Superelastic Graphene Aerogel/Poly(3,4-Ethylenedioxythiophene)/MnO2 Composite as Compression-Tolerant Electrode for Electrochemical Capacitors
Abstract
1. Introduction
2. Experimental Section
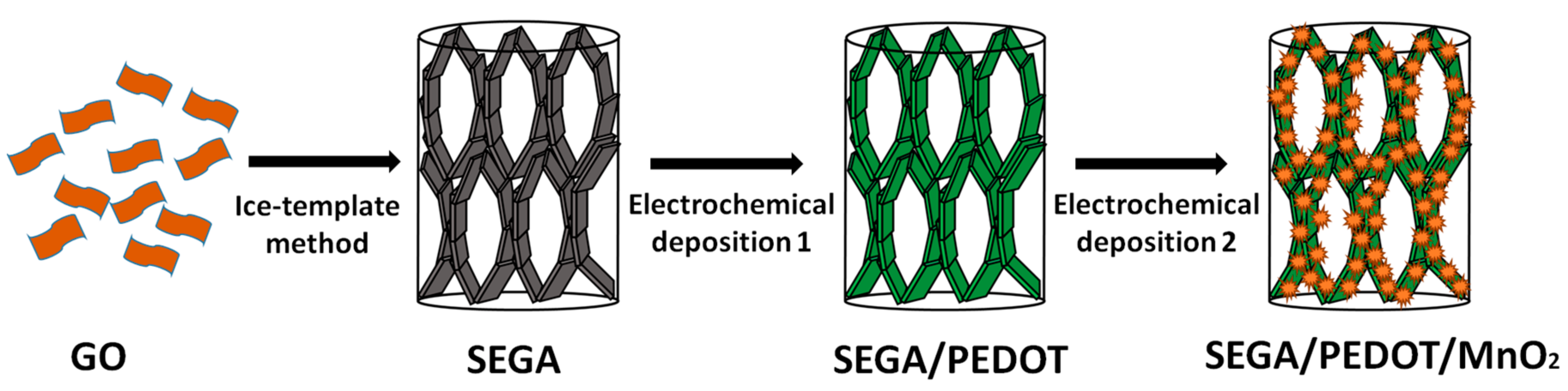
2.1. Preparation of Ultra-Compressible SEGA/PEDOT/MnO2 Composite
2.2. Characterizations
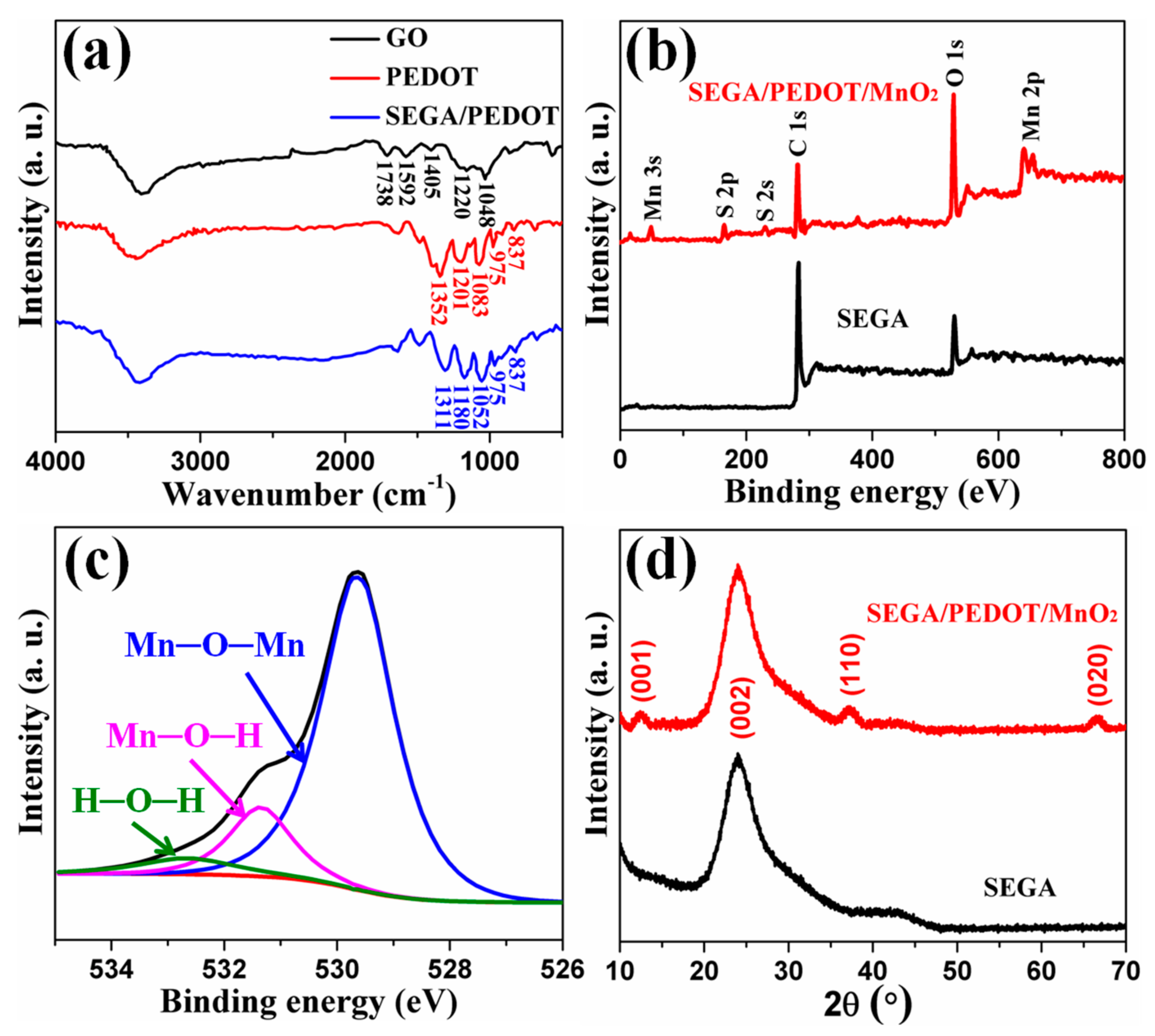
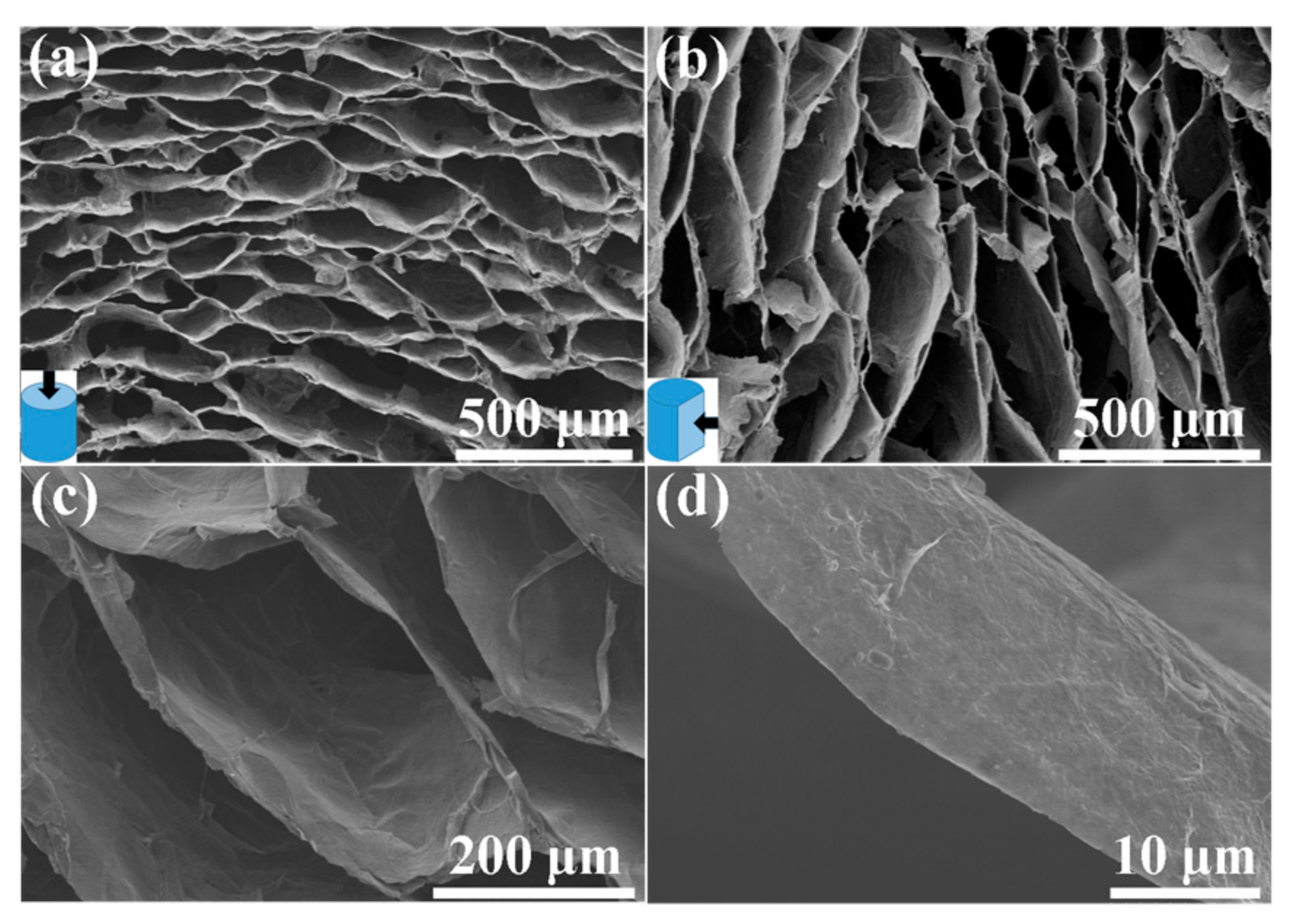
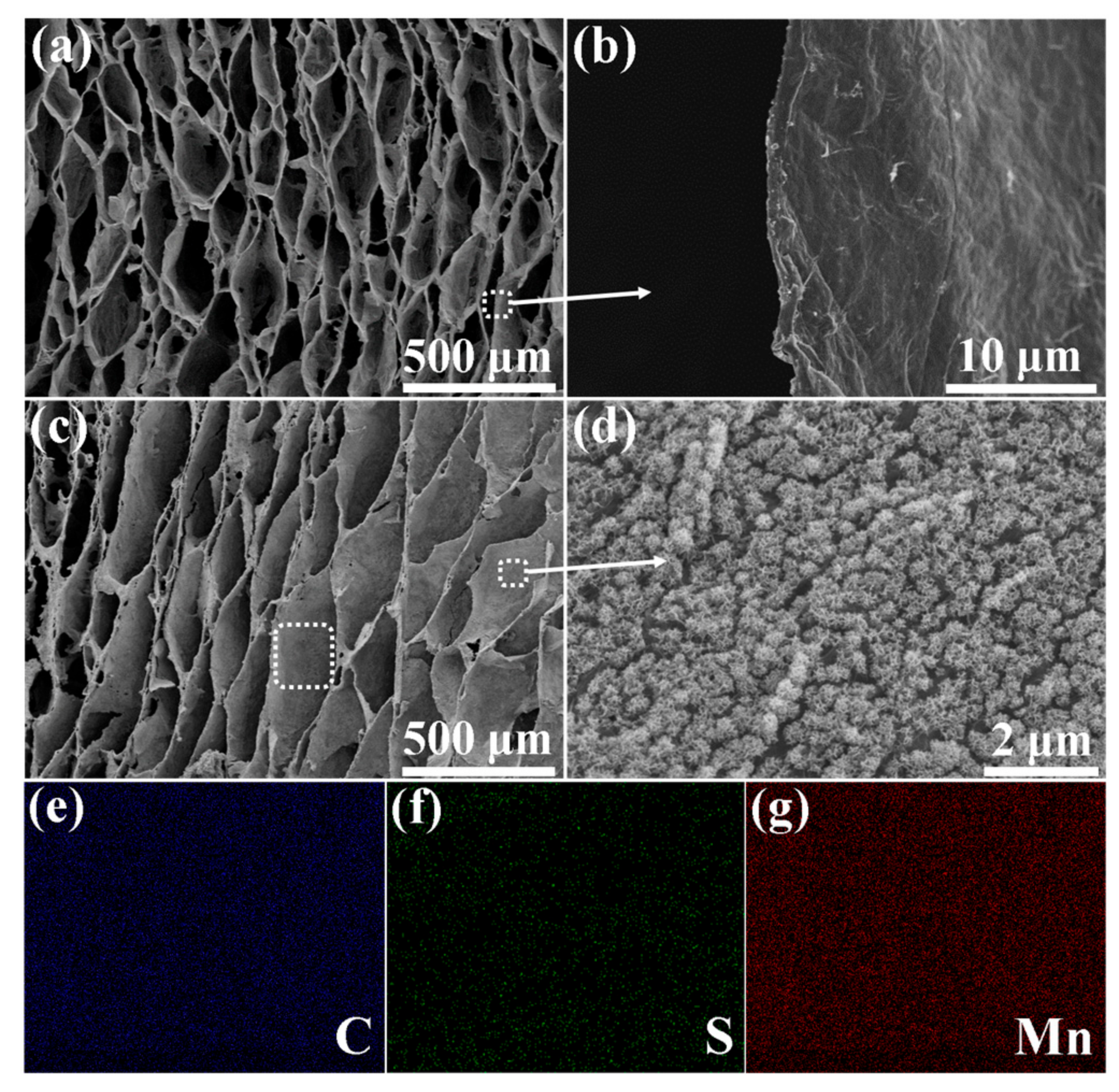
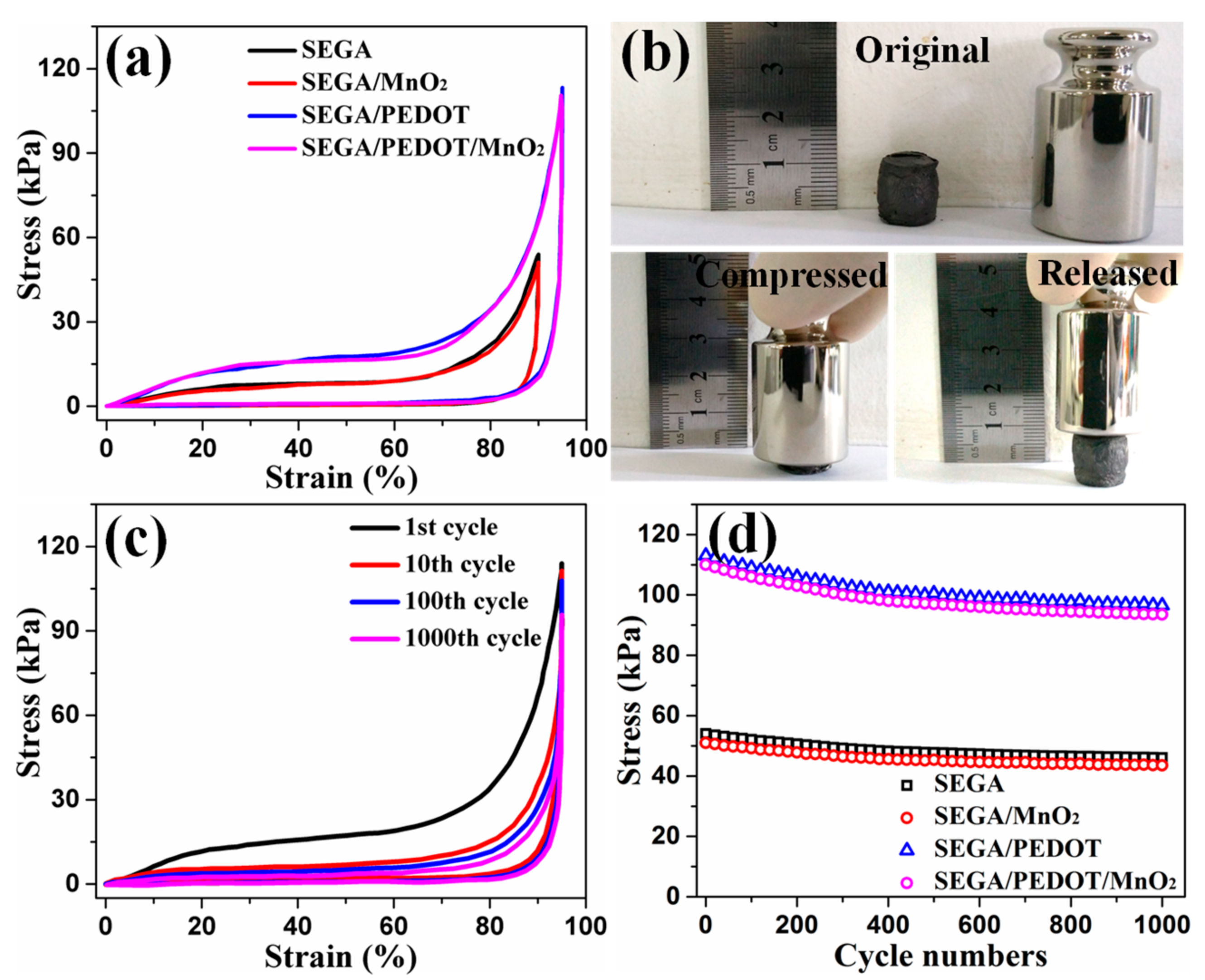
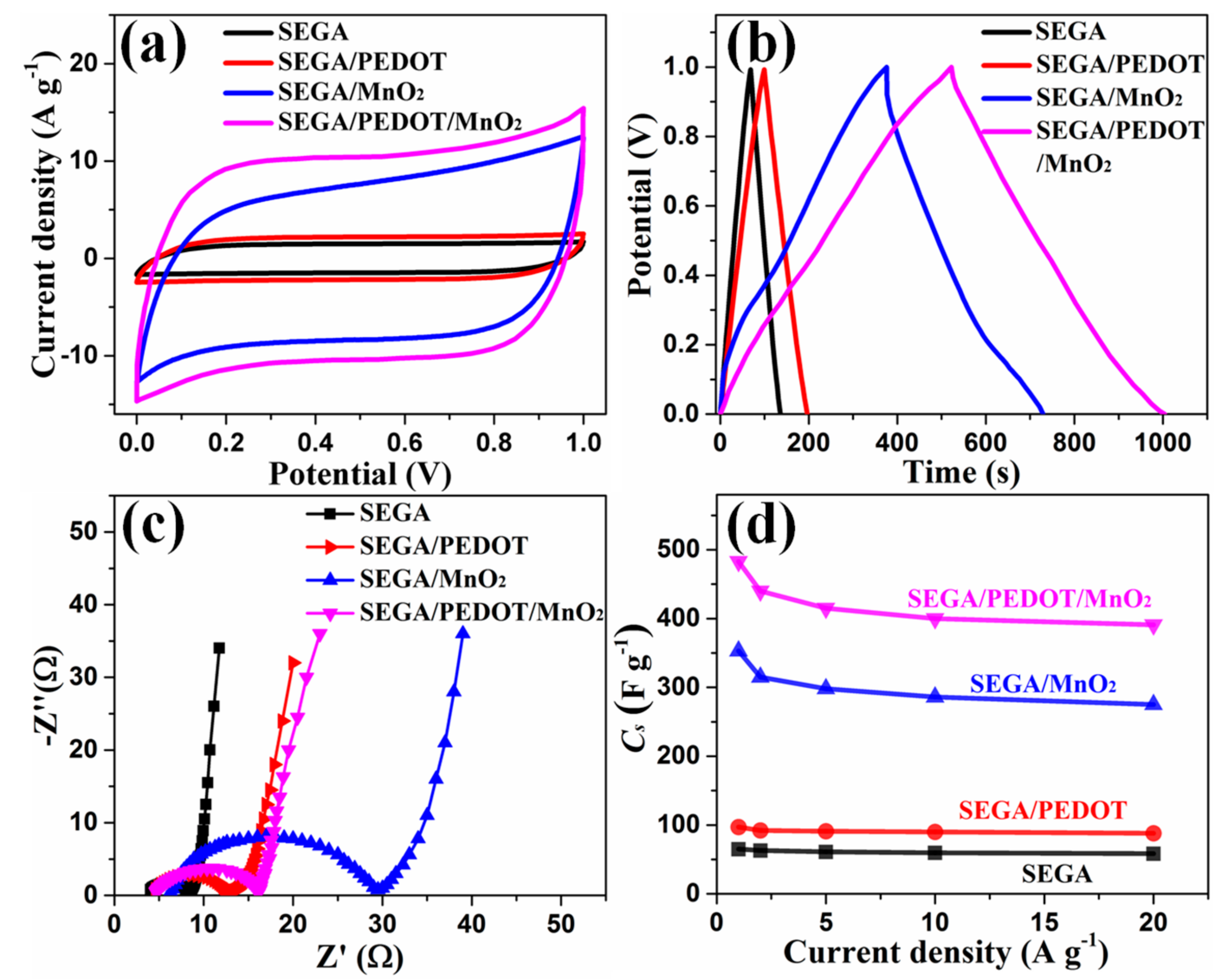
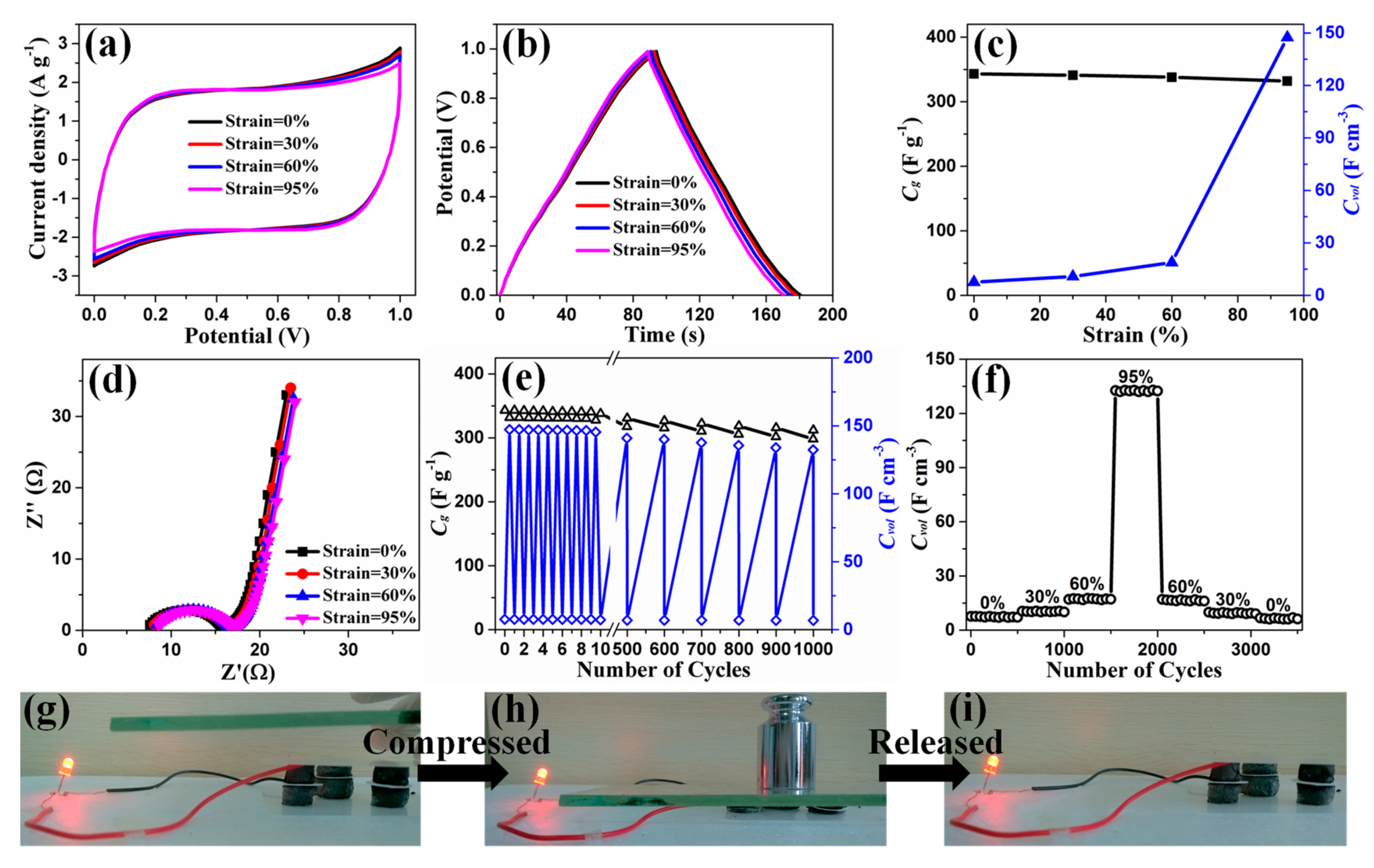
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.; Chen, J.; Huang, L.; Li, C.; Hong, J.D.; Shi, G. Highly compressible macroporous graphene monoliths via an improved hydrothermal process. Adv. Mater. 2014, 26, 4789–4793. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Bak, B.M.; Wie, J.J.; Kong, J.; Park, H.S. Reversibly compressible, highly elastic, and durable graphene aerogels for energy storage devices under limiting conditions. Adv. Funct. Mater. 2015, 25, 1053–1062. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Yu, N.; Hou, Z.; Hu, L.; Ma, Y.; Li, H.; Zhai, T. Smart supercapacitors with deformable and healable functions. J. Mater. Chem. A 2017, 5, 16–30. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xue, Y.; Roy, A.K.; Dai, L. Transparent and stretchable high-performance supercapacitors based on wrinkled graphene electrodes. ACS Nano 2014, 8, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, G.; Marais, A.; Karabulut, E.; Wagberg, L.; Cui, Y.; Hamedi, M.M. Self-assembled three-dimensional and compressible interdigitated thin-film supercapacitors and batteries. Nat. Commun. 2015, 6, 7259. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, T.; Jiang, M.; Duan, Y.; Zhang, J. Ambient pressure dried graphene aerogels with superelasticity and multifunctionality. J. Mater. Chem. A 2015, 3, 19268–19272. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, X.; Lin, D.; Chen, W.; Xiong, G.; Yu, Y.; Fisher, T.S.; Li, H. Hyperbolically patterned 3D graphene metamaterial with negative poisson’s ratio and superelasticity. Adv. Mater. 2016, 28, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, P.; Cheng, H.; Zhao, Y.; Zhang, Z.; Shi, G.; Qu, L. Solution-processed ultraelastic and strong air-bubbled graphene foams. Small 2016, 12, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. Flexible all-solid-state asymmetric supercapacitors based on free-standing carbon nanotube/graphene and Mn3O4 nanoparticle/graphene paper electrodes. ACS Appl. Mater. Interfaces 2012, 4, 7019–7025. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; She, P.; Xu, K.; Shang, Y.; Yin, S.; Liu, Z. A self-standing nanocomposite foam of polyaniline@reduced graphene oxide for flexible super-capacitors. Synth. Met. 2015, 209, 68–73. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Chen, I.W.; Lin, T.; Huang, F. A new tubular graphene form of a tetrahedrally connected cellular structure. Adv. Mater. 2015, 27, 5943–5949. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, J.Z.; Chang, S.L.Y.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Islam, M.F. Ultracompressible, high-rate supercapacitors from graphene-coated carbon nanotube aerogels. ACS Appl. Mater. Interfaces 2015, 7, 5612–5618. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, K.; Huang, Z.; Zhang, C.; Liu, T. Highly ordered graphene architectures by duplicating melamine sponges as a three-dimensional deformation-tolerant electrode. Nano Res. 2016, 9, 2938–2949. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Hu, Y.; Cheng, H.; Hu, C.; Jiang, C.; Jiang, L.; Cao, A.; Qu, L. Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes. Adv. Mater. 2013, 25, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, M.P.; Liu, S.; Islam, M.F. Superelastic pseudocapacitors from freestanding MnO2-decorated graphene-coated carbon nanotube aerogels. ACS Appl. Mater. Interfaces 2017, 9, 23810–23819. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Liu, F.; Xu, F.; Bi, H.; Du, Y.; Tang, Y.; Huang, F. Superelastic few-layer carbon foam made from natural cotton for all-solid-state electrochemical capacitors. ACS Appl. Mater. Interfaces 2015, 7, 25306–25312. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Ding, L.X.; Liu, G.; Chen, H.; Wang, S.; Wang, H. Freestanding, hydrophilic nitrogen-doped carbon foams for highly compressible all solid-state supercapacitors. Adv. Mater. 2016, 28, 5997–6002. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Peng, X.; Jin, H.; Li, T.; Zhang, C.; Gao, B.; Hu, B.; Huo, K.; Zhou, J. Freestanding mesoporous VN/CNT hybrid electrodes for flexible all-solid-state supercapacitors. Adv. Mater. 2013, 25, 5091–5097. [Google Scholar] [CrossRef] [PubMed]
- Han, M.G.; Foulger, S.H. 1-Dimensional structures of poly(3,4-ethylenedioxythiophene) (PEDOT): A chemical route to tubes, rods, thimbles, and belts. Chem. Commun. 2005, 3092–3094. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, W.; Su, X.; Xiao, S.; Xie, H.; Uher, C.; Tang, X. Chemical synthesis and enhanced electrical properties of bulk poly(3,4-ethylenedioxythiophene)/reduced graphene oxide nanocomposites. Synth. Met. 2017, 229, 65–71. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, W.; Li, G.; Wang, J.; Lu, Y. Graphene/poly(3,4-ethylenedioxythiophene) hydrogel with excellent mechanical performance and high conductivity. Carbon 2013, 59, 495–502. [Google Scholar] [CrossRef]
- Lv, P.; Feng, Y.Y.; Li, Y.; Feng, W. Carbon fabric-aligned carbon nanotube/MnO2/conducting polymers ternary composite electrodes with high utilization and mass loading of MnO2 for super-capacitors. J. Power Sources 2012, 220, 160–168. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, P.; Feng, Y.; Li, Y.; Feng, W. High-performance electrochemical capacitors using electrodeposited MnO2 on carbon nanotube array grown on carbon fabric. Electrochim. Acta 2012, 78, 515–523. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene-MnO2 composite as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Yan, J.; Wei, T.; Fan, Z.; Wei, F. Compressible aligned carbon nanotube/MnO2 as high-rate electrode materials for supercapacitors. J. Electroanal. Chem. 2012, 684, 32–37. [Google Scholar] [CrossRef]
- Cai, J.; Huang, Y.; Huang, B.; Zheng, S.; Guo, Y. Enhanced activity of Pt nanoparticle catalysts supported on manganese oxide-carbon nanotubes for ethanol oxidation. Int. J. Hydrogen Energy 2014, 39, 798–807. [Google Scholar] [CrossRef]
- Li, C.; Qiu, L.; Zhang, B.; Li, D.; Liu, C.Y. Robust vacuum-/air-dried graphene aerogels and fast recoverable shape-memory hybrid foams. Adv. Mater. 2016, 28, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, M.; Li, X.; Wang, C.; Gui, C.X.; Yu, Z.Z. Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids. Carbon 2016, 100, 456–464. [Google Scholar] [CrossRef]
- Qiu, L.; Coskun, M.B.; Tang, Y.; Liu, J.Z.; Alan, T.; Ding, J.; Van-Tan, T.; Li, D. Ultrafast Dynamic Piezoresistive Response of Graphene-Based Cellular Elastomers. Adv. Mater. 2016, 28, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Y.; Shi, E.; Shen, Q.; Shang, Y.; Wu, S.; Wei, J.; Wang, K.; Zhu, H.; Yuan, Q.; et al. Core-double-shell, carbon nanotube@polypyrrole@MnO2 sponge as freestanding, compressible supercapacitor electrode. ACS Appl. Mater. Interfaces 2014, 6, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Li, J.; Yuan, Y.; He, X.; Li, Y. Lightweight, superelastic, and mechanically flexible graphene/polyimide nanocomposite foam for strain sensor application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, D.; Wang, Y.; Cheng, C.; Zhou, K.; Ding, J.; Van-Tan, T.; Li, D. Mechanically robust, electrically conductive and stimuli-responsive binary network hydrogels enabled by superelastic graphene aerogels. Adv. Mater. 2014, 26, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Sang, X.; Liu, C.; Luo, T.; Peng, L.; Han, B.; Tan, X.; Ma, X.; Wang, D.; et al. Cellular graphene aerogel combines ultralow weight and high mechanical strength: A highly efficient reactor for catalytic hydrogenation. Sci. Rep. 2016, 6, 25830. [Google Scholar] [CrossRef] [PubMed]
| Materials | Uncompressed Capacitance | Maximum Compressive Strain | Compressed Capacitance | Mass content/Mass Loading of Pseudomaterials | Crystalline Phases of MnO2 | Test Condition | Reffrence |
|---|---|---|---|---|---|---|---|
| Cross-linked graphene aerogel | 90 F g−1 | 90% | 130 F g−1 | - | - | 10 mV s−1 | [2] |
| 0.94 F cm−3 | 13.6 F cm−3 | ||||||
| Graphene-carbon nanotube aerogel | 37 F g−1 | 90% | 45 F g−1 | - | - | 1 A g−1 | [17] |
| 0.5 F cm−3 | 6 F cm−3 | ||||||
| Melamine foam/graphene/polypyrrole sponge | 411 F g−1 | 75% | 329 F g−1 | 3.92 wt % | - | 10 mV s−1 | [18] |
| N.A. | |||||||
| Graphene/polypyrrole foam | 350 F g−1 | 50% | 350 F g−1 | N.A. | - | 1.5 A g−1 | [19] |
| 14 F cm−3 | 28 F cm−3 | 6.7–7.4 mg cm−2 | |||||
| Graphene-carbon nanotube/MnO2 aerogel | 98 F g−1 | 50% | 106 F g−1 | 17 wt % | Manganite and Ramsdelite | 2 mV s−1 | [20] |
| 1.5 F cm−3 | 3.3 F cm−3 | 0.4 mg cm−2 | |||||
| SEGA/PEDOT/MnO2 composite | 343 F g−1 | 95% | 332 F g−1 | 77.4 wt % | Birnessite-type | 1 A g−1 | This work |
| 7.6 F cm−3 | 147.4 F cm−3 | 8.6 mg cm−2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, P.; Wang, Y.; Ji, C.; Yuan, J. Superelastic Graphene Aerogel/Poly(3,4-Ethylenedioxythiophene)/MnO2 Composite as Compression-Tolerant Electrode for Electrochemical Capacitors. Materials 2017, 10, 1353. https://doi.org/10.3390/ma10121353
Lv P, Wang Y, Ji C, Yuan J. Superelastic Graphene Aerogel/Poly(3,4-Ethylenedioxythiophene)/MnO2 Composite as Compression-Tolerant Electrode for Electrochemical Capacitors. Materials. 2017; 10(12):1353. https://doi.org/10.3390/ma10121353
Chicago/Turabian StyleLv, Peng, Yaru Wang, Chenglong Ji, and Jiajiao Yuan. 2017. "Superelastic Graphene Aerogel/Poly(3,4-Ethylenedioxythiophene)/MnO2 Composite as Compression-Tolerant Electrode for Electrochemical Capacitors" Materials 10, no. 12: 1353. https://doi.org/10.3390/ma10121353
APA StyleLv, P., Wang, Y., Ji, C., & Yuan, J. (2017). Superelastic Graphene Aerogel/Poly(3,4-Ethylenedioxythiophene)/MnO2 Composite as Compression-Tolerant Electrode for Electrochemical Capacitors. Materials, 10(12), 1353. https://doi.org/10.3390/ma10121353




