Preparation of Advanced CuO Nanowires/Functionalized Graphene Composite Anode Material for Lithium Ion Batteries
Abstract
:1. Introduction
2. Experimental
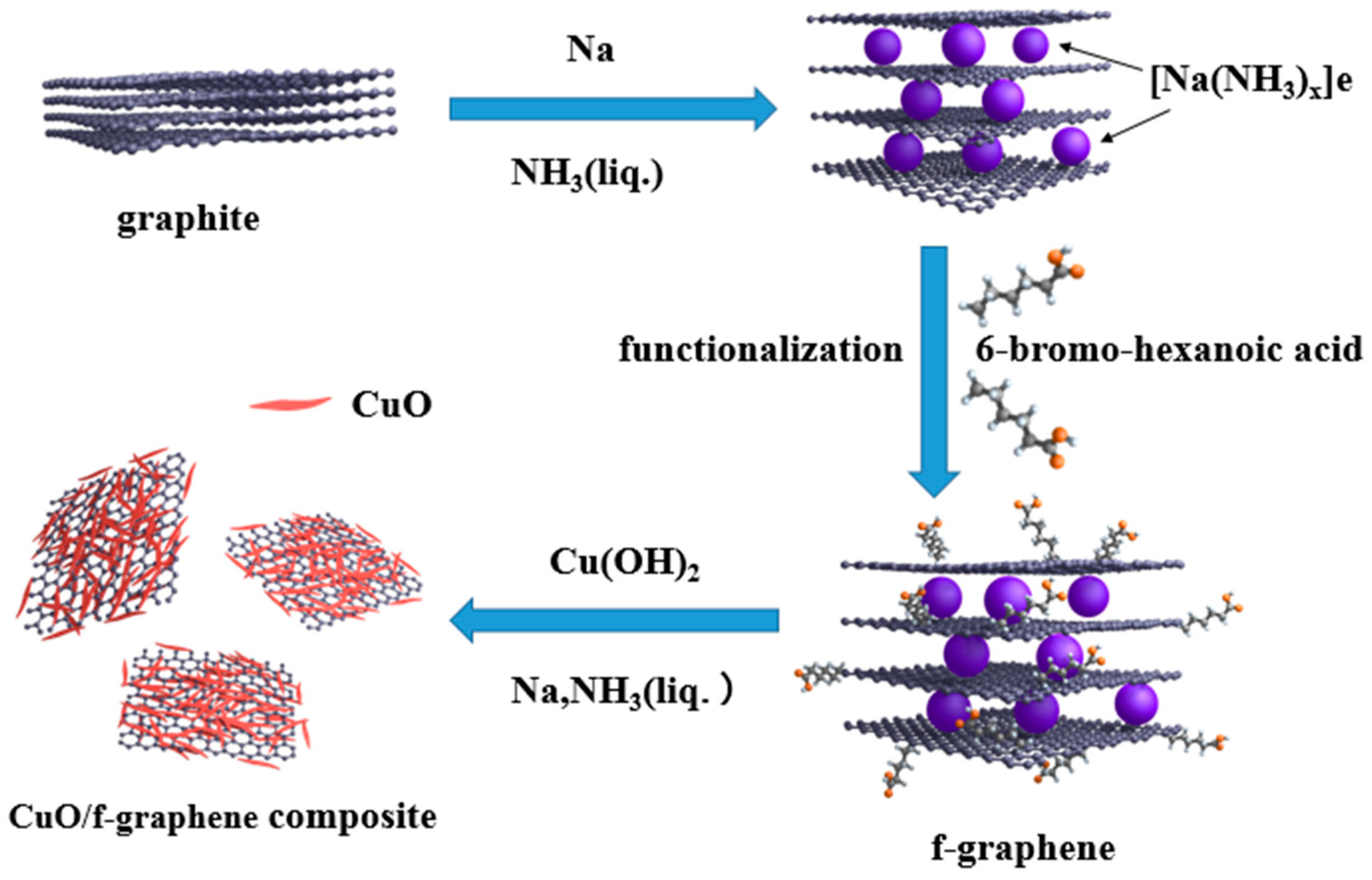
2.1. Synthesis of CuO/f-Graphene Composite
2.2. Materials Characterizations
2.3. Electrochemical Measurements
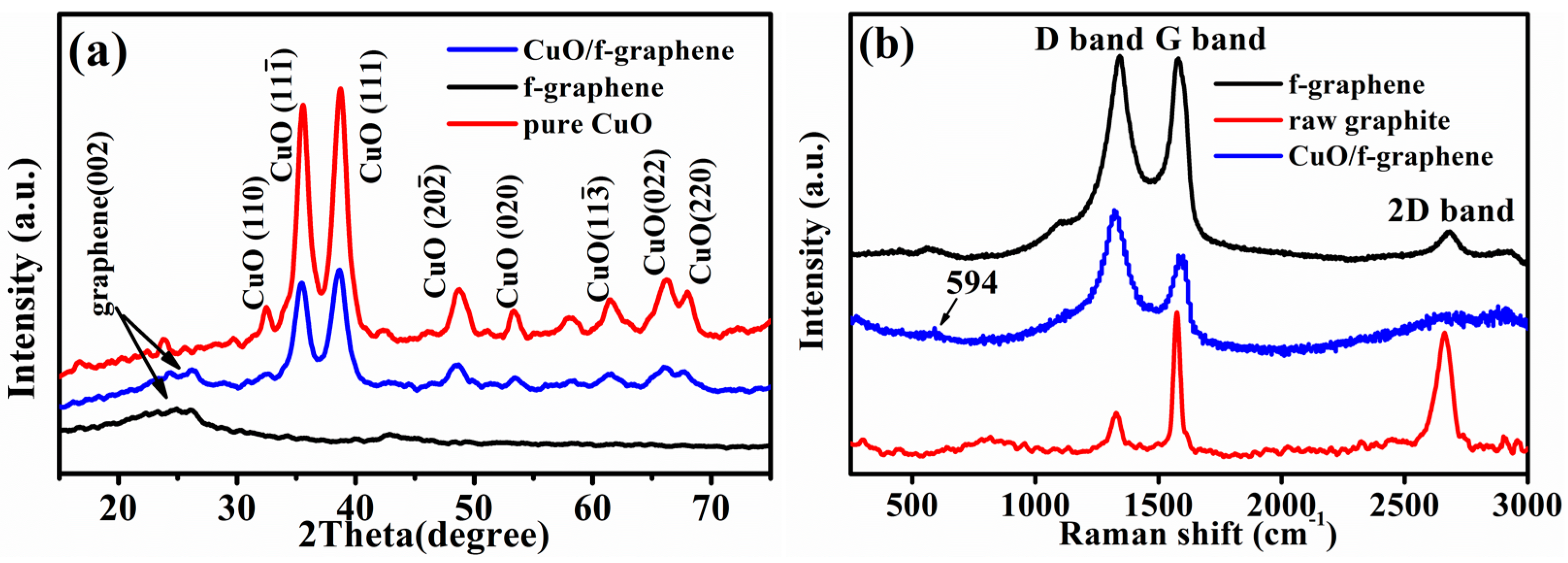
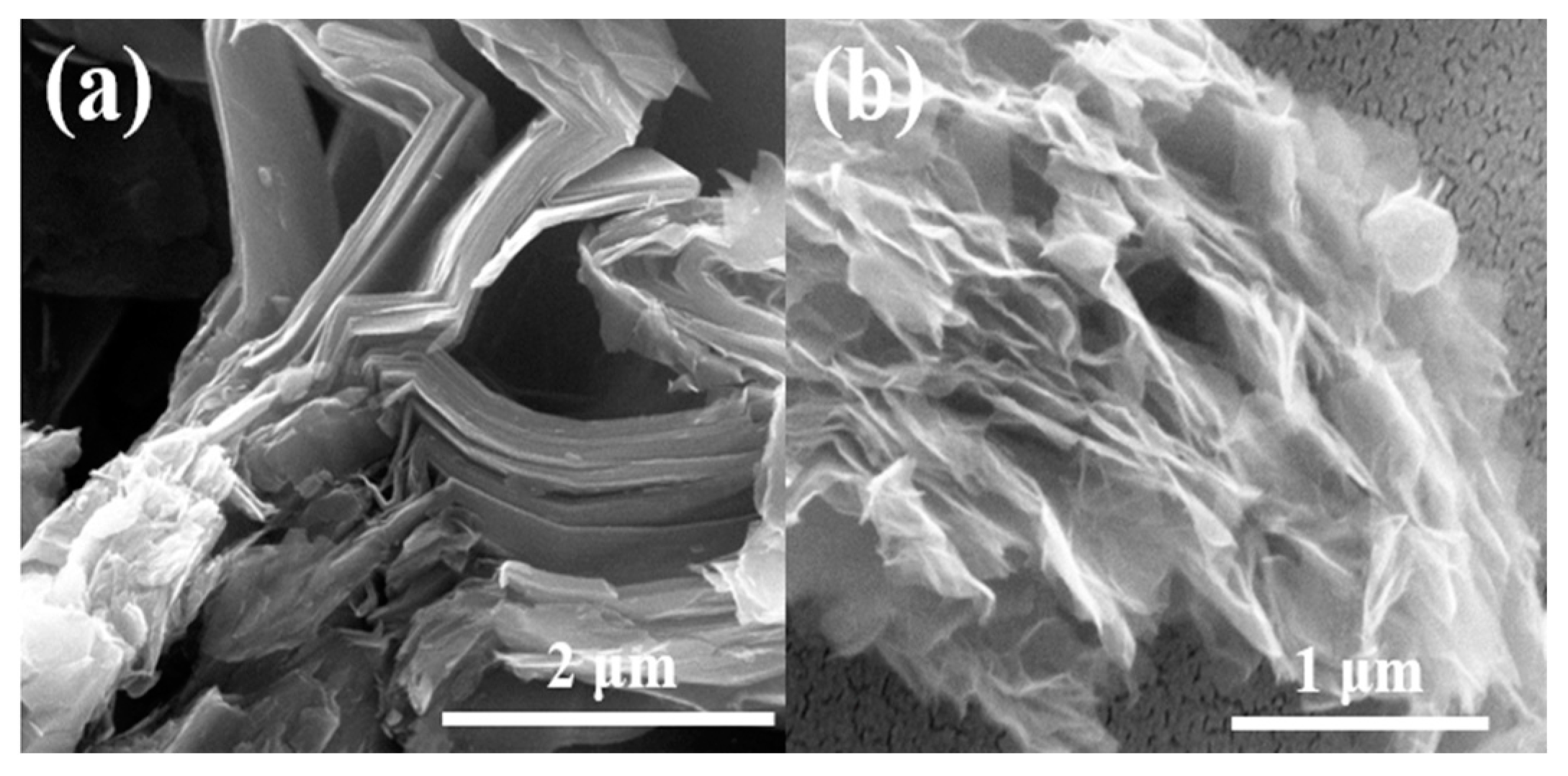
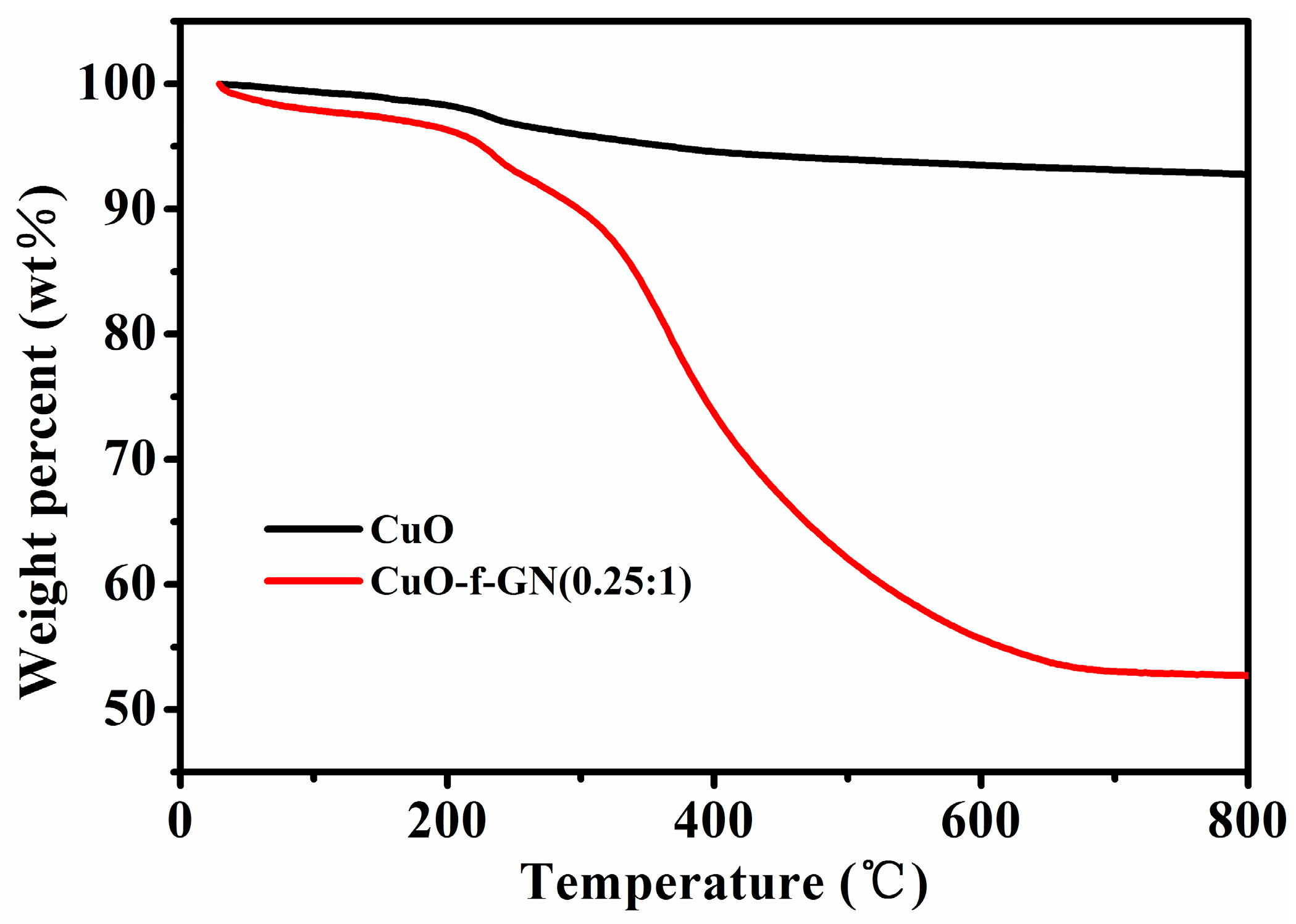
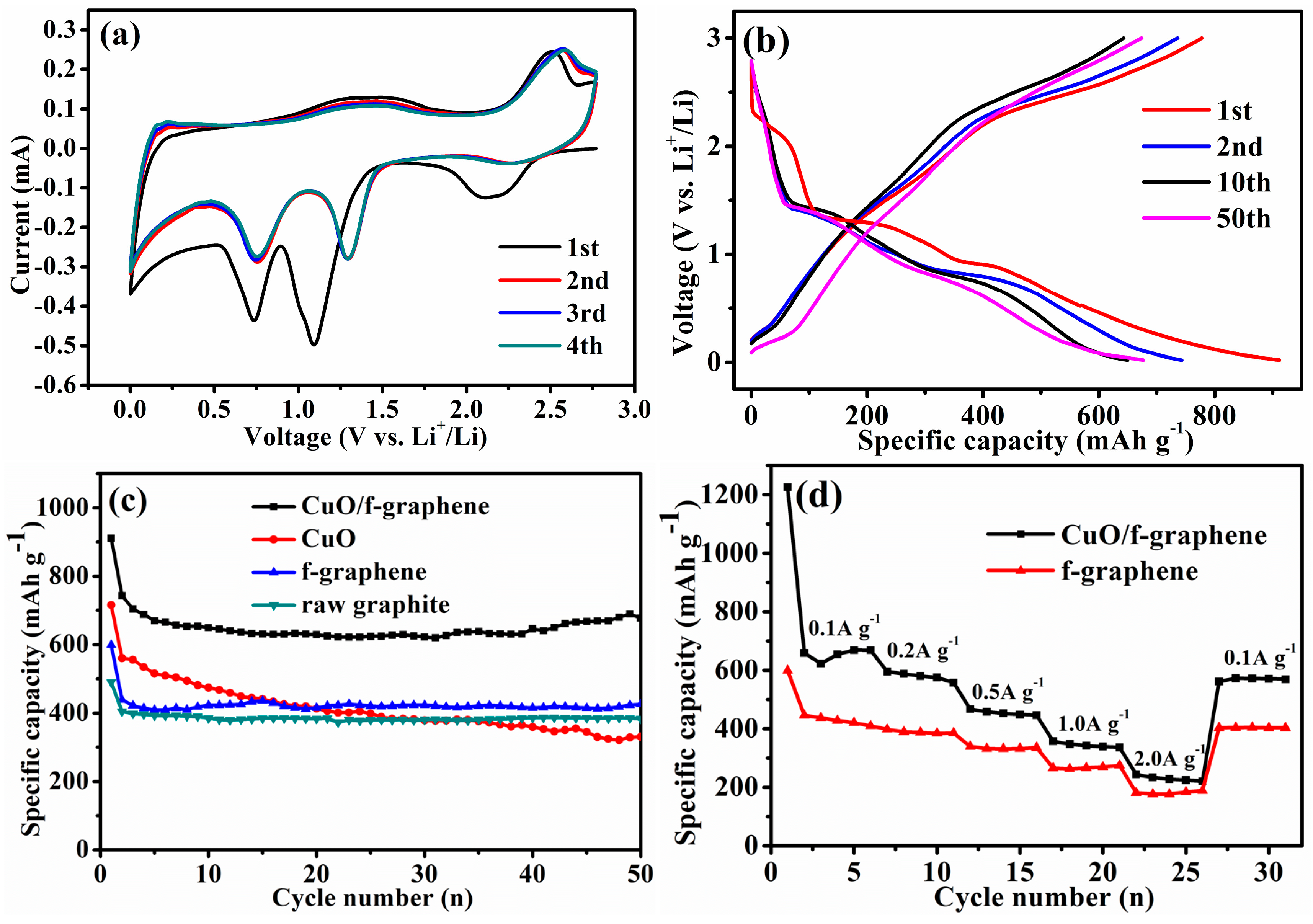
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiao, A.; Zhou, S.; Zuo, C.; Zhuan, Y.; Ding, X. Improved electrochemical performances of CuO nanotube array prepared via electrodeposition as anode for lithium ion battery. Mater. Res. Bull. 2015, 70, 795–798. [Google Scholar] [CrossRef]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fan, C.; Sun, C.; Ren, Z.; Fu, X.; Qian, G.; Wang, Z. Synthesis of different CuO nanostructures from Cu(OH)2 nanorods through changing drying medium for lithium-ion battery anodes. RSC Adv. 2015, 5, 28611–28618. [Google Scholar] [CrossRef]
- Zhao, H.; Qi, W.; Li, X.; Zeng, H.; Wu, Y.; Xiang, J.; Zhang, S.; Li, B.; Huang, Y. SnSb/TiO2/C nanocomposite fabricated by high energy ball milling for high-performance lithium-ion batteries. RSC Adv. 2016, 6, 32462–32466. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Wang, Z.; Xiong, X.; Hu, P.; Liu, Y.; Huang, Y. Controllable growth of TiO2-B nanosheet arrays on carbon nanotubes as a high-rate anode material for lithium-ion batteries. Carbon 2014, 69, 302–310. [Google Scholar] [CrossRef]
- Tian, L.; Zhuang, Q.; Li, J.; Wu, C.; Shi, Y.; Sun, S. The production of self-assembled Fe2O3–graphene hybrid materials by a hydrothermal process for improved Li-cycling. Electrochim. Acta 2012, 65, 153–158. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Hu, X.; Yuan, L.; Huang, Y. Synthesis of hierarchical MoS2 and its electrochemical performance as an anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 3498–3504. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Qie, L.; Jiang, Y.; Xiong, X.; Qiao, Y.; Huang, Y. Facile synthesis of sandwiched Zn2GeO4-graphene oxide nanocomposite as a stable and high-capacity anode for lithium-ion batteries. Nanoscale 2014, 6, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, F.; Tang, X.; Li, Q.; Wang, T.; Cao, G. Flexible CoO–graphene–carbon nanofiber mats as binder-free anodes for lithium-ion batteries with superior rate capacity and cyclic stability. J. Mater. Chem. A 2014, 2, 5890–5897. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhang, X.; Li, C.; Chen, J.; Wang, K. Graphene supported ZnO/CuO flowers composites as anode materials for lithium ion batteries. Mater. Lett. 2015, 152, 181–184. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Xu, Y.; Rajarajan, V.; Ouyang, T.; Chowdari, B.V.R. Template free facile molten synthesis and energy storage studies on MCo2O4(M = Mg, Mn) as anode for Li-ion batteries. ACS Sustain. Chem. Eng. 2015, 3, 3035–3042. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, X.; Shi, W. Free-standing graphene/carbon nanotubes/CuO aerogel paper anode for lithium ion batteries. Mater. Lett. 2016, 172, 72–75. [Google Scholar] [CrossRef]
- Xu, Y.; Jian, G.; Zachariah, M.R.; Wang, C. Nano-structured carbon-coated CuO hollow spheres as stable and high rate anodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 15486–15490. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Wan, W.; Zhou, J.; Chen, X. Copper oxide nanowire arrays synthesized by in-situ thermal oxidation as an anode material for lithium-ion batteries. Electrochim. Acta 2014, 132, 42–48. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, H. Three-dimensional CuO microflowers as anode materials for Li-ion batteries. Ceram. Int. 2015, 41, 8257–8260. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, C.; Jiahuan, F.; Loh, K.P.; Chowdari, B.V. Li-cycling properties of molten salt method prepared nano/submicrometer and micrometer-sized CuO for lithium batteries. ACS Appl. Mater. Interfaces 2013, 5, 4361–4366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Wang, Y.; Jiao, L. CuO quantum dots embedded in carbon nanofibers as binder-free anode for sodium ion batteries with enhanced properties. Small 2016, 12, 4865–4872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chao, D.; Sun, J.; Bacho, I.M.; Fan, Z.; Ng, C.F.; Xia, X.; Huang, H.; Zhang, H.; Shen, Z.X.; et al. Enhanced lithium storage performance of CuO nanowires by voating of graphene quantum dots. Adv. Mater. Interfaces 2015, 2, 239–245. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, C.; Wang, Y.; Qiu, L.; Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 2013, 341, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Wang, X.; Liu, M.; Ye, F.; Wang, J.; Qiu, Y.; Li, W.; Zhang, Y. Dense integration of graphene and sulfur through the soft approach for compact lithium/sulfur battery cathode. Nano Energy 2015, 12, 468–475. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Mai, Y.J.; Wang, X.L.; Xiang, J.Y.; Qiao, Y.Q.; Zhang, D.; Gu, C.D.; Tu, J.P. CuO/graphene composite as anode materials for lithium-ion batteries. Electrochim. Acta 2011, 56, 2306–2311. [Google Scholar] [CrossRef]
- Chen, H.; Feng, F.; Hu, Z.-L.; Liu, F.-S.; Gong, W.-Q.; Xiang, K.-X. Preparation of uniform flower-like CuO and flower-like CuO/graphene composite and their application in lithium ion batteries. Trans. Nonferr. Metal. Soc. 2012, 22, 2523–2528. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kang, J.; Paul, B.J.; Singh, N.K.; Song, J.; Kim, J. Facile approach to synthesize CuO/reduced graphene oxide nanocomposite as anode materials for lithium-ion battery. J. Power Sources 2013, 244, 435–441. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.-L.; Shu, C.-Y.; Guo, Y.-G.; Wang, C.-R. Synthesis of CuO/graphene nanocomposite as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar] [CrossRef]
- Guo, Z.; Reddy, M.V.; Goh, B.M.; San, A.K.P.; Bao, Q.; Loh, K.P. Electrochemical performance of graphene and copper oxide composites synthesized from a metal-organic framework (Cu-MOF). RSC Adv. 2013, 3, 19051–19056. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Zhu, D.; Xie, A.; Shen, Y. A novel porous CuO nanorod/rGO composite as a high stability anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 1833–1839. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Gu, L.; Wang, Y.; Ying, Y.; Mao, Y.; Sun, L.; Peng, X. Flexible CuO nanosheets/reduced-graphene oxide composite paper: Binder-free anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 9850–9855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, L.; Song, H.; Wu, B.; Chen, X. Durable high-rate performance of CuO hollow nanoparticles/graphene-nanosheet composite anode material for lithium-ion batteries. Electrochem. Commun. 2011, 13, 1357–1360. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolani, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An advanced lithium-ion battery based on a graphene anode and a lithium iron phosphate cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Wang, F.; Song, X.; Wang, Y.; Yang, S. CuO necklace: Controlled synthesis of a metal oxide and carbon nanotube heterostructure for enhanced lithium storage performance. J. Phys. Chem. C 2013, 117, 12346–12351. [Google Scholar] [CrossRef]
- Sun, H.; Song, X.; Xu, M.; Zhang, Y.; Que, W.; Yang, S. Functionalization of carbon nanotubes via Birch reduction chemistry for selective loading of CuO nanosheets. New J. Chem. 2015, 39, 4278–4283. [Google Scholar] [CrossRef]
- Park, G.D.; Kang, Y.C. Superior lithium-ion storage properties of mesoporous CuO-reduced graphene oxide composite powder prepared by a two-step spray-drying process. Chem.-Eur. J. 2015, 21, 9179–9184. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xue, D. A chemical reaction controlled mechanochemical route to construction of CuO nanoribbons for high performance lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 19708–19714. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hu, Y.; Cheng, B.; Chen, R.; Lv, H.; Ma, L.; Zhu, G.; Wang, Y.; Yan, C.; Tie, Z.; et al. Multi-yolk-shell copper oxide@carbon octahedra as high-stability anodes for lithium-ion batteries. Nano Energy 2016, 20, 305–314. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, W.J. Hierarchically mesoporous CuO/carbon nanofiber coaxial shell-core nanowires for lithium ion batteries. Sci. Rep. 2015, 5, 9754. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.Y.; Tu, J.P.; Qiao, Y.Q.; Wang, X.L.; Zhong, J.; Zhang, D.; Gu, C.D. Electrochemical impedance analysis of a hierarchical CuO electrode composed of self-assembled nanoplates. J. Phys. Chem. C 2011, 115, 2505–2513. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Su, Q.; Shi, J.; Liu, Y.; Du, G. Nanoleaf-on-sheet CuO/graphene composites: Microwave-assisted assemble and excellent electrochemical performances for lithium ion batteries. Electrochim. Acta 2014, 125, 615–621. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, B.; Zhou, J.; Xia, R.; Chu, Y.; Huang, J. Preparation of Advanced CuO Nanowires/Functionalized Graphene Composite Anode Material for Lithium Ion Batteries. Materials 2017, 10, 72. https://doi.org/10.3390/ma10010072
Zhang J, Wang B, Zhou J, Xia R, Chu Y, Huang J. Preparation of Advanced CuO Nanowires/Functionalized Graphene Composite Anode Material for Lithium Ion Batteries. Materials. 2017; 10(1):72. https://doi.org/10.3390/ma10010072
Chicago/Turabian StyleZhang, Jin, Beibei Wang, Jiachen Zhou, Ruoyu Xia, Yingli Chu, and Jia Huang. 2017. "Preparation of Advanced CuO Nanowires/Functionalized Graphene Composite Anode Material for Lithium Ion Batteries" Materials 10, no. 1: 72. https://doi.org/10.3390/ma10010072
APA StyleZhang, J., Wang, B., Zhou, J., Xia, R., Chu, Y., & Huang, J. (2017). Preparation of Advanced CuO Nanowires/Functionalized Graphene Composite Anode Material for Lithium Ion Batteries. Materials, 10(1), 72. https://doi.org/10.3390/ma10010072




