Abstract
This study used thermal cracking with hydrogen (HTC) to produce bio-fuel oil (BFO) from jatropha oil (JO) and to improve its quality. We conducted HTC with different hydrogen pressures (PH2; 0–2.07 MPa or 0–300 psig), retention times (tr; 40–780 min), and set temperatures (TC; 623–683 K). By applying HTC, the oil molecules can be hydrogenated and broken down into smaller molecules. The acid value (AV), iodine value, kinematic viscosity (KV), density, and heating value (HV) of the BFO produced were measured and compared with the prevailing standards for oil to assess its suitability as a substitute for fossil fuels or biofuels. The results indicate that an increase in PH2 tends to increase the AV and KV while decreasing the HV of the BFO. The BFO yield (YBFO) increases with PH2 and tr. The above properties decrease with increasing TC. Upon HTC at 0.69 MPa (100 psig) H2 pressure, 60 min time, and 683 K temperature, the YBFO was found to be 86 wt%. The resulting BFO possesses simulated distillation characteristics superior to those of boat oil and heavy oil while being similar to those of diesel oil. The BFO contains 15.48% light naphtha, 35.73% heavy naphtha, 21.79% light gas oil, and 27% heavy gas oil and vacuum residue. These constituents can be further refined to produce gasoline, diesel, lubricants, and other fuel products.
1. Introduction
Animal fats and vegetable oils have been widely used as alternative feedstocks for biodiesel production in order to reduce the dependence on fossil-fuel-based diesel [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. To increase usage of these feedstocks, much attention has been paid to its use in the production of diesel-like fuels and/or other value-added chemicals [15]. Common production methods are pyrolysis [16,17], catalytic pyrolysis [18,19,20,21,22], and hydrogenation [23].
In a study on the pyrolysis of babassu, piqui, and palm oils in a glass apparatus at 573–773 K, Alencar et al. [17] obtained mixtures of the major products (n-alkanes and 1-alkenes) at yields (v/v) of 94.46%, 68.20%, and 95.55%, respectively. Adebanjo et al. [16] performed pyrolysis of lard with continuous feeding into a fixed bed at 873–1073 K, using nitrogen as the carrier gas. This produced a diesel-like fuel with a cetane index of 46, specific gravity of 0.86, and heating value (HV) of 40 MJ/kg.
Catalysis has been incorporated into pyrolysis to enhance production. Dos Anjos et al. [19] investigated the decomposition of vapors of crude and pre-hydrogenated soybean oils by passing them through a solid acid, Al2O3–n, and a base, MgO, in a tubular reactor at 573–773 K. The crude oil gave oxygen-containing products and hydrocarbons (HCs) with a low mean molecular weight (MW), while the pre-hydrogenated oil produced HCs with a mean MW comparable to those of HCs in diesel. They also found that Al2O3 was better than MgO at producing a diesel-like fuel. Konar et al. [21] pyrolyzed dried raw sludge from Atlanta sewage over activated alumina at 723 K and 1 atm. The products consisted of low-viscosity liquids (10.7–67.5 wt%), non-condensable gases (12.1–15.6 wt%), semisolids, and water. The liquid products comprised mixtures of HCs containing mainly alkanes. Using a reactor with a fractionating packed column at 673 and 693 K, Dandik and Aksoy [18] studied the pyrolysis of used sunflower oil in the presence of sodium carbonate. An increase in the pyrolytic temperature and catalyst content enhanced the yields of liquid HCs and gases while reducing the formation of aqueous compounds, acids, and coke-residual oil. The major constituents of the liquid HCs and gases were C5–C11 and C1–C3 HCs, respectively. Lima et al. [22] conducted pyrolyses of soybean, palm, and castor oils in a 5 L batch reactor at 623–673 K. Gaseous products immediately produced during catalytic pyrolysis were then directly fed into a fritted-bottom glass-tube deoxygenating reactor packed with HZSM-5 zeolite. The yields of product fractions at distillation temperatures (DTs) of <353, 353–413, 413–473, and >473 K were 7–10, 9–15, 9–20, and 60–75 wt%, respectively. Instead of applying conventional transesterification, pyrolysis using Pd/C catalyst was used by Ito et al. [20] to convert waste animal fat and cooking oil into light-oil HCs in an autoclave reactor at 633–693 K. This approach enhanced the selectivity for light oil at 453–623 K.
Catalytic hydrogenation of jatropha oil (JO) at 10.34 MPa (1500 psig) H2 pressure (PH2) and 613–653 K was studied by Kumar et al. [23]. When sulfided Ni–Mo/Al2O3, Ni–W/SiO2–Al2O3 and Co–Mo/Al2O3 were used, 98%, 81% and 49% yields of C15–C18 diesel range HCs were obtained, respectively. The use of Co–Mo/Al2O3 also yielded 36% kerosene. Co-processing a mixture of JO with refinery gas oil while using sulfided Ni–Mo/Al2O3 resulted in a diesel yield of 88%–92%. These results indicate that sulfided Ni–Mo/Al2O3 is a suitable catalyst for hydro-processing.
Kumar et al. [23] conducted catalytic hydrogenation of JO at high PH2, and Ito et al. [20] studied the catalytic pyrolysis of triglycerides without hydrogen. The present study, on the other hand, performed non-catalytic hydrogenation at low to moderate PH2, which can save on catalyst and H2 while maintaining the hydrogenation process. Here we also determined the feasibility of processing JO via thermal cracking with hydrogen (HTC) for bio-fuel oil (BFO) production and the role of hydrogenation, which may compete with thermal cracking. The effects of hydrogen on the yield and key properties of the resulting BFO were also addressed. Simulated distillation of the BFO was carried out to analyze its fuel content, and the results were compared against those of various fuels.
2. Results and Discussion
2.1. Characteristics of Jatropha Oil
The acid value (AV), iodine value (IV), kinematic viscosity (KV), and density (ρLO) of the JO used are about 36.07 mg KOH/g, 113.8 g I2/100 g, 33.56 mm2/s, and 917.8 kg/m3, respectively, which are similar to those obtained by Andrade-Tacca et al. [3,4]. Except for the IV (<120 g I2/100 g max), the other properties do not meet the standards for biodiesel. The AV of JO (36.07 mg KOH/g) indicates that it contains about 18.04 wt% free fatty acids (FFAs), which is substantially high. Moreover, the JO contains unsaturated bonds, as reflected by its IV. These properties need to be improved to allow the value-added use of JO. The HV of JO (37.46 MJ/kg or 34.38 MJ/L), however, is much higher than that of coal (24.17 MJ/kg, dry basis) [24] and similar to that of diesel (35.15 MJ/L) [25].
2.2. Thermal Cracking of Jatropha Oil
Table 1 presents the yield and properties of the liquid product BFO (YBFO) obtained from JO thermal cracking at 683 K set temperature (TC) and 60 min retention time (tr) for run 1. The reactions involved can be found in studies by Ito et al. [20], who investigated biodiesel production from waste animal fats and cooking oils using pyrolysis. The reaction products are triacylglycerol (TG), diglyceride (DG), monoglyceride (MG), FFAs, HCs, organic gases, and carbon dioxide. Cleavage of the ester bond generates unsaturated and saturated FFAs, while breakage of the unsaturated bonds forms short-chain HCs and FFAs. Decarboxylation of the FFAs then yields light-oil HCs while releasing CO2. Further decomposition of the HCs may produce some organic gases. Thus, chain-breaking and decomposition reactions of the unsaturated and saturated fractions take place during thermal cracking. Both condensable and non-condensable fragments are formed. The BFO obtained is essentially the pyrolysis oil.

Table 1.
Yield and properties of BFO obtained from thermal cracking of JO at TC = 683 K. YBFO: yield of jatropha oil (JO) derived bio-fuel oil (BFO); TC: setting temperature; tr: retention time; PH2: H2 pressure; IV: iodine value; KV: kinematic viscosity; ρLO; density; HV: heating value; and N/A: not applicable.
YBFO is maintained at 72.5 wt% after thermal cracking. About 27.5 wt% of the JO decomposes into gases. The increase in AV of the BFO produced by JO thermal cracking (from 36.07 to 46.48 KOH/g) is due to FFA formation during thermal breakage of the ester bonds of glycerides. The decrease in IV (113.8 to 77.49 g I2/100 g) is attributed to the cleavage of double bonds of unsaturated glycerides and fatty acids. The decrease in KV from 33.56 to 1.76 mm2/s and ρLO from 917.8 to 863.6 kg/m3 results from the formation of short-chain HCs and FFAs. All of these results are consistent with the findings of Ito et al. [20] in a study on waste animal fats and cooking oils. The decomposition of the volatile matter and light components of the JO subjected to carbonization via thermal cracking also results in an increase in HV from 37.46 to 39.15 MJ/kg.
2.3. Thermal Cracking of Jatropha Oil with Hydrogen
2.3.1. Effects of PH2
The performance of HTC processing of JO at PH2 of 0–2.07 MPa (0–300 psig) at TC of 683 K and tr of 60 min is summarized Table 2. Hydrogenation has functions of: (1) saturating the unsaturated bonds, which decreases the IV; (2) assisting in bond breaking of long-chain molecules, thus forming smaller fragments; and (3) inhibiting carbonization. Thermal cracking of BFO increases its AV and HV while reducing the IV, KV, and ρLO, as noted in Section 2.2. Hydrogenation during thermal cracking may have both enhancing and inhibiting effects. Saturation of unsaturated bonds via hydrogenation facilitates breakdown of saturated components during thermal cracking. However, radicals formed by thermal cracking may be attacked by hydrogen, as indicated by Ito et al. [20]. The hydrogen may be derived from the feed or may be abstracted from alkyl HCs. Thus, an excess of hydrogen may inhibit the effectiveness of radicals formed by thermal cracking. The increase in AV with PH2 is due to the assistance of hydrogenation in thermal cracking, which breaks ester bonds and forms more FFAs. IV generally decreases with increasing PH2 as hydrogenation saturates the unsaturated bonds that otherwise need to be broken down via thermal cracking. At a high PH2 (2.07 MPa or 300 psig), however, the inhibitory effect of hydrogen on radicals reduces the propagation of decomposition reactions that decrease IV, resulting in an IV of 76.67 g I2/100 g. This value is higher than that obtained at a PH2 of 1.38 MPa (200 psig), 54.62 g I2/100 g. The inhibitory effect of hydrogen on radicals also causes a high KV (4.08 mm2/s) and a high ρLO (874.6 kg/m3) at 2.07 MPa (300 psig) H2 pressure. The low HV (30.30 MJ/kg) at 2.07 MPa (300 psig) H2 pressure is due to the retardation of carbonization by H2. The presence of H2 leads to retention of more HCs in the liquid state, thus giving a YBFO of about 86–89 wt%, which is higher than that obtained in the absence of H2 (72.5 wt%).

Table 2.
Yields and properties of BFO obtained from treating JO via thermal cracking with hydrogen (HTC) at various PH2.
2.3.2. Effects of tr
Table 3 illustrates the time variation of YBFO during the HTC of JO at TC of 683 K and PH2 of 2.07 MPa (300 psig). YBFO increases from 80 to 93 wt% as tr increases from 40 to 80 min, as more HCs form.

Table 3.
Yields and properties of BFO obtained from treating JO via HTC at various tr.
One may refer to the studies of Ito et al. [20] on the pyrolysis of waste animal fats and cooking oils to understand the effects of tr on YBFO and the properties of the liquid BFO product. Their results indicate that increasing the pyrolysis time reduces the yields of TG, DG, MG, and FFAs while increasing those of HCs, organic gases, and CO2. The decrease in AV, IV, KV, ρLO, and HV with the increase in YBFO and with the increase in tr from 40 to 80 min for the HTC of JO in the present study are consistent with the time-dependent trends of TG, DG, MG, FFAs, and HCs reported by Ito et al. [20]. An increasing tr enhances the decomposition reactions of starting and intermediate compounds and the formation of final products. Hydrogenation also inhibits carbonization, lowering the HV as tr increases from 40 to 80 min.
At an intermediate tr (60 min), hydrogenation is dominant, enhancing the breakage of ester bonds in the formation of FFAs via thermal cracking. This results in an AV (85.09 mg KOH/g) higher than that obtained at 40 min (79.79 mg KOH/g). With further increase in tr to 80 min, thermal cracking becomes dominant, thus lowering the AV via decarboxylation of FFAs. The inhibitory effect of hydrogenation on radicals at 60 min is more severe than that at 40 min, resulting in hindered cleavage of double bonds during thermal cracking. This results in an increase in the IV as tr increases from 40 to 60 min. However, the domination of thermal cracking at 80 min contributes to further breakage of double bonds, thus reducing the IV. The inhibitory effect of hydrogenation on radicals at 60 min and the enhancement of thermal cracking at 80 min also explain the increase in KV and ρLO as tr increases from 40 to 60 min, which is in contrast to a decrease as tr increases from 60 to 80 min. The decrease in HV with increasing tr from 40 to 60 min and from 60 to 80 min may be attributed to the inhibitory effect of hydrogenation and to the enhancement of thermal cracking with carbonization, respectively.
2.3.3. Effects of TC
The YBFO for the HTC of JO at TC at 623–683 K at PH2 of 2.07 MPa (300 psig) and tr of 80 min is shown in Table 4. With high PH2 and long tr, which result in a high YBFO (93–94 wt%), the effect of TC on YBFO is insignificant. However, its effects on the formation of different product species are significant. A higher TC induces vigorous thermal cracking, facilitating the decarboxylation of FFAs and thereby reducing the AV. It also promotes the cleavage of double bonds, thus decreasing the IV. In addition, the higher thermal energy at a higher TC enhances the decomposition of large molecules to small ones through bond breaking. This then generally lowers the KV and ρLO. The decrease in HV (from 41.47 to 37.13 MJ/kg) with increasing TC (623–683 K) may arise from the inhibitory effect of hydrogenation on the carbonization, which is more pronounced at a higher T, thus reducing the HV.

Table 4.
Yields and properties of BFO obtained from treating JO via HTC at various TC.
2.3.4. Simulated Distillation Characteristic of Bio-Fuel Oil
Figure 1 compares the SDCs of the BFO derived from the HTC of JO at TC of 683 K, tr of 60 min, and PH2 of 0.69 MPa (100 psig) with those of various fuels. The comparison indicates that the BFO from HTC possesses SDCs close to those of diesel, while being superior to those of heavy and boat oils. About 57.52% of the BFO constituents have boiling points in the range of 366–573 K.
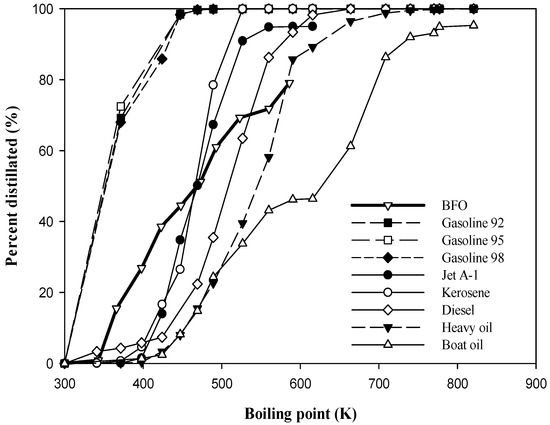
Figure 1.
Simulated distillation characteristic of BFO for HTC of JO at TC = 683 K, tr = 60 min and PH2 = 0.69 MPa (100 psig) comparing with those of different fuels. BFO: this study; fuels other than BFO: Chang et al. [26].
More detailed classifications based on the fractionating temperature are presented in Table 5. The amounts of light naphtha, heavy naphtha, light gas oil, and heavy gas oil with vacuum residue are about 15.48%, 35.73%, 21.79%, and 27%, respectively. Thus, the BFO obtained can be further refined to value-added fuels and chemicals.

Table 5.
Fuel contents of BFO obtained from treating JO via HTC at TC = 683 K, tr = 60 min and PH2 = 0.69 MPa (100 psig). HCs: hydrocarbons.
2.4. Comparison of Results with Those of Others
A comparison of some of the results of this work with those of others is presented in Table 6. The main products obtained by HTC are C6–C16 (heavy naphtha and light gas oil, at about 57.52 wt%), while those of other studies are HCs in various carbon fractions or diesel-like fuels.

Table 6.
Comparison of some results of this work with those of others. DG: diglyceride; FFA: free fatty acid; HTC: Thermal cracking with hydrogen; MG: monoglyceride; TG: triacylglycerol; Py: pyrolysis; CPy: catalytic pyrolysis; pre H2: pre-hydrogenation; DT: distillation temperature; YHC: yield of HC; CHy: catalytic hydrogenation; NiMo: Sulfide Ni-Mo/Al2O3; Ni-W: sulfide Ni-W/SiO2-Al2O3; and CoMo: sulfided Co-Mo/Al2O3.
The YBFO obtained with HTC in the present work (86 wt%) is comparable to or better than that obtained using pyrolysis and catalytic pyrolysis; however, it is less than that of a process of Kumar et al. [23], which uses sulfided Ni–Mo/Al2O3 catalysts for treating JO (98%) and a mixture of JO and gas oil (88%–92%). The degree of deoxygenation of the BFO produced from JO by HTC is worth examining, as this parameter is important for its proper use. A higher degree of deoxygenation gives better fuel properties and a lower oxygen content. Although we did not perform deoxygenation analyses in the present study, the work of Huang [27] concerning the hydrogenation and upgrade of tung oil is a useful reference. Upon catalytic hydrogenation using MoS2/γ–Al2O3 in a continuous continuous-flow process through a packed bed, the dry-basis oxygen content of tung oil (16.01 wt%) decreases to that of BFO derived from the tung oil at 623–673 K (0.24–0.36 wt%); that is, extensive deoxygenation was achieved. Thus, it is expected that HTC would can also reduce the oxygen content of the BFO derived from JO. That said, further study may help in understanding the effect of HTC on the deoxygenation of JO.
3. Experimental Methods
3.1. Materials
The JO used was supplied by Ozone Environmental Technology Co. (Yi-Lan, Taiwan) and was imported from Indonesia. Hydrogen and nitrogen of 99.995% purity were provided by Ching-Fong Co. (Taipei, Taiwan). Other chemicals that were used include isopropyl alcohol, toluene, acetic acid, cyclohexane, Wijs solution, KI, and Na2S2O3.
3.2. Equipments and Procedures
An autoclave reactor (HP/HT 4570 bench top reactor; Parr Instrument Co., Moline, IL, USA) with a volume of 600 mL, maximum pressure of 20.67 MPa (3000 psig), and maximum temperature of 773 K (500 °C) was used for the batch-wise HTC of JO. The reaction system, shown as a schematic diagram in Figure 2, features a temperature controller, pressure gauge, and circulating cooling bath. The TC variation in the reactor during heating, constant-temperature reaction, and cooling at TC values of 623, 652, and 683 K is presented in Figure 3. The trends of these values are similar and consistent, indicating good temperature control. A lower TC at the same tr means that the plateau in the TC is reached more quickly, and that cooling down to end the reaction at room temperature is likewise swifter. A time of 28–33 min is required for heating, and about 81–96 min is needed for cooling at a rate of about 4 K/min.
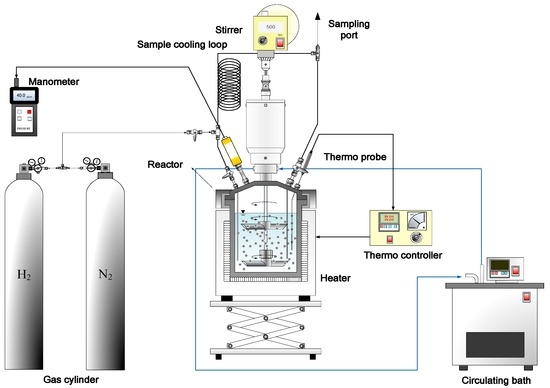
Figure 2.
Schematic diagram of the HTC reaction system.
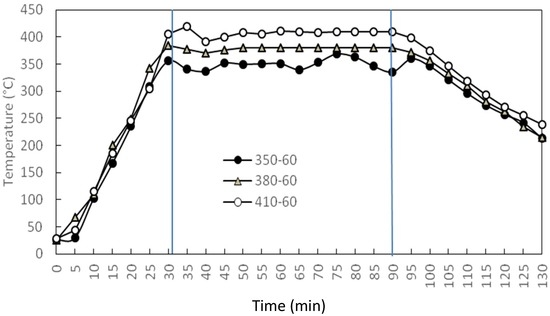
Figure 3.
Time (t) variations of temperature (T) during heating, constant-temperature reaction and cooling for three TC. Retention time = 60 min; 350-60: TC in °C-t in min.
JO (100 mL) was injected into the reactor. Nitrogen was then introduced for about 1 min to purge the residual air. This was followed by charging with hydrogen at PH2 of 0, 0.69, 1.38, or 2.07 MPa (0, 100, 200, and 300 psig, respectively). A PH2 of 0 psig was used in the case of thermal cracking without hydrogen. The stirring speed was held at 600 rpm. tr values during the constant-temperature reaction period were 40, 60, and 80 min.
3.3. Analyses
The AV was determined according to the method BS EN 14104 [28], which uses an automatic potentiometric titrator (KEN AT-510; Kyoto Electronics Manufacturing Co., Shinjuku-ku, Tokyo, Japan). The chemicals used included isopropyl alcohol and toluene. The IV was also measured using the KEN AT-510 and the reagents acetic acid, cyclohexane, Wijs solution, KI, and Na2S2O3, in accordance with the procedure of BS EN 14111 [29]. The KV was analyzed at 313 K (40 °C) using a Firstek B801-2 (Taipei, Taiwan) on the basis of BS EN ISO 3104 [30]. The viscosity tubes 100 T803 (with coefficient of viscometer CV = 0.01574 cSt/s) and 100 T 851 (CV = 0.01398 cSt/s), which were supplied by Cannon Instrument Co. (State College, PA, USA), were used for samples with different ranges of viscosity. The process time for each sample flowing through the viscometer was multiplied by the CV to obtain the KV. Measurement of ρLO was conducted using the DMA 35 Anton Parr density meter (Anton Paar Benelux, Oosterhout, The Netherlands) set at API Density B at 15 °C (288 K), in accordance with the Chinese National Standard CNS 14474 [31]. Analysis of the HV was performed using a calorimeter (oxygen bomb plain jacket calorimeter, model 1341; Parr Instrument Co., Moline, IL, USA) according to NIEA R214.01C [32]. The SDCs were deduced by gas chromatography using a flame ionization detector (5890 Series II; Hewlett Packard Inc., Wilmington, DE, USA) and a Supelco fused-silica capillary column (SBR-5, Supelco, Bellefonte, PA, USA).
4. Conclusions
- (1)
- Thermal cracking of JO can produce a BFO with lower IV, KV, and ρLO and higher HV compared with those of JO.
- (2)
- An increase in PH2 and tr increases the YBFO during HTC treatment of JO.
- (3)
- A higher TC generally results in lower AV, IV, KV, ρLO, and HV at the same retention time.
- (4)
- At 683 K, 60 min, and 0.69 MPa (100 psig) H2, the major constituent of the resulting BFO is heavy naphtha (about 35.73 wt%).
- (5)
- The BFO obtained via HTC exhibits SDCs better than those of boat oil and heavy oil, while being similar to those of diesel oil.
Acknowledgments
The authors are grateful for the financial support for this research provided by the Ministry of Science and Technology (formerly the National Science Council) of Taiwan.
Author Contributions
Yi-Yu Wang, Chia-Chi Chang and Ching-Yuan Chang conceived and designed the experiments; Yi-Yu Wang, Yi-Hung Chen, Li-Xuan Huang, Min-Yi Tsai and Michael Huang performed the experiments; Yi-Yu Wang, Yi-Hung Chen and Li-Xuan Huang analyzed the data; Yen-Hau Chen, Je-Lueng Shie, Cesar Augusto Andrade-Taccaa, Do Van Manh contributed reagents/materials/analysis tools; Yi-Yu Wang, Chia-Chi Chang, Ching-Yuan Chang and Min-Hao Yuan wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AV | Acid value | mg KOH/g |
| CV | Coefficient of viscometer | cSt/s |
| DT | Distillation temperature | K |
| HV | Heating value | MJ/kg or kcal/kg |
| IV | Iodine value | g I2/100 g |
| KV | Kinematic viscosity | mm2/s |
| P | Gas pressure during HTC | kPa or psi |
| PH2 | Hydrogen pressure | kPa, or psi (1 psi = 6.89 kPa) |
| T | Temperature | K or °C |
| TC | Setting temperature for HTC | K or °C |
| T | Time | min |
| tr | Retention time at constant setting temperature TC | min |
| YBFO | Yield of jatropha oil derived bio-fuel oil (BFO) | wt% |
| YHC | Yield of HC | wt% |
| ρLO | Density | kg/m3 |
Abbreviation
| BFO | Bio-fuel oil |
| CPy | Catalytic pyrolysis |
| DG | Diglyceride |
| FFA | Free fatty acid |
| HC | Hydrocarbon |
| HTC | Thermal cracking with hydrogen |
| JO | Jatropha oil |
| MG | Monoglyceride |
| MW | Molecular weight |
| TG | Triacylglycerol |
References
- Achten, W.M.J.; Verchot, L.; Franken, Y.J.; Mathijs, E.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Machado, Y.L.; Torres, A.E.B.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; Firmiano, L.R.; Parente, E.J.S., Jr. Properties of biodiesel oils formulated using different biomass sources and their blends. Renew. Energy 2009, 34, 857–859. [Google Scholar] [CrossRef]
- Andrade-Tacca, C.A.; Chang, C.C.; Chen, Y.H.; Manh, D.V.; Chang, C.Y.; Ji, D.R.; Tseng, J.Y.; Shie, J.L. Esterification of jatropha oil via ultrasonic irradiation with auto-induced temperature-rise effect. Energy 2014, 71, 346–354. [Google Scholar] [CrossRef]
- Andrade-Tacca, C.A.; Chang, C.C.; Chen, Y.H.; Manh, D.V.; Chang, C.Y. Esterification of jatropha oil by sequential ultrasonic irradiation with auto-induced temperature rise and dosing of methanol and sulfuric acid catalyst. J. Taiwan Inst. Chem. Eng. 2014, 45, 1523–1531. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar]
- Canoira, L.; Rodríguez-Gamero, M.; Querol, E.; Alcántara, R.; Lapuerta, M.; Oliva, F. Biodiesel from low-grade animal fat: Production process assessment and biodiesel properties characterization. Ind. Eng. Chem. Res. 2008, 47, 7997–8004. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, J.H.; Chang, C.Y.; Chang, C.C. Biodiesel production from tung (Vernicia montana) oil and its blending properties in different fatty acid compositions. Bioresour. Technol. 2010, 101, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chiang, T.H.; Chen, J.H. An optimum biodiesel combination: Jatropha and soapnut oil biodiesel blends. Fuel 2012, 92, 377–380. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.T.; Park, J.H.; Park, S.H.; Park, D.H. Estimating and improving cold filter plugging points by blending biodiesels with different fatty acid contents. Biotechnol. Bioprocess Eng. 2008, 13, 505–510. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, S.C.; Wu, T.Y.; Yang, P.M.; Jhang, S.R.; Lin, J.F. Energy-saving and rapid transesterification of jatropha oil using a microwave heating system with ionic liquid catalyst. J. Taiwan Inst. Chem. Eng. 2015, 49, 72–78. [Google Scholar] [CrossRef]
- Lu, H.; Chen, M.; Jiang, W.; Liang, B. Biodiesel processes and properties from Jatropha curcas L. oil. J. Biobased Mater. Bioenergy 2011, 5, 546–551. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Serra, T.M.; Barbosa, D.C.; Wolf, C.R. Biodiesel production from vegetable oil mixtures: Cottonseed, soybean and castor oils. Energy Fuel 2007, 21, 3746–3747. [Google Scholar] [CrossRef]
- Zarei, A.; Amin, N.A.S.; Talebian-Kiakalaieh, A.; Zain, N.A.M. Immobilized lipase-catalyzed transesterification of jatropha curcas oil: Optimization and modeling. J. Taiwan Inst. Chem. Eng. 2014, 45, 444–451. [Google Scholar] [CrossRef]
- Campbell, I.M. Biomass, Catalysts and Liquid Fuels; Holt, Rinehart and Winston: London, UK, 1983. [Google Scholar]
- Adebanjo, A.O.; Dalai, A.K.; Bakhshi, N.N. Production of diesel like fuel and other value-added chemicals from the pyrolysis of animal fat. Energy Fuel 2005, 19, 1735–1741. [Google Scholar] [CrossRef]
- Alencar, J.W.; Alves, P.B.; Craveiro, A.A. Pyrolysis of tropical vegetable-oils. J. Agric. Food Chem. 1983, 31, 1268–1270. [Google Scholar] [CrossRef]
- Dandik, L.; Aksoy, H.A. Pyrolysis of used sunflower oil in the presence of sodium carbonate by using fractionating pyrolysis reactor. Fuel Process. Technol. 1998, 57, 81–92. [Google Scholar] [CrossRef]
- Dos Anjos, J.R.S.; Gonzalez, W.D.A.; Lam, Y.L.; Frety, R. Catalytic decomposition of vegetable oil. Appl. Catal. 1983, 5, 299–308. [Google Scholar] [CrossRef]
- Ito, T.; Sakurai, Y.; Kakuta, Y.; Sugano, M.; Hirano, K. Biodiesel production from waste animal fats using pyrolysis method. Fuel Process. Technol. 2012, 94, 47–52. [Google Scholar] [CrossRef]
- Konar, S.K.; Boocock, D.G.B.; Mao, V.; Liu, J. Fuels and chemicals from sewage-sludge: 3. Hydrocarbon liquids from the catalytic pyrolysis of sewage-sludge lipids over activated alumina. Fuel 1994, 73, 642–646. [Google Scholar] [CrossRef]
- Lima, D.G.; Soares, V.C.D.; Ribeiro, E.B.; Carvalho, D.A.; Cardoso, E.C.V.; Mundim, K.C.; Rubim, J.C.; Suarez, P.A.Z. Diesel-like fuel obtained by pyrolysis of vegetable oils. J. Anal. Appl. Pyrolysis 2004, 71, 987–996. [Google Scholar] [CrossRef]
- Kumar, R.; Rana, B.S.; Tiwari, R.; Verma, D.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Hydroprocessing of jatropha oil and its mixtures with gas oil. Green Chem. 2010, 12, 2232–2239. [Google Scholar] [CrossRef]
- Chen, Y.H. The Torrefaction of Waste Bamboo Chopsticks to Manufacturing Solid Biofuel. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2016. [Google Scholar]
- Heating Values of Energy Products; 2013 Energy Statistics Handbook; Taiwan Bureau of Energy (TBOE): Taipei, Taiwan, 2014.
- Chang, C.C.; Chen, C.P.; Yang, C.S.; Chen, Y.H.; Huang, M.; Chang, C.Y.; Shie, J.L.; Yuan, M.H.; Chen, Y.H.; Ho, C.F.; et al. Conversion of waste bamboo chopsticks to bio-oil via catalytic hydrothermal liquefaction using K2CO3. Sustain. Environ. Res. 2016. [Google Scholar] [CrossRef]
- Huang, L.X. Hydrogenation and Upgrading of Bio-Oil. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2016. [Google Scholar]
- Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Acid Value; BS EN 14104:2003; British Standards Institution (BSI): London, UK, 2003.
- Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Iodine Value; BS EN 14111:2003; British Standards Institution (BSI): London, UK, 2003.
- Methods of Test for Petroleum and Its Products. Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity; BS EN ISO 3104:1996; British Standards Institution (BSI): London, UK, 1996.
- Taiwan Bureau of Standards, Metrology & Inspection (TBOS). CNS (Chinese National Standards)-14474 Method of Test for Density and Relative Density of Liquids by Digital Density Meter. Available online: http://www.bsmi.gov.tw/wSite/mp?mp=2 (accessed on 1 February 2016).
- Standard Test Method for Gross Calorific Value of Coal and Coke by the Adiabatic Bomb Calorimeter; NIEA R214.01C; Environmental Analysis Laboratory, Taiwan Environmental Protection Agency: Taoyuan, Taiwan, 2005.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).