Coelectrodeposition of Ternary Mn-Oxide/Polypyrrole Composites for ORR Electrocatalysts: A Study Based on Micro-X-ray Absorption Spectroscopy and X-ray Fluorescence Mapping
Abstract
:1. Introduction
2. Results and Discussion
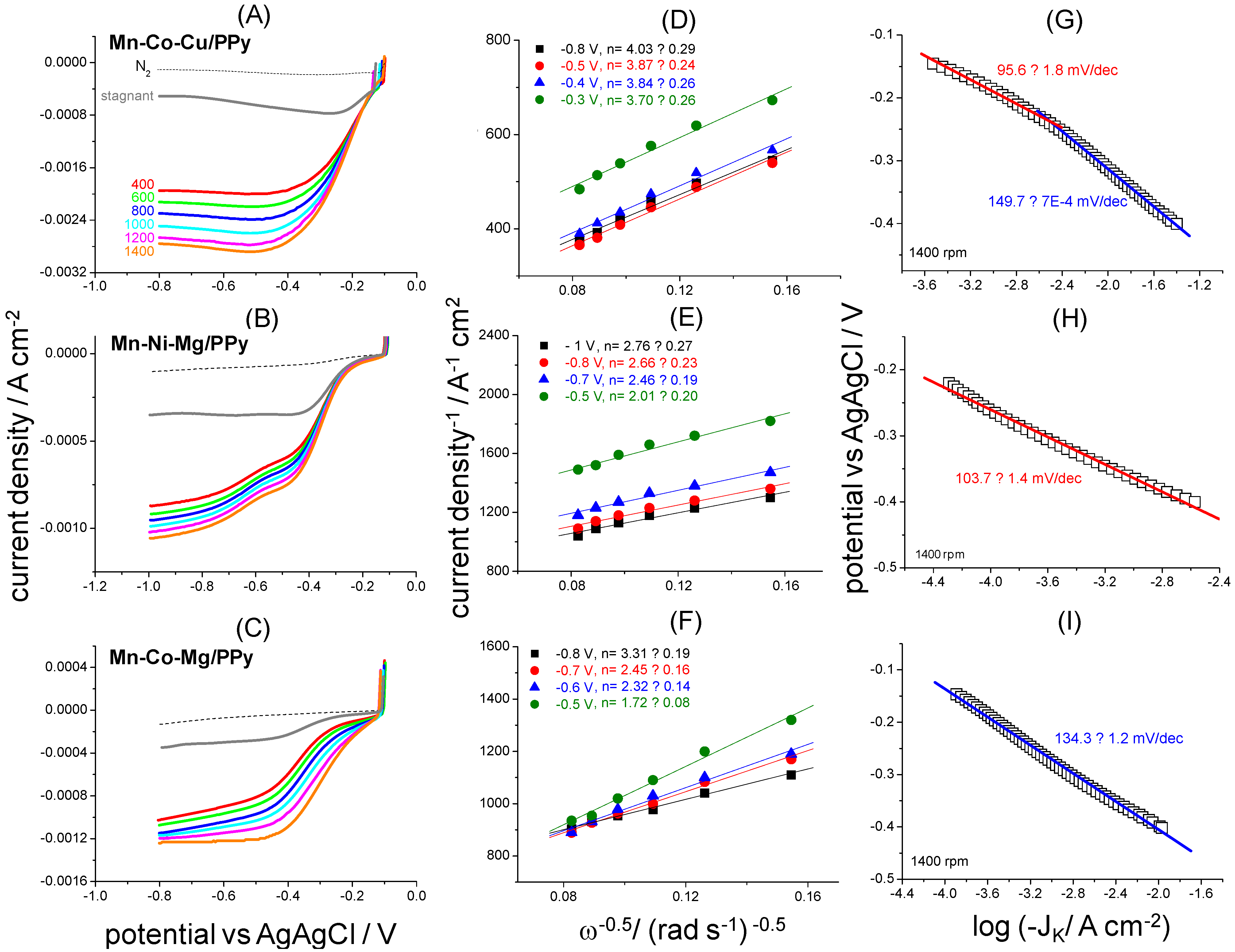
2.1. ORR Electrocatalytic Activity
| Electrocatalyst | E1/2/V | Eonset/V | J at 0.3 V/mA cm−2 | JL/mA cm−2 |
|---|---|---|---|---|
| Mn–Co–Cu/PPy | −0.22 | −0.122 | −2.23 | −2.87 |
| Mn–Ni–Mg/PPy | −0.34 | −0.237 | −0.213 | −0.74 |
| Mn–Co–Mg/PPy | −0.28 | −0.148 | −0.75 | −1.2 |
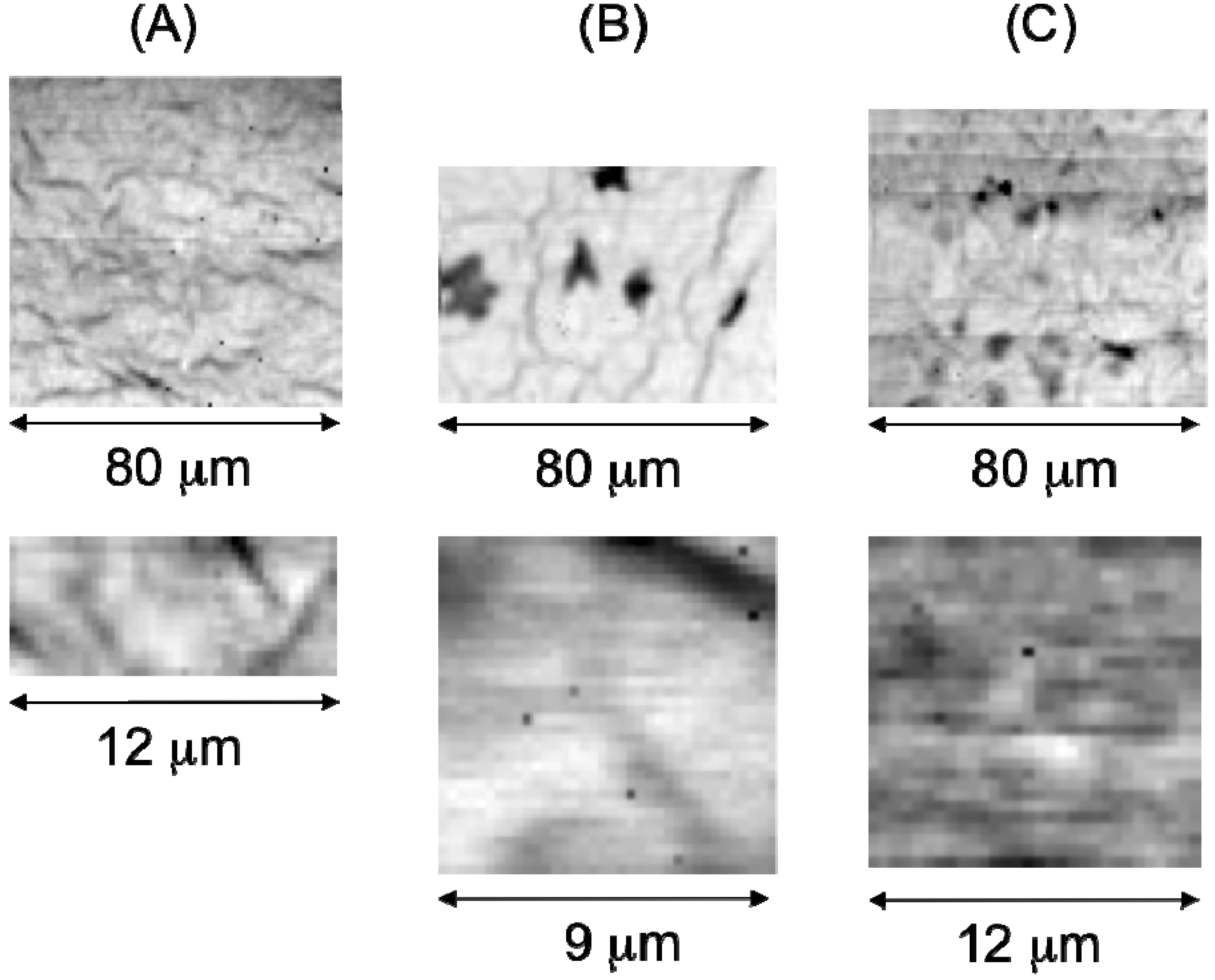
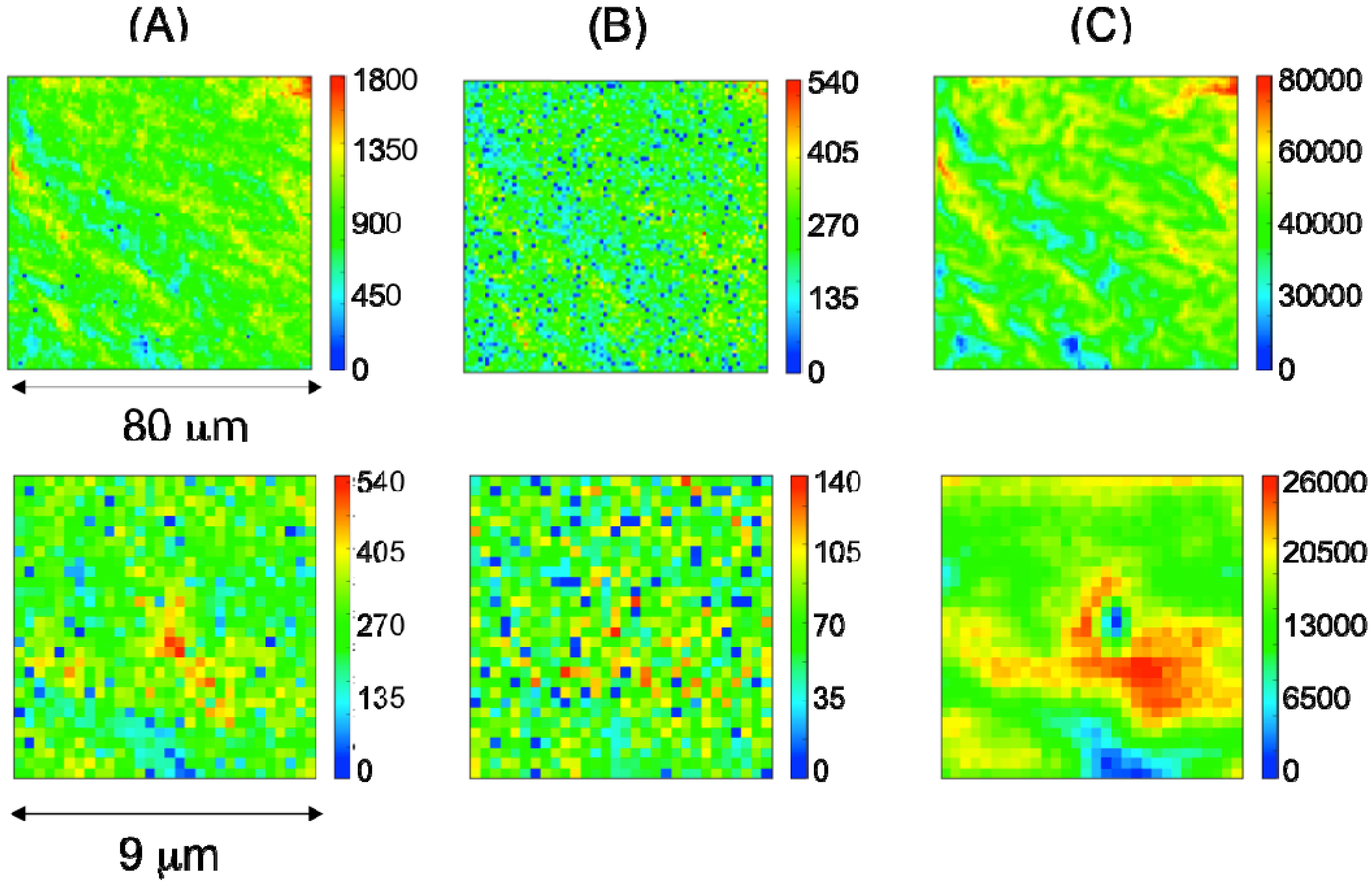
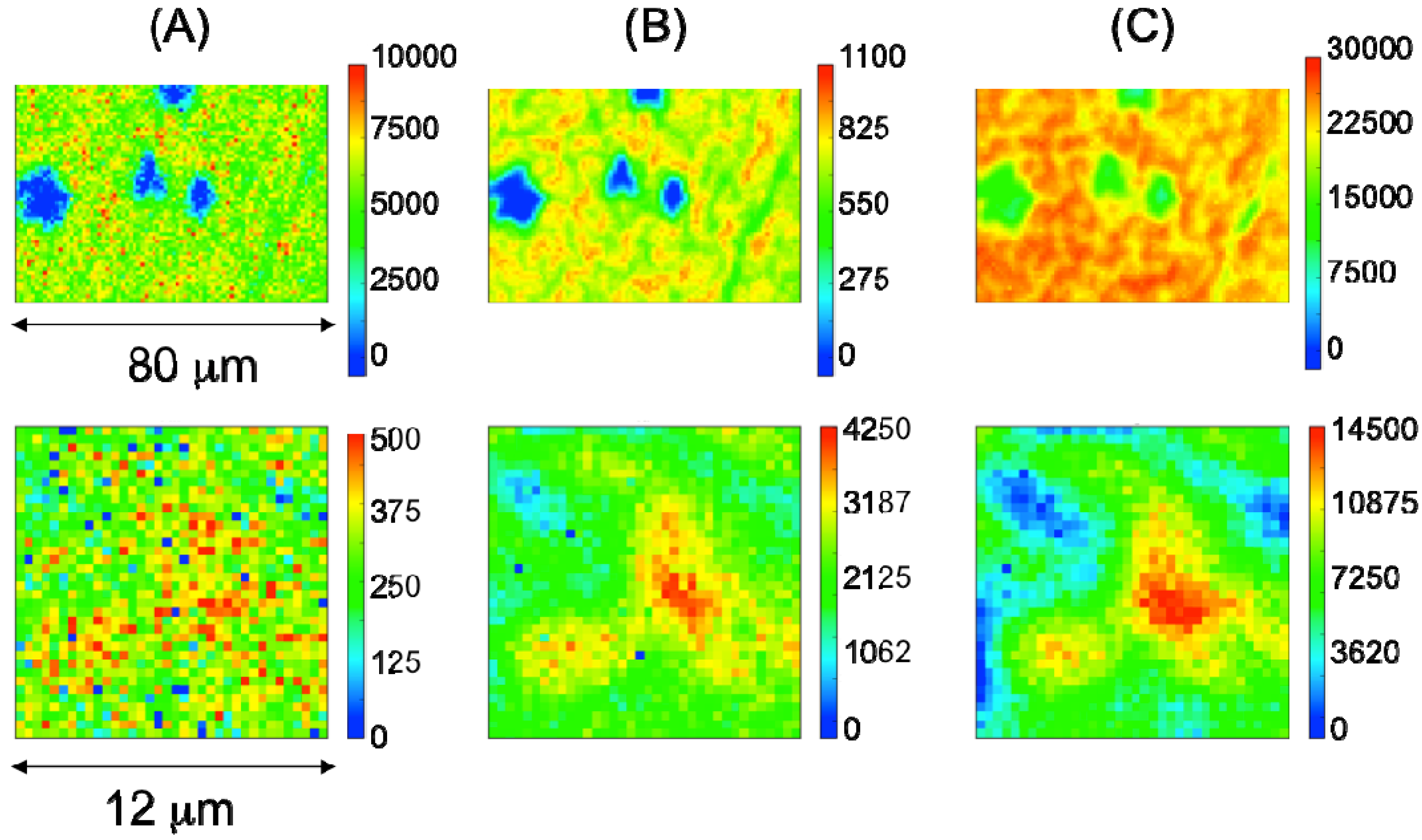
2.2. Characterization of Ternary Mn-Based/PPy Electrodeposited Composites by Low Energy X-ray Fluorescence Microspectroscopy

2.3. Characterization of Ternary Mn-based/PPy Electrodeposited Composites by Soft X-ray Micro-XAS Spectroscopy
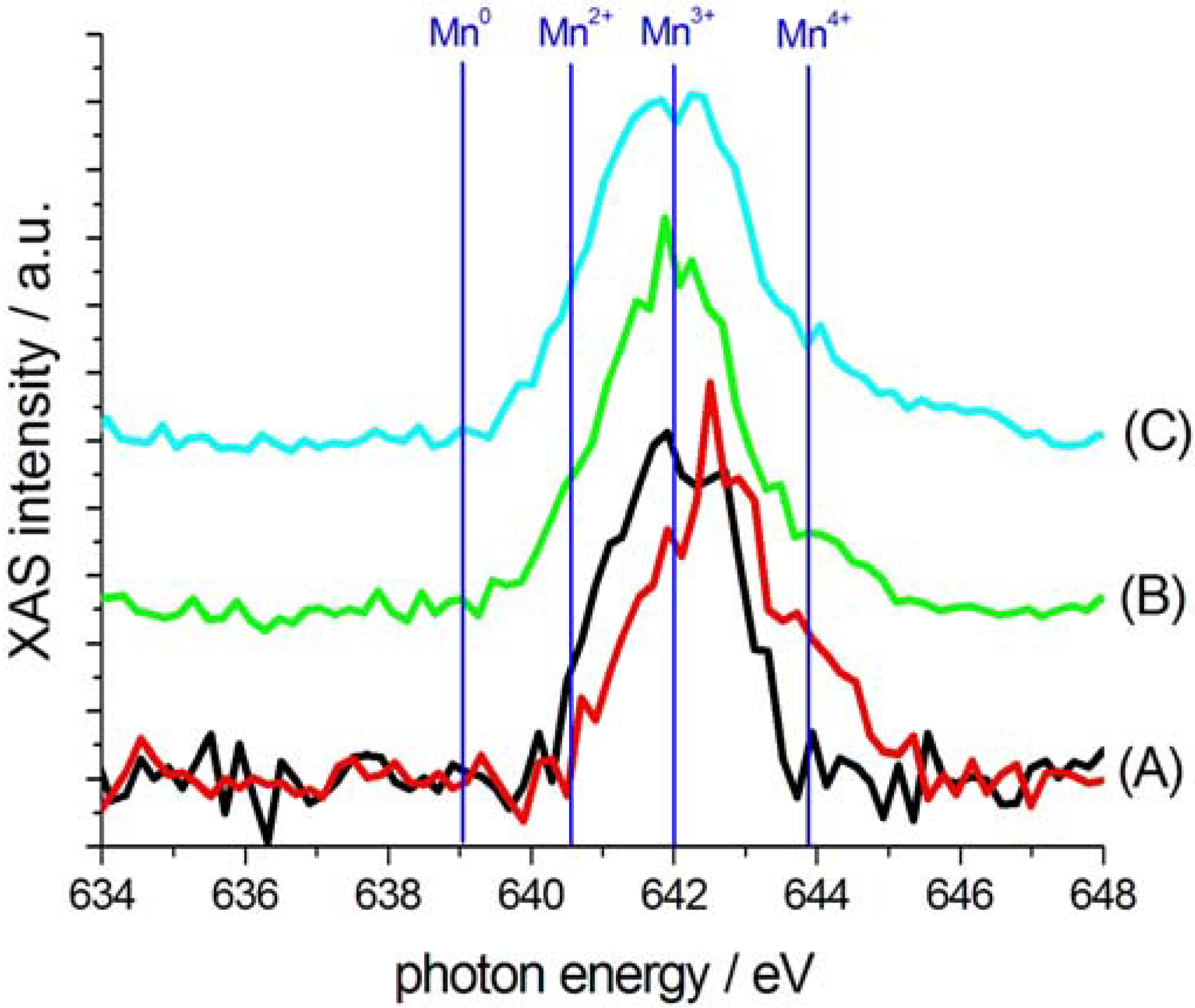
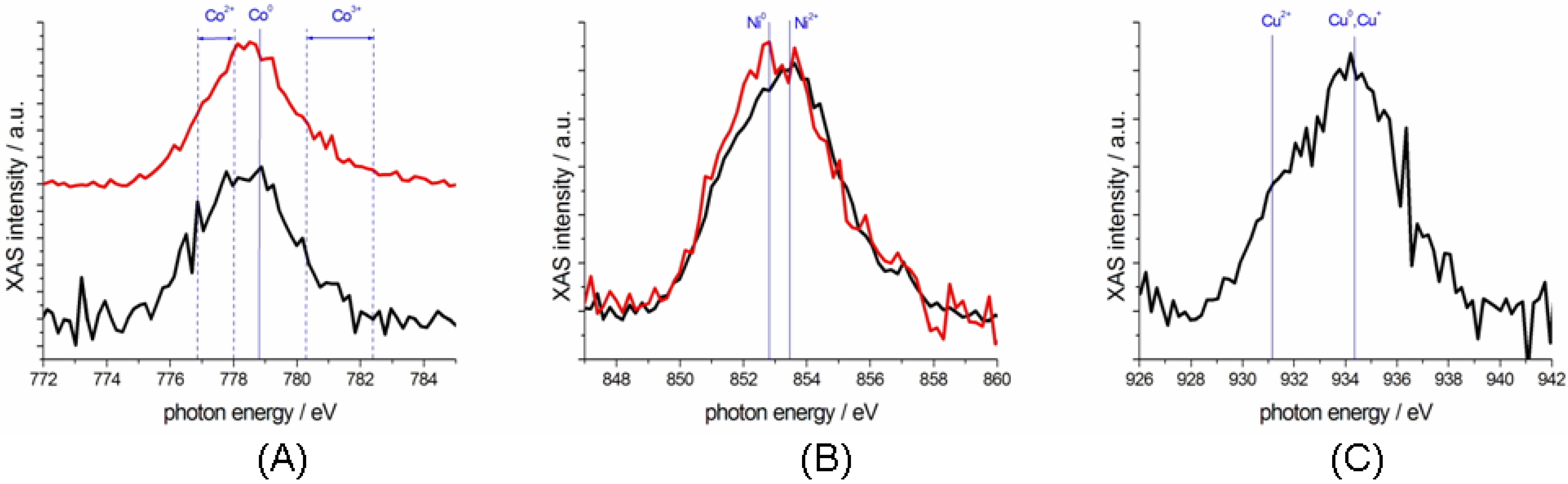
3. Experimental Section
3.1. Materials and Electrodes
3.2. Coelectrodeposition (CECD) Process
3.3. ORR Electrocatalytic Activity Evaluation
3.4. Soft X-ray Absorption and Fluorescence Mapping
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, X.; Ding, X.-L.; Wang, C.-Y.; Ma, Z.-F. Use of polypyrrole in catalysis for low temperature fuel cells. Energy Environ. Sci. 2013, 6, 1105–1124. [Google Scholar] [CrossRef]
- Nam, D.-H.; Lim, S.-J.; Kim, M.-J.; Kwon, H.-S. Facile synthesis of SnO2-polypyrrole hybrid nanowires by cathodic electrodeposition and their application to Li-ion battery anodes. RSC Adv. 2013, 3, 16102–16108. [Google Scholar] [CrossRef]
- Rapecki, T.; Donten, M.; Stojek, Z. Electrodeposition of polypyrrole—Au nanoparticles composite from one solution containing gold salt and monomer. Electrochem. Commun. 2010, 12, 624–627. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P.; Trasatti, S. Electrocatalytic activity for hydrogen evolution of polypyrrole films modified with noble metal particles. Mater. Chem. Phys. 2006, 98, 165–171. [Google Scholar] [CrossRef]
- Andreoli, E.; Rooney, D.A.; Redington, W.; Gunning, R.; Breslin, C.B. Electrochemical Deposition of Hierarchical Micro/Nanostructures of Copper Hydroxysulfates on Polypyrrole_Polystyrene Sulfonate Films. J. Phys. Chem. C 2011, 115, 8725–8734. [Google Scholar] [CrossRef]
- Ilieva, M.; Tsakova, V. Copper modified poly(3,4-ethylenedioxythiophene) Part I. Galvanostatic experiments. Synth. Metals 2004, 141, 281–285. [Google Scholar] [CrossRef]
- Ilieva, M.; Tsakova, V. Copper modified poly(3,4-ethylenedioxythiophene) Part II. Potentiostatic experiments. Synth. Metals 2004, 141, 287–292. [Google Scholar] [CrossRef]
- Jung, Y.; Singh, N.; Choi, K.-S. Cathodic Deposition of Polypyrrole Enabling the One-Step Assembly of Metal-Polymer Hybrid Electrodes. Angew. Chem. Int. Ed. 2009, 48, 8331. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hong, M.K.; Chung, J.K.; Park, S.Y. Electrochemical properties of Ni(OH)2/polypyrrole composite electrode prepared by electrodeposition for pseudo-capacitor. J. Ceram. Process. Res. 2013, 13, s274–s277. [Google Scholar]
- Nam, D.H.; Kim, M.J.; Lim, S.J.; Kwon, H.S. Single-step synthesis of polypyrrole nanowires by cathodic electropolymerization. J. Mater. Chem. A 2013, 1, 8061–8068. [Google Scholar] [CrossRef]
- Nam, D.-H.; Lim, S.-J.; Kim, M.-J.; Kwon, H.-S. One-step synthesis of a Si/CNT-polypyrrole composite film by electrochemical deposition. RSC Adv. 2014, 4, 10212–10215. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative study of Conductive Polymers by the ESCR Model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Bozzini, B.; Bocchetta, P.; Gianoncelli, A.; Mele, C.; Kiskinova, M. Electrodeposition of Co/CoO nanoparticles onto graphene for ORR electrocatalysis: A study based on micro-X-ray absorption spectroscopy and X-ray fluorescence mapping. Acta Chim. Slov. 2014, 61, 263–271. [Google Scholar] [PubMed]
- Otero, T.F.; Costa, S.O.; Ariza, M.J.; Marquez, M. Electrodeposition of Cu on deeply reduced polypyrrole electrodes at very high cathodic potentials. J. Mater. Chem. 2005, 15, 1662–1667. [Google Scholar] [CrossRef]
- Bocchetta, P.; Gianoncelli, A.; Abyaneh, M.K.; Kiskinova, M.; Jezeršek, D.; Mele, C.; Bozzini, B. Electrosynthesis of Co/PPy nanocomposites for ORR electrocatalysis: A study based on quasi-in situ X-ray absorption, fluorescence and in situ Raman spectroscopy. Electrochim. Acta 2014, 137, 535–545. [Google Scholar] [CrossRef]
- Tsakova, V. How to affect number, size, and location of metal particles deposited in conducting polymer layers. J. Solid State Electrochem. 2008, 12, 1421–1434. [Google Scholar] [CrossRef]
- Bouzek, K.; Holzhauser, P.; Kodym, R.; Moravcova, S.; Paidar, M. Utilization of Nafion®/conducting polymer composite in the PEM type fuel cells. J. Appl. Electrochem. 2007, 37, 137–145. [Google Scholar] [CrossRef]
- Yang, C.H.; Wen, T.C. Electrodeposited platinum nanoparticles in a sulfonate-polyaniline film for the electrosorption of methanol and sorbitol. Electrochim. Acta 1998, 44, 207–218. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.-M.; Yu, L.; Lu, Z.; Zhou, Q. In situ AFM study of electrochemical sythesis of polypyrrole/Au nanocomposite. Electrochem. Commun. 2008, 10, 1340–1434. [Google Scholar] [CrossRef]
- Guo, Z.; Shin, K.; Karki, A.B.; Young, D.P.; Kaner, R.B.; Hahn, H.T. Fabrication and characterization of iron oxide nanoparticles filled polypyrrole nanocomposites. J. Nanopart. Res. 2009, 11, 1441–1452. [Google Scholar] [CrossRef]
- Elliott, C.M.; Kopelove, A.B.; Albery, W.J.; Chen, Z. Nonaqueous electrochemistry of polypyrrole/polystyrenesulfonate composire films: Voltammetric, coulometric, EPR, and a.c. impedance studies. J. Phys. Chem. 1991, 95, 1743–1747. [Google Scholar] [CrossRef]
- Deng, J.; Peng, Y.; He, C.; Long, X.; Li, P.; Chan, A.S.C. Magnetic and conducting Fe3O4-polypyrrole nanoparticles with core shell structure. Polym. Int. 2003, 52, 1182–1187. [Google Scholar] [CrossRef]
- Ispas, A.; Peipmann, R.; Adolphi, B.; Efimov, I.; Bund, A. Electrodeposition of pristine and composite poly(3,4-ethylenedioxythiophene) layers studied by electro-acoustic impedance measurement. Electrochim. Acta 2011, 56, 3500–3506. [Google Scholar] [CrossRef]
- Ispas, A.; Peipmann, R.; Bund, A.; Efimov, I. On the P-doping of PEDOT layers in various ionic liquids studied by EQCM and acoustic impedance. Electrochim. Acta 2009, 54, 4668–4675. [Google Scholar] [CrossRef]
- Weidlich, C.; Mangold, K.M.; Jüttner, K. EQCN study of the ion exchange behaviour of polypyrrole with different counterions in differente electrolytes. Electrochim. Acta 2005, 50, 1547–1552. [Google Scholar] [CrossRef]
- El Mouahid, O.; Rakotondrainibe, A.; Crouigneau, V.; Léger, J.M.; Lamy, C.A. UV-visible study of the electropolymerization of CoTAPP at vitreous carbon and investigation of its catalytic activity towards the electroreduction of dioxygen. J. Electroanal. Chem. 1998, 455, 209–222. [Google Scholar] [CrossRef]
- Doherty, W.J., III; Wysocki, R.J., Jr.; Armstrong, N.R.; Saavedra, S.S. Potential-Modulated, Attenuated Total Reflectance Spectroscopy of Poly(3,4-ethylenedioxythiophene) and Poly(3,4-ethylenedioxythiophene Methanol) Copolymer Films on Indium-Tin Oxide. J. Phys. Chem. B 2006, 110, 4900–4907. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, M.T.; de Souza, L.M.M.; Ticianelli, E.A. Spectroscopic ellipsometry investigation of the redox process of polypyrrole in several aqueous solutions. Surf. Sci. 1998, 409, 465–473. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Domenech, S.C.; Lacaze, P.C. Synthesis and characterization of polypyrrole/TiO2 composites on mild steel. J. Appl. Electrochem. 2001, 31, 49–56. [Google Scholar] [CrossRef]
- Luo, X.J.; Xia, W.B.; Gao, J.L.; Zhang, S.Y.; Li, Y.L.; Tang, S.L.; Du, Y.W. Enhanced magnetic performance of metal-organic nanowire arrays by FeCo/polypyrrole co-electrodeposition. J. Appl. Phys. 2013, 113, 17B908. [Google Scholar] [CrossRef]
- Montoya, P.; Jaramillo, F.; Calderón, J.; de Torresi, S.C.; Torresi, R. Evidence of redox interactions between polypyrrole and Fe3O4 in polypyrrole-Fe3O4 composite films. Electrochim. Acta 2010, 55, 6116–6122. [Google Scholar] [CrossRef]
- Watanabe, N.; Morais, J.; Accione, S.B.B.; Morrone, Â.; Schmidt, J.E.; Martins Alves, M.C. Electronic, Structural, and Magnetic Properties of Cobalt Aggregates Embedded in Polypyrrole. J. Phys. Chem. B 2004, 108, 4013–4017. [Google Scholar] [CrossRef]
- Zu, T.M.; Yen, S.J.; Chen, E.C.; Sung, T.W.; Chiang, R.K. Conducting and magnetic behaviors of monodispersed iron oxide/polypyrrole nanocomposites synthesized by in situ chemical oxidative polymerization. J. Polym. Sci. A 2007, 45, 4647–4655. [Google Scholar]
- Malik, M.A.; Kuleszka, P.J.; Wlodarczyk, R.; Wittstock, G.; Szargan, R.; Bala, H.; Galus, Z. Formation of ultra-thin prussian blue layer of carbon steel that promotes adherence of hybrid polypyrrole based protective coating. J. Solid State Electrochem. 2005, 9, 403–411. [Google Scholar] [CrossRef]
- Li, S.; Qiu, Y.; Guo, X. Comparison of PPy/sulphate-copper complexes synthesized by the pulse method and the traditional DC method. React. Funct. Polym. 2009, 69, 743–749. [Google Scholar] [CrossRef]
- Ikeda, O.; Okabayashi, K.; Tamura, H. Electrocatalytic reduction of oxygen on cobalt-doped polypyrrole films. Chem. Lett. 1983, 12, 1821–1824. [Google Scholar] [CrossRef]
- Masa, J.; Schilling, T.; Bron, M.; Schumann, W. Electrochemical synthesis of metal-polypyrrole composites and their activation for electrocatalytic reduction of oxygen by thermal treatement. Electrochim. Acta 2012, 60, 410–418. [Google Scholar] [CrossRef]
- Chipara, M.; Skomski, R.; Sellmyer, D.J. Electrodeposition and magnetic properties of polypyrrole-Fe nanocomposites. Mater. Lett. 2007, 61, 2412–2415. [Google Scholar] [CrossRef]
- Haseko, Y.; Shrestha, N.K.; Teruyama, S.; Saji, T. Reversal pulsing electrodeposition of Ni/polypyrrole composite film. Electrochim. Acta 2006, 51, 3652–3657. [Google Scholar] [CrossRef]
- Bozzini, B.; Bocchetta, P.; Gianoncelli, A.; Kourousias, G.; Kiskinova, M.; dal Zilio, S. In situ soft X-ray fluorescence and absorption microspectroscopy: A study of Mn–Co/polypyrrole electrodeposition. J. Vac. Sci. Technol. A 2015, in press. [Google Scholar] [CrossRef]
- Gianoncelli, A.; Sgura, I.; Bocchetta, P.; Lacitignola, D.; Bozzini, B. High-lateral resolution X-ray fluorescence microspectroscopy and dynamic mathematical modelling as tools for the study of electrodeposited electrocatalysts. X-RAY Spectrom. 2015, in press. [Google Scholar] [CrossRef]
- Bozzini, B.; Gianoncelli, A.; Bocchetta, P.; dal Zilio, S.; Kourousias, G. Fabrication of a sealed electrochemical microcell for in situ soft X-ray microspectroscopy and testing with in situ Co-polypyrrole composite electrodeposition for Pt-free oxygen electrocatalysis. Anal. Chem. 2014, 86, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Caramia, V.; Bozzini, B. Materials-science aspects of Zinc—Air batteries: A review. Mater. Renew. Sustain. Energy 2014, 3, 1–12. [Google Scholar]
- Bocchetta, P.; Amati, M.; Bozzini, B.; Catalano, M.; Gianoncelli, A.; Gregoratti, A.; Taurino, A.; Kiskinova, M. Quasi-in-situ Single-Grain Photoelectron Microspectroscopy of Co/PPy Nanocomposites under Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 19621–19629. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.V.R.; Fianchini, M.; Rajapakse, R.M.G. Greener Method for High-Quality Polypyrrole. Polymer 2006, 47, 7349–7354. [Google Scholar] [CrossRef]
- Nguyen-Cong, H.; El-Abbassi, K.; Gautier, J.L.; Chartier, P. Oxygen reduction on oxide/polypyrrole composite electrodes: Effect of doping anions. Electrochim. Acta 2005, 50, 1369–1376. [Google Scholar] [CrossRef]
- An, K.H.; Jeon, K.K.; Heo, J.K.; Lim, S.C.; Bae, D.J.; Lee, Y.H. High-capacitance supercapacitor using a nanocomposite electrode of single-walled carbon nanotube and polypyrrole. J. Electrochem. Soc. 2002, 149, A1058–A1062. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Vondrák, J.; Klápšte, B.; Velická, J.; Sedlaříková, M.; Novák, V.; Reiter, J. Electrochemical Activity of Manganese Oxide/Carbon-based Electrocatalysts. J. New Mater. Electrochem. Syst. 2005, 8, 209–212. [Google Scholar]
- Vondrák, J.; Klápšte, B.; Velická, J.; Sedlaříková, M.; Novák, V.; Reiter, J. Carbon/Manganese oxide based fuel cell electrocatalyst using “flywheel” principle. J. New Mater. Electrochem. Syst. 2005, 8, 1–4. [Google Scholar]
- Mao, L.; Zhang, D.; Sotomura, T.; Nakatsu, K.; Koshiba, N.; Ohsaka, T. Mechanistic study of the reduction of oxygen in air electrode with manganese oxides as electrocatalysts. Electrochim. Acta 2003, 48, 1015–1021. [Google Scholar] [CrossRef]
- Roche, I.; Scott, K. Carbon-supported manganese oxide nanoparticles as electrocatalysts for oxygen reduction reaction (ORR) in neutral solution. J. Appl. Electrochem. 2008, 39, 197–204. [Google Scholar] [CrossRef]
- Cao, R.; Lee, J.-S.; Liu, M.; Cho, J. Recent Progress in Non-Precious Catalysts for Metal-Air Batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhang, X.G.; Wang, Y.G. A new air electrode based on carbon nanotubes and Ag-MnO2 for metal air electrochemical cells. Carbon 2004, 42, 3097–3102. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2001; pp. 335–344. [Google Scholar]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- De Koninck, M.; Marsan, B. MnxCu1−xCo2O4 used as bifunctional electrocatalyst in alkaline medium. Electrochim. Acta 2008, 53, 7012–7021. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Investigations of the catalytic properties of manganese oxides for the oxygen reduction reaction in alkaline media. J. Electroanal. Chem. 2006, 590, 152–160. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic activity of manganese oxides prepared by thermal decomposition for oxygen reduction. Electrochim. Acta 2007, 52, 3732–3738. [Google Scholar] [CrossRef]
- Benedetto, B.; Lacitignola, D.; Sgura, I. Spatio-Temporal Organisation in Alloy Electrodeposition: A Morphochemical Mathematical Model and its Experimental Validation. J. Solid State Electrochem. 2013, 17, 467–479. [Google Scholar]
- Lacitignola, D.; Bozzini, B.; Sgura, I. Spatio-temporal organization in a morphochemical electrodeposition model: Hopf and Turing instabilities and their interplay. Eur. J. Appl. Math. 2015, 26, 143–173. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, F.-B.; Xia, X.-H. Bioinspired copper catalyst effective for both reduction and evolution of oxygen. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaulich, B.; Susini, J.; David, C.; di Fabrizio, E.; Morrison, G.; Charalambous, P.; Thieme, J.; Wilhein, T.; Kovac, J.; Bacescu, D.; et al. Proceedings of 8th International Conference X-ray Microscopy; Aoki, S., Kagoshima, Y., Suzuki, Y., Eds.; IPAP: Tokyo, Japan, 2005; Volume 7, pp. 22–25. [Google Scholar]
- Kaulich, B.; Thibault, P.; Gianoncelli, A.; Kiskinova, M. Transmission and emission X-ray microscopy: Operation modes, contrast mechanisms and applications. J. Phys. Condensed Matter 2011, 23. [Google Scholar] [CrossRef] [PubMed]
- Gianoncelli, A.; Kaulich, B.; Alberti, R.; Klatka, T.; Longoni, A.; de Marco, A.; Marcello, A.; Kiskinova, M. Simultaneous soft X-ray transmission and emission microscopy. Nuclear. Intrum. Methods Phys. Res. Sect. A 2009, 608, 195–198. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzini, B.; Bocchetta, P.; Gianoncelli, A. Coelectrodeposition of Ternary Mn-Oxide/Polypyrrole Composites for ORR Electrocatalysts: A Study Based on Micro-X-ray Absorption Spectroscopy and X-ray Fluorescence Mapping. Energies 2015, 8, 8145-8164. https://doi.org/10.3390/en8088145
Bozzini B, Bocchetta P, Gianoncelli A. Coelectrodeposition of Ternary Mn-Oxide/Polypyrrole Composites for ORR Electrocatalysts: A Study Based on Micro-X-ray Absorption Spectroscopy and X-ray Fluorescence Mapping. Energies. 2015; 8(8):8145-8164. https://doi.org/10.3390/en8088145
Chicago/Turabian StyleBozzini, Benedetto, Patrizia Bocchetta, and Alessandra Gianoncelli. 2015. "Coelectrodeposition of Ternary Mn-Oxide/Polypyrrole Composites for ORR Electrocatalysts: A Study Based on Micro-X-ray Absorption Spectroscopy and X-ray Fluorescence Mapping" Energies 8, no. 8: 8145-8164. https://doi.org/10.3390/en8088145







