Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Pillared Clays
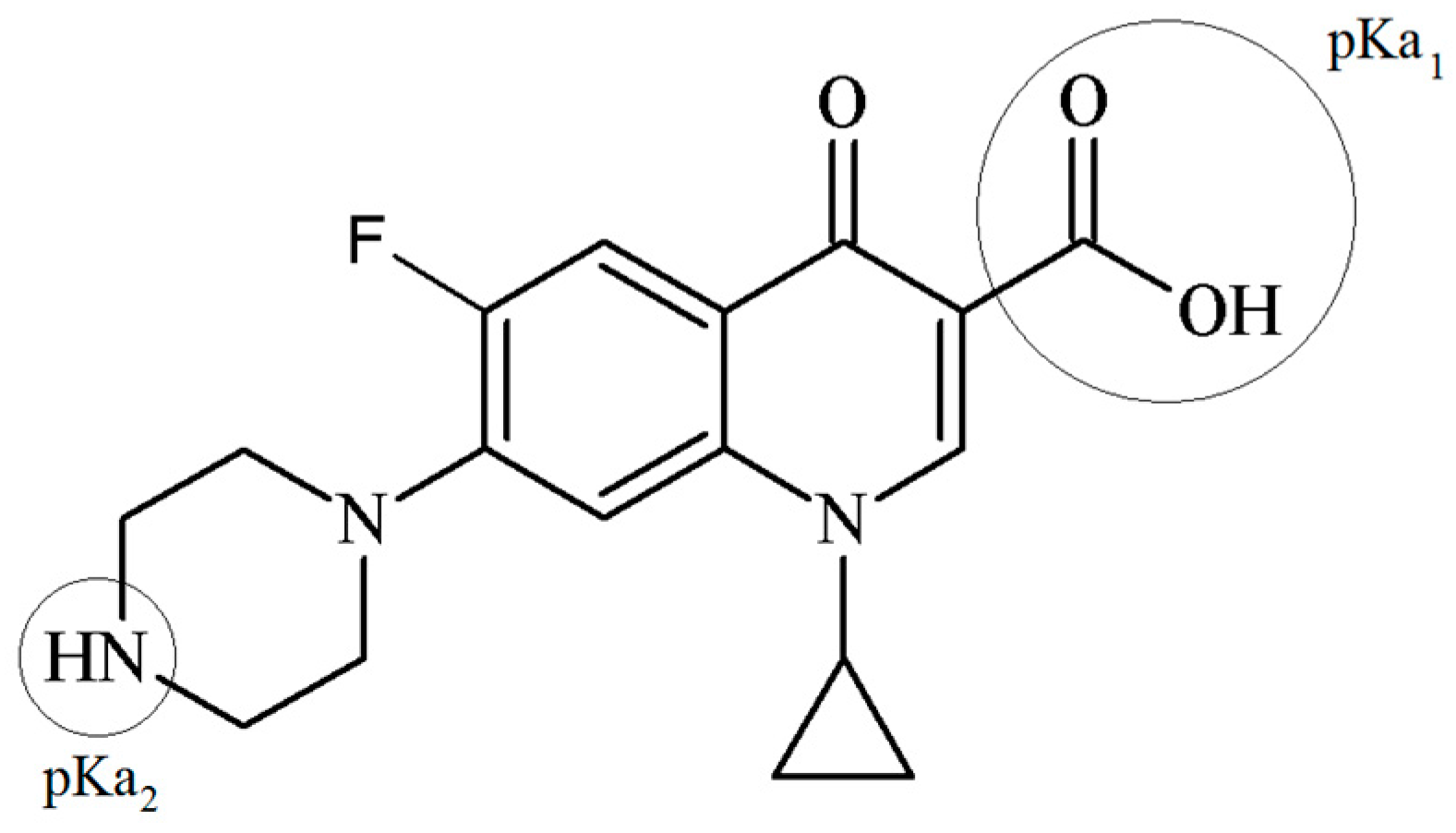
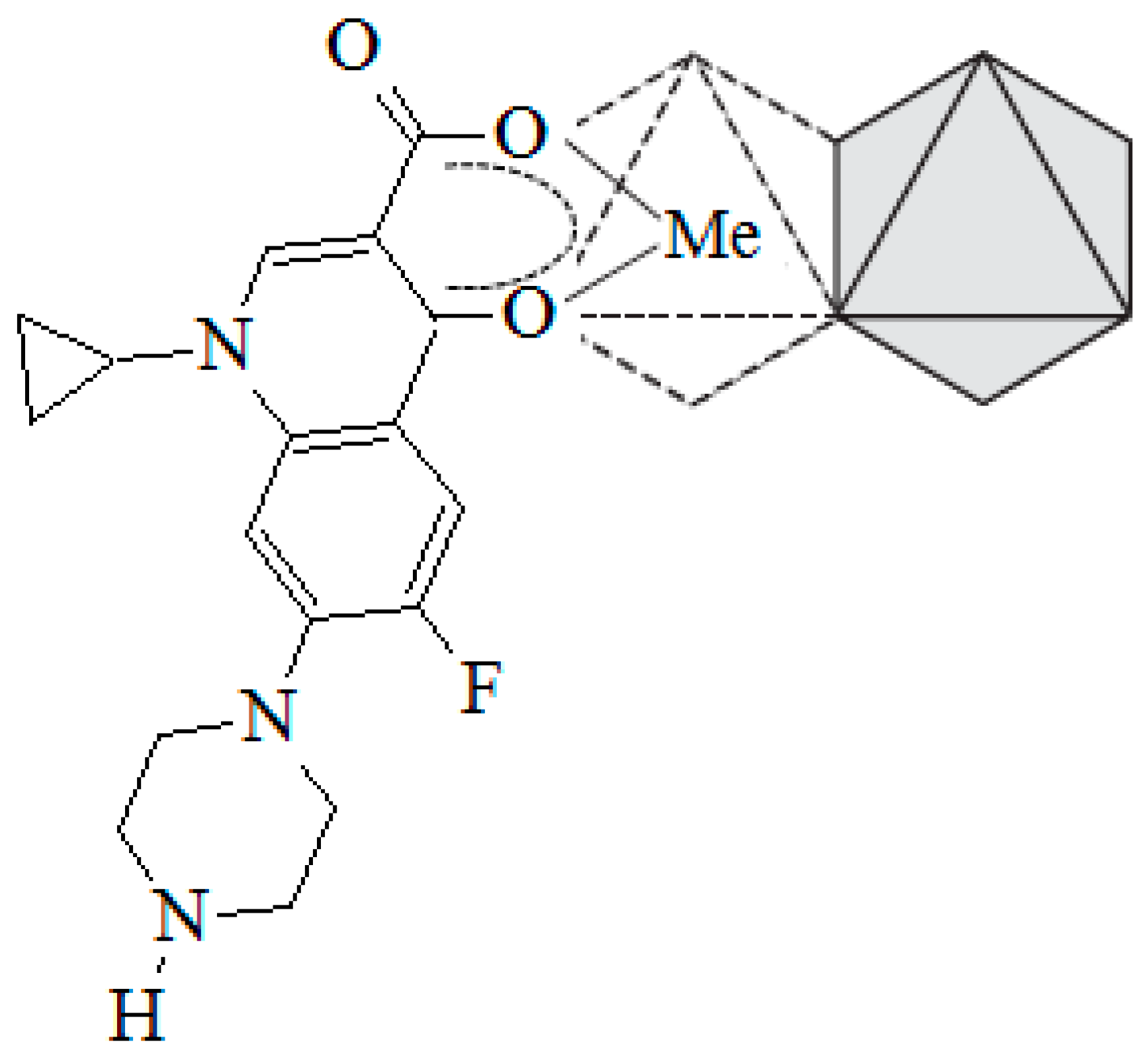
2.2. Adsorptive: Ciprofloxacin
2.3. CPX Adsorption Studies
2.4. Modelling Methods
3. Results and Discussion
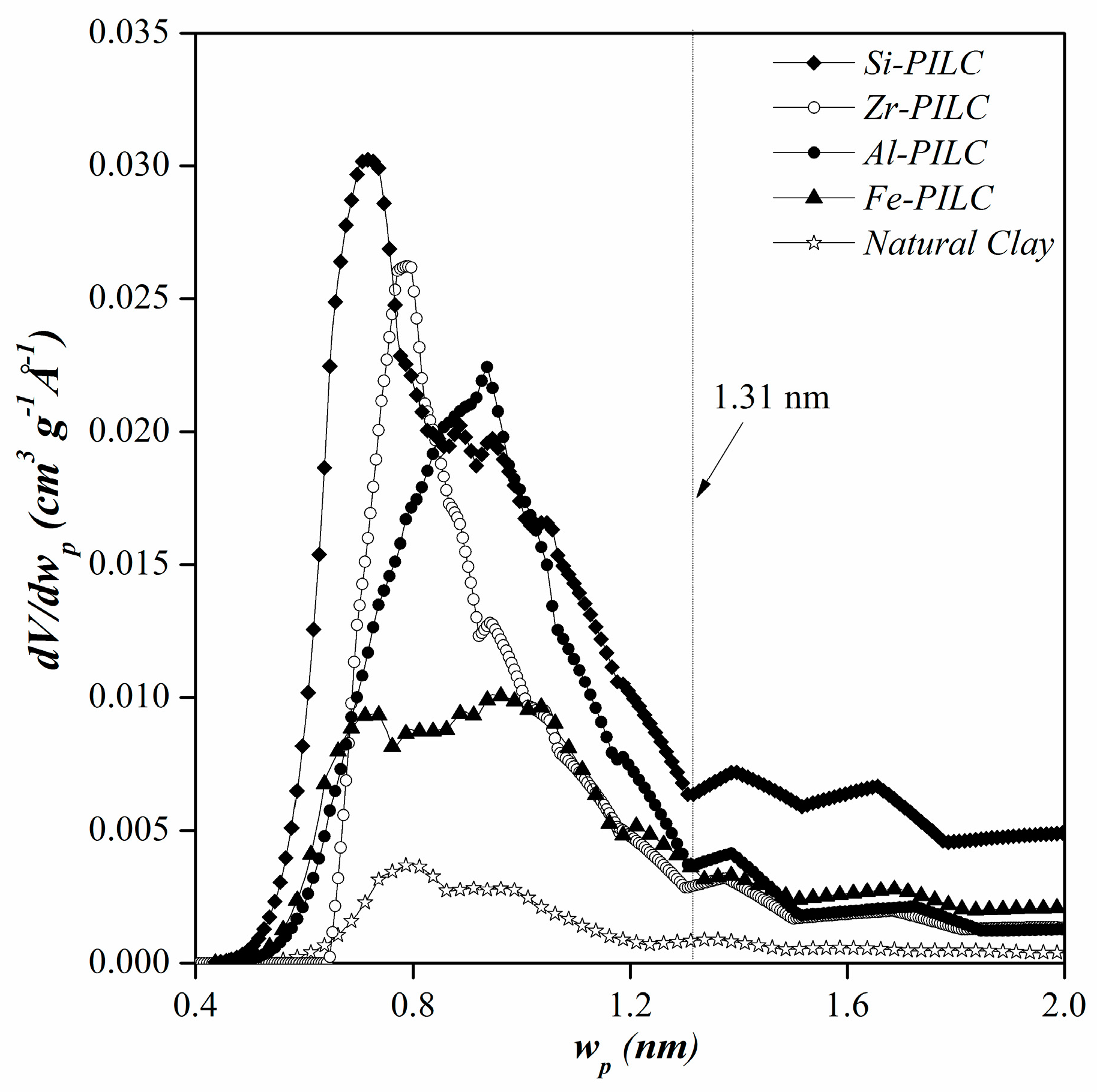
3.1. Adsorbents Characterization
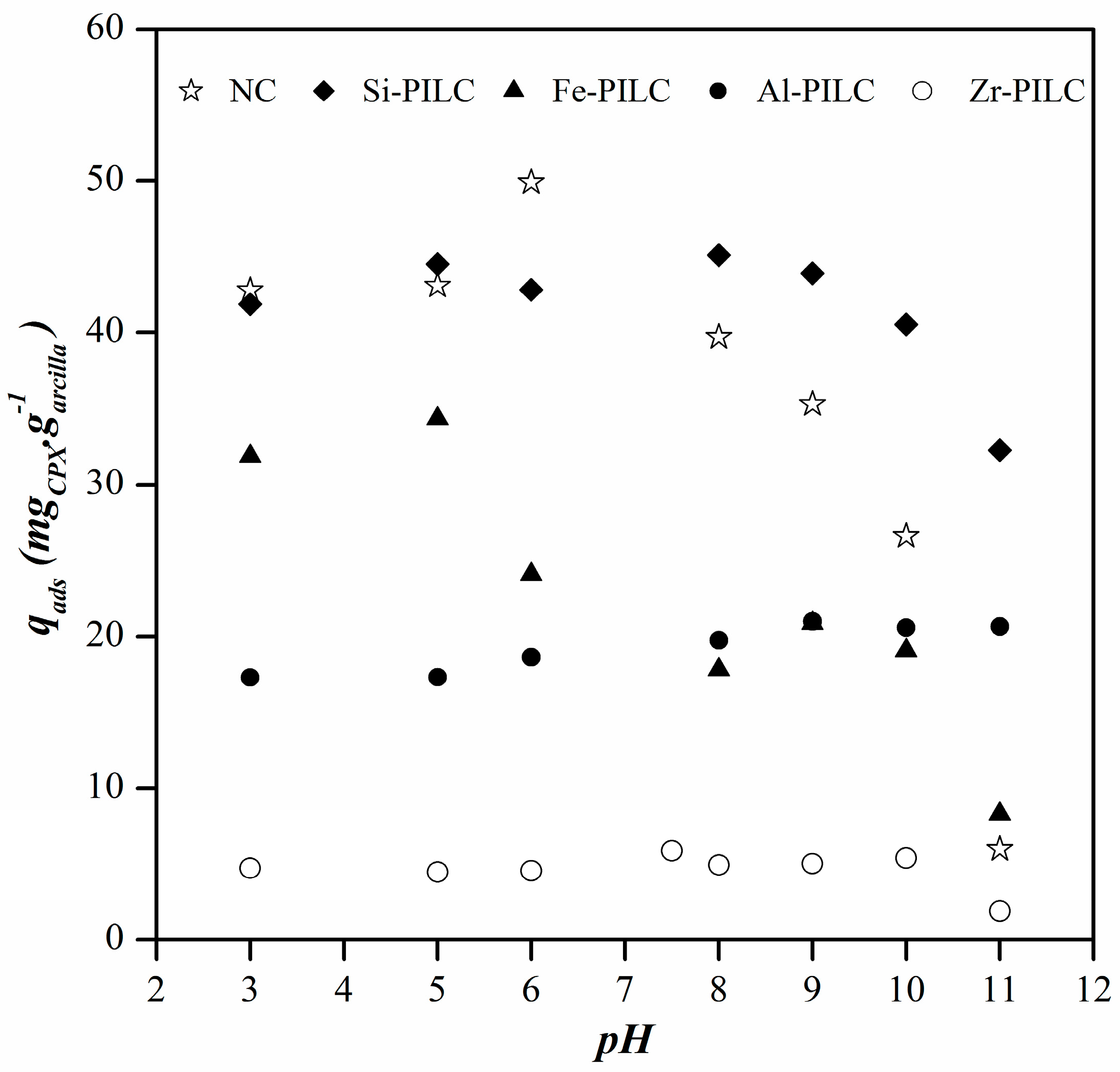
3.2. Effect of pH Media on Adsorption
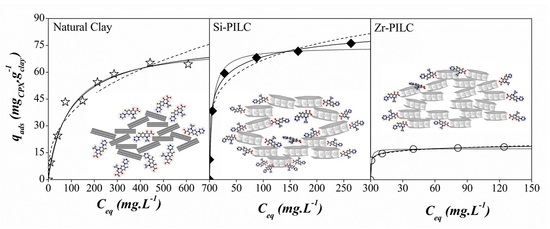
3.3. Adsorption Isotherms
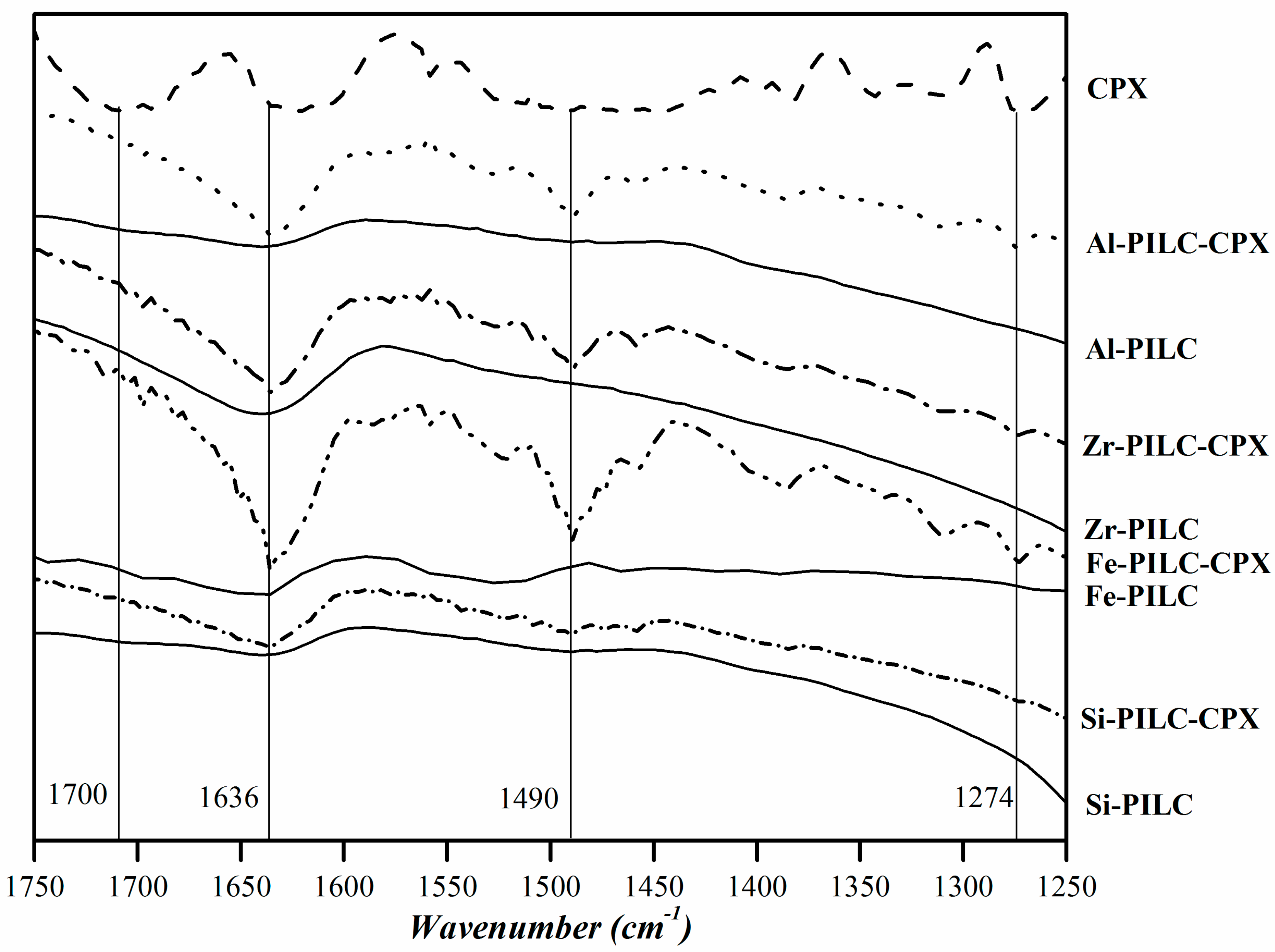
3.4. Evidences of CPX Interactions with PILC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dietrich, D.R.; Hitzfeld, B.C.; O’Brien, E. Toxicology and Risk assessment of pharmaceuticals. In Organic Pollutants in the Water Cycle; Reemtsma, T., Jekel, M., Eds.; John Wiley & Sons: Weinheim, Germany, 2006; pp. 287–309. ISBN 9783527608775. [Google Scholar]
- Halling-Sørensen, B.; Holten Lützhøft, H.C.; Andersen, H.R.; Ingerslev, F. Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 2000, 46, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Removal of Emerging contaminants from water and wastewater by adsorption process. In Emerging Compounds Removal from Wastewater. Natural and Solar Based Treatments; Lofrano, G., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 15–37. [Google Scholar]
- Balarak, D.; Mostafapour, F.K.; Bazrafshan, E.; Saleh, T.A. Studies on the adsorption of amoxicillin on multi-wall carbon nanotubes. Water Sci. Technol. 2017, 75, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Calisto, V.; Ferreira, C.I.; Oliveira, J.A.; Otero, M.; Esteves, V.I. Adsorptive removal of pharmaceuticals from water by commercial and waste-based carbons. J. Environ. Manag. 2015, 152, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, F.; Lam, F.L.; Wang, Y.; Liu, Z.; Dai, H. Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon. J. Colloid Interface Sci. 2015, 460, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Liang, Z.; Zhaob, Z.; Sun, T.; Shi, W.; Cui, F. Adsorption of quinolone antibiotics in spherical mesoporous silica: Effects of the retained template and its alkyl chain length. J. Hazard. Mater. 2016, 305, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Karthileyan, G. Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides. Environ. Sci. Technol. 2005, 39, 9166–9173. [Google Scholar] [CrossRef] [PubMed]
- Genç, N.; Dogan, E.C.; Yurtsever, M. Bentonite for ciprofloxacin removal from aqueous solution. Water Sci. Technol. 2013, 68, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-T.; Chang, P.-H.; Wang, Y.-S.; Tsai, Y.; Jean, J.-S.; Li, Z.; Krukowski, K. Removal of ciprofloxacin from water by birnessite. J. Hazard. Mater. 2013, 250–251, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Roca Jalil, M.E.; Baschini, M.; Sapag, K. Influence of pH and antibiotic solubility on the removal of ciprofloxacin from aqueous media using montmorillonite. Appl. Clay Sci. 2015, 114, 69–76. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Z.; Jiang, W.-T. Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl. Clay Sci. 2011, 53, 723–728. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Z.; Jiang, W.-T.; Jean, J.-S.; Liu, C.-C. Cation Exchange interaction between antibiotic ciprofloxacin and montmorillonite. J. Hazard. Mater. 2010, 183, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Mabrouki, H.; Akretche, D.E. Diclofenac potassium removal from water by adsorption on natural and Pillared Clay. Desalin. Water Treat. 2016, 57, 6033–6043. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.; Valles, J.; Baschini, M.; Avena, M. Tetracycline adsorption on montmorillonite: Effects of pH and ionic strength. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Wu, H.; Xie, H.; He, G.; Guan, Y.; Zhang, Y. Effects of the pH and anions on the adsorption of tetracycline on iron-montmorillonite. Appl. Clay Sci. 2016, 119, 161–169. [Google Scholar] [CrossRef]
- Al-Khalisy, R.S.; Al-Haidary, A.M.A.; Al-Dujaili, A.H. Aqueous phase adsorption of cephalexin onto bentonite and activated carbon. Sep. Sci. Technol. 2010, 45, 1286–1294. [Google Scholar] [CrossRef]
- Gil, A.; Korili, S.A.; Vicente, M.A. Recent Advances in the control and characterization of the porous structure of pillared clay catalysts. Catal. Rev. 2008, 50, 153–221. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.C.C.; Albeniz, S.; Korili, S.A. Removal of dyes from wastewaters by adsorption on Pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Hou, M.-F.; Ma, C.-X.; Zhang, W.-D.; Tang, X.-Y.; Fan, Y.-N.; Wan, H.-F. Removal of rhodamine B using iron-pillared bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Dong, C.; Wei, H.; Yuan, W.; Li, K. Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): Kinetics, equilibrium and mechanism. Appl. Clay Sci. 2015, 118, 301–307. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noelite, B.F.; Anirudhan, T.S. Removal of Vanadium (IV) from aqueous solutions by adsorption process with aluminum-pillared bentonite. Ind. Eng. Chem. Res. 2005, 44, 6676–6684. [Google Scholar] [CrossRef]
- Mishra, T.; Mahato, D.K. A comparative study on enhanced arsenic (V) and arsenic (III) removal by iron oxide and manganese oxide pillared clays from ground water. J. Environ. Chem. Eng. 2016, 4, 1224–1230. [Google Scholar] [CrossRef]
- Molu, Z.B.; Yurdakoç, K. Preparation and characterization of aluminum Pillared K10 and KSF for adsorption of thimethoprim. Microporous Mesoporous Mater. 2010, 127, 50–60. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Baschini, M.; Rodríguez-Castellón, E.; Infantes-Molina, A.; Sapag, K. Effect of the Al/clay ratio on the thiabendazole removal by aluminum pillared clays. Appl. Clay Sci. 2014, 87, 245–263. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Vieria, R.S.; Azevedo, D.; Baschini, M.; Sapag, M. Improvement in the adsorption of thiabendazole by using aluminum pillared clays. Appl. Clay Sci. 2013, 71, 55–63. [Google Scholar] [CrossRef]
- Yan, L.G.; Xu, Y.Y.; Yu, H.Q.; Xin, X.D.; Wei, Q.; Du, B. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron–aluminum pillared bentonites. J. Hazard. Mat. 2010, 179, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Matsumoto, H.; Yamanaka, S. Preparation of new silica sol-based Pillared clays with high Surface area and high thermal stability. Chem. Mater. 1997, 9, 2013–2018. [Google Scholar] [CrossRef]
- Yamanaka, S.; Doi, T.; Sako, S.; Hattori, M. High surface area solids obtained by intercalation of iron oxide pillars in montmorillonite. Mater. Res. Bull. 1984, 19, 161–168. [Google Scholar] [CrossRef]
- Farfan-Torres, E.M.; Dedeycker, O.; Grange, P. Zirconium pillared clays. Influence of basic polymerization of the precursor on their structure and stability. Stud. Surf. Sci. Catal. 1991, 63, 337–343. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K.; Llewellyn, P.; Maurin, G. Adsorption by powders and porous solids. In Principles Methodology and Applications, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013; ISBN 978-0-08-097035-6. [Google Scholar]
- Villarroel-Rocha, J.; Barrera, D.; García Blanco, A.A.; Roca Jalil, M.E.; Sapag, K. Importance of the α-plot Method in the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2013, 31, 165–183. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Suchithra, P.S. Equilibrium, kinetic and thermodynamic modeling for the adsorption of heavy metals onto chemically modified hydrotalcite. Indian J. Chem. Technol. 2010, 17, 247–259. [Google Scholar]
- Dahlquist, F.W. The Meaning of Scatchard and Hill Plots in: Methods of Enzymology; Hirs, C.H.W., Timasheff, S.N., Eds.; Academic Press: New York, NY, USA, 1978; Volume 48, pp. 270–299. [Google Scholar]
- Gerente, C.; Couespel du Mesnil, P.; Andrès, Y.; Thibault, J.-F.; Le Cloirec, P. Removal of metal ions from aqueous solution on low cost natural polysaccharides Sorption mechanism approach. React. Funct. Polym. 2000, 46, 135–144. [Google Scholar] [CrossRef]
- Gezici, O.; Kara, H.; Ayar, A.; Topkafa, M. Sorption behavior of Cu(II) ions on insolubilized humic acid under acidic conditions: An application of Scatchard plot analysis in evaluating the pH dependence of specific and nonspecific bindings. Sep. Purif. Technol. 2007, 55, 132–139. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Acid activation of Clay Minerals. In Handbook of Clay Science Developments in Clay Science, Part A: Fundamentals, 2nd ed.; Bergaya, F.G., Lagaly, G., Eds.; Elsevier Ltd. Press: Amsterdam, The Netherlands, 2013; Volume 2, pp. 385–408. ISBN 9780080993713. [Google Scholar]
- Mendioroz, S.; Pajares, A.; Benito, I.; Pesquera, C.; González, F.; Blanco, C. Texture evolution of montmotillonite under progressive acid treatment: Change from H3 to H2 type of hysteresis. Langmuir 1987, 3, 676–681. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral organic interactions. In Handbook of Clay Science-Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Press: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 435–505. ISBN 978-0-08-044183-2. [Google Scholar]
- Marco-Brown, J.L.; Barbosa-Lema, C.M.; Torres Sanchez, R.M.; Mercader, R.C.; dos Santos Afonso, M. Adsorption of picloram herbicide on iron oxide pillared montmorillonite. Appl. Clay Sci. 2012, 58, 25–33. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Stumm, W. Chemistry of the Solid-Water Interface. Processes at the Mineral-water and Particle-Water Interface in Natural Systems; John Wiley & Son Inc. Press: New York, NY, USA, 1992; ISBN 0471576727. [Google Scholar]
- Li, Z.; Hong, H.; Liao, L.; Ackley, C.J.; Schulz, L.A.; MacDonald, R.A.; Emard, S.M. A mechanistic study of ciprofloxacin removal by kaolinite. Colloid Surface B 2011, 88, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustafa, J.; Tashtoush, B. Iron (II) and iron (III) perchlorate complexes of ciprofloxacin and norfloxacin. J. Coord. Chem. 2003, 56, 113–124. [Google Scholar] [CrossRef]

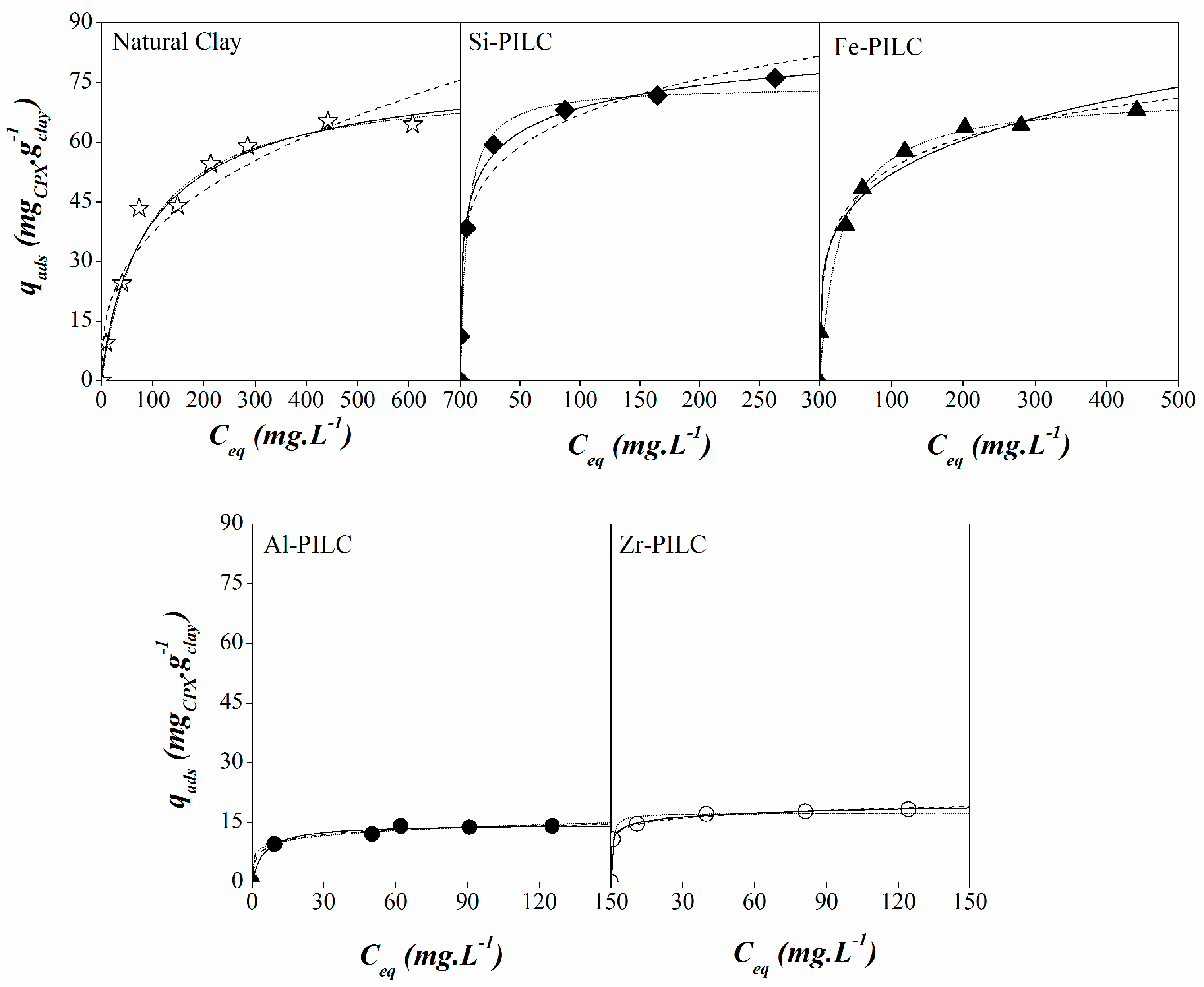

| Materials | SBET (m2 g−1) | VT (cm3 g−1) | Vµp (cm3 g−1) |
|---|---|---|---|
| Natural Clay | 67 | 0.10 | 0.01 |
| Al-PILC | 322 | 0.18 | 0.12 |
| Si-PILC | 519 | 0.31 | 0.19 |
| Zr-PILC | 231 | 0.16 | 0.07 |
| Fe-PILC | 206 | 0.17 | 0.07 |
| Models | Units | NC | Si-PILC | Fe-PILC | Al-PILC | Zr-PILC |
|---|---|---|---|---|---|---|
| Freundlich model | kF (mg g−1(L mg−1)n) | 6.98 | 28.88 | 18.98 | 6.86 | 11.22 |
| n | 2.75 | 5.49 | 4.57 | 6.45 | 9.48 | |
| R2 | 0.962 | 0.979 | 0.986 | 0.986 | 0.997 | |
| Langmuir model | qm (mg g−1) | 75.73 | 74.12 | 72.09 | 14.48 | 17.34 |
| K (L mg −1) | 0.01 | 0.19 | 0.03 | 0.20 | 1.78 | |
| R2 | 0.993 | 0.971 | 0.966 | 0.986 | 0.975 | |
| Sips model | qm (mg g−1) | 80.82 | 100.60 | 122.10 | 17.78 | 25.20 |
| b (L mg −1) | 0.01 | 0.07 | 0.01 | 0.14 | 0.36 | |
| n | 1.13 | 2.56 | 2.76 | 2.07 | 3.85 | |
| R2 | 0.993 | 0.996 | 0.991 | 0.987 | 0.999 |
| Pillared Clays | Vµp (<2 nm) | Vmp (2–10 nm) | Vmp (10–50 nm) | VT |
|---|---|---|---|---|
| Si-PILC | 0.09 | 0.18 | 0.04 | 0.31 |
| Fe-PILC | 0.03 | 0.08 | 0.06 | 0.17 |
| Al-PILC | 0.10 | 0.04 | 0.04 | 0.18 |
| Zr-PILC | 0.06 | 0.05 | 0.06 | 0.17 |
| Adsorbent | qm,CPX (pH) (mg g−1) | Reference |
|---|---|---|
| Aluminum hydrous oxide | 14.72 (7) | [14] |
| Iron hydrous oxide | 25.76 (7) | |
| Ca2+-montmorillonite (Saz) | 330 (4–5.5) | [19] |
| Activated carbon | 231 (≈7) | [8] |
| Carbon nanotubes | 135 (≈7) | |
| Carbon xerogel | 112 (≈7) | |
| kaolinite | 6.99 (5–6) | [53] |
| Illite | 33 (4–5.5) | [18] |
| Rectorie | 135 (4–5.5) | |
| Bentonite | 147 (4.5) | [15] |
| Birnessite | 80.96 (5–6) | [16] |
| Montmorillonite | 332.8 (3) 138.7 (6) 71.6 (7.5) 80.82 (10) | [17] |
| Graphene Oxide | 379 (5) | [11] |
| CMK-3 CMK-3 modified Bamboo-based carbon Bamboo-based carbon modified | 281.47 (<7) 369.34 (<7) 153.17 (<7) 237.44 (<7) | [10] |
| Multi-walled nanotubes | 194 (4) | [12] |
| Si-PILC | 100.6 (10) | This work |
| Fe-PILC | 122.1 (10) | This work |
| Al-PILC | 17.78 (10) | This work |
| Zr-PILC | 25.20 (10) | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roca Jalil, M.E.; Baschini, M.; Sapag, K. Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays. Materials 2017, 10, 1345. https://doi.org/10.3390/ma10121345
Roca Jalil ME, Baschini M, Sapag K. Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays. Materials. 2017; 10(12):1345. https://doi.org/10.3390/ma10121345
Chicago/Turabian StyleRoca Jalil, Maria Eugenia, Miria Baschini, and Karim Sapag. 2017. "Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays" Materials 10, no. 12: 1345. https://doi.org/10.3390/ma10121345
APA StyleRoca Jalil, M. E., Baschini, M., & Sapag, K. (2017). Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays. Materials, 10(12), 1345. https://doi.org/10.3390/ma10121345