Plastic Deformation of Pressured Metallic Glass
Abstract
1. Introduction
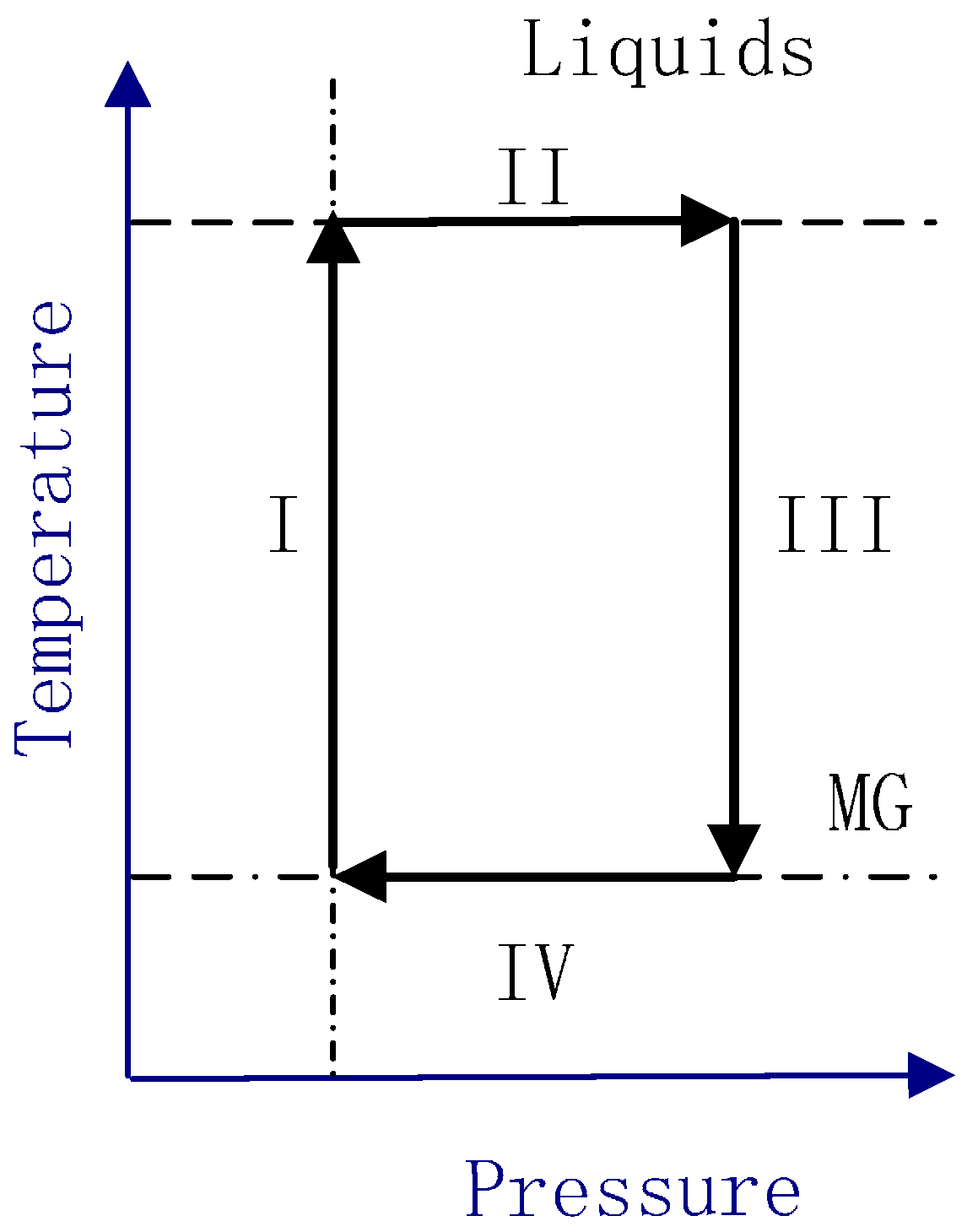
2. Method
3. Results and Discussion
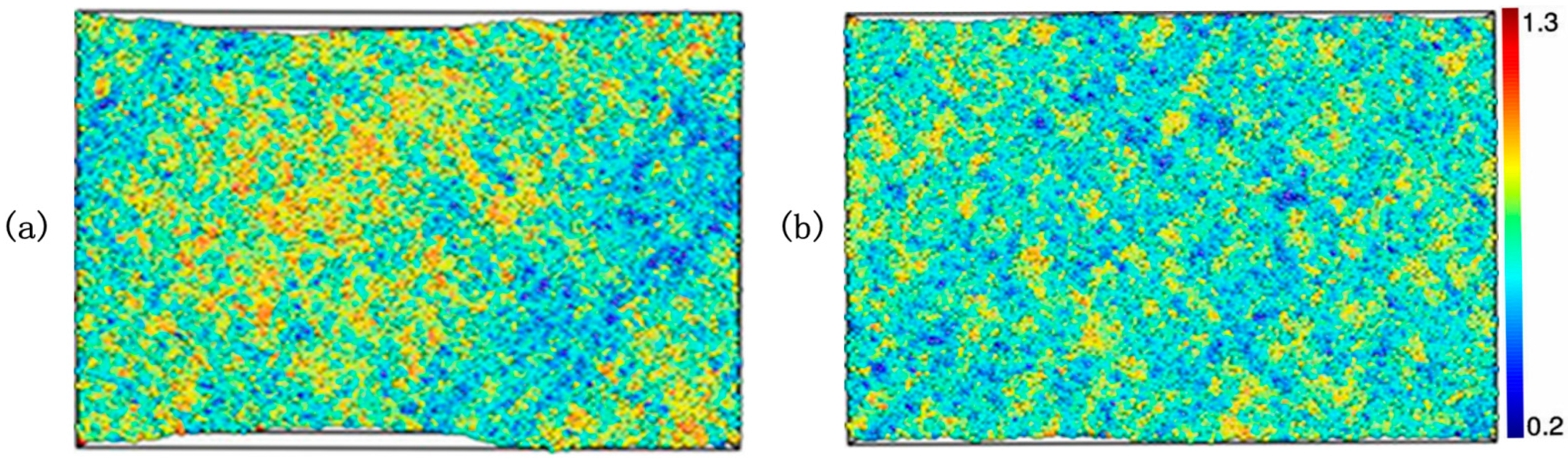
3.1. Part A: Structural and Dynamical Heterogeneity in Liquid and Glass
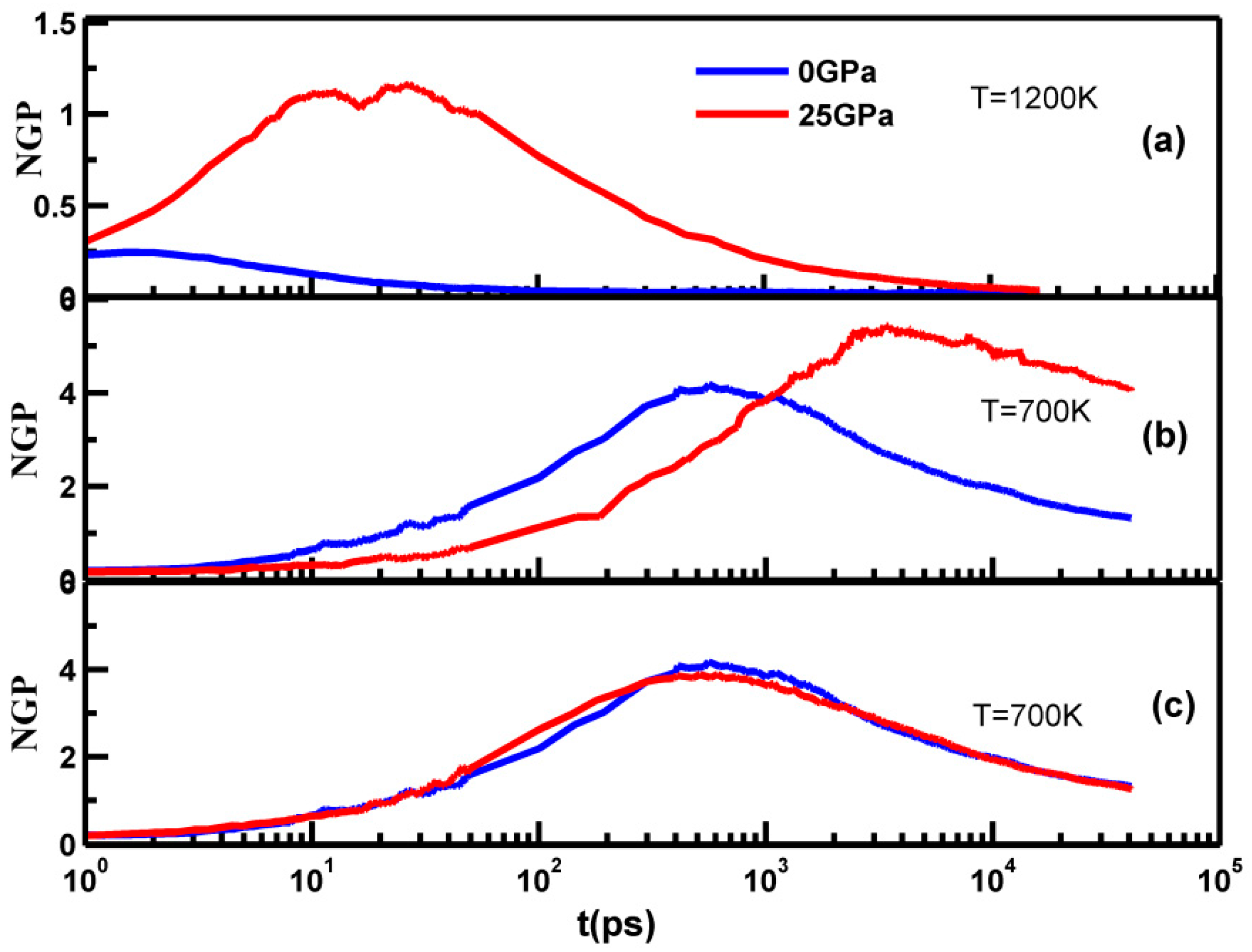
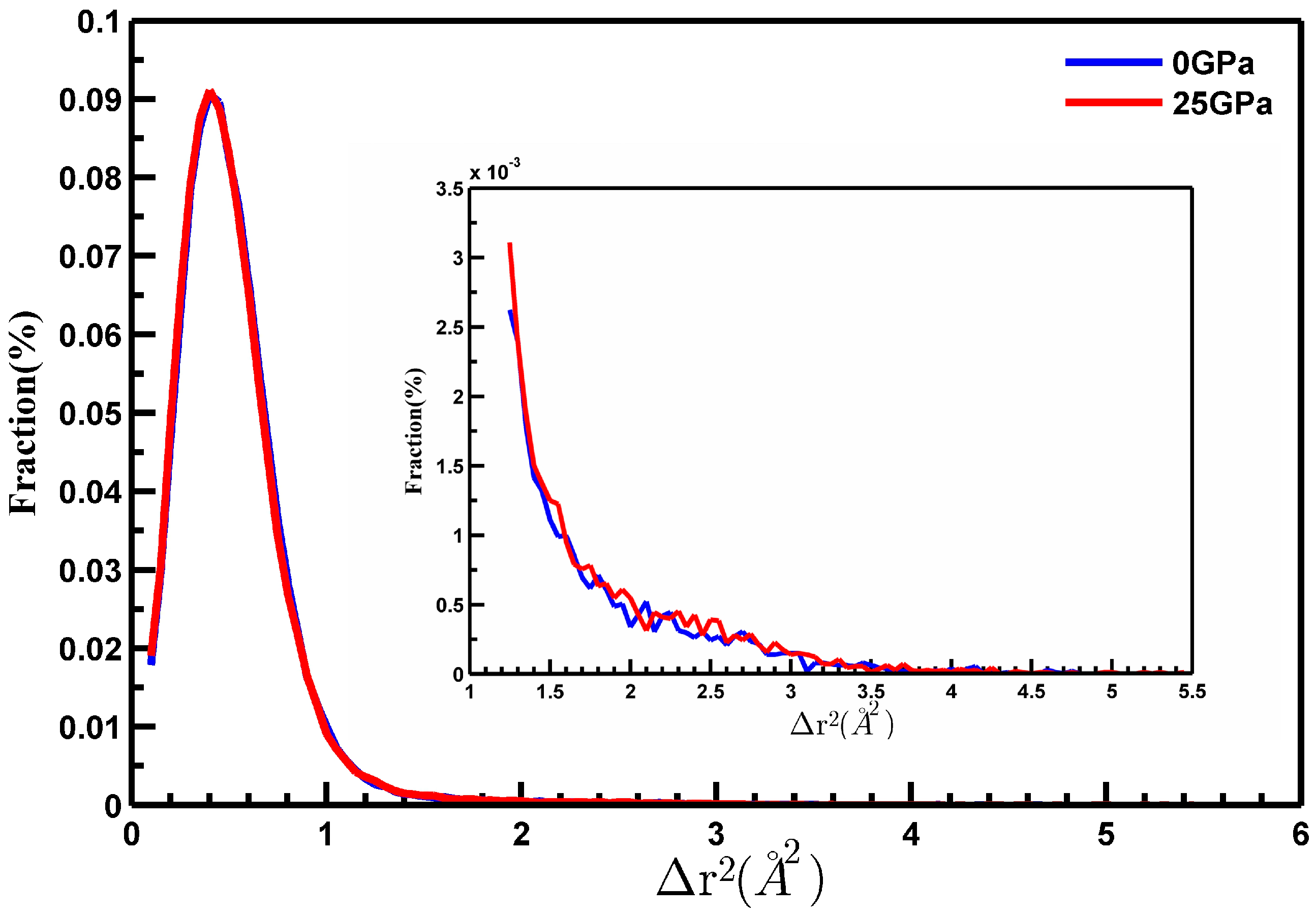
3.2. Part B: Dynamic Heterogeneity during Deformation
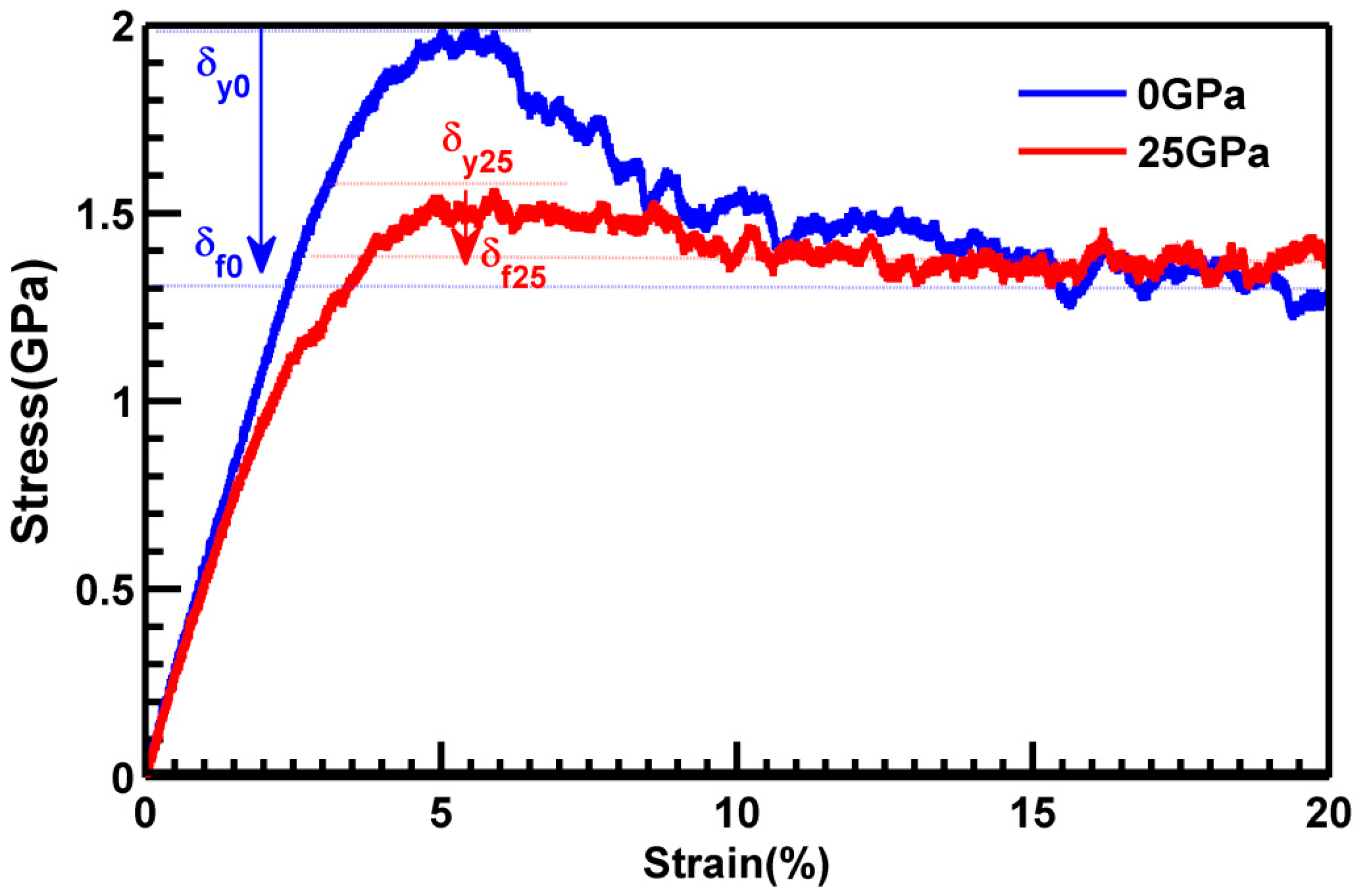
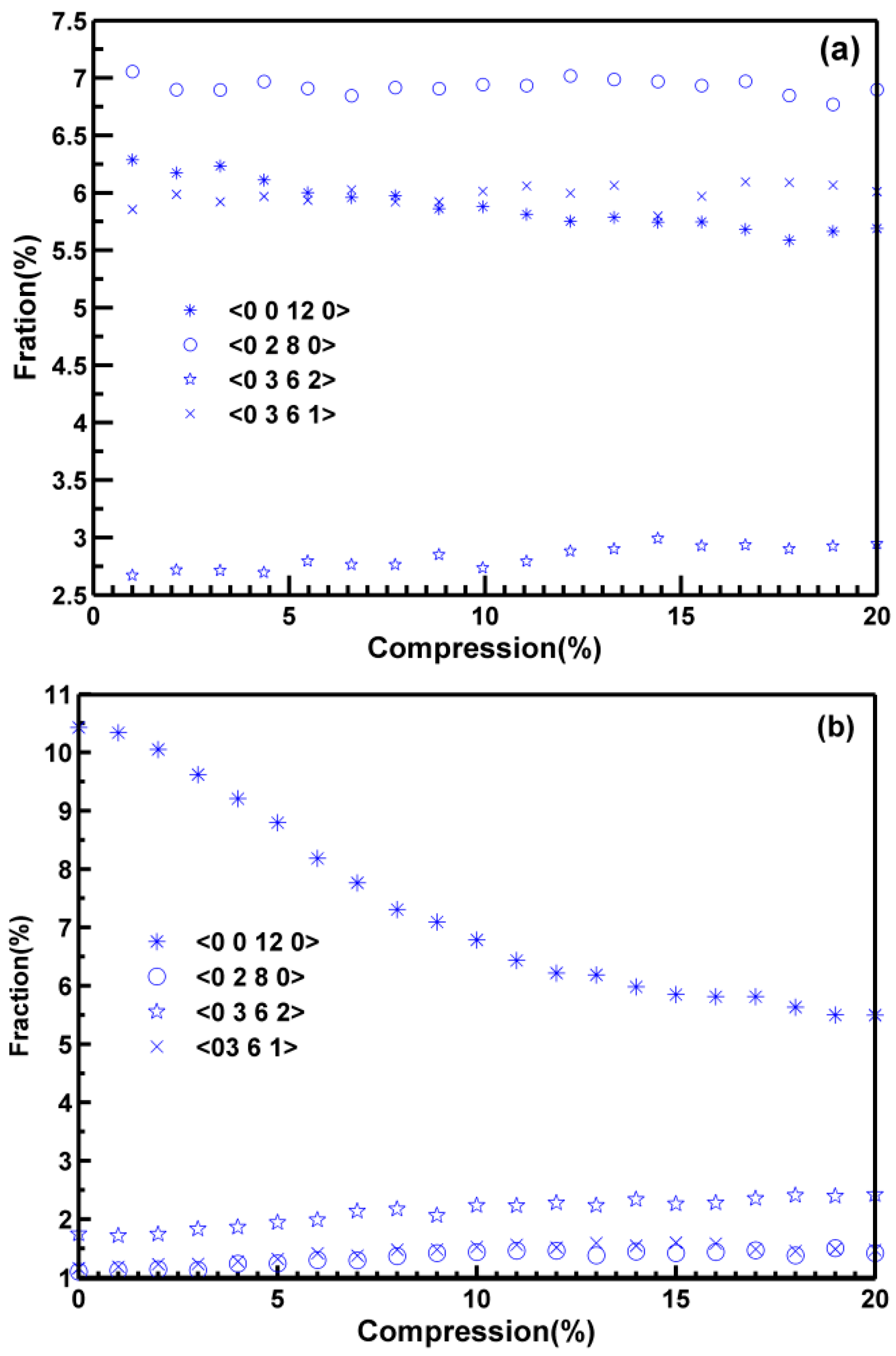
3.3. Part CStructural Evolution during Deformation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kramer, J. Über nichtleitende metallmodifikationen. Annl. Phys. 1934, 19, 37–64. [Google Scholar] [CrossRef]
- Johnson, W.L.; Samwer, K. A universal criterion for plastic yielding of metallic glasses with a (T/Tg) 2/3 temperature dependence. Phys. Rev. Lett. 2005, 95, 195501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Q.; Sheng, H.W.; Ma, E. Relationship between structure, dynamics, and mechanical properties in metallic glass-forming alloys. Phys. Rev. B 2008, 78, 014207. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Launey, M.E.; Garrett, G.; Schramm, J.P.; Hofmann, D.C.; Johnson, W.L.; Ritchie, R.O. A damage-tolerant glass. Nat. Mater. 2011, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S. The influence of structural relaxation on the density and Young’s modulus of metallic glasses. J. Appl. Phys. 1978, 49, 3289. [Google Scholar] [CrossRef]
- Slipenyuk, A.; Eckert, J. Correlation between enthalpy change and free volume reduction during structural relaxation of Zr55Cu30Al10Ni5 metallic glass. J. Scr. Mater. 2004, 50, 39–44. [Google Scholar] [CrossRef]
- Ding, J.; Cheng, Y.Q.; Ma, E. Sample size matters for Al88Fe7Gd5 metallic glass: Smaller is stronger. Acta Mater. 2014, 69, 343. [Google Scholar] [CrossRef]
- Cohen, M.H.; Turnbull, D. Molecular transport in liquids and glasses. J. Chem. Phys. 1959, 31, 1164. [Google Scholar] [CrossRef]
- Pronin, A.; Kondrin, M.V.; Lyapin, A.G.; Brazhkin, V.V.; Volkov, A.A.; Lunkenheimer, P.; Loidl, A. Glassy dynamics under super high pressure. Phys. Rev. E 2010, 81, 041503. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, S.; Paluch, M.; Ziolo, J.; Roland, C.M. On the pressure dependence of the fragility of glycerol. J. Phys. 2009, 21, 332101. [Google Scholar] [CrossRef] [PubMed]
- Paluch, M.; Casalini, R.; Hensel-Bielowka, S.; Roland, C.M. Effect of pressure on the α relaxation in glycerol and xylitol. J. Chem. Phys. 2002, 116, 9839. [Google Scholar] [CrossRef]
- Wakeda, M.; Saida, J.; Li, J.; Ogata, S. Controlled rejuvenation of amorphous metals with thermal processing. Sci. Rep. 2015, 5, 10545. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, N.; Lo, Y.C.; Wakeda, M.; Ogata, S. Properties of high-density, well-ordered, and high-energy metallic glass phase designed by pressurized quenching. Appl. Phys. Lett. 2016, 109, 091906. [Google Scholar] [CrossRef]
- Casalini, R.; Roland, C.M. Scaling of the supercooled dynamics and its relation to the pressure dependence of the dynamic crossover and the fragility of glass formers. Phys. Rev. B 2005, 71, 014210. [Google Scholar] [CrossRef]
- Roland, C.M.; Hensel-Bielowka, S.; Paluch, M.; Casalini, R. Super cooled dynamics of glass-forming liquids and polymers under hydrostatic pressure. Rep. Prog. Phys. 2005, 68, 1405. [Google Scholar] [CrossRef]
- Miyazaki, N.; Wakeda, M.; Wang, Y.J.; Ogata, S. Prediction of pressure-promoted thermal rejuvenation in metallic glasses. NPJ Comput. Mater. 2016, 2, 16013. [Google Scholar] [CrossRef]
- Bordat, P.; Affouard, F.; Descamps, M.; Ngai, K. Does the interaction potential determine both the fragility of a liquid and the vibrational properties of its glassy state? Phys. Rev. Lett. 2005, 93, 105502. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Guan, P.F.; Wang, Q.; Yang, Y.; Bai, H.Y.; Wang, W.H. Pressure effects on structure and dynamics of metallic glass-forming liquid. J. Chem. Phys. 2017, 146, 024507. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.Z.; Hao, S.G.; Kramer, M.J.; Ho, K.M. Structural heterogeneity and medium-range order in ZrxCu100−x metallic glasses. Phys. Rev. B 2009, 80, 184201. [Google Scholar] [CrossRef]
- Lad, K.N.; Jakse, N.; Pasturel, A. Signatures of fragile-to-strong transition in a binary metallic glass-forming liquid. J. Chem. Phys. 2012, 136, 104509. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, C.M.; Lee, K.R.; Ma, E.; Lee, J.C. Networked interpenetrating connections of icosahedra: Effects on shear transformations in metallic glass. Acta Mater. 2011, 59, 159–170. [Google Scholar] [CrossRef]
- Ding, J.; Asta, M.; Ritchie, R.O. Anomalous structure-property relationships in metallic glasses through pressure-mediated glass formation. Phys. Rev. B 2016, 93, 140204. [Google Scholar] [CrossRef]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, P.J.; Nelson, D.R.; Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 1983, 28, 784. [Google Scholar] [CrossRef]
- Ding, J.; Patinet, S.; Falk, M.L.; Cheng, Y.Q.; Ma, E. Soft spots and their structural signature in a metallic glass. Proc. Natl. Aced. Sci. USA 2014, 111, 14052. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Wang, W.H.; Bai, H.Y.; Samwer, K. The beta-relaxation in metallic glasses. Natl. Sci. Rev. 2014, 1, 429. [Google Scholar] [CrossRef]
- Zhu, F.; Nguyen, H.K.; Song, S.X.; Aji, D.P.; Hirata, A.; Wang, H.; Nakajima, K.; Chen, M.W. Intrinsic correlation between β-relaxation and spatial heterogeneity in a metallic glass. Nat. Commun. 2016, 7, 11516. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E.; Sheng, H.W. Atomic level structure in multicomponent bulk metallic glass. Phys. Rev. Lett. 2009, 102, 245501. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.B.; Xiong, H.L.; Ahmad, A.S.; Li, A.G.; Yang, K.; Glazyrin, K.; Liermann, H.P.; Franz, H.; Wang, D.X.; Cao, Q.P. Atomic structure of Pd81Si19 glassy alloy under high pressure. Acta Mater. 2014, 81, 420–427. [Google Scholar] [CrossRef]
- Wang, B.; Shang, B.S.; Gao, X.Q.; Wang, W.H.; Bai, H.Y.; Pan, M.X.; Guan, P.F. Pressure-Induced structural and optical properties of organometal halide perovskite-based formamidinium lead bromide. Phys. Chem. Lett. 2016, 7, 02466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.Z.; Zhang, F.; Mendelev, M.L.; Kramer, M.J.; Ho, K.M. Strong correlations of dynamical and structural heterogeneities with localized soft modes in a Cu-Zr metallic glass. Appl. Phys. Lett. 2014, 105, 151910. [Google Scholar] [CrossRef]
- Donati, C.; Glotzer, S.C.; Poole, P.H.; Kob, W.; Plimpton, S.J. Spatial correlations of mobility and immobility in a glass-forming Lennard-Jones liquid. Phys. Rev. E 1999, 60, 3107. [Google Scholar] [CrossRef]
- Hu, Y.C.; Li, F.X.; Li, M.Z.; Bai, H.Y.; Wang, W.H. Five-Fold symmetry as indicator of dynamic arrest in metallic glass-forming liquids. Nat. Commun. 2015, 6, 8310. [Google Scholar] [CrossRef] [PubMed]
- Debenedetti, P.G.; Stillinger, F.H. Super cooled liquids and the glass transition. Nature 2001, 410, 259. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Richert, R.; Maasz, R.; Samwer, K. Strain induced fragility transition in metallic glass. Nat. Commun. 2015, 6, 7179. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.X.; Song, K.K.; Wang, L.; Sopu, D.; Pauly, S.; Eckert, J. Correlation between structural heterogeneity and plastic deformation for phase separating FeCu metallic glasses. Sci. Rep. 2016, 6, 34340. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wong, C.H. Interpenetrating networks in Zr–Cu–Al and Zr–Cu metallic glasses. Intermetallics 2011, 22, 13. [Google Scholar] [CrossRef]
- Park, K.W.; Fleury, E.; Seok, H.K.; Kim, Y.C. Deformation behaviors under tension and compression: Atomic simulation of CuZr metallic glass. Intermetallics 2011, 19, 03024. [Google Scholar] [CrossRef]
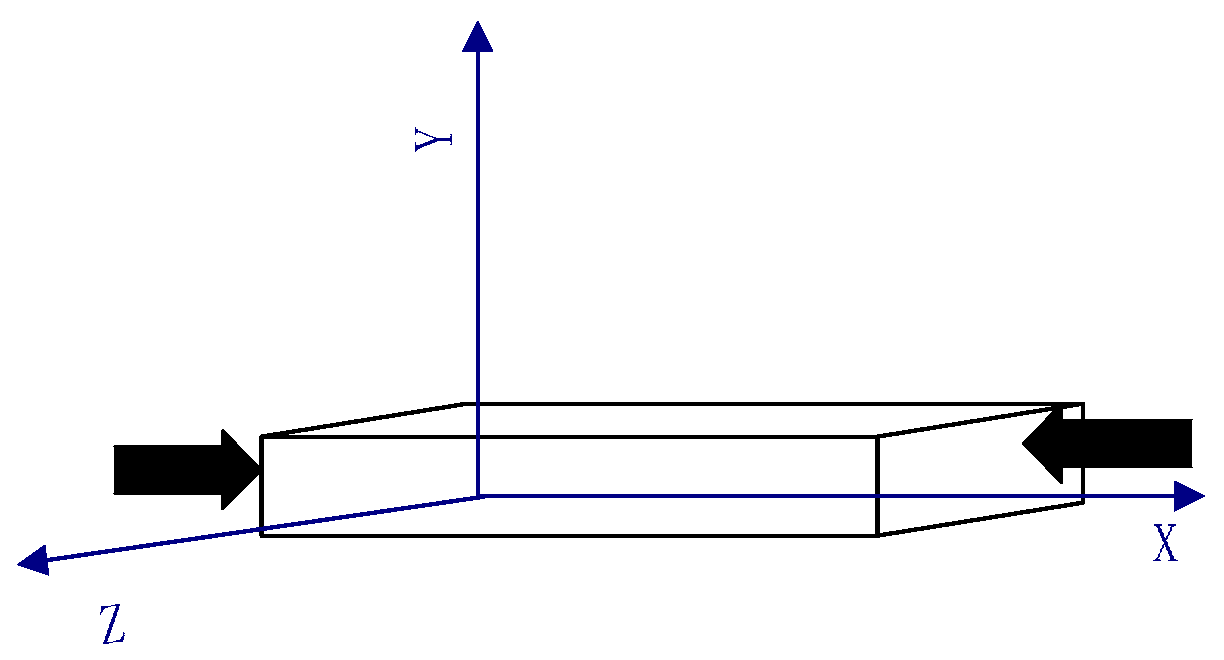



| Direction of Box | Samples Quenched at 0 GPa | Samples Quenched at 25 GPa |
|---|---|---|
| x- | 0.0517 | 0.0359 |
| y- | 0.0525 | 0.0326 |
| z- | 0.032 | 0.0187 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Peng, C.; Zhang, Z.; Wang, P.; Yuan, S.; Wang, L. Plastic Deformation of Pressured Metallic Glass. Materials 2017, 10, 1361. https://doi.org/10.3390/ma10121361
Cheng Y, Peng C, Zhang Z, Wang P, Yuan S, Wang L. Plastic Deformation of Pressured Metallic Glass. Materials. 2017; 10(12):1361. https://doi.org/10.3390/ma10121361
Chicago/Turabian StyleCheng, Yun, Chuanxiao Peng, Zhenting Zhang, Pengfei Wang, Shengzhong Yuan, and Li Wang. 2017. "Plastic Deformation of Pressured Metallic Glass" Materials 10, no. 12: 1361. https://doi.org/10.3390/ma10121361
APA StyleCheng, Y., Peng, C., Zhang, Z., Wang, P., Yuan, S., & Wang, L. (2017). Plastic Deformation of Pressured Metallic Glass. Materials, 10(12), 1361. https://doi.org/10.3390/ma10121361





