Synthesis of a Novel Polyethoxysilsesquiazane and Thermal Conversion into Ternary Silicon Oxynitride Ceramics with Enhanced Thermal Stability
Abstract
:1. Introduction
2. Experimental Section
2.1. Precursor Synthesis
2.2. Pyrolysis and Heat Treatment
2.3. Characterizations
3. Results and Discussion
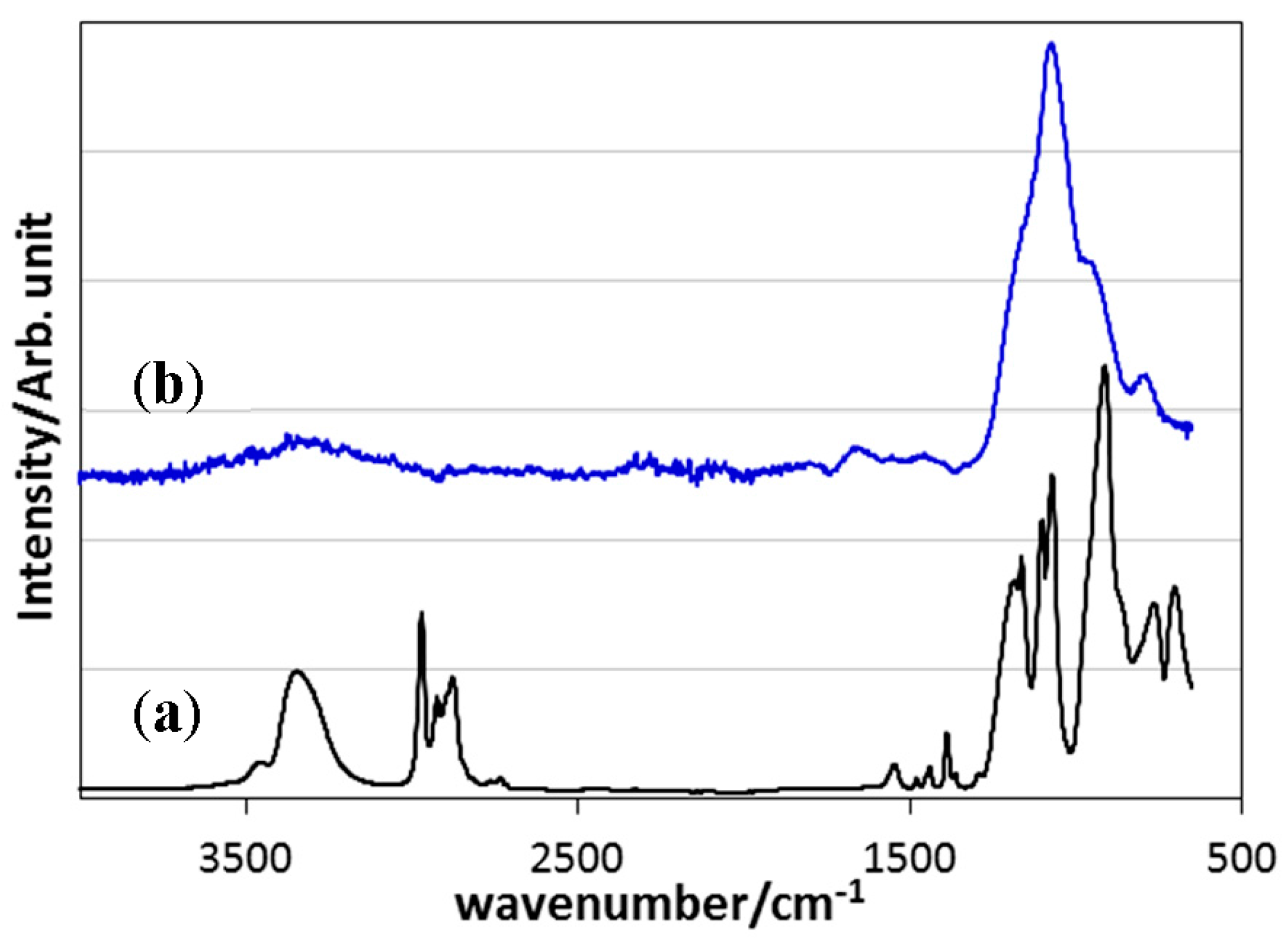
3.1. Chemical Structure of EtOSZ
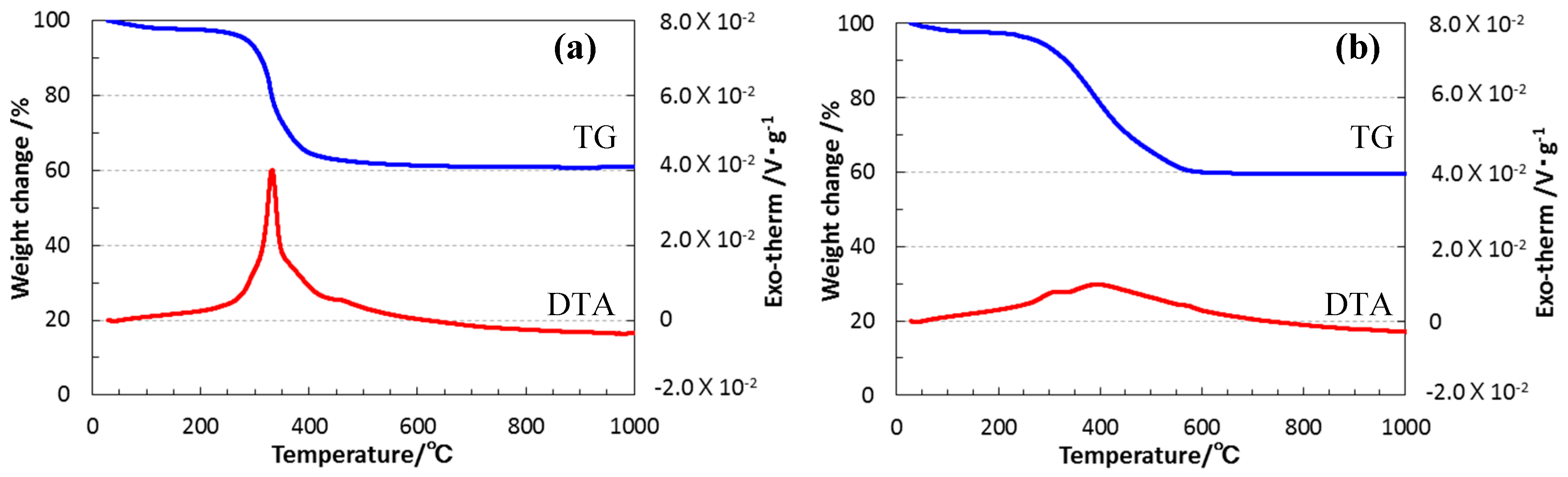
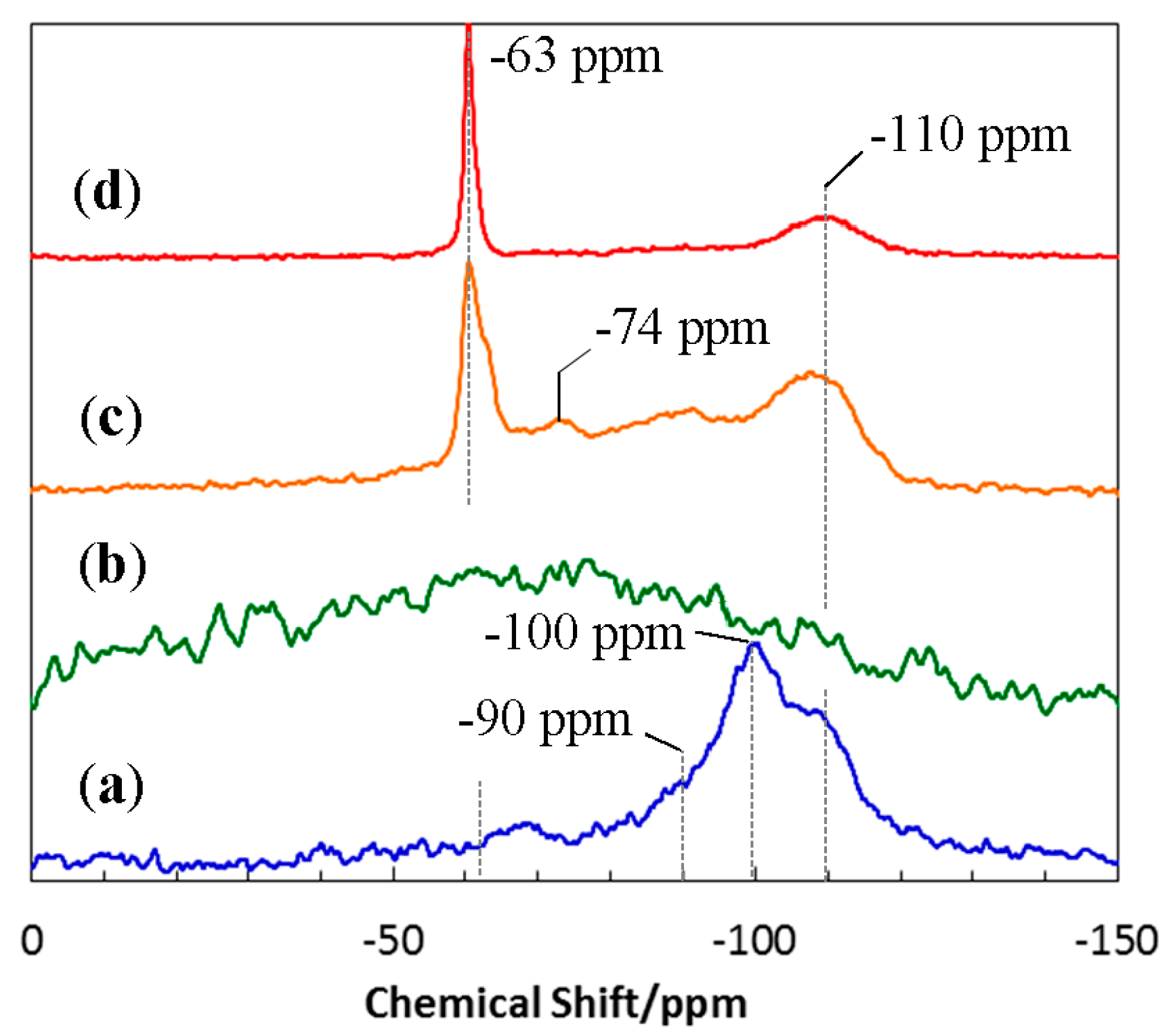
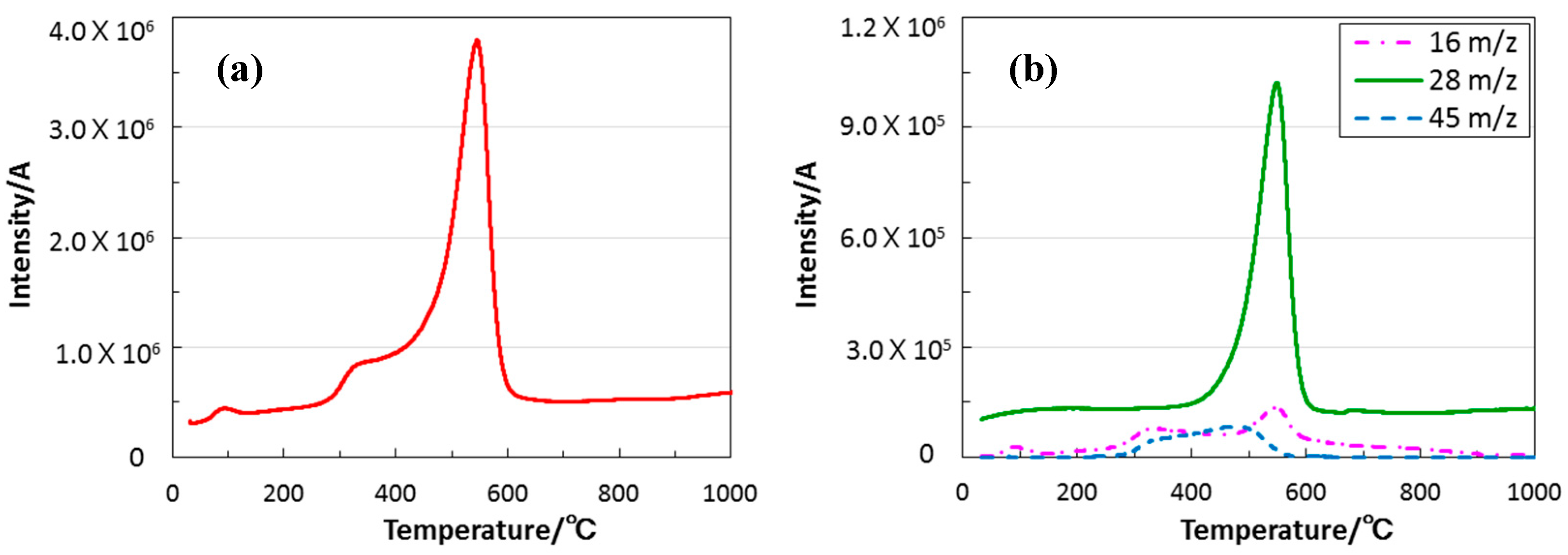
3.2. Conversion to Inorganic Compound
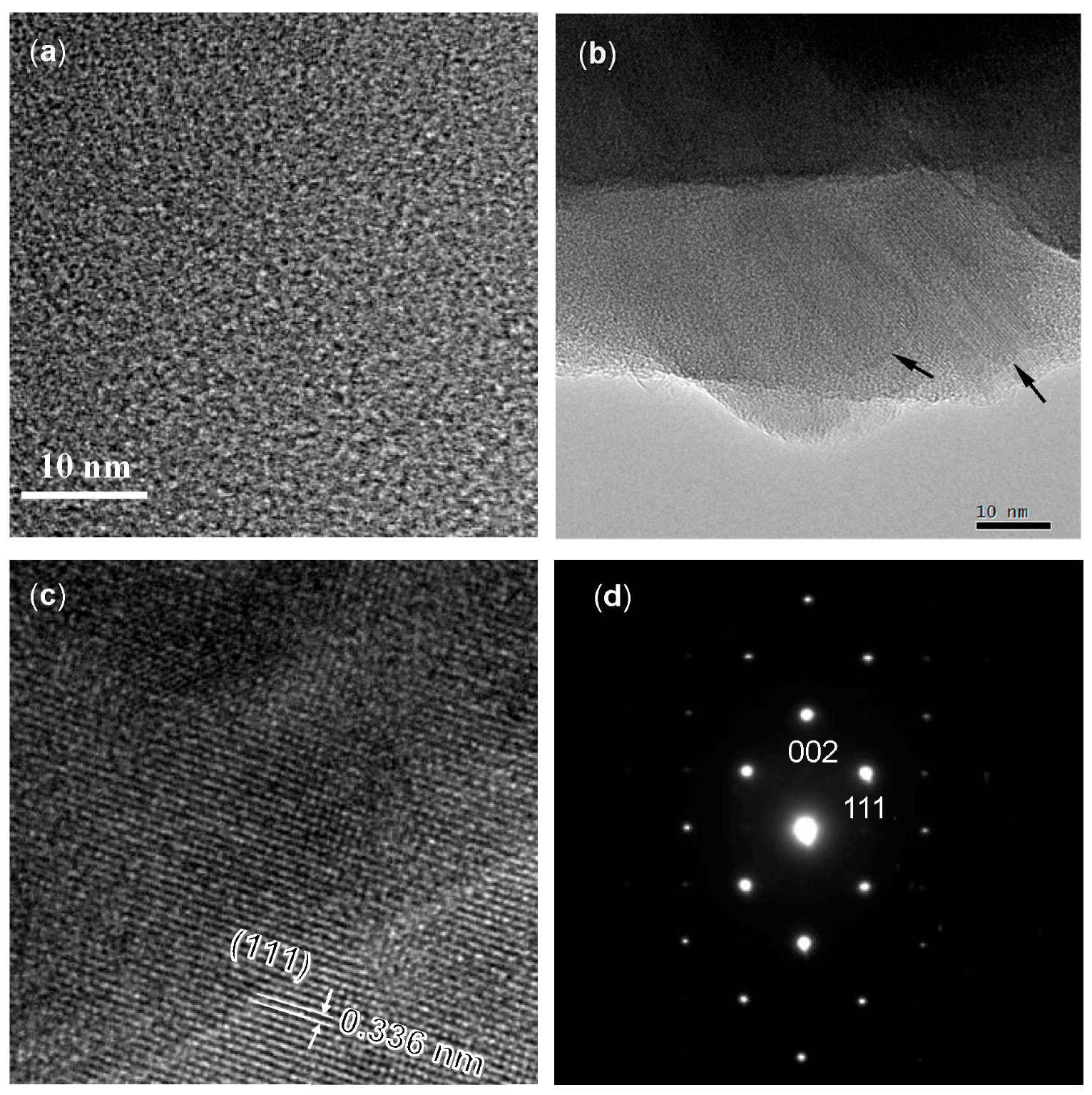
3.3. Crystallization Behavior of EtOSZ-Derived Amorphous Si-O-N in N2
4. Summary
- (1)
- ATR-IR, 13C- and 29Si-NMR spectroscopic analyses revealed that the synthesized polymer was composed of EtOSi(NH)3 unit, and polyethoxysilsesquiazane was successfully synthesized in a good yield via simple two-steps reaction, stoichiometric reaction of SiCl4 with EtOH to afford EtOSiCl3, followed by ammonolysis at −78 °C.
- (2)
- Under an inert atmosphere, thermal decomposition of EtOSZ mainly proceeded at around 200 to 600 °C, and the resulting ceramic yield after heating to 1000 °C was 58%.
- (3)
- The simultaneous TG-MS analyses for the thermal decomposition identified ethylene as a main gaseous species that was formed in-situ, and it was clarified that cleavage of oxygen-carbon bond of the EtO group in the EtOSZ contributed to the formation of the quaternary amorphous Si–O–C–N with extremely low carbon content (1.1 wt %) after pyrolysis at 800 °C in N2.
- (4)
- Additional heat treatment up to 1400 °C of the 800 °C-pyrolyzed EtOSZ resulted in the further reduction of the carbon content to afford oxygen rich Si–O–N amorphous ceramics.
- (5)
- The EtOSZ-derived Si–O–N was found to keep an amorphous state up to 1400 °C in N2, then Si2N2O crystallization started during heat treatment from 1400 to 1600 °C.
Author Contributions
Conflicts of Interest
References
- Larker, R. Reaction sintering and properties of silicon oxynitride densified by hot isostatic pressing. J. Am. Ceram. Soc. 1992, 75, 62–66. [Google Scholar] [CrossRef]
- Ohashi, M.; Kanzaki, S.; Tabata, H. High-Temperature Flexural Strength of Hot-Pressed Silicon Oxynitride Ceramics. J. Mater. Sci. Lett. 1988, 7, 339–340. [Google Scholar] [CrossRef]
- Ohashi, M.; Kanzaki, S.; Tabata, H. Processing, Mechanical Properties, and Oxidation Behavior of Silicon Oxynitride Ceramics. J. Am. Ceram. Soc. 1991, 74, 109–114. [Google Scholar] [CrossRef]
- Li, S.Q.; Pei, Y.C.; Yu, C.Q.; Li, J.L. Mechanical and Dielectric Properties of Porous Si2N2O-Si3N4 in Situ Composites. Ceram. Int. 2009, 35, 1851–1854. [Google Scholar]
- Washbum, M.E. Silicon Oxynitride Refractories. Am. Ceram. Soc. Bull. 1967, 46, 667–671. [Google Scholar]
- Li, X.M.; Zhang, L.; Yin, X.W. Study on in-Situ Reaction Synthesis and Mechanical Properties of Si2N2O Ceramic. Ceram. Int. 2013, 39, 3035–3041. [Google Scholar] [CrossRef]
- Bolech, M.; Metselaar, R.; van Dijen, F.K.; Blomer, F.; de With, G.; Ramaekers, P.R.J. Carbothermal Preparation of Si2N2O Powder. In High Technology Ceramics; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; pp. 527–533. [Google Scholar]
- Zhou, Y.; Liu, Q.; Zhou, H.; Zhuang, J. Yttrium Oxide-Assisted CRN Synthesis of Silicon Oxynitride Powders with Controlled Morphology. J. Am. Ceram. Soc. 2013, 96, 3650–3655. [Google Scholar] [CrossRef]
- Pradeilles, N.; Record, M.C.; Marin-Ayral, R.M.; Linde, A.; Studenikin, L.A.; Grachev, V.V. Influence of Thermal Conditions on the Combustion Synthesis of Si2N2O Phase. Mater. Res. Bull. 2008, 43, 463–472. [Google Scholar] [CrossRef]
- Radwan, M.; Kashiwagi, T.; Miyamoto, Y. New Synthesis Route for Si2N2O Ceramics Based on Desert Sand. J. Eur. Ceram. Soc. 2003, 23, 2337–2341. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, J.; Li, Z.; Zhou, Y. Low-temperature synthesis/densification and properties of Si2N2O prepared with Li2O additive. J. Eur. Ceram. Soc. 2007, 27, 4767–4772. [Google Scholar] [CrossRef]
- Kijaszek, W.; Oleszkiewicz, W.; Zakrzewski, A.; Patela, S.; Tłaczała, M. Investigation of optical properties of silicon oxynitride films deposited by RF PECVD method. Mater. Sci.-Pol. 2016, 34, 868–871. [Google Scholar] [CrossRef]
- Lipiński, M.; Kaminski, A.; Lelievre, J.-F.; Lemiti, M.; Fourmond, E.; Zięba, P. Investigation of graded index SiOxNy antireflection coating for silicon solar cell manufacturing. Phys. Status Solidi C 2007, 4, 1566–1569. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Friis, P.; Hubner, J.; Petersen, N.; Jørgensen, A.M.; Telleman, P.; Kutter, J.P. Ultraviolet transparent silicon oxynitride waveguides for biochemical microsystems. Opt. Lett. 2001, 26, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Sabac, A.; Gorecki, C.; Jozwik, M.; Nieradko, L.; Meunier, C.; Gut, K. Technology and performances of silicon oxynitride waveguides for optomechanical sensors fabricated by plasma-enhanced chemical vapour deposition. J. Eur. Opt. Soc. 2007, 2, 07026. [Google Scholar] [CrossRef]
- Aparicio, F.J.; Froner, E.; Rigo, E.; Gandolfi, D.; Scarpa, M.; Han, B.; Ghulinyan, M.; Pucker, G.; Pavesi, L. Silicon oxynitride waveguides as evanescent-field-based fluorescent biosensors. J. Phys. D Appl. Phys. 2014, 47, 405401. [Google Scholar] [CrossRef]
- Hiranaka, K.; Yamaguchi, T. Amorphous Silicon Thin-Film Transistors with SiOxNy/SiNx Gate Insulators. Jpn. J. Appl. Phys. 1990, 29, 229–235. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.; Son, H.; Hwang, S.; Jang, K.; Lee, J.; Lee, K.; Park, H.; Kim, K.; Yi, J.; et al. Fabrication and characterization of metal-oxide-nitride-oxynitride-polysilicon nonvolatile semiconductor memory device with silicon oxynitride (SiOxNy) as tunneling layer on glass. J. Appl. Phys. 2007, 102, 094502. [Google Scholar] [CrossRef]
- Heo, S.; Lee, J.; Kim, S.H.; Yun, D.-J.; Park, J.-B.; Kim, K.; Kim, N.J.; Kim, Y.; Lee, D.; Kim, K.-S.; et al. Device performance enhancement via a Si-rich silicon oxynitride buffer layer for the organic photodetecting device. Sci. Rep. 2017, 7, 1516. [Google Scholar] [CrossRef] [PubMed]
- Kroke, E.; Li, Y.-L.; Konetschny, C.; Lecomte, E.; Fasel, C.; Riedel, R. Silazane derived ceramics and related materials. Mater. Sci. Eng. 2000, R26, 97–199. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derivedceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar]
- Yu, G.-E.; Edirisinghe, M.; Finich, D.; Ralph, B.; Parrick, J. Synthesis of silicon oxynitride from a polymeric precursor, Part IV Pyrolysis of the copolymers. J. Mater. Sci. 1995, 30, 5371–5380. [Google Scholar] [CrossRef]
- Gunji, T.; Suzuki, Y.; Abe, Y. Solid State NMR Analysis on the Conversion Process of Poly(Si-isocyanato-Si-methylpolysilazane) into Silicon Nitride Oxide. Nippon Kagaku Kaishi 2000, 2000, 871–875. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Kroke, E.; Herkenhoff, S. In situ synthesis of Si2N2O/Si3N4 composite ceramics using polysilyloxycarbodiimide precursors. J. Eur. Ceram. Soc. 2013, 33, 2181–2189. [Google Scholar] [CrossRef]
- Wang, K.; Günthner, M.; Motz, G.; Flinn, B.D.; Bordia, R.K. Control of Surface Energy of Silicon Oxynitride Films. Langmuir 2013, 29, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Seyferth, D.; Wiseman, G.; Prud’homme, C. A liquid silazane precursor to silicon nitride. J. Am. Ceram. Soc. 1983, 66, C-13–C-14. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. A kinetic study of the thermal and thermal oxidative degradations of new bridged POSS/PS nanocomposites. Polym. Degrad. Stab. 2013, 98, 2564–2570. [Google Scholar] [CrossRef]
- Sokri, M.N.M.; Onishi, T.; Mouline, Z.; Daiko, Y.; Honda, S.; Iwamoto, Y. Polymer-derived amorphous silica-based inorganic-organic hybrids having alkoxy groups: Intermediates for synthesizing microporous amorphous silica materials. J. Ceram. Soc. Jpn. 2015, 123, 732–738. [Google Scholar] [CrossRef]
- Levy, G.C.; Cargioli, J.D. Nuclear Magnetic Resonance Spectroscopy of Nuclei Other than Protons; Axenrod, T., Webb, G.A., Eds.; Wiley: New York, NY, USA, 1974; Chapter 17; pp. 251–274. [Google Scholar]
- Engelhardt, G.; Jancke, H.; Magi, M.; Pehk, T.J.; Lippma, E. Über die 1H-, 13C- und 29Si-NMR chemischen Verschiebungen einiger linearer, verzweigter und cyclischer Methylsiloxan-Verbindungen. J. Organomet. Chem. 1971, 28, 293–300. [Google Scholar] [CrossRef]
- Dupree, R.; Lewis, M.H.; Smith, M.E. High-Resolution Silicon-29 Nuclear Magnetic Resonance in the Y-Si-O-N System. J. Am. Chem. Soc. 1988, 110, 1083–1087. [Google Scholar] [CrossRef]
- Dupree, R.; Lewis, M.H.; Smith, M.E. High-Resolution NMR Study of the La-Si-Al-O-N System. J. Am. Chem. Soc. 1989, 111, 5125–5132. [Google Scholar] [CrossRef]
- Weeren, R.V.; Leone, E.A.; Curran, S.; Klein, L.C.; Danforth, S.C. Synthesis and Characterization of Amorphous Si2N2O. J. Am. Ceram. Soc. 1994, 77, 2699–2702. [Google Scholar] [CrossRef]
- Donely, M.S.; Baer, D.R.; Stoebe, T.G. Nitrogen 1s Charge Referencing for Si, N, and Related Compounds. Surf. Interface Anal. 1988, 11, 335–340. [Google Scholar] [CrossRef]
- Belyi, V.I.; Vasilyeva, L.L.; Ginorker, A.S.; Gritsenko, V.A.; Repinsky, S.M.; Sinitsa, S.P.; Sinirnova, T.P.; Edelman, F.L. Silicon Nitride in Electronics. In Materials Science Monographs; Edelman, F.L., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1988; Volume 134, pp. 138–187. [Google Scholar]
- Sjöberg, J.; Helgesson, G.; Idrestedt, I. Refinement of the structure of Si2N2O. Acta Crystallogr. 1991, C47, 2438–2441. [Google Scholar] [CrossRef]

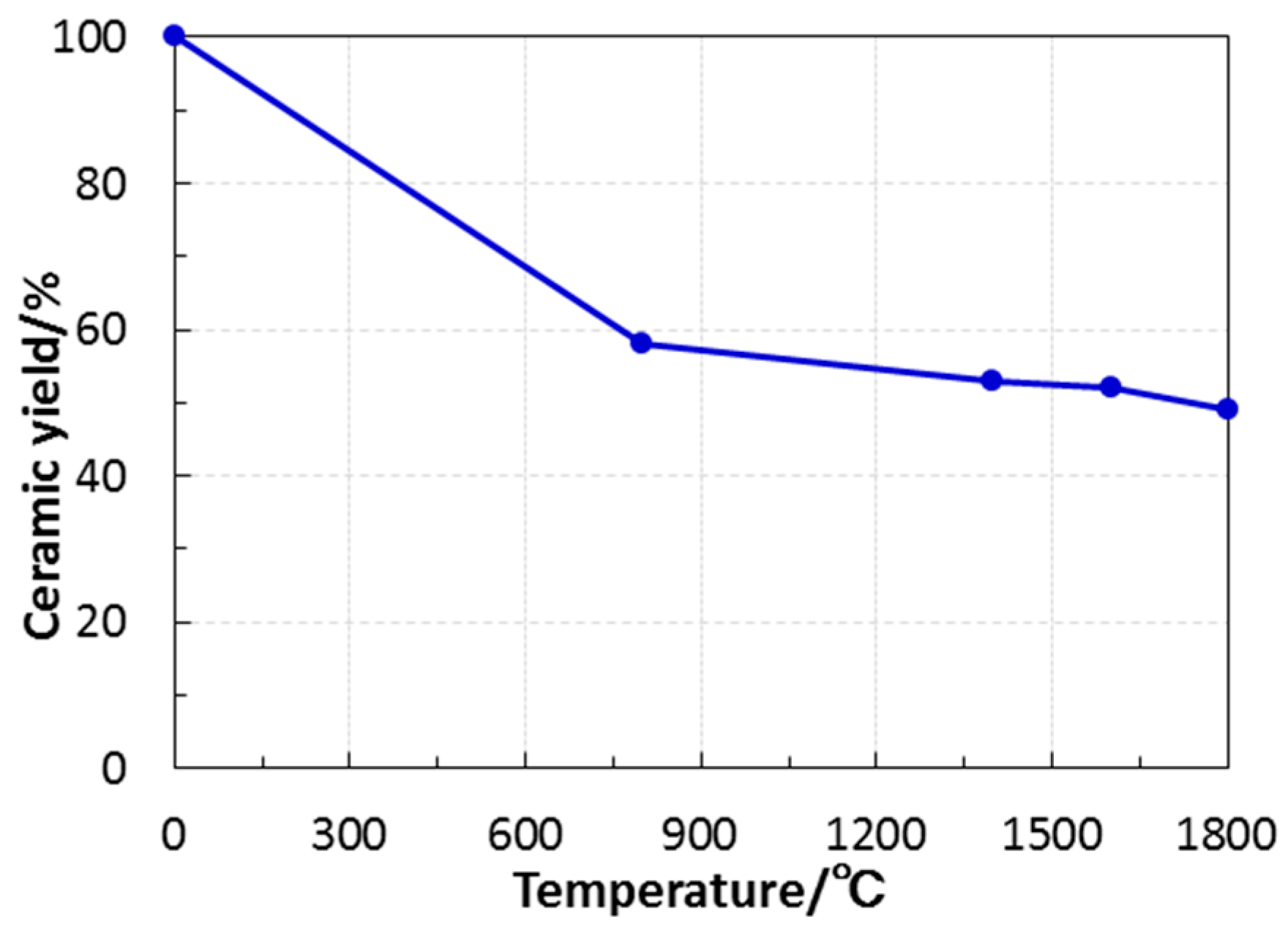
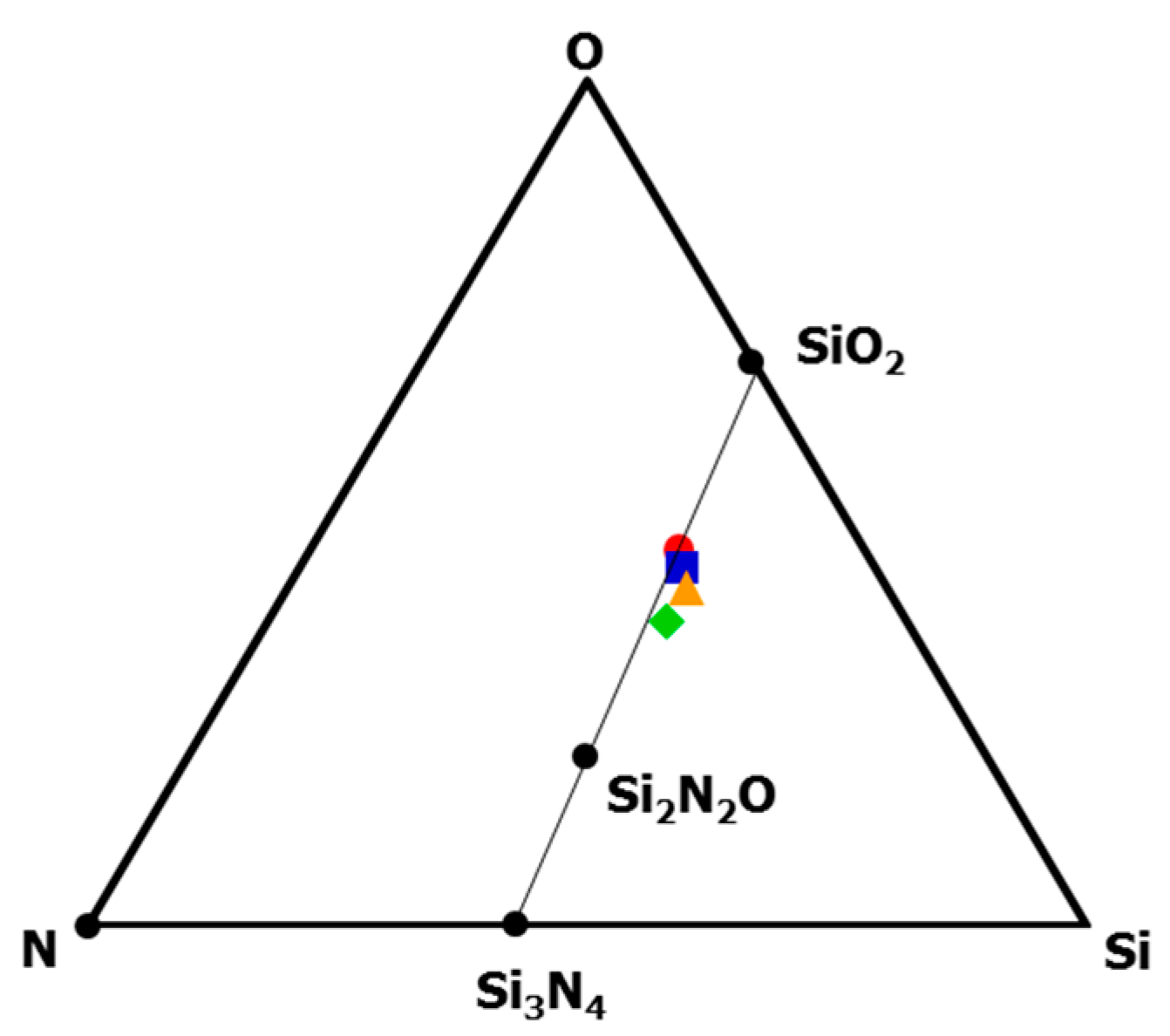
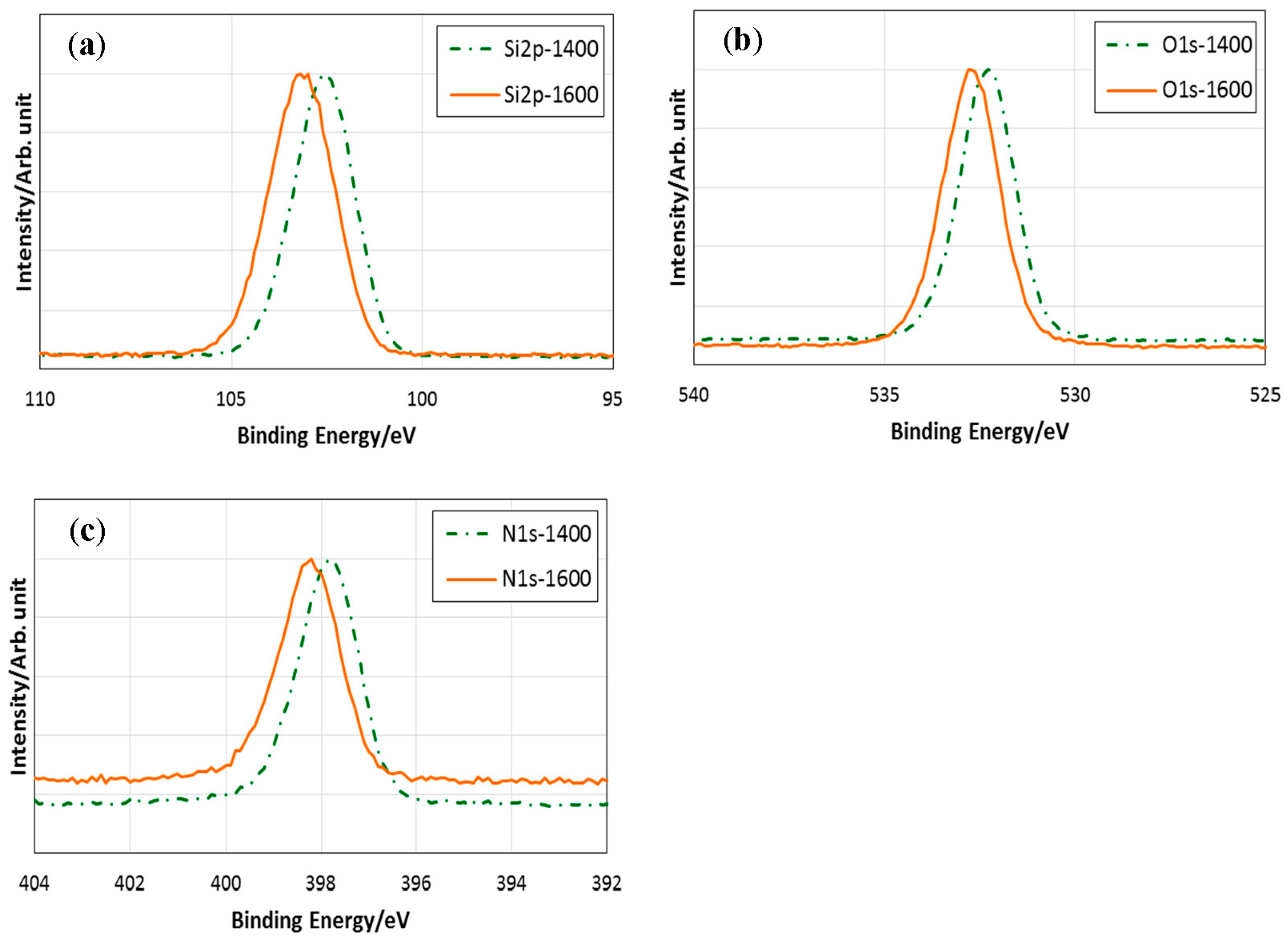
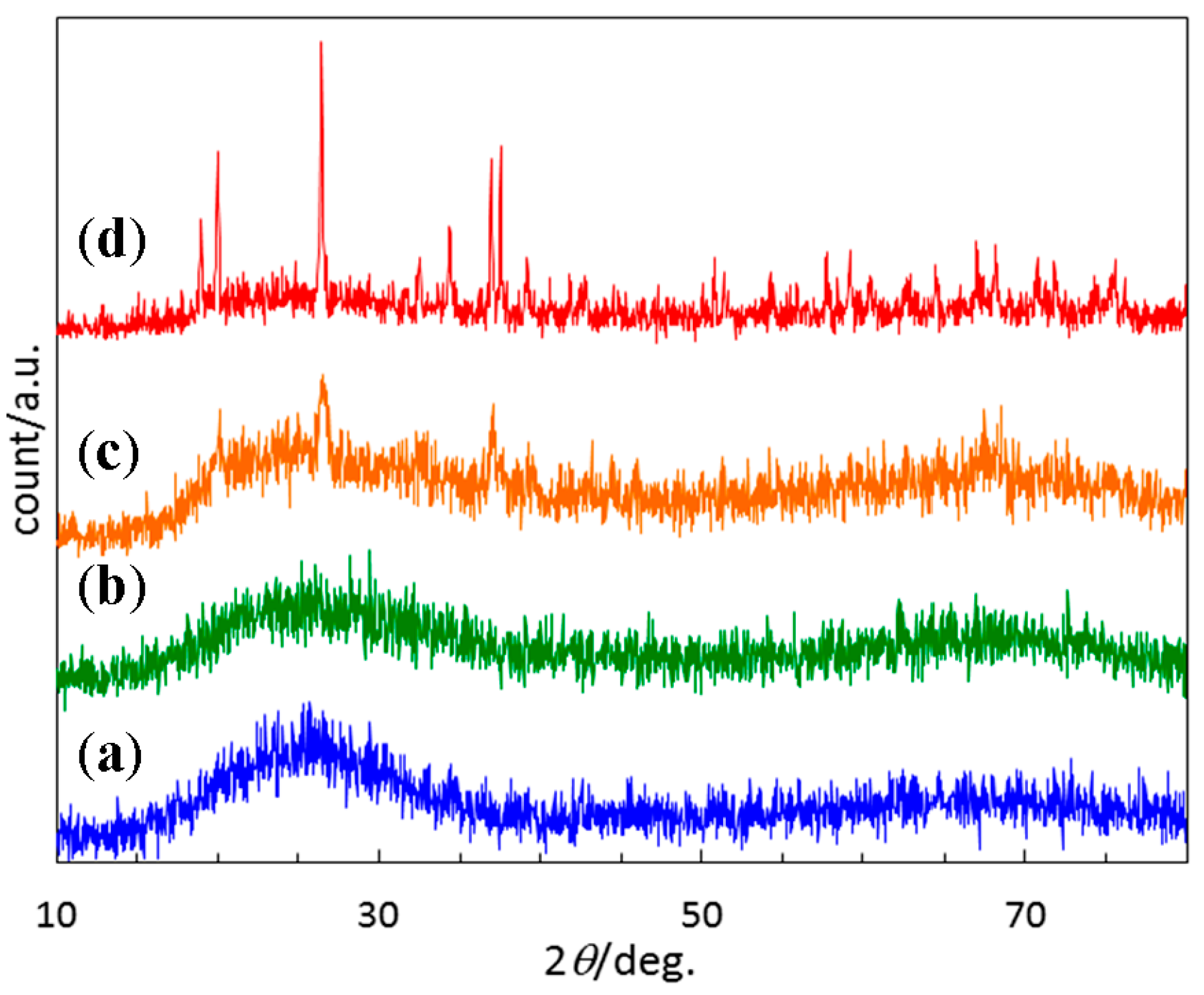
| Sample | Composition/wt % | Empirical Ratio | ||||
|---|---|---|---|---|---|---|
| Si | C | O | N | H | ||
| As-synthesized | 29.3 | 25.1 | 16.8 | 22.0 | 6.80 | Si1.0C2.0O1.0N1.5H6.5 |
| 800 °C-pyrolysed | 51.3 | 1.10 | 32.4 | 13.5 | 1.69 | Si1.0C0.05O1.1N0.5H0.9 |
| 1400 °C-heat treated | 54.7 | 0.41 | 29.4 | 15.5 | 0.03 | Si1.0C0.01O0.9N0.6H0.0 |
| 1600 °C-heat treated | 55.1 | 0.30 | 31.8 | 12.8 | 0.01 | Si1.0C0.01O1.0N0.5H0.0 |
| 1800 °C-heat treated | 56.2 | 0.04 | 33.2 | 10.6 | 0.01 | Si1.0C0.0O1.0N0.4H0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwase, Y.; Horie, Y.; Daiko, Y.; Honda, S.; Iwamoto, Y. Synthesis of a Novel Polyethoxysilsesquiazane and Thermal Conversion into Ternary Silicon Oxynitride Ceramics with Enhanced Thermal Stability. Materials 2017, 10, 1391. https://doi.org/10.3390/ma10121391
Iwase Y, Horie Y, Daiko Y, Honda S, Iwamoto Y. Synthesis of a Novel Polyethoxysilsesquiazane and Thermal Conversion into Ternary Silicon Oxynitride Ceramics with Enhanced Thermal Stability. Materials. 2017; 10(12):1391. https://doi.org/10.3390/ma10121391
Chicago/Turabian StyleIwase, Yoshiaki, Yoji Horie, Yusuke Daiko, Sawao Honda, and Yuji Iwamoto. 2017. "Synthesis of a Novel Polyethoxysilsesquiazane and Thermal Conversion into Ternary Silicon Oxynitride Ceramics with Enhanced Thermal Stability" Materials 10, no. 12: 1391. https://doi.org/10.3390/ma10121391
APA StyleIwase, Y., Horie, Y., Daiko, Y., Honda, S., & Iwamoto, Y. (2017). Synthesis of a Novel Polyethoxysilsesquiazane and Thermal Conversion into Ternary Silicon Oxynitride Ceramics with Enhanced Thermal Stability. Materials, 10(12), 1391. https://doi.org/10.3390/ma10121391





