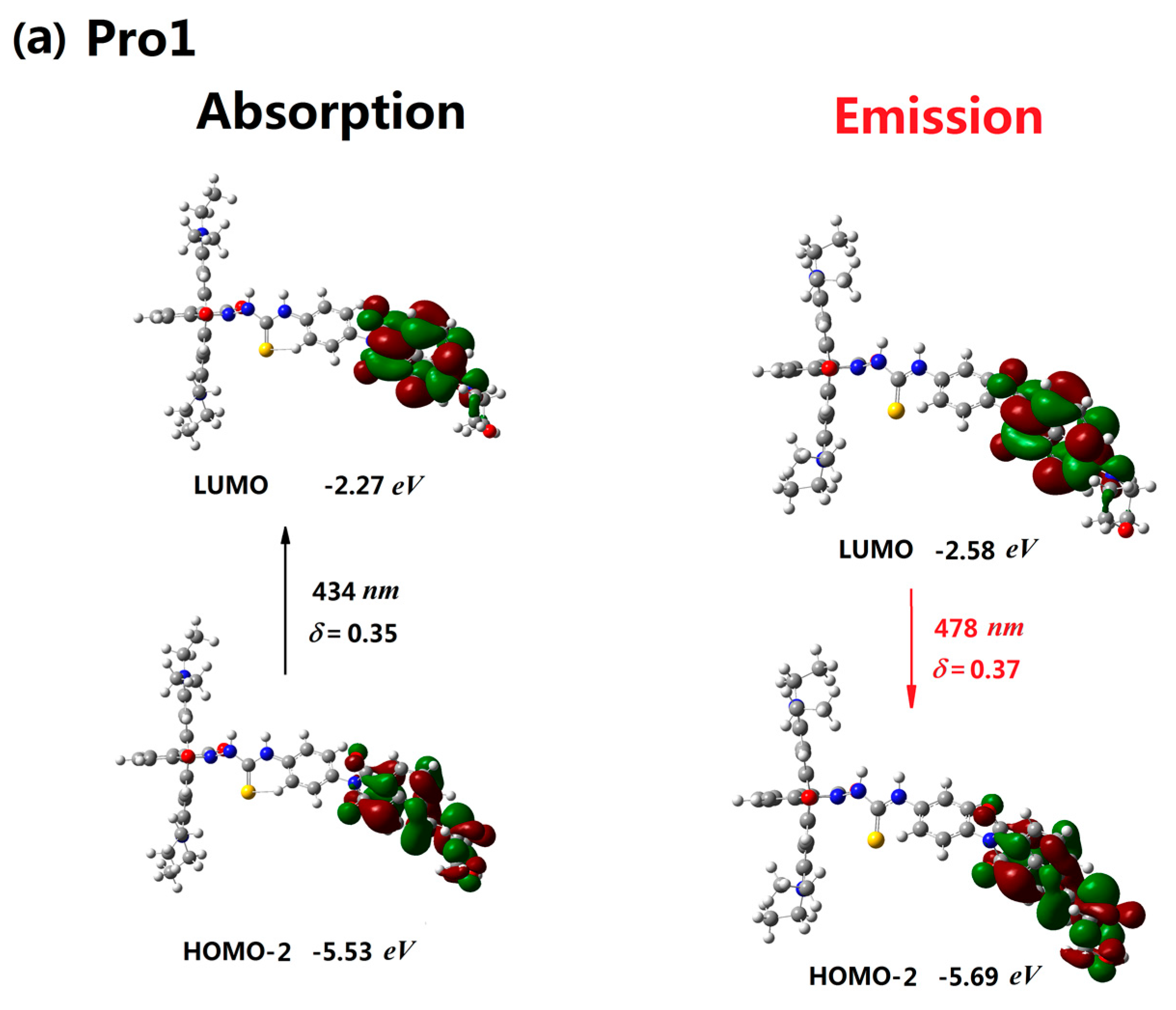
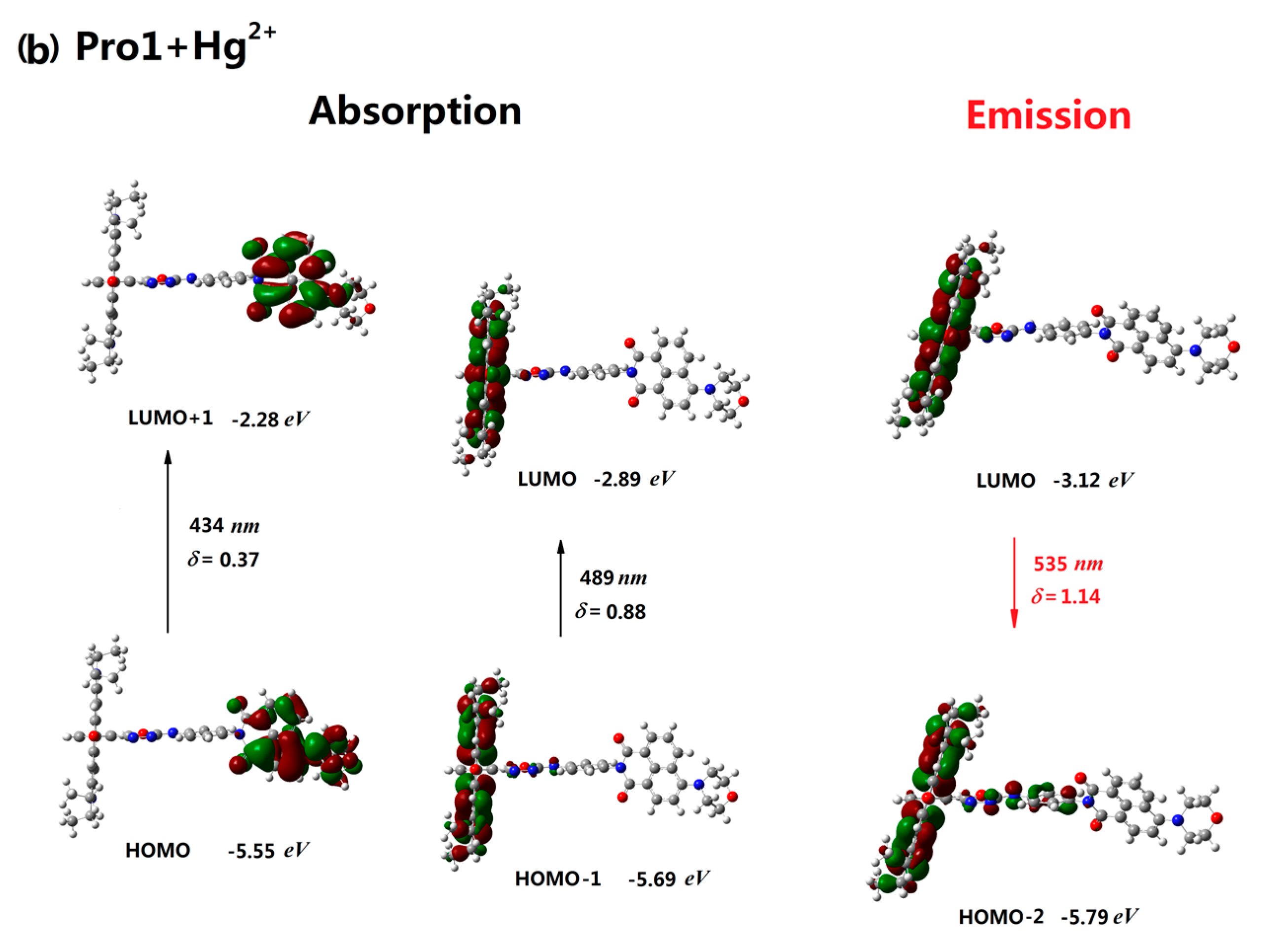
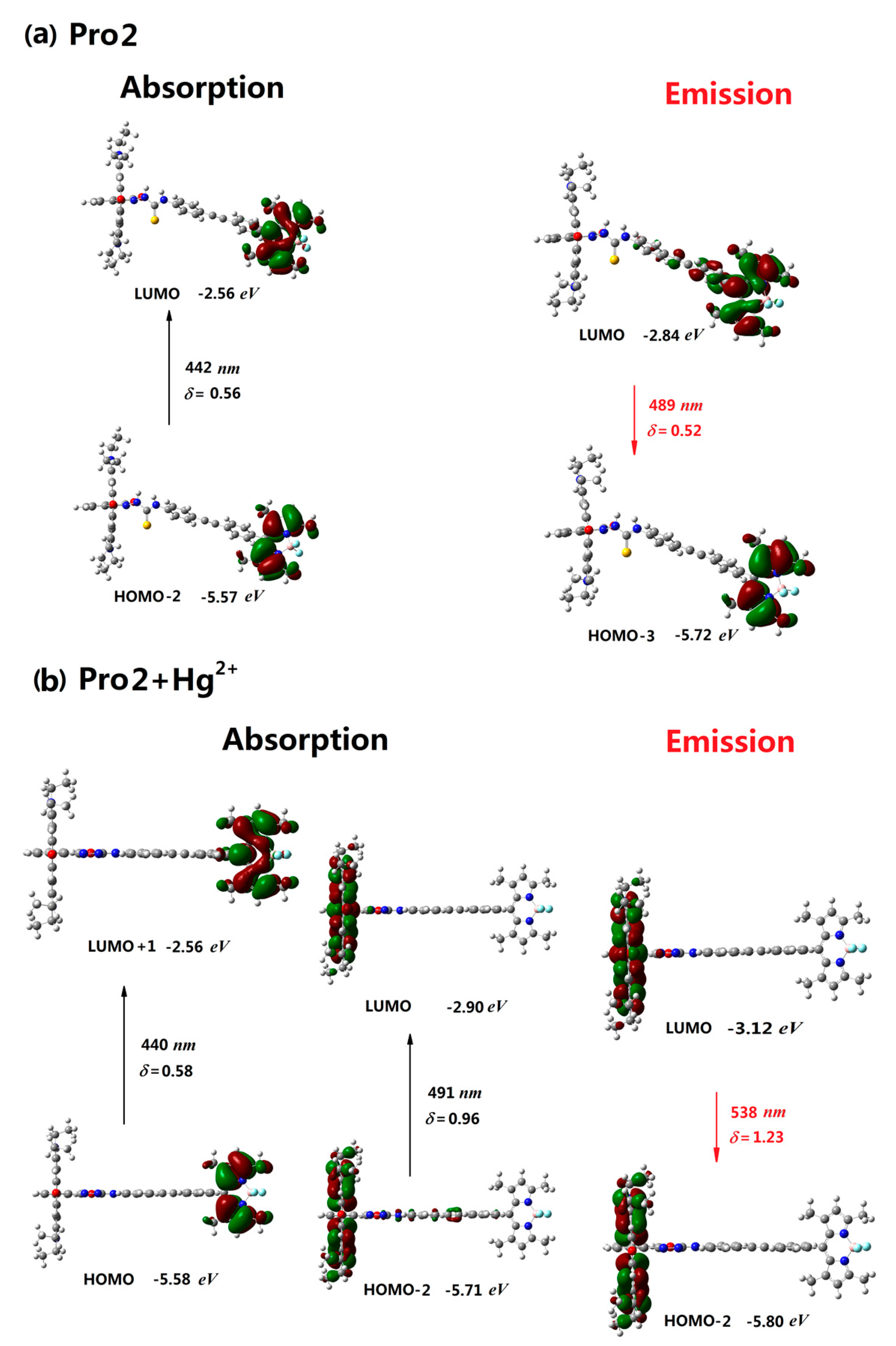

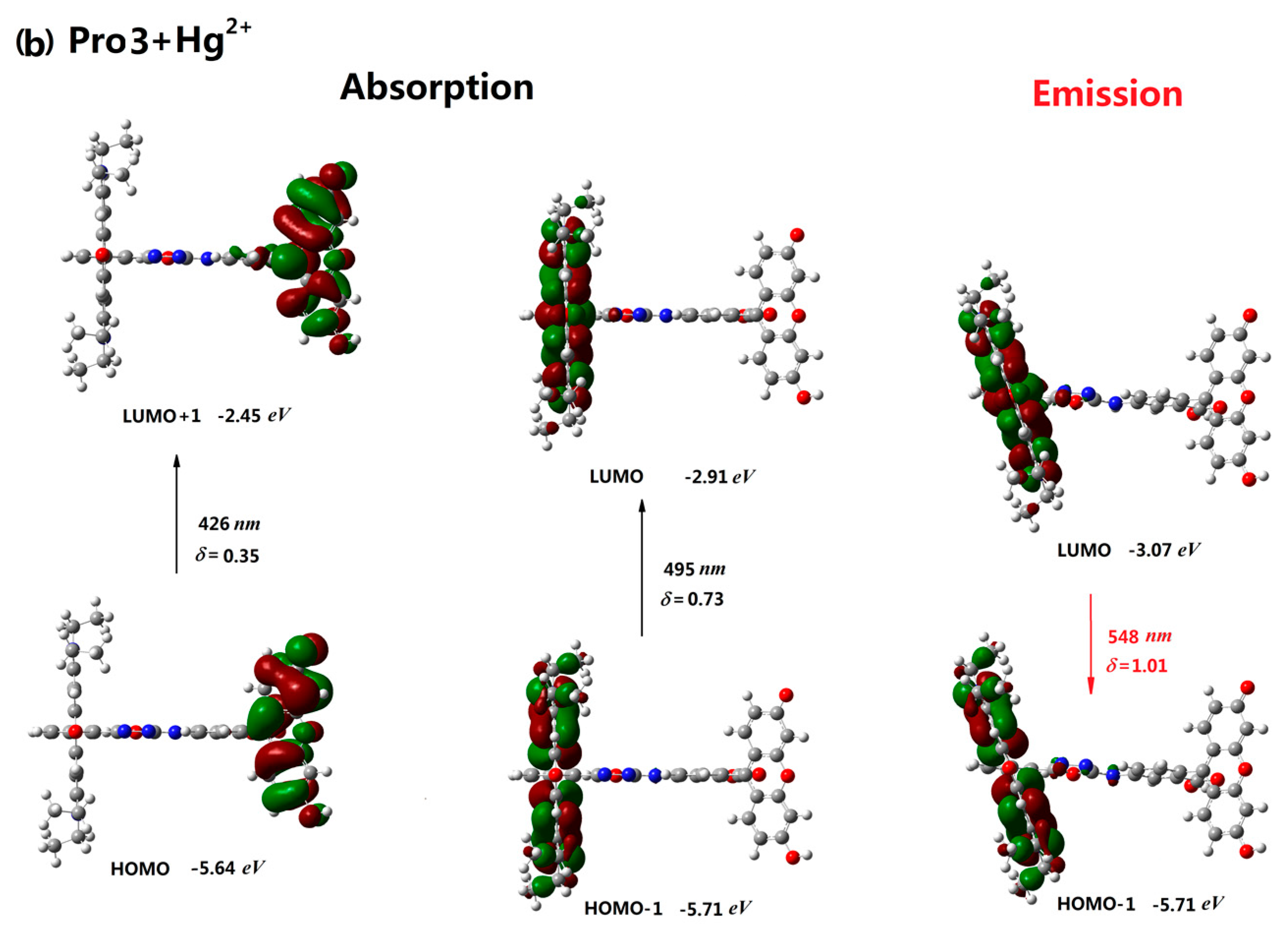
| Molecule | Eopa (eV) | λopa (nm) | δopa (a.u.) | Transition Nature |
|---|---|---|---|---|
| Pro1 | 2.85 | 434 | 0.35 | H-2 → L 98% |
| Pro1+Hg2+ | 2.53, 2.85 | 489, 434 | 0.88, 0.37 | H-1 → L 77%, H → L+1 98% |
| Pro2 | 2.80 | 442 | 0.56 | H-2 → L 98% |
| Pro2+Hg2+ | 2.52, 2.81 | 491, 440 | 0.96, 0.58 | H-2 → L 98%, H → L+1 98% |
| Pro3 | 2.83 | 436 | 0.39 | H-2 → L 85% |
| Pro3+Hg2+ | 2.50, 2.90 | 495, 426 | 0.73, 0.35 | H-1 → L 84%, H → L+1 68% |
| Molecule | Etpa (eV) | λtpa (nm) | σtpa (GM) | Molecule | Etpa (eV) | λtpa (nm) | σtpa (GM) |
|---|---|---|---|---|---|---|---|
| Pro1 | 2.71 | 912 | 0.00 | Pro1+Hg2+ | 2.37 | 1043 | 9.18 |
| 2.85 | 868 | 534.72 | 2.38 | 1039 | 26.68 | ||
| 2.87 | 861 | 2.83 | 2.53 | 977 | 231.27 | ||
| 3.13 | 790 | 17.81 | 2.85 | 868 | 566.42 | ||
| 3.36 | 736 | 6.15 | 3.08 | 802 | 2502.89 | ||
| Pro2 | 2.52 | 981 | 2.15 | Pro2+Hg2+ | 2.20 | 1124 | 6.35 |
| 2.67 | 926 | 0.04 | 2.40 | 1030 | 0.00 | ||
| 2.75 | 899 | 108.08 | 2.52 | 981 | 225.73 | ||
| 2.80 | 883 | 33.21 | 2.65 | 933 | 39.48 | ||
| 3.08 | 802 | 0.93 | 2.81 | 880 | 33.51 | ||
| Pro3 | 2.51 | 985 | 12.10 | Pro3+Hg2+ | 2.33 | 1061 | 0.00 |
| 2.68 | 922 | 10.21 | 2.50 | 989 | 142.91 | ||
| 2.83 | 873 | 89.22 | 2.58 | 958 | 98.54 | ||
| 2.88 | 858 | 15.81 | 2.85 | 867 | 89.89 | ||
| 2.99 | 827 | 49.43 | 2.90 | 852 | 3.09 |
| Molecule | Eope (eV) | λope (nm) | δope (a.u.) | Transition Nature |
|---|---|---|---|---|
| Pro1 | 2.59 | 478 | 0.37 | L → H-2 98% |
| Pro1+Hg2+ | 2.31 | 535 | 1.14 | L → H-2 98% |
| Pro2 | 2.53 | 489 | 0.52 | L → H-3 87% |
| Pro2+Hg2+ | 2.30 | 538 | 1.23 | L → H-2 98% |
| Pro3 | 2.46 | 504 | 0.64 | L → H-2 98% |
| Pro3+Hg2+ | 2.26 | 548 | 1.01 | L → H-1 98% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, W. Energy Donor Effect on the Sensing Performance for a Series of FRET-Based Two-Photon Fluorescent Hg2+ Probes. Materials 2017, 10, 108. https://doi.org/10.3390/ma10020108
Zhang Y, Hu W. Energy Donor Effect on the Sensing Performance for a Series of FRET-Based Two-Photon Fluorescent Hg2+ Probes. Materials. 2017; 10(2):108. https://doi.org/10.3390/ma10020108
Chicago/Turabian StyleZhang, Yujin, and Wei Hu. 2017. "Energy Donor Effect on the Sensing Performance for a Series of FRET-Based Two-Photon Fluorescent Hg2+ Probes" Materials 10, no. 2: 108. https://doi.org/10.3390/ma10020108
APA StyleZhang, Y., & Hu, W. (2017). Energy Donor Effect on the Sensing Performance for a Series of FRET-Based Two-Photon Fluorescent Hg2+ Probes. Materials, 10(2), 108. https://doi.org/10.3390/ma10020108






