Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity
Abstract
1. Introduction
2. Application of ZnO NPs
3. Exposure Routes
4. Genotoxicity of ZnO NPs
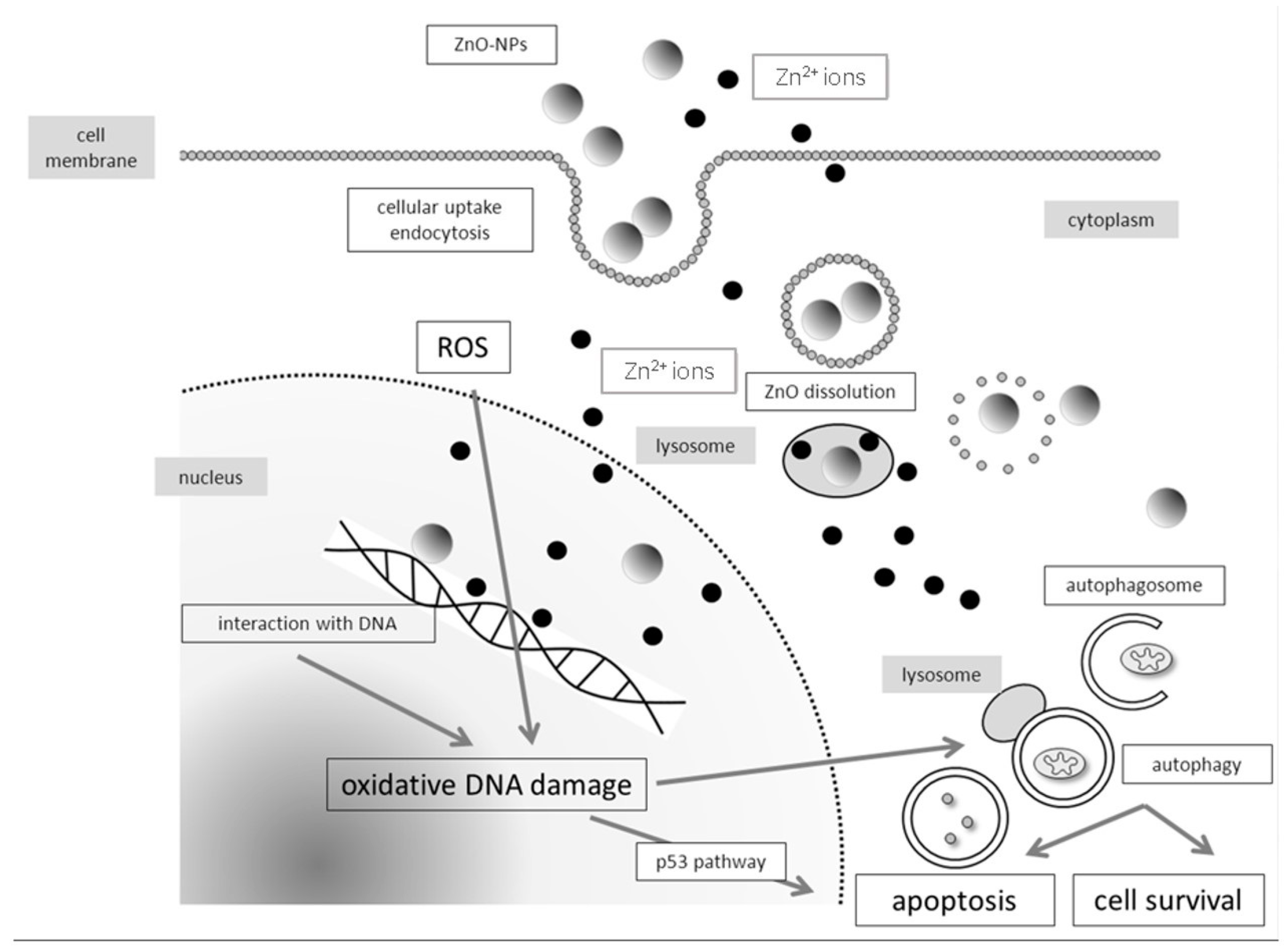
4.1. Molecular Mechanisms of Genotoxicity and Evaluation of Oxidative DNA Damage
4.2. In Vivo Studies
5. Summary
6. Conclusions and Recommendations for Future Research
Author Contributions
Conflicts of Interest
References
- Official Journal of the European Union. COMMISSION RECOMMENDATION of 18 October 2011 on the Definition of Nanomaterial (Text with EEA Relevance) (2011/696/EU). Available online: https://ec.europa.eu/research/industrial_technologies/pdf/policy/commission-recommendation-on-the-definition-of-nanomater-18102011_en.pdf (accessed on 11 December 2017).
- Shi, H.B.; Magaye, R.; Castranova, V.; Zhao, J.S. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Klingshirn, C. ZnO: Material, physics and applications. Chemphyschem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Gamer, A.O.; Leibold, E.; van Ravenzwaay, B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol. In Vitro 2006, 20, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Innes, B.; Roberts, M.S.; Tsuzuki, T.; Robertson, T.A.; McCormick, P. Human skin penetration of sunscreen nanoparticles: In Vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol. Phys. 2007, 20, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Otberg, N.; Richter, H.; Weigmann, H.J.; Lindemann, U.; Schaefer, H.; Sterry, W. Investigation of follicular penetration of topically applied substances. Skin Pharmacol. Appl. 2001, 14, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Vermylen, J.; Nemmar, A.; Nemery, B.; Hoylaerts, M.F. Ambient air pollution and acute myocardial infarction. J. Thromb. Haemost. 2005, 3, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.M.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care 2001, 164, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.J.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Koedrith, P.; Seo, Y.R. Current investigations into the genotoxicity of zinc oxide and silica nanoparticles in mammalian models in vitro and in vivo: Carcinogenic/genotoxic potential, relevant mechanisms and biomarkers, artifacts, and limitations. Int. J. Nanomed. 2014, 9, 271–286. [Google Scholar]
- Liu, J.; Feng, X.L.; Wei, L.M.; Chen, L.J.; Song, B.; Shao, L.Q. The toxicology of ion-shedding zinc oxide nanoparticles. Crit. Rev. Toxicol. 2016, 46, 348–384. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Biological reactivity of zinc oxide nanoparticles with mammalian test systems: An overview. Nanomedicine 2015, 10, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R.L.M.; Benfenati, E.; Leszczynski, J.; Cronin, M.T.D. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale 2015, 7, 2154–6398. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Webb, T.R.; Sayes, C.M.; Colvin, V.L.; Reed, K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol. Sci. 2006, 91, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Moller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Manshian, B.; Jenkins, G.J.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. In Vitro 2011, 25, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Costa, C.; Kilic, G.; Costa, S.; Pasaro, E.; Laffon, B.; Teixeira, J.P. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ. Int. 2013, 55, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Singh, S.K.; Chauhan, L.K.; Das, M.; Tripathi, A.; Dwivedi, P.D. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol. Lett. 2014, 227, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Gohla, A.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Nanoparticle-induced photocatalytic head and neck squamous cell carcinoma cell death is associated with autophagy. Nanomedicine 2014, 9, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Vessoni, A.T.; Filippi-Chiela, E.C.; Menck, C.F.M.; Lenz, G. Autophagy and genomic integrity. Cell Death Differ. 2013, 20, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Das, I.; Mehta, R.K.; Sahu, R.; Sonawane, A. Zinc-Oxide Nanoparticles Exhibit Genotoxic, Clastogenic, Cytotoxic and Actin Depolymerization Effects by Inducing Oxidative Stress Responses in Macrophages and Adult Mice. Toxicol. Sci. 2016, 150, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.; Repar, N.; Marusic, N.; Drasler, B.; Romih, T.; Hocevar, S.; Drobne, D. Comparative in vitro genotoxicity study of ZnO nanoparticles, ZnO macroparticles and ZnCl2 to MDCK kidney cells: Size matters. Toxicol. In Vitro 2017, 40, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Khoo, S.P.K.; Heng, B.C.; Setyawati, M.I.; Tan, E.C.; Zhao, X.X.; Xiong, S.J.; Fang, W.R.; Leong, D.T.; Loo, J.S.C. The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials 2011, 32, 8218–8225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.B.; Ma, Y.F. Irradiation-Enhanced Cytotoxicity of Zinc Oxide Nanoparticles. Int. J. Toxicol. 2014, 33, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wang, S.G.; Xia, Q.S.; He, W.W.; Yin, J.J.; Fu, P.P.; Li, J.H. Phototoxicity of Zinc Oxide Nanoparticles in HaCaT Keratinocytes-Generation of Oxidative DNA Damage During UVA and Visible Light Irradiation. J. Nanosci. Nanotechnol. 2013, 13, 3880–3888. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Akca, H.; Kaya, B.; Burgucu, D.; Tokgun, O.; Turna, F.; Aksakal, S.; Vales, G.; Creus, A.; Marcos, R. Zinc oxide nanoparticles: Genotoxicity, interactions with UV-light and cell-transforming potential. J. Hazard. Mater. 2014, 264, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Santra, C.R.; Ghosh, A.N.; Karmakar, P. Differential Toxicity of Rod and Spherical Zinc Oxide Nanoparticles on Human Peripheral Blood Mononuclear Cells. J. Biomed. Nanotechnol. 2014, 10, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Casey, P.S.; McCall, M.J.; Fenech, M. Effects of Surface Chemistry on Cytotoxicity, Genotoxicity, and the Generation of Reactive Oxygen Species Induced by ZnO Nanoparticles. Langmuir 2010, 26, 15399–15408. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, L.Z.; Xing, B.S. Sorption of phenanthrene by nanosized alumina coated with sequentially extracted humic acids. Environ. Sci. Pollut. Res. 2010, 17, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative stress and genotoxicity in human liver cells (HepG2). J. Biomed. Nanotechnol. 2011, 7, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Kansara, K.; Senapati, V.A.; Shanker, R.; Dhawan, A.; Kumar, A. Cell cycle dependent cellular uptake of zinc oxide nanoparticles in human epidermal cells. Mutagenesis 2016, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.F.; Baumgartner, A.; Cemeli, E.; Fletcher, J.N.; Anderson, D. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp-2 cells. Nanomedicine 2010, 5, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; De Berardis, B.; Ammendolia, M.G.; Barone, F.; Condello, G.; Degan, P.; Meschini, S. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol. In Vitro 2016, 35, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Toduka, Y.; Toyooka, T.; Ibuki, Y. Flow cytometric evaluation of nanoparticles using side-scattered light and reactive oxygen species-mediated fluorescence-correlation with genotoxicity. Environ. Sci. Technol. 2012, 46, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Gaiser, B.K.; Hutchison, G.R.; Stone, V. An in vitro liver model—Assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part. Fibre Toxicol. 2012, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO(2) nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Kang, T.; Lu, F.; Zhang, Z.; Shen, H.; Liu, M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 2012, 7, 602. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Yong, L.Q.; Hande, M.P.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int. J. Nanomed. 2017, 12, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, S.K.; Anderson, D.; Tobin, D.J.; Dhawan, A. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J. Nanosci. Nanotechnol. 2011, 11, 3782–3788. [Google Scholar] [CrossRef] [PubMed]
- Kleinsasser, N. Toxicological evaluation of inhalation noxae: Test methods, assessment of toxic action and hazard potential, threshold limit values. Laryngo-Rhino-Otologie 2004, 83, S36–S53. [Google Scholar] [PubMed]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Froelich, K.; Hagen, R.; Kleinsasser, N. Functional responses of human adipose tissue-derived mesenchymal stem cells to metal oxide nanoparticles in vitro. J. Biomed. Nanotechnol. 2013, 9, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Zimmermann, F.Z.; Scherzed, A.; Friehs, G.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Repetitive exposure to zinc oxide nanoparticles induces dna damage in human nasal mucosa mini organ cultures. Environ. Mol. Mutagen. 2011, 52, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Sinha, S.; Jothiramajayam, M.; Jana, A.; Nag, A.; Mukherjee, A. Cyto-genotoxicity and oxidative stress induced by zinc oxide nanoparticle in human lymphocyte cells in vitro and Swiss albino male mice in vivo. Food Chem. Toxicol. 2016, 97, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Branica, G.; Mladinic, M.; Omanovic, D.; Zeljezic, D. An alternative approach to studying the effects of ZnO nanoparticles in cultured human lymphocytes: Combining electrochemistry and genotoxicity tests. Arhiv za higijenu rada i toksikologiju 2016, 67, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Kim, S.W.; An, Y.J. No evidence of the genotoxic potential of gold, silver, zinc oxide and titanium dioxide nanoparticles in the SOS chromotest. J. Appl. Toxicol. 2013, 33, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, S.Y.; Koedrith, P.; Lee, J.Y.; Kim, K.M.; Oh, J.M.; Yang, S.I.; Kim, M.K.; Lee, J.K.; Jeong, J.; et al. Lack of genotoxic potential of ZnO nanoparticles in in vitro and in vivo tests. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 761, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Annangi, B.; Hernandez, A.; Creus, A.; Marcos, R. A comprehensive study of the harmful effects of ZnO nanoparticles using Drosophila melanogaster as an in vivo model. J. Hazard. Mater. 2015, 296, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Size-Dependent Effect of Zinc Oxide on Toxicity and Inflammatory Potential of Human Monocytes. J. Toxicol. Environ. Health 2014, 77, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Bayat, N.; Rajapakse, K.; Marinsek-Logar, R.; Drobne, D.; Cristobal, S. The effects of engineered nanoparticles on the cellular structure and growth of Saccharomyces cerevisiae. Nanotoxicology 2014, 8, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Alarifi, S.; Kumar, S.; Ahamed, M.; Siddiqui, M.A. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat. Toxicol. 2012, 124, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Shen, C.C.; Cheng, Y.W.; Huang, S.H.; Wu, C.C.; Kao, C.C.; Liao, J.W.; Kang, J.J. Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology 2012, 6, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Baky, N.A.; Faddah, L.M.; Al-Rasheed, N.M.; Fatani, A.J. Induction of inflammation, DNA damage and apoptosis in rat heart after oral exposure to zinc oxide nanoparticles and the cardioprotective role of alpha-lipoic acid and vitamin E. Drug Res. 2013, 63, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136–137, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Ma-Hock, L.; Van Ravenzwaay, B.; Schulz, M.; Wiench, K.; Champ, S.; Schulte, S.; Wohlleben, W.; Oesch, F. Gene toxicity studies on titanium dioxide and zinc oxide nanomaterials used for UV-protection in cosmetic formulations. Nanotoxicology 2010, 4, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Li, M.; Cui, Y.B.; Li, D.S.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Stoeger, T.; van den Brule, S.; Saber, A.T.; Beyerle, A.; Vietti, G.; Mortensen, A.; Szarek, J.; Budtz, H.C.; Kermanizadeh, A.; et al. Acute and subacute pulmonary toxicity and mortality in mice after intratracheal instillation of ZnO nanoparticles in three laboratories. Food Chem. Toxicol. 2015, 85, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.S.; Prasad, D.N.; Singh, S.B.; Kohli, E. Chronic exposure of zinc oxide nanoparticles causes deviant phenotype in Drosophila melanogaster. J. Hazard. Mater. 2017, 327, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, P.; Pandey, A.K.; Dhawan, A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat. Res. 2012, 745, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Manzo, S.; Schiavo, S.; Oliviero, M.; Toscano, A.; Ciaravolo, M.; Cirino, P. Immune and reproductive system impairment in adult sea urchin exposed to nanosized ZnO via food. Sci. Total Environ. 2017, 599, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Boran, H.; Ulutas, G. Genotoxic effects and gene expression changes in larval zebrafish after exposure to ZnCl2 and ZnO nanoparticles. Dis. Aquat. Org. 2016, 117, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Annangi, B.; Rubio, L.; Alaraby, M.; Bach, J.; Marcos, R.; Hernandez, A. Acute and long-term in vitro effects of zinc oxide nanoparticles. Arch. Toxicol. 2016, 90, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.R.; Inostroza-Blancheteau, C.; Rubio, L.; Marcos, R. Genotoxic and oxidative stress potential of nanosized and bulk zinc oxide particles in Drosophila melanogaster. Toxicol. Ind. Health 2016, 32, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.; Oliviero, M.; Miglietta, M.; Rametta, G.; Manzo, S. Genotoxic and cytotoxic effects of ZnO nanoparticles for Dunaliella tertiolecta and comparison with SiO2 and TiO2 effects at population growth inhibition levels. Sci. Total Environ. 2016, 550, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.; Felder, E.; Tahir, M.N.; Kaltbeitzel, A.; Heinrich, U.R.; Brochhausen, C.; Mailander, V.; Tremel, W.; Brieger, J. Genotoxic effects of zinc oxide nanoparticles. Nanoscale 2015, 7, 8931–8938. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, S.; Ali, D.; Alkahtani, S.; Verma, A.; Ahamed, M.; Ahmed, M.; Alhadlaq, H.A. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int. J. Nanomed. 2013, 8, 983–993. [Google Scholar]
- Uzar, N.K.; Abudayyak, M.; Akcay, N.; Algun, G.; Ozhan, G. Zinc oxide nanoparticles induced cyto- and genotoxicity in kidney epithelial cells. Toxicol. Mech. Methods 2015, 25, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.D.; de Rezende, A.A.A.; Santos, D.V.; de Oliveria, P.F.; Nicolella, H.D.; Tavares, D.C.; Silva, A.C.A.; Dantas, N.O.; Spano, M.A. Assessment of the genotoxic potential of two zinc oxide sources (amorphous and nanoparticles) using the in vitro micronucleus test and the in vivo wing somatic mutation and recombination test. Food Chem. Toxicol. 2015, 84, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Mechanistic Investigation of the Biological Effects of SiO2, TiO2, and ZnO Nanoparticles on Intestinal Cells. Small 2015, 11, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Goswami, M.; Yadav, K.; Chaudhary, D. Oxidative Stress and Nano-Toxicity Induced by TiO2 and ZnO on WAG Cell Line. PLoS ONE 2015, 10, e0127493. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Casey, P.S.; McCall, M.J.; Fenech, M. Size-dependent cytotoxicity and genotoxicity of ZnO particles to human lymphoblastoid (WIL2-NS) cells. Environ. Mol. Mutagen. 2015, 56, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Senapati, V.A.; Kumar, A.; Gupta, G.S.; Pandey, A.K.; Dhawan, A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: A mechanistic approach. Food Chem. Toxicol. 2015, 85, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Kaya, N.; Kaya, B. Genotoxic effects of zinc oxide and titanium dioxide nanoparticles on root meristem cells of Allium cepa by comet assay. Turk. J. Biol. 2014, 38, 31–39. [Google Scholar] [CrossRef]
- Roszak, J.; Catalan, J.; Jarventaus, H.; Lindberg, H.K.; Suhonen, S.; Vippola, M.; Stepnik, M.; Norppa, H. Effect of particle size and dispersion status on cytotoxicity and genotoxicity of zinc oxide in human bronchial epithelial cells. Mutat. Res. Genet. Toxicol. Environ. 2016, 805, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, R.C.; Osman, I.F.; Amani, A.; De Matas, M.; Anderson, D. The effect of zinc oxide and titanium dioxide nanoparticles in the Comet assay with UVA photoactivation of human sperm and lymphocytes. Nanotoxicology 2009, 3, 33–39. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, M.; Mukherjee, A.; Chattopadhyay, D.; Acharya, K. Biosynthesis and safety evaluation of ZnO nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.A.; Gade, W.N.; Deobagkar, D.D. Epigenetic modulation upon exposure of lung fibroblasts to TiO2 and ZnO nanoparticles: Alterations in DNA methylation. Int. J. Nanomed. 2016, 11, 4509–4519. [Google Scholar]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Vranic, S.; Boland, S.; Moreau, K.; Baeza-Squiban, A.; Gaiser, B.K.; Andrzejczuk, L.A.; Stone, V. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: Cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity. BMC Nephrol. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Zijno, A.; De Angelis, I.; De Berardis, B.; Andreoli, C.; Russo, M.T.; Pietraforte, D.; Scorza, G.; Degan, P.; Ponti, J.; Rossi, F.; et al. Different mechanisms are involved in oxidative DNA damage and genotoxicity induction by ZnO and TiO2 nanoparticles in human colon carcinoma cells. Toxicol. In Vitro 2015, 29, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Shalini, D.; Senthilkumar, S.; Rajaguru, P. Effect of size and shape on toxicity of zinc oxide (ZnO) nanomaterials in human peripheral blood lymphocytes. Toxicol. Mech. Methods 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, A.; Kwiatkowski, D.; Czarny, P.; Milczarek, J.; Toma, M.; Korycinska, A.; Szemraj, J.; Sliwinski, T. Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles. Toxicol. Mech. Methods 2015, 25, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Dommershausen, N.; Schulz, M.; Landsiedel, R.; Reichardt, P.; Krause, B.C.; Tentschert, J.; Luch, A. Genotoxicity testing of different surface-functionalized SiO2, ZrO2 and silver nanomaterials in 3D human bronchial models. Arch. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.; Gandhi, D.; Tarale, P.; Bafana, A.; Pandey, R.A.; Sivanesan, S. Oxidative Stress and Genotoxicity of Zinc Oxide Nanoparticles to Pseudomonas Species, Human Promyelocytic Leukemic (HL-60), and Blood Cells. Biol. Trace Elem. Res. 2017, 178, 218–227. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of Nanomaterial(s) | In Vivo | Exposure | Methods | Results | Reference |
|---|---|---|---|---|---|
| ZnO NPs: average size 10–20 nm | Earthworm Eisenia fetida (Savigny, 1826) | 0.1, 0.5, 1.0, 5.0 g/kg for 7 days | Comet assay | DNA damages were observed at dosages greater than 1.0 g/kg | [66] |
| ZnO NPs: average size 12 ± 3 nm | Cells of bronchoalveolar lavage fluid, day 1 and 3 after ZnO exposure, in female wild-type C57BL/6JBonTac (C57) mice | Intratracheal instillation of 2, 6, 18 μg ZnO NPs | Comet assay | DNA damage was dose dependent. However, three days post-exposure genotoxicity decreased | [67] |
| ZnO NPs: average size 22 nm | Freshwater snail Lymnaea luteola (L. luteola) | 10, 21.33, and 32 µg/mL for 96 h | Comet assay | Comet assay revealed DNA damage after treatment with ZnO NPs | [61] |
| ZnO NPs: average size 28 ± 5 nm Zeta potential −22 mV | Drosophila melanogaster | Food containing 0.1 mM, 1 mM, and 10 mM of ZnO NPs throughout the entire life cycle from egg to egg stage | TUNEL (TdT-mediated dUTP-biotin nick end labeling) assay ROS detection assay | ZnO NPs exposure induced a increase of DNA fragmentation and phenotypic changes, which were transmitted to the offspring | [68] |
| ZnO NPs: average size 30 nm | Cells of liver and kidney of mice after oral exposure | 50 and 300 mg/kg of ZnO for 14 days | Comet assay | The Comet assay revealed a significant increase in the Fpg-specific DNA lesions in liver and kidney cells | [69] |
| ZnO-NPs: average size: ~70 nm Zeta potential +5.8 mV | MRC5 human lung fibroblasts, Drosophila melanogaster | 0, 1, 10, 25, 50, 75, and 100 μg/mL for 24, 48 and 72 h | Comet assay ROS detection assay | Significant genotoxicity was induced by ZnO NPs | [49] |
| ZnO NPs: average size <100 nm | Human peripheral blood mononuclear cells (PBMCs) and Swiss albino male mice | Cell treatment: 0, 25, 50, and 100 μg/mL for 3 h Animal treatment: 25, 50, and 100 mg/kg body weight 18 h before sacrifice | Comet assay Chromosome aberration assay Micronucleus assay | Apoptosis mediated by ROS generation, reduced mitochondrial membrane potential (MMP) in bone marrow cells, a G0/G1 cell cycle arrest, and chromosomal aberration with micronuclei formation | [54] |
| ZnO NPs: average size 100 nm Surface area: 15–25 m2/g 14 nm Surface area: 30 ± 5 m2/g | Sea urchin | 1 mg/kg food for three weeks | Comet assay | ZnO NPs 100 nm provoked in adult echinoids damages to immune cells and transmissible effects to offspring, ZnO NPs 14 nm provoked nucleus damages in immune cells and malformed larvae | [70] |
| ZnO NPs: average size 72 ± 46 nm Zeta potential −13.3 ± 2.3 mV ZnO microparticles particles (MPs) | Madin–Darby canine kidney (MDCK) cells | 1, 5, 10, 15, 30, and 60 μg/mL ZnO for 24 h | Comet assay Cytokinesis-block micronucleus assay | ZnO NPs significantly elevated DNA and chromosomal damage, whereas equimolar concentrations of ZnO MPs did not | [30] |
| ZnO NPs: average size <100 nm | Broodstock zebrafish larvae, Danio rerio | 0.2, 1, 2, 4, 6 mg/L for 96 h | Comet assay | Comet assay revealed significant DNA damage after ZnO NPs exposure | [71] |
| ZnO NPs: average size 20 nm (+) charge: 35 ± 5, 20 nm (−) charge: 28 ± 8, 70 nm (+) charge: 70 ± 19, 70 nm (−) charge: 72 ± 11 nm; Hydrodynamic size of ZnO nanoparticles: 20 nm (+) charge: 200 to 400 nm, 20 nm (−) charge: 180–300, 70 nm (+) charge: 300–900 nm, 70 nm (−) charge: 200–500 nm; zeta potential: 20 nm (+) charge: +25.9 mV, 20 nm (−) charge: −38.5 mV, 70 nm (+) charge: +25.9 mV, 70 nm (−) charge: −40.6 mV | SD rat: liver and stomach cells | 500, 1000, and 2000 mg/kg body weights, three times by gavage at 0, 24, and 45 h | Bacterial mutagenicity assay in vitro chromosomal aberration test in vivo comet assay in vivo micronucleus test | Surface modified ZnO NPs did not induce genotoxicity in vitro and in vivo | [57] |
| ZnO NPs: average size 104.17 ± 66.77 nm | Mouse embryonic fibroblast (MEF Ogg1+/+) and mouse embryonic fibroblast knockout (MEF Ogg1−/−) cell lines | Sub-toxic dose (1 μg/mL) for 12 weeks, Short-term exposure (0.3125 to 40 μg/mL) for 48 h | Comet assay | Short-term ZnO NPs exposure induce ROS, genotoxicity, and oxidative DNA damage. No effects after long-term exposure | [72] |
| ZnO NPs: average size 106.55 ± 64.79 nm Zeta potential: −21.00 ± 0.80 mV ZnO NPs bulk: average size 4.2 μm | Haemolymph cells from Drosophila melanogaster | 6, 12, 24, mM for 24 h | Wing-spot test Comet assay | No increases in the frequency of mutant spots was detected Significant increase in DNA damage was observed | [73] |
| ZnO NPs: average size 200–250 nm Zeta potential −0.56 mV | Mice and cells isolated from mice | 0–500 µg/mL for 24 h Mice were treated with 200 and 500 mg/kg bodyweight of ZnO NPs | Comet assay Micronucleus Assay | The comet assay revealed severe DNA damage in peripheral blood and bone marrow cells. Moreover, DNA repair mechanism were inhibited | [29] |
| ZnO NPs: average size 291.66 ± 6.59 nm Zeta potential −11.40 ± 0.26 mV | Drosophila melanogaster | 0.02, 0.1, 0.2, 1 and 2 mg/g of food media | The wing-spot assay Comet assay | ZnO NPs were not genotoxic | [58] |
| ZnO NPs: average size 470 ± 45 nm Zeta potential: −10.35 ± 0.83 mV ZnO NPs: average size 1040 ± 70 nm Zeta potential: −10.51 ± 1.43 mV | Dunaliella tertiolecta | 0.1, 2, 5, 10, 25, 50 mg/L for 24 and 72 h | Comet assay | Genotoxic action was evident only starting from 5 mg/L | [74] |
| ZnO NPs: average size 15–18 nm | Cell line (A549) | 0.1, 10, 100 μg/mL | γH2AX immunofluorescence assay | Foci analyses showed the induction of DNA double strand breaks by ZnO NPs. Reduction of DNA damage was achieved by the treatment with the ROS scavenger N-acetyl-l-cysteine | [75] |
| ZnO NPs: average size 15–25 nm | Human neuroblastoma SHSY5Y cell line | 20, 30, 40 μg/mL for 3 h and 6 h | Micronuclei evaluation by flow cytometry γH2AX assay Comet assay Oxidative DNA damage | Micronuclei were induced by ZnO NPs, H2AX phosphorylation and DNA damage were observed in all cases | [24] |
| ZnO NPs: average size 17 nm Zeta potential: −14.0 mV | Human malignant melanoma skin (A375) cell line | 5, 10, 20 μg/mL for 24 and 48 h | Comet assay | ZnO NPs induced DNA damage. A gradual nonlinear increase in cell DNA damage was observed as concentration and duration of ZnO nanoparticle exposure increased | [76] |
| ZnO NPs: average size 10–50 nm | Rat kidney epithelial cell line (NRK-52E) | 25.0–100.0 mg/mL for cytotoxicity assays and 12.5–50.0 mg/mL for genotoxicity assay | Comet assay | ZnO NPs caused statistically significant DNA damage | [77] |
| ZnO NPs: average size 20 nm | Chinese hamster lung fibroblasts (V79 cells) | 30.0, 60.0, 120.0 μM for 3 h | Cytokinesis-block micronucleus Assay somatic mutation and Recombination test micronucleus assay | ZnO NPs increase the frequency of micronuclei, results were not dose related | [78] |
| ZnO NPs: average size 19.6 ± 5.8 nm | Primary mouse embryo fibroblasts (PMEF) | 5 and 10 μg/mL for 24 h | Comet assay | ZnO NPs caused statistically significant DNA damage | [79] |
| ZnO NPs: average size 25.8 ± 8.9 nm Zeta potential: +17.4 mV | Human intestinal carcinoma epithelial cell lines, SW480 and DLD-1 and the normal human intestinal mucosa epithelial cell line, NCM460 | Cell exposure concentrations 62.5, 250, and 1000 μM for 12 or 24 h | Oxidative stress measurement Cell cycle analysis | The elevated ROS levels induce significant damage to the DNA of the cells, resulting in cell-cycle arrest and subsequently cell death | [80] |
| ZnO NPs: average size 25.12 ± 9.2 nm | Cell line from gill tissue of Wallago attu (WAG) | 0, 12.5, 25, 50 mg/L for 24 h | Comet assay Micronucleus assay | ZnO NPs induced DNA damage in a dose dependent manner | [81] |
| ZnO-S ZnO NPs-S: average size 26 ± 9 nm Zeta potential: +19.2 ± 0.3 mV ZnO NPs-M average size 78 ± 25 nm Zeta potential: +20.0 ± 0.6 mV ZnO NPs-L: average size 147 ± 53 nm Zeta potential: +21.1 ± 0.4 mV | Human lymphoblastoid (WIL2-NS) cells | 10 mg/L for 24 h | Genotoxicity-cytokinesis-block micronucleus (CBMN) Cytome Assay | Genotoxicity was significantly enhanced in the presence of the medium-sized and large-sized particles | [82] |
| ZnO NPs: average size 30 nm Zeta potential: −13.4 mV | Human monocytic cell line (THP-1) | 0.5, 1, 5, 10, 15, 20 μg/mL for 3 h | Comet assay micronucleus assays | ZnO NPs induced an enhanced DNA damage and micronucleated cells | [83] |
| ZnO NPs: average size 30 nm Zeta potential: −26 mV | Human epidermal cell line (A431) | 0.008–20 μg/mL for 3, 6, 24, 48 h | Comet assay | ZnO NPs induced an enhanced DNA damage | [39] |
| ZnO NPs: average size 29 ± 10 nm | WIL2-NS human lymphoblastoid cells | 10 μg/mL for 24 h | Comet assay | PMAA-coated ZnO had significant genotoxicity compared to uncoated ZnO | [37] |
| ZnO NPs: average size <35 nm | Human lymphocyte | 1.0, 2.5, 5, and 7.5 μg/mL over 2 weeks | Comet assay Comet-FISH | ZnO NPs induced DNA damage | [55] |
| ZnO NPs: average size ≤35 nm Zeta potential: +46.2 mV ZnO NPs: average size 50–80 nm Zeta potential: −23 mV | Human embryonic kidney (HEK293) and mouse embryonic fibroblast (NIH/3T3) cells | 10, 100, 1000 μg/mL for 1 h | Comet assay Micronucleus assay | ZnO NPs induced a significant of DNA damage with and without enzymes. The frequency of micronuclei was enhanced as well | [35] |
| ZnO NPs: average size ≤35 nm Zeta potential: +46.2 mV ZnO NPs: average size 50–80 nm Zeta potential: −23 mV | Allium cepa root meristem cells | 10, 100, 1000 μg/mL for 1 h | Comet assay | ZnO NPs were genotoxic in a dose dependent manner | [84] |
| ZnO NPs: average size (given by producer) nanosized (30–35 nm) fine (150–300 nm) | human bronchial epithelial BEAS-2B cells | 0.5–3.0 μg/cm2 for 48 h Comet assay 3 h to 6 h | Comet assay | ZnO NPs exposure induced DNA damage, fine ZnO did not induced DNA damages | [85] |
| ZnO NPs: average size 40–70 nm | human peripheral lymphocytes, human sperm cells | 11.5, 46.2, 69.4, 93.2 μg/mL for 30 min, simultaneous or pre-irradiation with UV light | Comet assay | ZnO NPs are capable of inducing genotoxic effects on human sperm and lymphocytes. The effect is enhanced by UV | [86] |
| ZnO NPs: average size 50–70 nm | human colon carcinoma cells (LoVo) | Treatment concentration and duration was not unique e.g., cell death assay: 5 μg/cm2 ZnO NPs for 2, 4, and 6 h Zn2+ ions release: 5 and 10 μg/cm2 for 30 min, 1 h, 2 h, 4 h, 6 h, 24 h | DNA damage assessment by 8-oxodG steady-state levels and γ-H2AX histone phosphorylation | ZnO NPs entered LoVo cells. The simultaneous presence of ZnO NPs and Zn(2+) ions in the LoVo cells induced severe DNA damage | [43] |
| ZnO NPs: average size 75 ± 5 nm | Human lymphocyte cells | 0, 125, 500, 1000 μg/mL for 3 h | Comet assay | 1000 μg/mL ZnO NPs induced significant genotoxic effects | [87] |
| ZnO NPs: average size 86 ± 41 nm; mean lateral diameter: 42 ± 21 nm | Primary human nasal mucosa cells | 0.01, 0.1, 5, 10, 50 μg/mL for 24 h | Comet assay | ZnO NPs induced DNA damage in a dose dependent manner | [23] |
| ZnO NPs: average size <100 nm Zeta potential −33.8 ± 10 | Saccharomyces cerevisiae cells | GreenScreen assay Comet assay | GreenScreen assay: No genotoxic effects could be measured Comet assay: ZnO NPs were genotoxic | [60] | |
| ZnO NPs: average size <100 nm (given by producer) | lung fibroblast (MRC5) cell line | 0, 0.125, 0.25, 0.5, 1, 2, 4, and 8 µg/mL for 24, 48, and 72 h Colony-forming assay: cells were treated for 10 days. | Immunochemical assay DNA methyltransferase activity Quantification of the 5-mC content in genomic DNA | dose-related decrease in global DNA methylation and DNA methyltransferase activity direct correlation between the concentration of NPs, global methylation levels, and expression levels of Dnmt1, 3A, and 3B genes upon exposure | [88] |
| ZnO NPs: average 20–200 nm, Zeta potential: 26.9 mV | A549 cells | 1, 20, 40 μg/cm (=2, 40, 80 μg/mL) for 4 h; fpg-sensitive sites: 20 and 40 μg/cm after 4 h | Comet assay | ZnO NPs induced DNA damage | [89] |
| ZnO NPs: average size NM-110: 70–100 nm; NM-111: 58–93 nm Zeta potential: NM-110: −11.5 mV; NM-111: −11.4 mV | HK2-cells | Ten concentrations between 0.16 and 80 μg/cm for 4 h | Comet assay | Increase of tail DNA following nanomaterials exposure | [90] |
| ZnO NPs: average size 45–170 nm Zeta potential: −15.6 ± 2.4 mV | Human colon carcinoma (Caco-2) cells | CBMN assay: 6.4, 12.8, 22.4, 64.0 μg/mL for 6 or 24 h Comet assay: 6.4, 16.0 μg mL−1 for 24 h | CBMN assay Comet assay | ZnO NPs induced DNA damage | [91] |
| ZnO NPs: average size 120 ± 2.6 nm | Root cells of Allium cepa | 25, 50, 75, 100 μg/mL for 4 h | Analysis of mitotic index, micronuclei index and chromosomal aberration index | Dose dependent depression of mitotic index, an increase of pyknotic cells, an increase of micronuclei index and chromosomal aberration index | [48] |
| ZnO NPs: average size NM-110: 20–250/50–350 nm; NM-111: 20–200/10–450 nm | Human hepatoblastoma C3A cells, in vitro | NM concentrations between 0.16 μg cm−2 and 80 μg/cm for 4 h | Comet assay | significant increase in percentage tail DNA | [45] |
| ZnO NPs: average size 64–510 nm Zeta potential: −25.30 mV | human peripheral blood lymphocytes | 50–1000 µg/mL for 24 h (cytotoxicity) 25, 50 and 100 µg/mL for 4 h (genotoxicity) | Comet assay | The smaller NPs are more genotoxic, treatment with vitamin C or quercetin significantly reduces the genotoxicity | [92] |
| human peripheral blood lymphocytes | 0.01–10 mM for 4, 8, 24 h | Comet assay | ZnO NPs induced DNA damage in a dose dependent manner | [93] | |
| ZnO NPs: average size 250–970 nm Zeta potential 20 mV | human bronchial cells (3D model) | 30 μL of a 1.06 mg/mL suspension with a dosage of 50 µg/cm2 for 24 to 72 h | Comet assay | ZnO NPs were genotoxic in a dose-dependent manner | [94] |
| ZnO NPs (50 wt %) were purchased From Sigma-Aldrich (St. Louis, MO, USA). No data about particle size | human promyelocytic leukemia (HL-60) cells, and peripheral blood mononuclear cells (PBMC) | 0, 0.05, 5, 10, 15, and 20 mg/L for 24 h | Comet assay | ZnO NPs were genotoxic in a dose-dependent manner | [95] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherzad, A.; Meyer, T.; Kleinsasser, N.; Hackenberg, S. Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity. Materials 2017, 10, 1427. https://doi.org/10.3390/ma10121427
Scherzad A, Meyer T, Kleinsasser N, Hackenberg S. Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity. Materials. 2017; 10(12):1427. https://doi.org/10.3390/ma10121427
Chicago/Turabian StyleScherzad, Agmal, Till Meyer, Norbert Kleinsasser, and Stephan Hackenberg. 2017. "Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity" Materials 10, no. 12: 1427. https://doi.org/10.3390/ma10121427
APA StyleScherzad, A., Meyer, T., Kleinsasser, N., & Hackenberg, S. (2017). Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity. Materials, 10(12), 1427. https://doi.org/10.3390/ma10121427





