Preparation and Investigation of Foaming Amphiphilic Fluorinated Nanoparticles for Enhanced Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
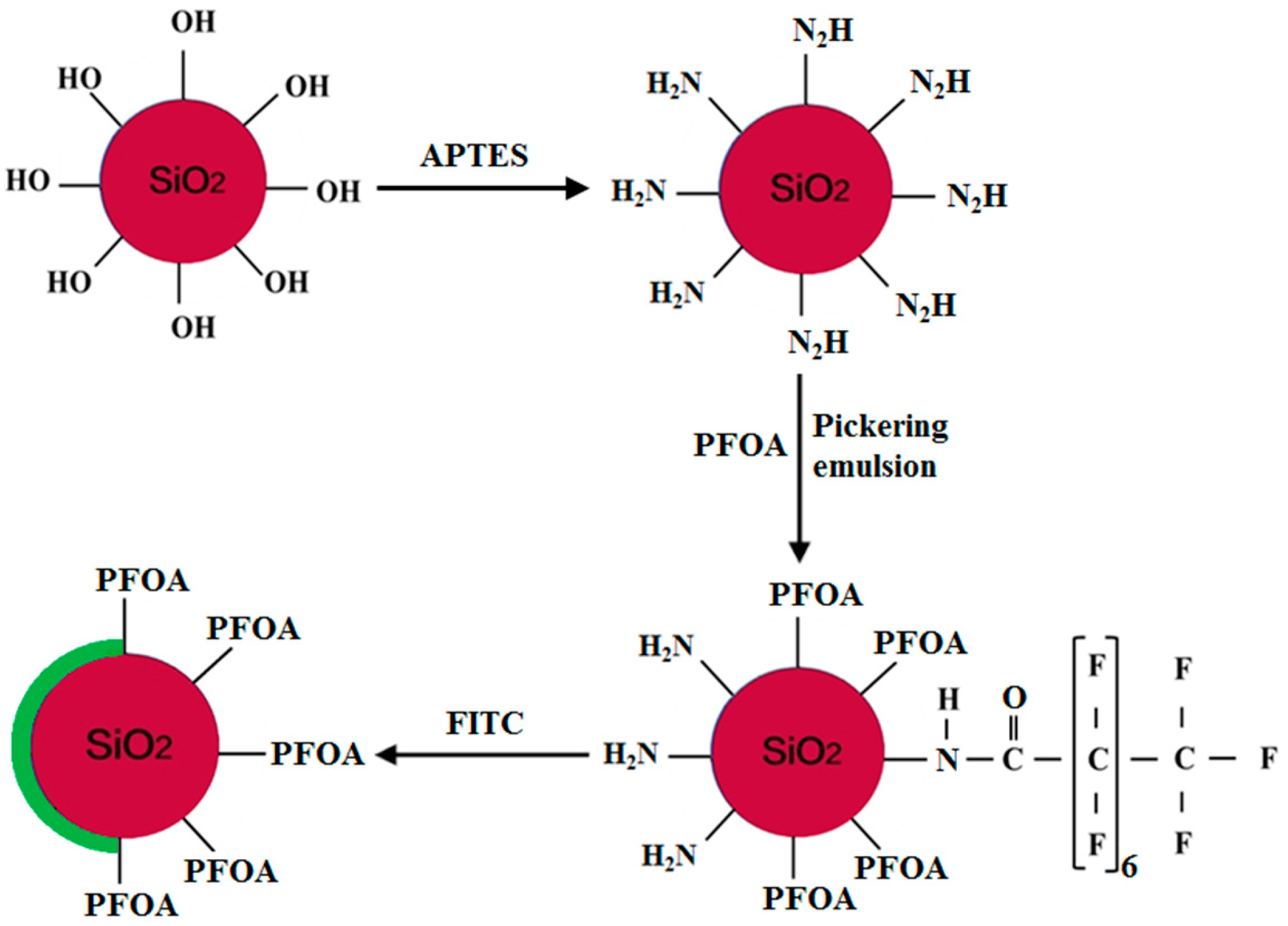
2.2. Preparation of the Amphiphilic Fluorinated Nanoparticles
2.3. Preparation of the Fluorescent Amphiphilic Fluorinated Nanoparticles
2.4. Characterization of the Modified Nanoparticles
2.5. Characterization of the Interfacial Properties
2.6. Characterization of the Foaming Properties
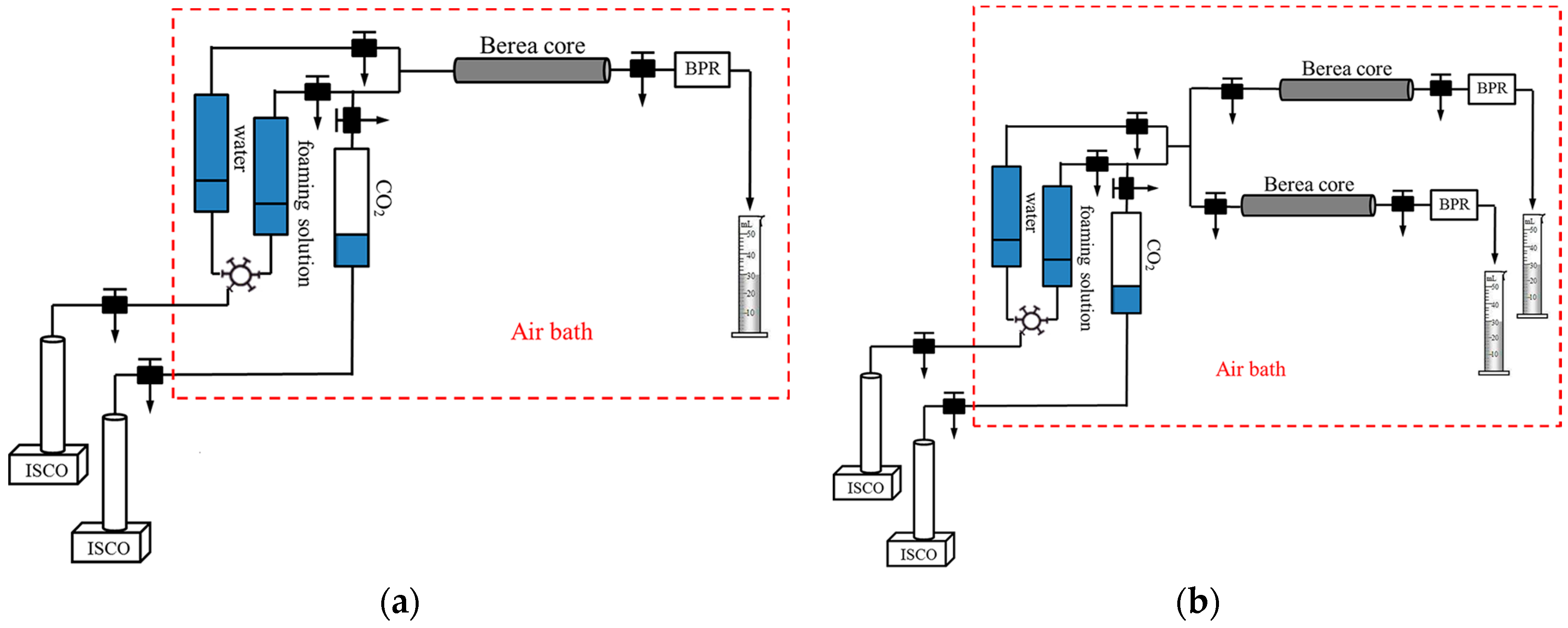
2.7. Plugging Capacity
2.8. Enhanced Oil Recovery
3. Results
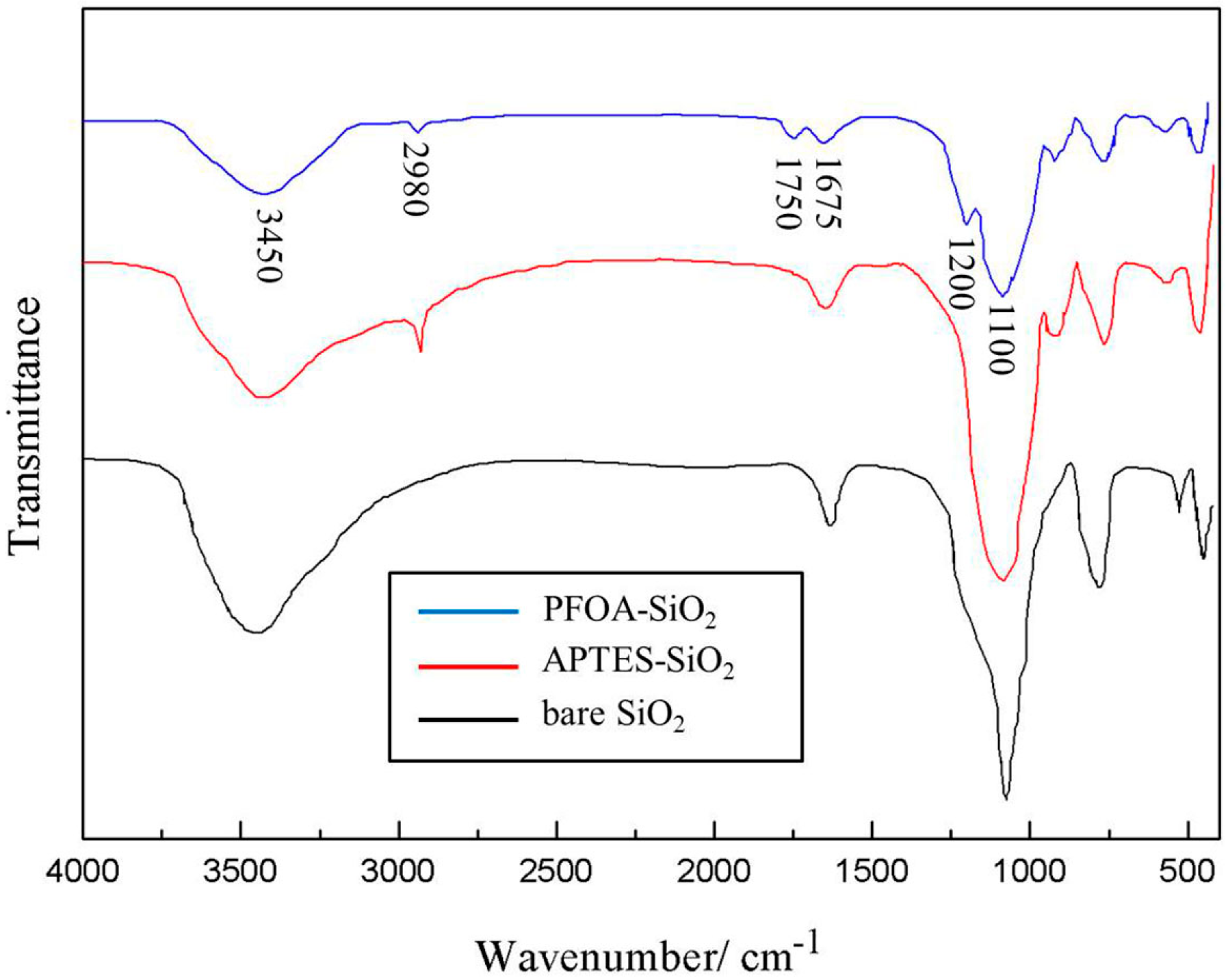
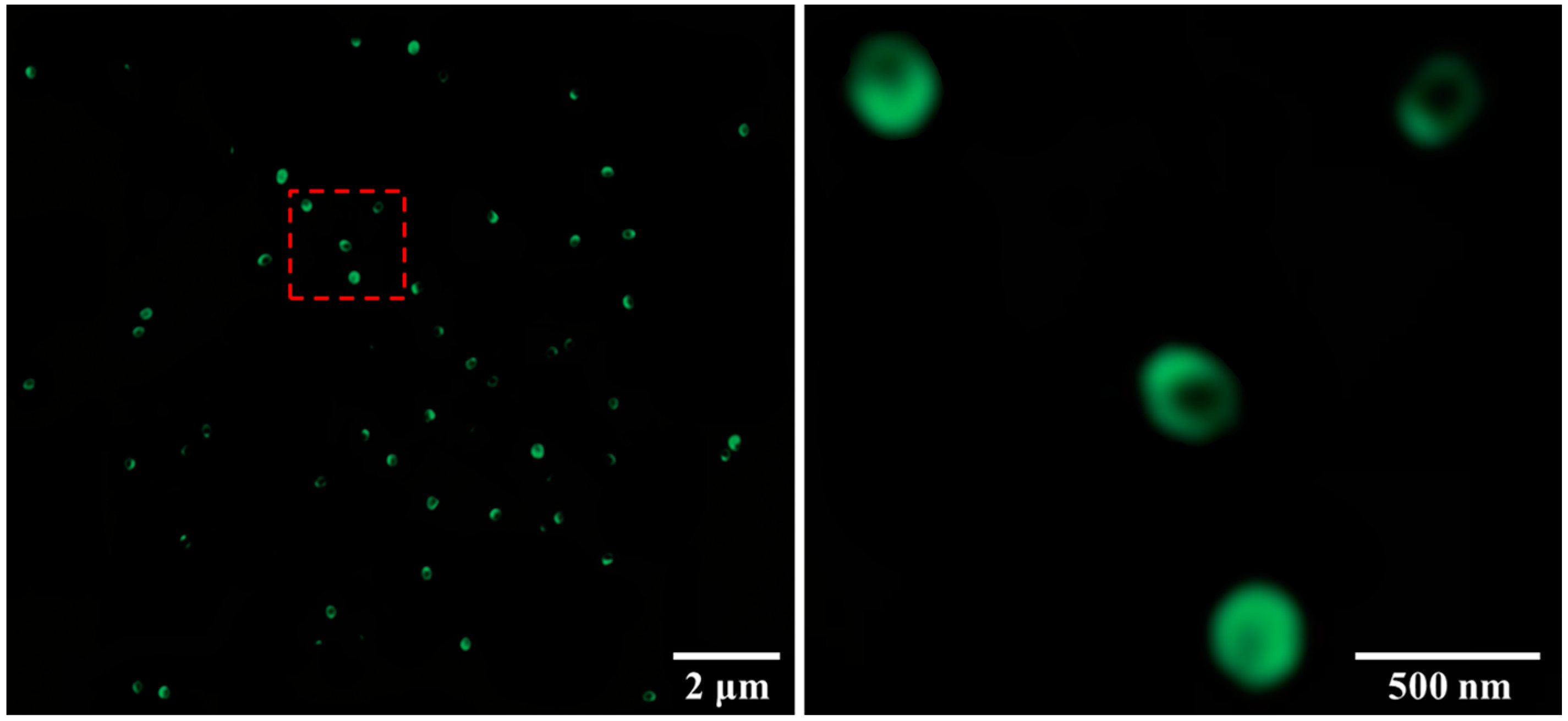
3.1. Characterization of the Amphiphilic Fluorinated Nanoparticles
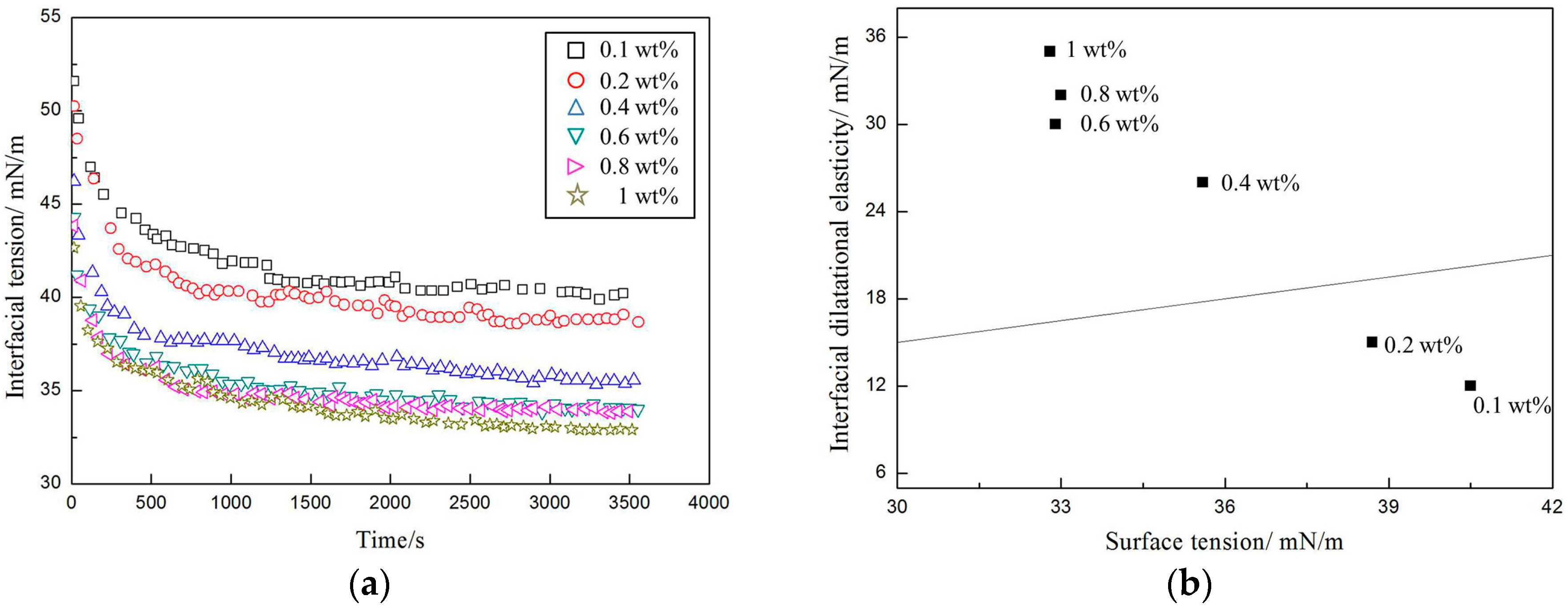
3.2. Interfacial Properties of the Amphiphilic Fluorinated Nanoparticles
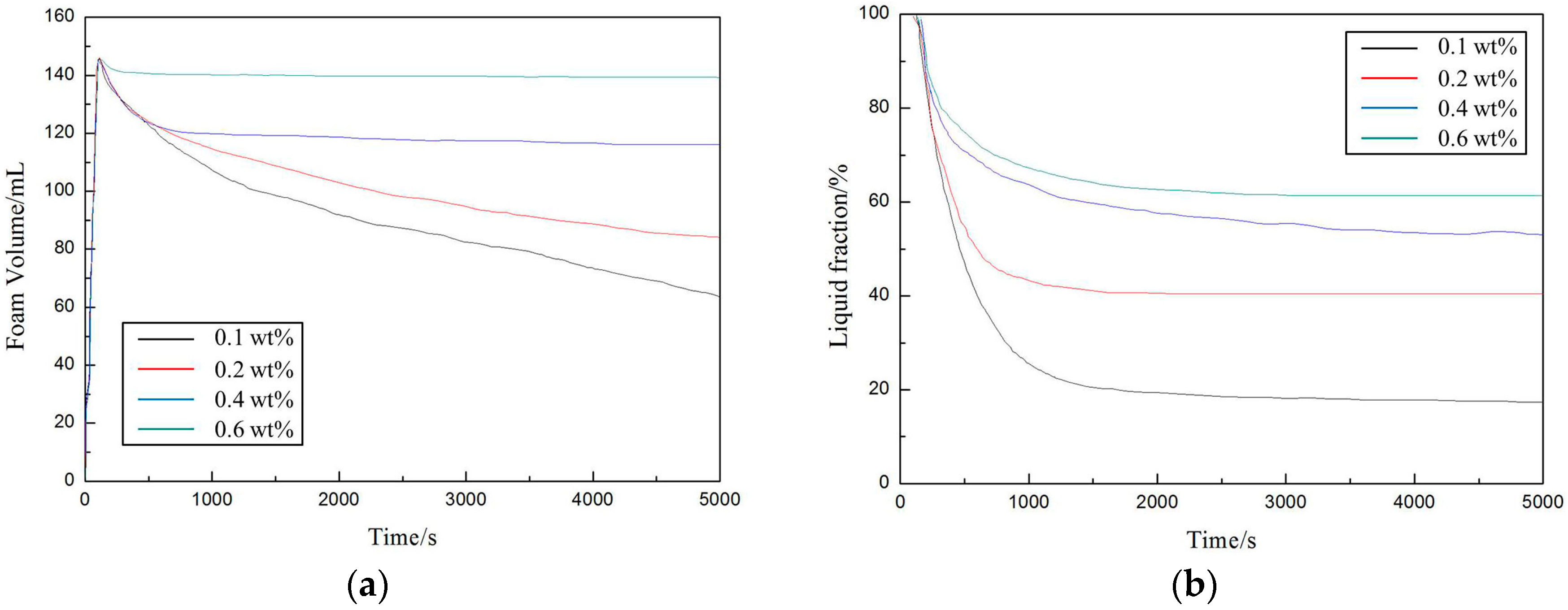
3.3. Foaming Properties of the Amphiphilic Fluorinated Nanoparticles
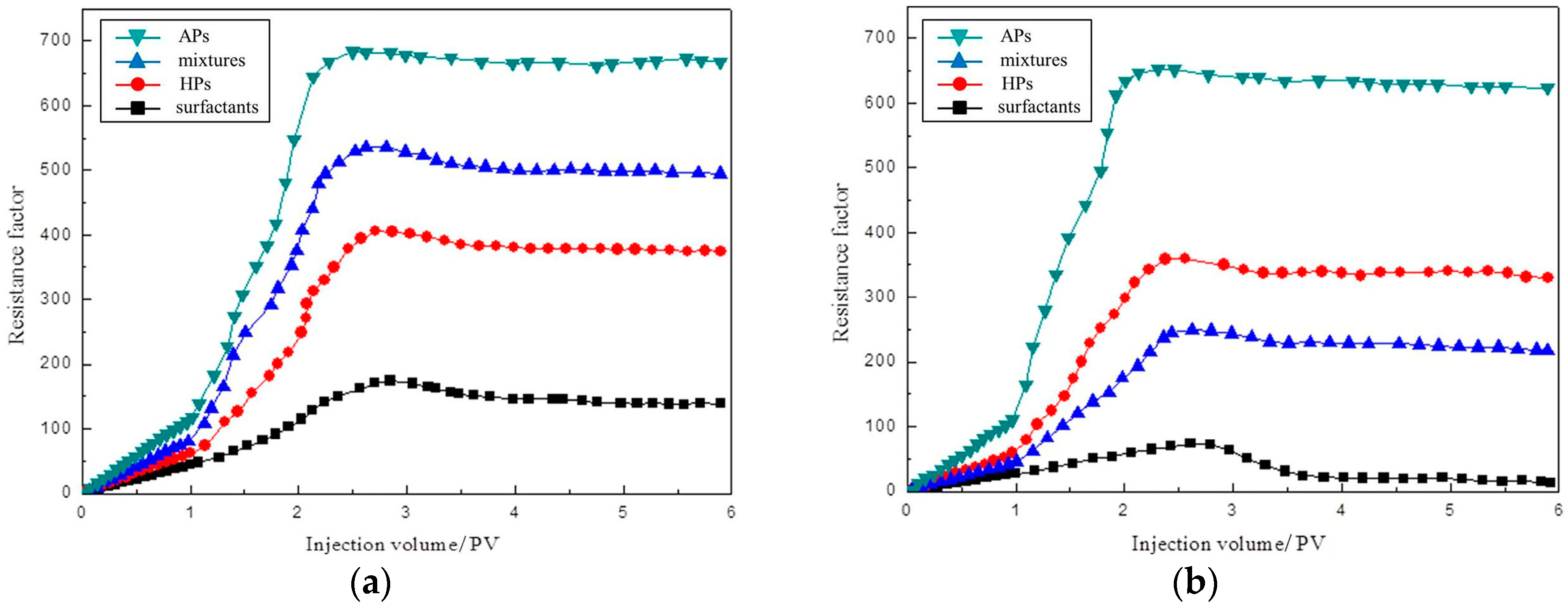
3.4. Plugging Capacity
3.5. Enhanced Oil Recovery
4. Discussion
4.1. Preparation of the Amphiphilic Fluorinated Nanoparticles
4.2. The Interfacial Properties and Foaming Properties of the Amphiphilic Fluorinated Nanoparticles
4.3. The Plugging Capacity and Enhanced Oil Recovery
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Soponronnarit, S. Drying characteristics and quality of banana foam mat. J. Food Eng. 2008, 86, 573–583. [Google Scholar] [CrossRef]
- Moody, C.A.; Field, J.A. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000, 34, 3864–3870. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.E.; Rajaei, K.; Junin, R. Assessing the effects of nanoparticle type and concentration on the stability of CO2 foams and the performance in enhanced oil recovery. Colloids Surf. A 2016, 511, 222–231. [Google Scholar] [CrossRef]
- Gu, S.; Li, W.; Luo, W.; Chang, Z. Novel application of silicate sol to improve the stability of sodium dodecylsulfate foams used for enhanced oil recovery. Chem. Technol. Fuels Oil 2016, 52, 386–395. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z.; Miao, Q.; Deng, Z.; Zhu, Y. Foams stabilized by in situ-modified nanoparticles and anionic surfactants for enhanced oil recovery. Energy Fuels 2017, 31, 4721–4730. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Ultrastable particle–stabilized foams. Angew. Chem. Int. Ed. 2006, 45, 3526–3530. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem. Int. Ed. 2005, 117, 3788–3791. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Stabilization of foams with inorganic colloidal particles. Langmuir 2006, 22, 10983–10988. [Google Scholar] [CrossRef] [PubMed]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Britan, A.; Liverts, M.; Ben-Dor, G.; Koehler, S.A.; Bennani, N. The effect of fine particles on the drainage and coarsening of foam. Colloids Surf. A 2009, 344, 15–23. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R. Phase inversion of particle-stabilized materials from foams to dry water. Nat. Mater. 2006, 5, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S.; Ettelaie, R. Foam stability: Proteins and nanoparticles. Curr. Opin. Colloid Interface Sci. 2004, 9, 314–320. [Google Scholar] [CrossRef]
- Stocco, A.; Drenckhan, W.; Rio, E.; Langevin, D.; Binks, B.P. Particle-stabilised foams: An interfacial study. Soft Matter 2009, 5, 2215–2222. [Google Scholar] [CrossRef]
- Stocco, A.; Rio, E.; Binks, B.P.; Langevin, D. Aqueous foams stabilized solely by particles. Soft Matter 2011, 7, 1260–1267. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Pickering emulsions stabilized by monodisperse latex particles: Effects of particle size. Langmuir 2001, 17, 4540–4547. [Google Scholar] [CrossRef]
- Melle, S.; Lask, M.; Fuller, G.G. Pickering emulsions with controllable stability. Langmuir 2005, 21, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Müller, A.H. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Kirkland, M.; Rodrigues, J.A. Origin of stabilisation of aqueous foams in nanoparticle-surfactant mixtures. Soft Matter 2008, 4, 2373–2382. [Google Scholar] [CrossRef]
- Carn, F.; Colin, A.; Pitois, O.; Vignes-Adler, M.; Backov, R. Foam drainage in the presence of nanoparticle–surfactant mixtures. Langmuir 2008, 25, 7847–7856. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.G.; Yang, L.L.; Cui, Y.Z.; Binks, B.P. Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant. Langmuir 2008, 26, 4717–4724. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Wang, B.; Wang, Y.; He, Y.F.; Song, P.; Wang, R.M. An efficient strategy for preparation of polymeric Janus particles with controllable morphologies and emulsifiabilities. Colloids Surf. A 2016, 503, 94–100. [Google Scholar] [CrossRef]
- Passas-Lagos, E.; Schuth, F. Amphiphilic Pickering Emulsifiers Based on Mushroom-Type Janus Particles. Langmuir 2015, 31, 7749–7757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Park, B.J.; Tu, F.; Lee, D. Amphiphilic Janus particles at fluid interfaces. Soft Matter 2013, 9, 6604–6617. [Google Scholar] [CrossRef]
- Schröder, J.H.; Doroshenko, M.; Pirner, D.; Mauer, M.E.; Förster, B.; Boyko, V.; Förster, S. Interfacial stabilization by soft Janus nanoparticles. Polymer 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Binks, B.P.; Fletcher, P.I. Particles adsorbed at the oil-water interface: A theoretical comparison between spheres of uniform wettability and “Janus” particles. Langmuir 2001, 17, 4708–4710. [Google Scholar] [CrossRef]
- Pan, M.; Rosenfeld, L.; Kim, M.; Xu, M.; Lin, E.; Derda, R.; Tang, S.K. Fluorinated pickering emulsions impede interfacial transport and form rigid interface for the growth of anchorage-dependent cells. ACS Appl. Mater. Interfaces 2014, 6, 21446–21453. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lyu, F.; Tang, S.K. Fluorinated pickering emulsions with nonadsorbing interfaces for droplet-based enzymatic assays. Anal. Chem. 2015, 87, 7938–7943. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Kim, M.; Blauch, L.; Tang, S.K. Surface-functionalizable amphiphilic nanoparticles for pickering emulsions with designer fluid-fluid interfaces. RSC Adv. 2016, 6, 39926–39932. [Google Scholar] [CrossRef]
- Arnedo-Sánchez, L.; Bhowmik, S.; Hietala, S.; Puttreddy, R.; Lahtinen, M.; De Cola, L.; Rissanen, K. Rapid self-healing and anion selectivity in metallosupramolecular gels assisted by fluorine-fluorine interactions. Dalton Trans. 2017, 46, 7309–7316. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Tyowua, A.T. Influence of the degree of fluorination on the behaviour of silica particles at air-oil surfaces. Soft Matter 2013, 9, 834–845. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Conroy, M.W.; Dougherty, J.A.; Otto, N.; Williams, B.A.; Ananth, R.; Fleming, J.W. Bubble coarsening dynamics in fluorinated and non-fluorinated firefighting foams. Colloids Surf. A 2015, 470, 268–279. [Google Scholar] [CrossRef]
- Vikingstad, A.K.; Aarra, M.G. Comparing the static and dynamic foam properties of a fluorinated and an alpha olefin sulfonate surfactant. J. Petrol. Sci. Eng. 2009, 65, 105–111. [Google Scholar] [CrossRef]
- Hong, L.; Jiang, S.; Granick, S. Simple method to produce Janus colloidal particles in large quantity. Langmuir 2006, 22, 9495–9499. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, E.; Salami-Kalajahi, M.; Hosseini, M.S.; Aghjeh, M.K.R. A temperature-controlled method to produce Janus nanoparticles using high internal interface systems: Experimental and theoretical approaches. Colloids Surf. A 2016, 506, 56–62. [Google Scholar] [CrossRef]
- Berger, S.; Synytska, A.; Ionov, L.; Eichhorn, K.J.; Stamm, M. Stimuli-responsive bicomponent polymer janus particles by “grafting from”/“grafting to” approaches. Macromolecules 2008, 41, 9669–9676. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. Res. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Guignot, S.; Faure, S.; Vignes-Adler, M.; Pitois, O. Liquid and particles retention in foamed suspensions. Chem. Eng. Sci. 2010, 65, 2579–2585. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Khalil, M.; Liu, N.; Lee, R. Effect of particle hydrophobicity on CO2 foam generation and foam flow behavior in porous media. Fuel 2014, 126, 104–108. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Foams stabilized by in-situ surface-activated nanoparticles in bulk and porous media. SPE J. 2016, 21, 121–130. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bryant, S.L.; Huh, C.; Johnston, K.P. Carbon dioxide-in-water foams stabilized with nanoparticles and surfactant acting in synergy. AIChE J. 2013, 59, 3490–3501. [Google Scholar] [CrossRef]

| Foam Systems | Composition |
|---|---|
| traditional surfactants | 0.4 wt % CAB |
| homogeneous modified particles | 0.4 wt % H30 |
| mixtures of particles and surfactants | 0.3 wt % DDAB + 0.1 wt % T30 |
| amphiphilic fluorinated nanoparticles | 0.4 wt % amphiphilic nanoparticles |
| Injection Mode | Sand Pack | K (μm2) | Oil Saturation | Oil Recovery before Injection of Foam | Oil Recovery after Injection of Foam | Enhanced Oil Recovery | ||
|---|---|---|---|---|---|---|---|---|
| Respectively | Total | Respectively | Total | |||||
| surfactants | high | 0.615 | 72.6% | 48.3% | 34.7% | 4.9% | 45.8% | 11.1% |
| low | 0.044 | 73.8% | 21.2% | 17.3% | ||||
| homogeneous particles | high | 0.624 | 71.9% | 47.6% | 35.3% | 11.0% | 56.5% | 21.2% |
| low | 0.046 | 73.1% | 22.9% | 31.4% | ||||
| mixtures of particles and surfactants | high | 0.591 | 71.9% | 47.8% | 34.5% | 9.9% | 49.8% | 15.4% |
| low | 0.044 | 72.5% | 21.1% | 20.9% | ||||
| amphiphilic particles | high | 0.607 | 71.4% | 48.6% | 34.9% | 16.3% | 67.8% | 32.9% |
| low | 0.045 | 73.4% | 21.2% | 49.4% | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, G.; Lu, C.; Pei, C.; Wang, Y. Preparation and Investigation of Foaming Amphiphilic Fluorinated Nanoparticles for Enhanced Oil Recovery. Materials 2017, 10, 1403. https://doi.org/10.3390/ma10121403
Wang K, Wang G, Lu C, Pei C, Wang Y. Preparation and Investigation of Foaming Amphiphilic Fluorinated Nanoparticles for Enhanced Oil Recovery. Materials. 2017; 10(12):1403. https://doi.org/10.3390/ma10121403
Chicago/Turabian StyleWang, Keliang, Gang Wang, Chunjing Lu, Cuiying Pei, and Ying Wang. 2017. "Preparation and Investigation of Foaming Amphiphilic Fluorinated Nanoparticles for Enhanced Oil Recovery" Materials 10, no. 12: 1403. https://doi.org/10.3390/ma10121403
APA StyleWang, K., Wang, G., Lu, C., Pei, C., & Wang, Y. (2017). Preparation and Investigation of Foaming Amphiphilic Fluorinated Nanoparticles for Enhanced Oil Recovery. Materials, 10(12), 1403. https://doi.org/10.3390/ma10121403





