Surface Modification of Carbon Nanotubes with an Enhanced Antifungal Activity for the Control of Plant Fungal Pathogen
Abstract
:1. Introduction
2. Results
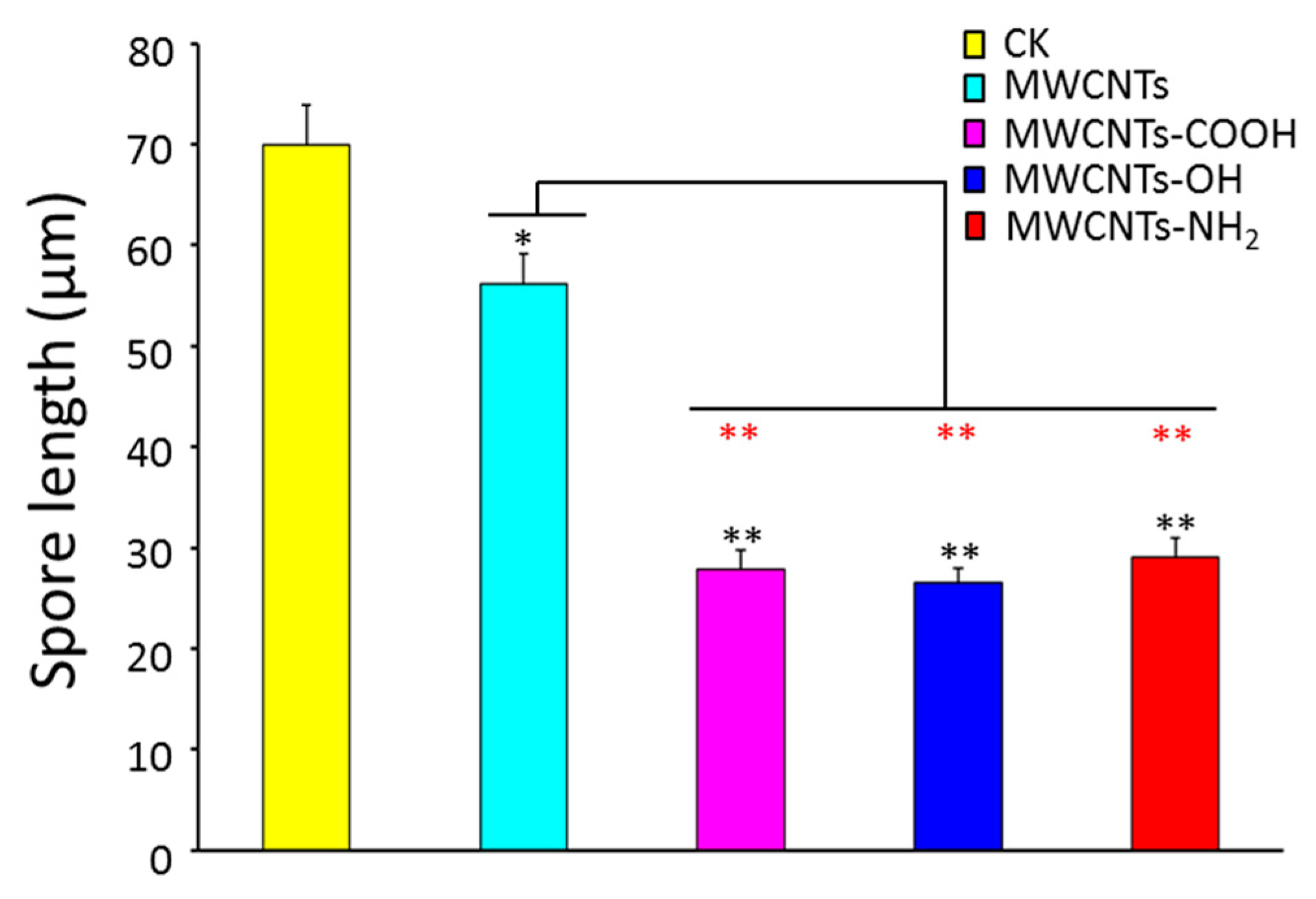
2.1. Surface Modification Effects of MWCNTs on Spore Length
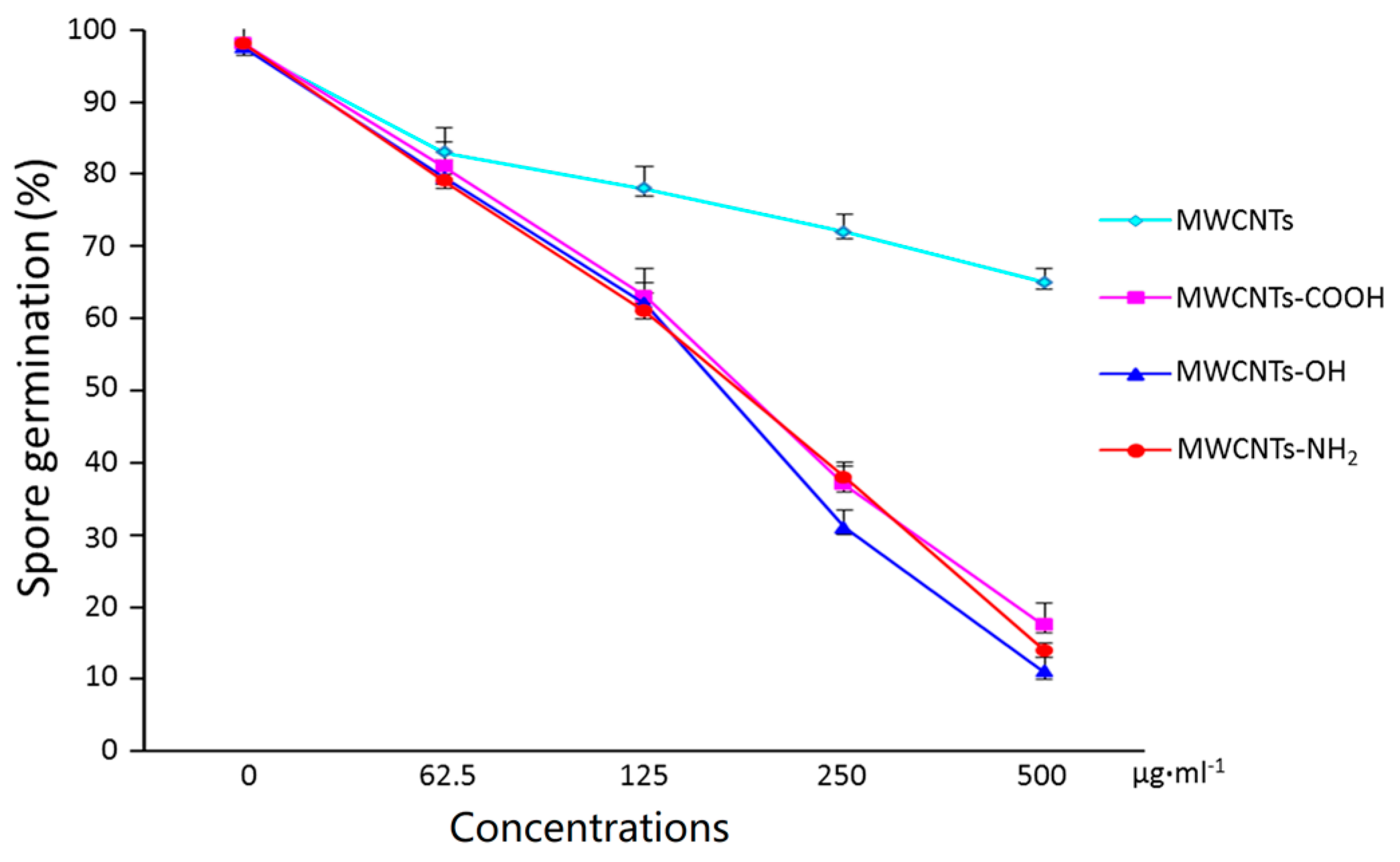
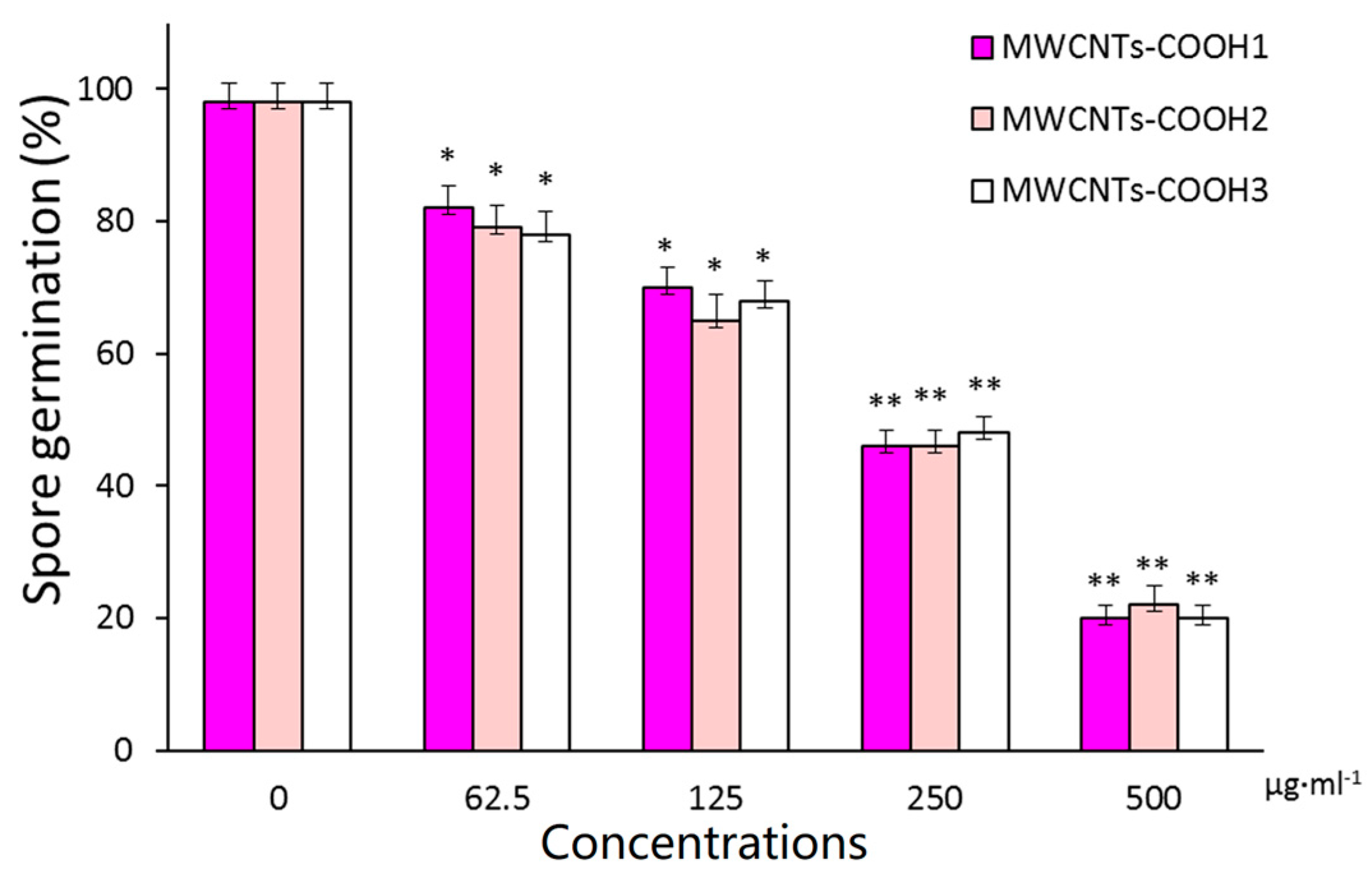
2.2. Surface Modification Effects of MWCNTs on Spore Germination
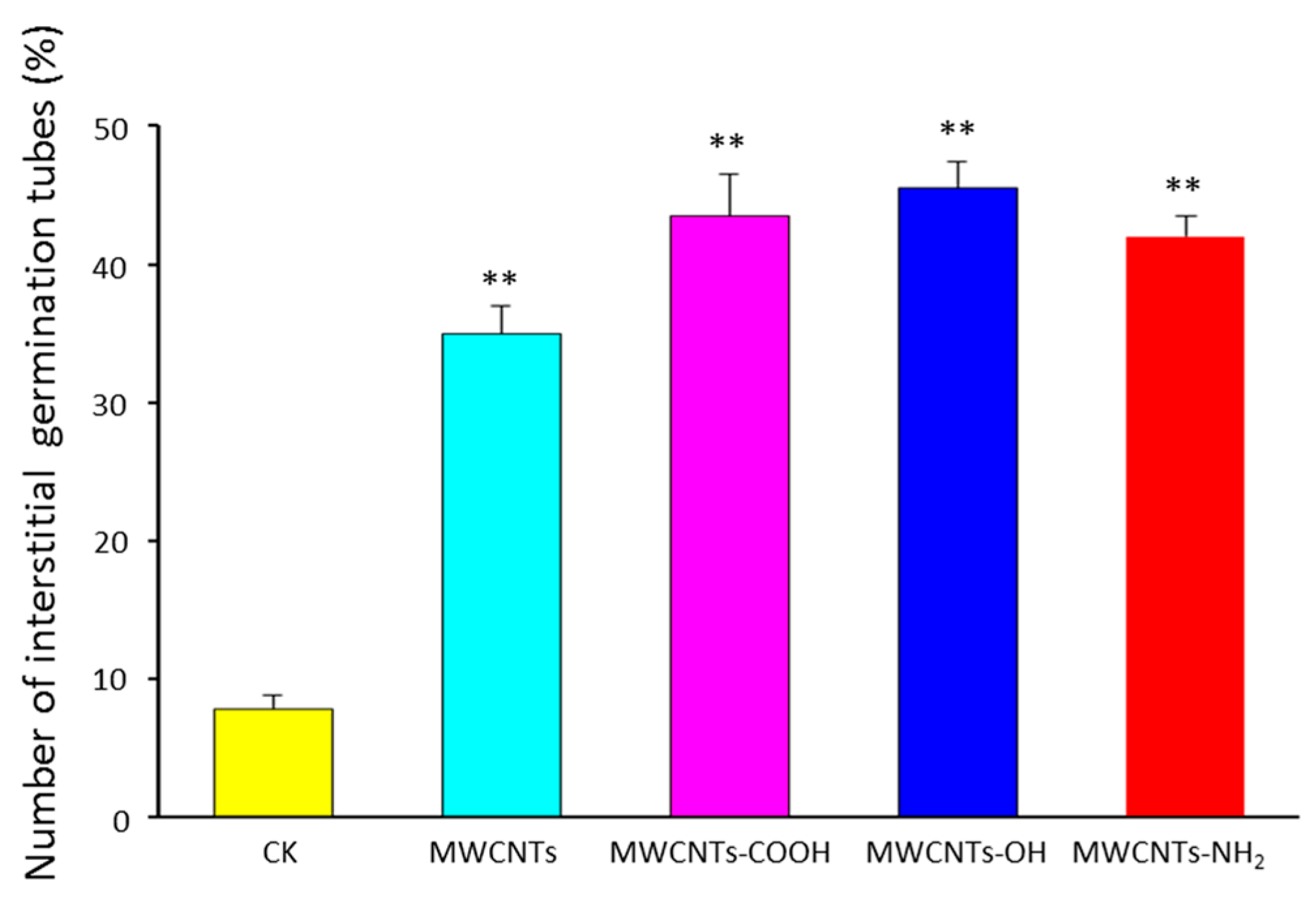
2.3. Surface Modification Effects of MWCNTs on Germination Pattern of Spores
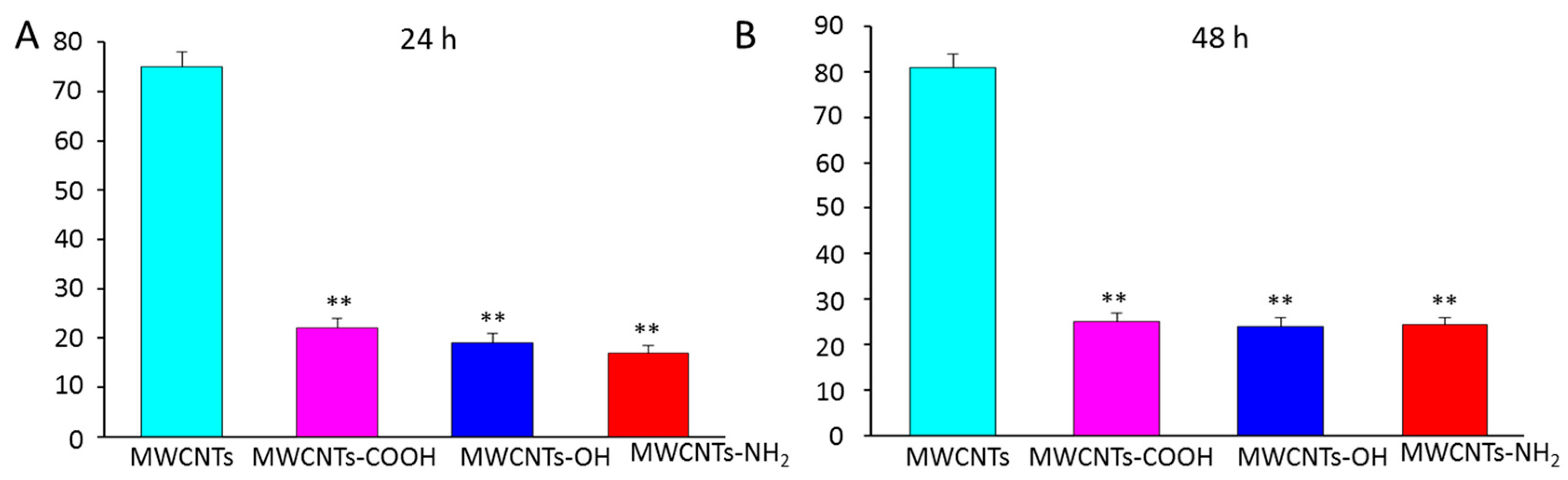
2.4. Surface Modification Effects of MWCNTs on Persistence of Antifungal Activity
2.5. Length Effect of MWCNTs on Antifungal Activity
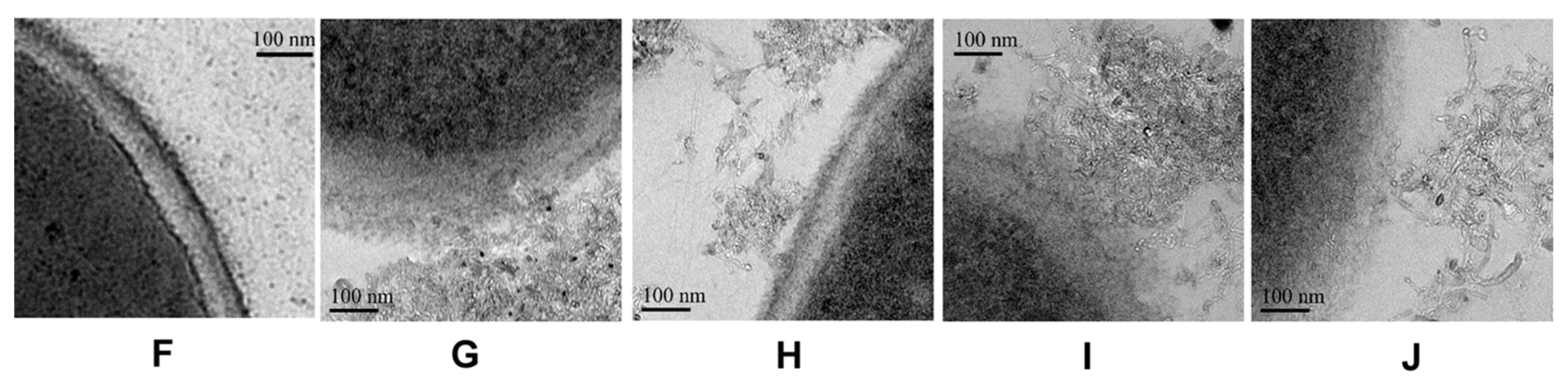
2.6. The Antifungal Mechanism of Surface Modification MWCNTs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Fungal Strains
4.3. Spore Germination and CNTs Treatment
4.4. Morphological Observation by TEM
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Min, H.W.; Giraldo, J.P.; Kwak, S.Y.; Koman, V.B.; Sinclair, R.; Lew, T.T.S.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2017, 16, 264–272. [Google Scholar] [CrossRef]
- Oliveira, S.F.; Bisker, G.; Bakh, N.A.; Gibbs, S.L.; Landry, M.P.; Strano, M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon 2015, 95, 767–779. [Google Scholar] [CrossRef]
- Polizu, S.; Savadogo, O.; Poulin, P.; Yahia, L.H. Applications of carbon nanotubes-based biomaterials in biomedical nanotechnology. J. Nanosci. Nanotechnol. 2006, 6, 1883–1904. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Usui, Y.; Aoki, K.; Narita, N.; Shimizu, M.; Hara, K.; Ogiwara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Carbon nanotubes: Biomaterial applications. Chem. Soc. Rev. 2009, 38, 897–1903. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, W.; Jie, W.; Chan, L.; Liang, X.J.; Yin, M. Terrylenediimide-based intrinsic theranostic nanomedicines with high photothermal conversion efficiency for photoacoustic imaging-guided cancer therapy. ACS Nano 2017, 11, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.Y.; Wong, M.H.; Tts, L.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R. Nanosensor technology applied to living plant systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Son, Y.; Yoon, T.K.; Kim, S.; Kim, W. The effect of multi-walled carbon nanotubes on soil microbial activity. Ecotoxicol. Environ. Saf. 2011, 74, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Son, Y.; Yoon, T.K.; Kang, Y.J.; Kim, W.; Chung, H. High concentrations of single-walled carbon nanotubes lower soil enzyme activity and microbial biomass. Ecotoxicol. Environ. Saf. 2013, 88, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.M.; Limmer, A.; Giersig, M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Brady-Estevez, A.S.; Kang, S.; Elimelech, M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter. Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster “nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle, L.D.; Van Tassel, P.R. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid). Nanoscale 2010, 2, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Oyelami, A.O.; Semple, K.T. Impact of carbon nanomaterials on microbial activity in soil. Soil Biol. Biochem. 2015, 86, 172–180. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B 2013, 103, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zhang, Q.; Mu, Q.; Bai, Y.; Li, L.; Zhou, H.; Butch, E.R.; Powell, T.B.; Snyder, S.E.; Jiang, G.; et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity. ACS Nano 2011, 5, 4581–4591. [Google Scholar] [CrossRef] [PubMed]
- Ali-Boucetta, H.; Nunes, A.; Sainz, R.; Herrero, M.A.; Tian, B.; Prato, M.; Bianco, A.; Kostarelos, K. Asbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalization. Angew. Chem. Int. Ed. 2013, 52, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tam, U.C.; Czlapinski, J.L.; Lee, G.S.; Rabuka, D.; Zettl, A.; Bertozzi, C.R. Interfacing carbon nanotubes with living cells. J. Am. Chem. Soc. 2006, 128, 6292–6293. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fu, C.; Lu, Q. Advanced technology for functionalization of carbon nanotubes. Prog. Nat. Sci. 2009, 19, 801–810. [Google Scholar] [CrossRef]
- Gu, L.; Luo, P.G.; Wang, H.; Meziani, M.J.; Lin, Y.; Veca, L.M.; Cao, L.; Lu, F.; Wang, X.; Quinn, R.A.; et al. Single-walled carbon nanotube as a unique scaffold for the multivalent display of sugars. Biomacromolecules 2008, 9, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.R.; Yang, L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.M.; Hashmi, S.M.; Sommer, T.J.; Elimelech, M.; Zimmerman, J.B. Impact of surface functionalization on bacterial cytotoxicity of single-walled carbon nanotubes. Environ. Sci. Technol. 2012, 46, 6297–6305. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.D. Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia 2005, 97, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lafon, A.; Seo, J.A.; Han, K.H.; Yu, J.H.; d’Enfert, C. The heterotrimeric G-protein GanB(alpha)-SfaD(beta)-GpgA(gamma) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans. Genetics 2005, 171, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial cytotoxicity of carbon-based nanomaterials: Implications for river water and wastewater effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, H.; Liu, X.; Gu, X.; Chen, K.; Lu, D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012, 14, 841. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.B.; Li, H.P.; Zhang, J.B.; Song, B.; Chen, F.F.; Duan, X.J.; Xu, H.Q.; Liao, Y.C. Disruption of the chitin synthase gene CHS1 from Fusarium asiaticum results in an altered structure of cell walls and reduced virulence. Fungal Genet. Biol. 2010, 47, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.F.; Zhang, J.B.; Song, X.S.; Yang, J.; Li, H.P.; Tang, H.R.; Liao, Y.C. Combined Metabonomic and quantitative real-time PCR analyses reveal systems metabolic changes of Fusarium graminearum induced by Tri5 gene deletion. J. Proteome Res. 2011, 10, 2273–2285. [Google Scholar] [CrossRef] [PubMed]

| Items | Purity (%) | Functional Groups Content (%) | ID (nm) | OD (nm) | Length (μm) |
|---|---|---|---|---|---|
| MWCNTs-OH | >95 | 3.70 | 3–5 | 8–15 | ~50 |
| MWCNTs-NH2 | >95 | 0.45 | 3–5 | 8–15 | ~50 |
| MWCNTs-COOH1 | >95 | 3.82 | 3–5 | 8–15 | ~50 |
| MWCNTs-COOH2 | >95 | 2.56 | 2–5 | <8 | 10–30 |
| MWCNTs-COOH3 | >95 | 3.86 | 2–5 | <8 | 0.2–2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhou, Z.; Chen, F. Surface Modification of Carbon Nanotubes with an Enhanced Antifungal Activity for the Control of Plant Fungal Pathogen. Materials 2017, 10, 1375. https://doi.org/10.3390/ma10121375
Wang X, Zhou Z, Chen F. Surface Modification of Carbon Nanotubes with an Enhanced Antifungal Activity for the Control of Plant Fungal Pathogen. Materials. 2017; 10(12):1375. https://doi.org/10.3390/ma10121375
Chicago/Turabian StyleWang, Xiuping, Zilin Zhou, and Fangfang Chen. 2017. "Surface Modification of Carbon Nanotubes with an Enhanced Antifungal Activity for the Control of Plant Fungal Pathogen" Materials 10, no. 12: 1375. https://doi.org/10.3390/ma10121375
APA StyleWang, X., Zhou, Z., & Chen, F. (2017). Surface Modification of Carbon Nanotubes with an Enhanced Antifungal Activity for the Control of Plant Fungal Pathogen. Materials, 10(12), 1375. https://doi.org/10.3390/ma10121375






