Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review
Abstract
:1. Introduction
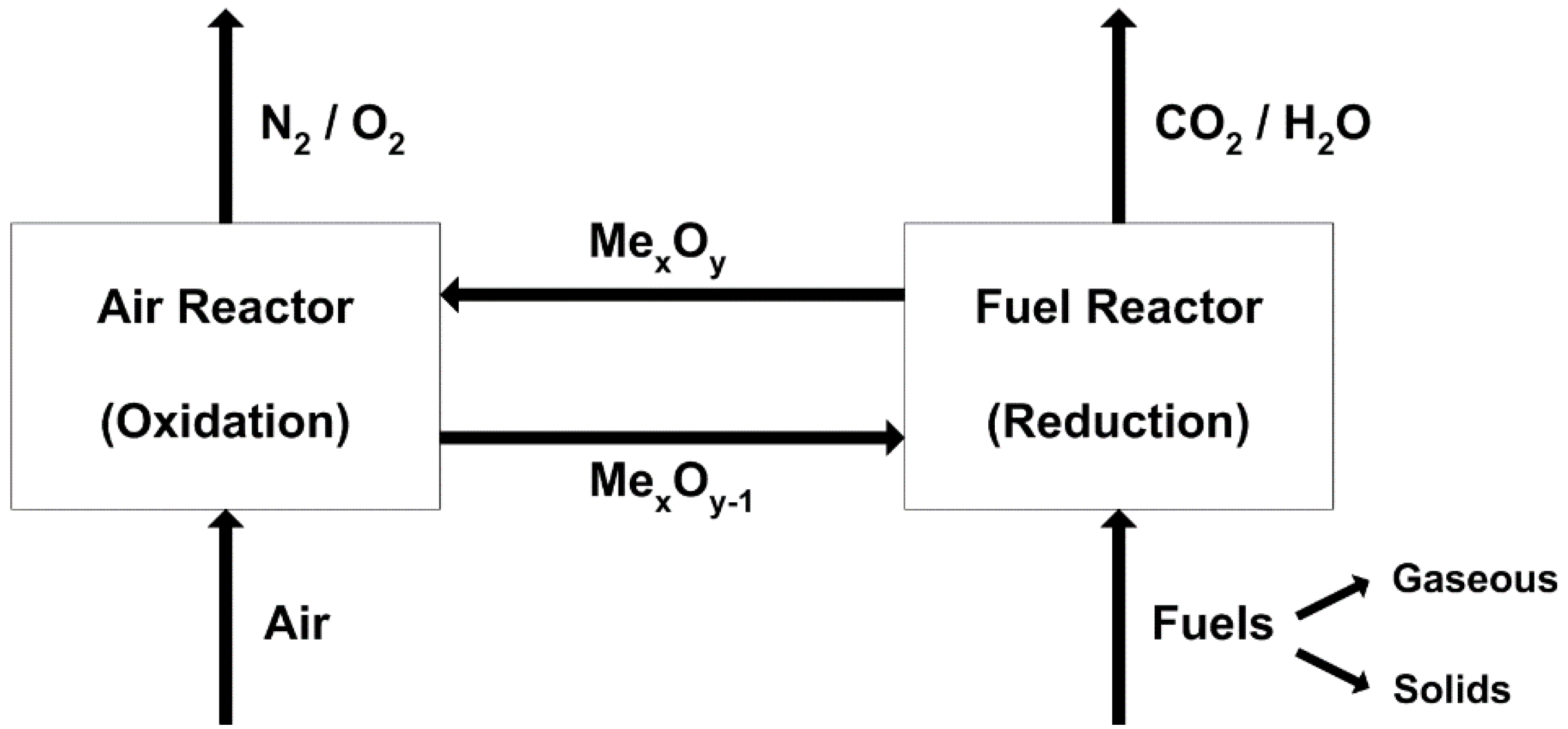
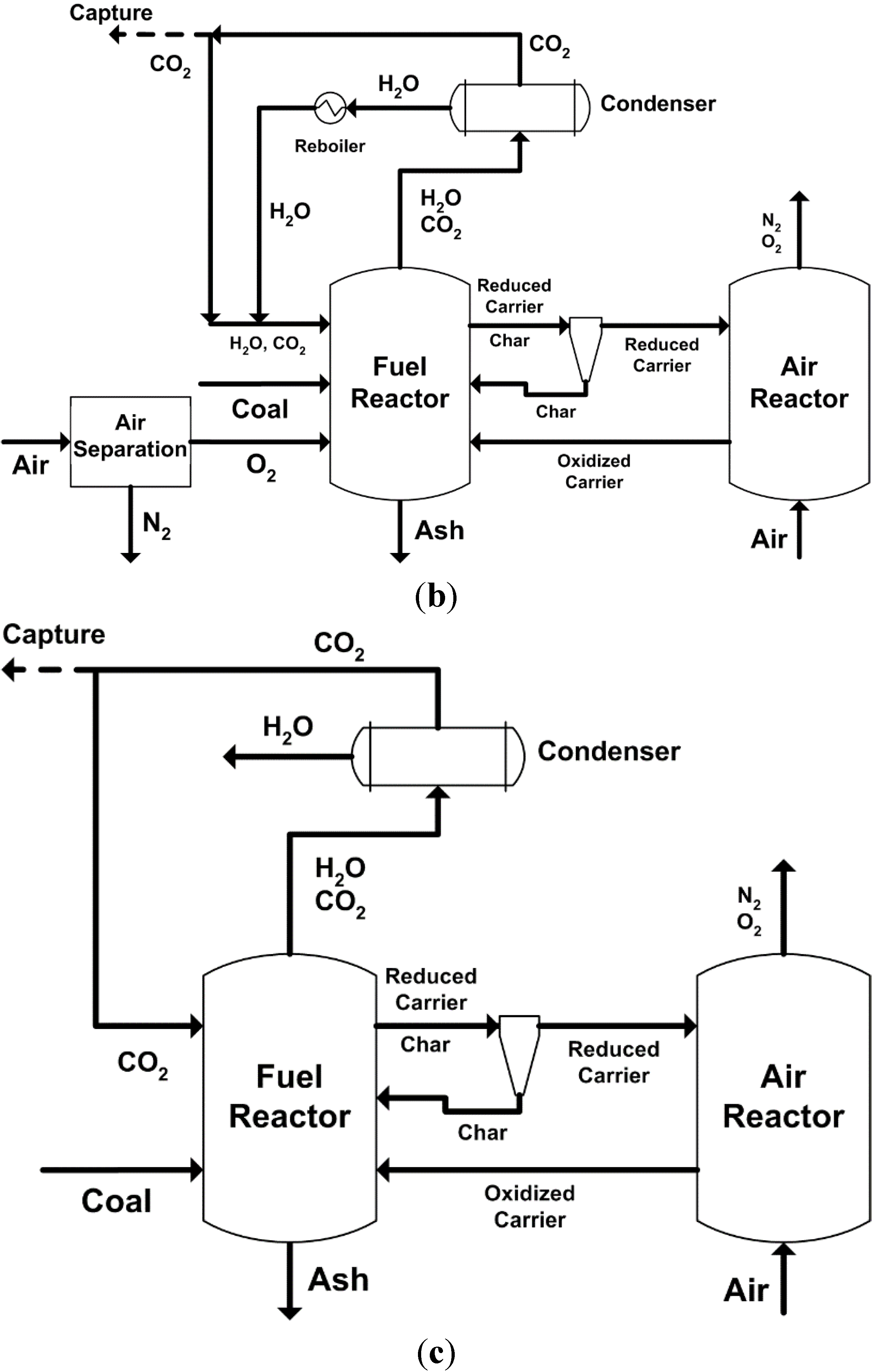
2. Major Proposed Solid Fuel Chemical-Looping Combustion (CLC) and Gasification (CLG)
2.1. Major Proposed Solid Fuel Chemical-Looping Combustion Processes

ΔH°298 = 131.1 kJ/mol
ΔH°298 = 172.5 kJ/mol
ΔH°900 = 262.1 kJ/mol O2
ΔH°900 = 193.1 kJ/mol O2
ΔH°900 = 406.7 kJ/mol O2
ΔH°900 = −132.9 kJ/mol O2
ΔH°900 = −201.9 kJ/mol O2
ΔH°900 = 11.7 kJ/mol O2
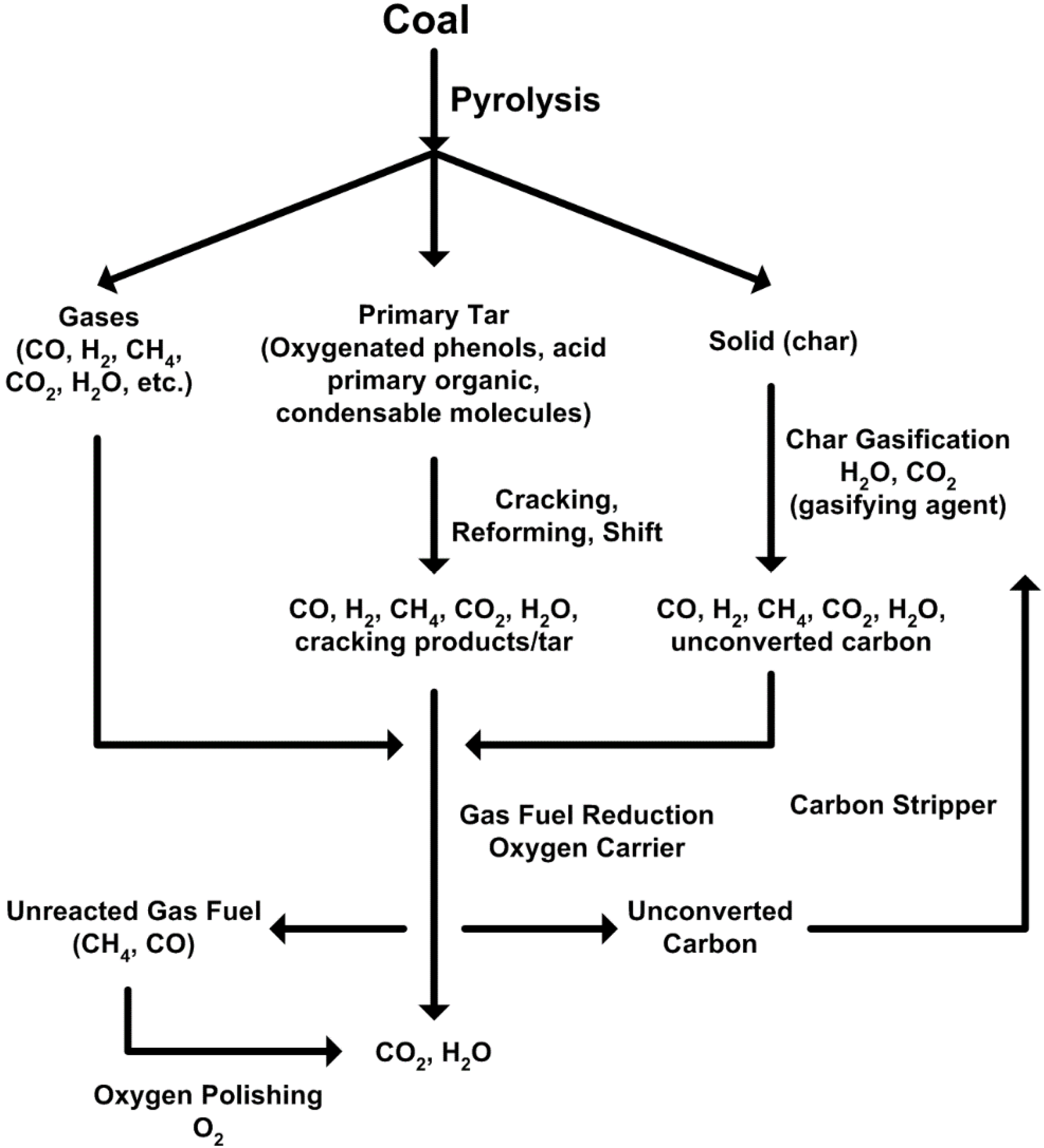
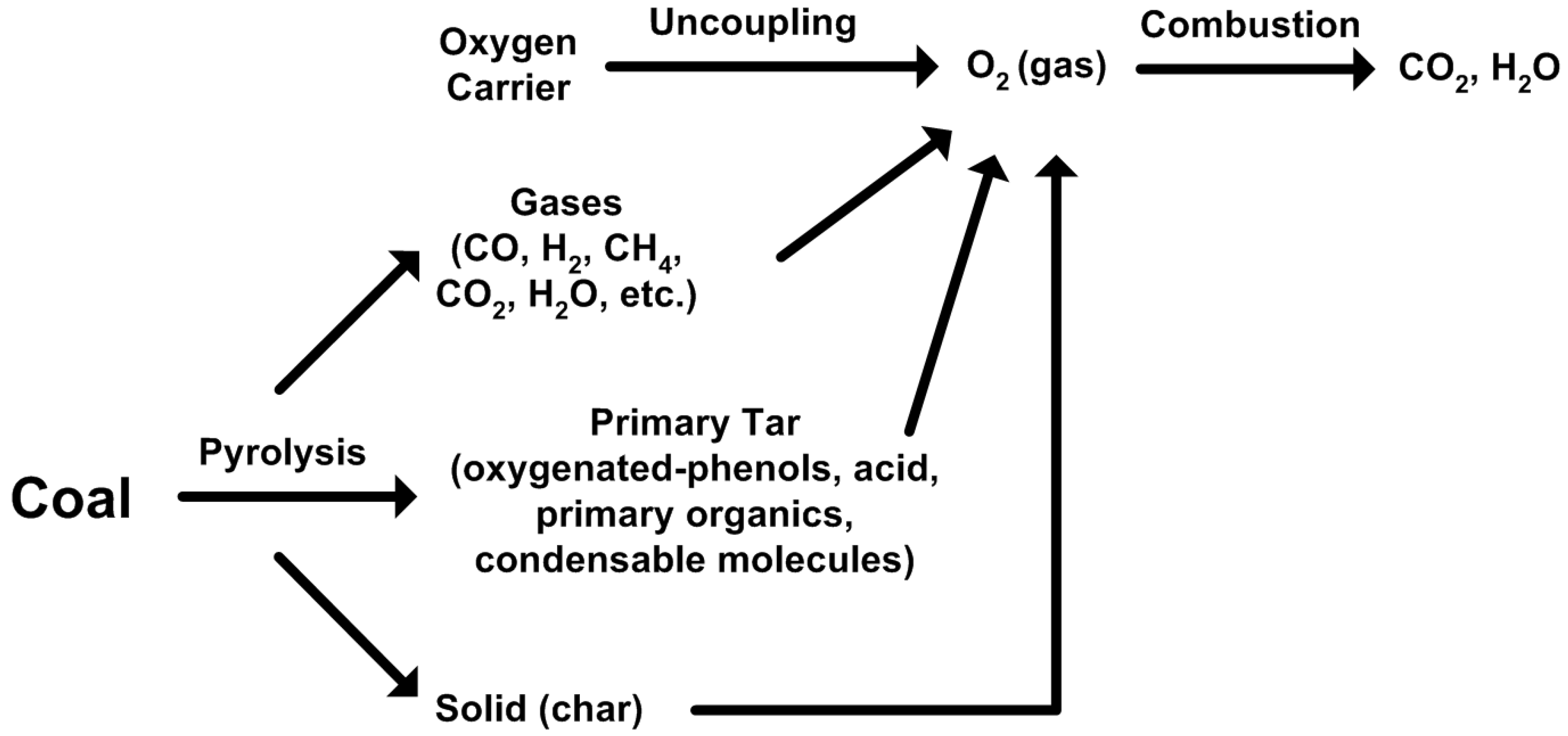
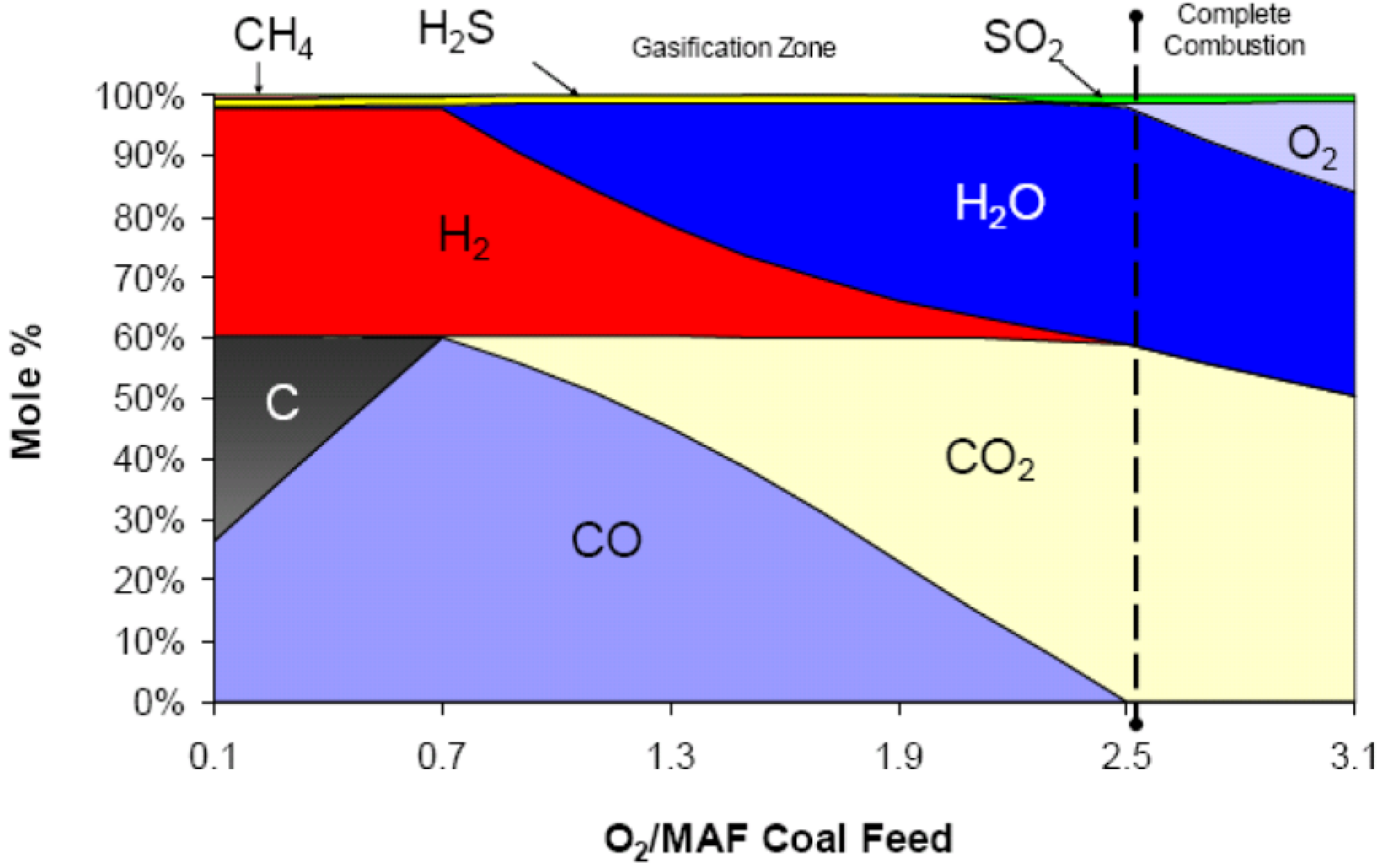
2.2. Major Proposed Solid Fuel Chemical-Looping Gasification Processes
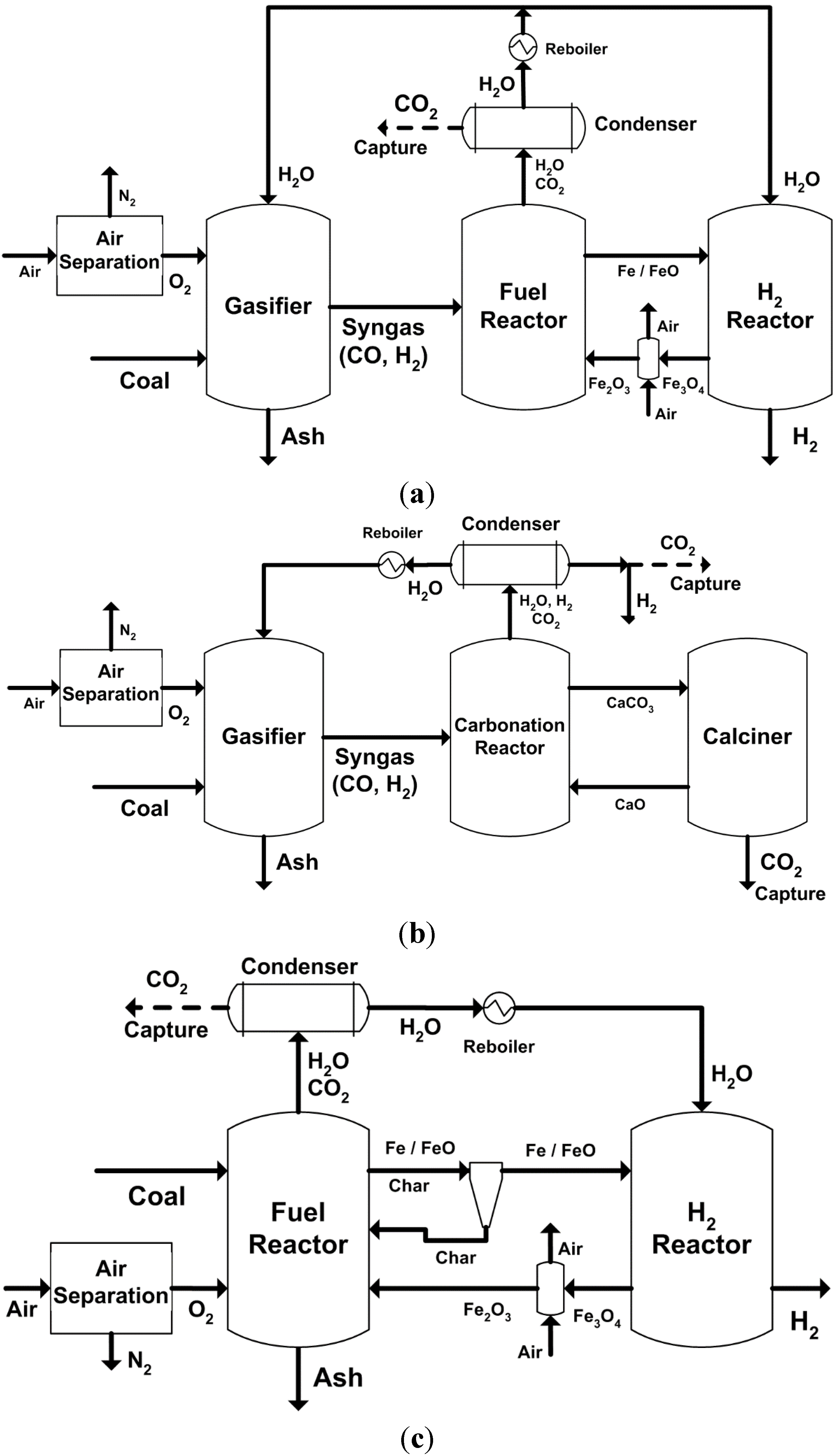
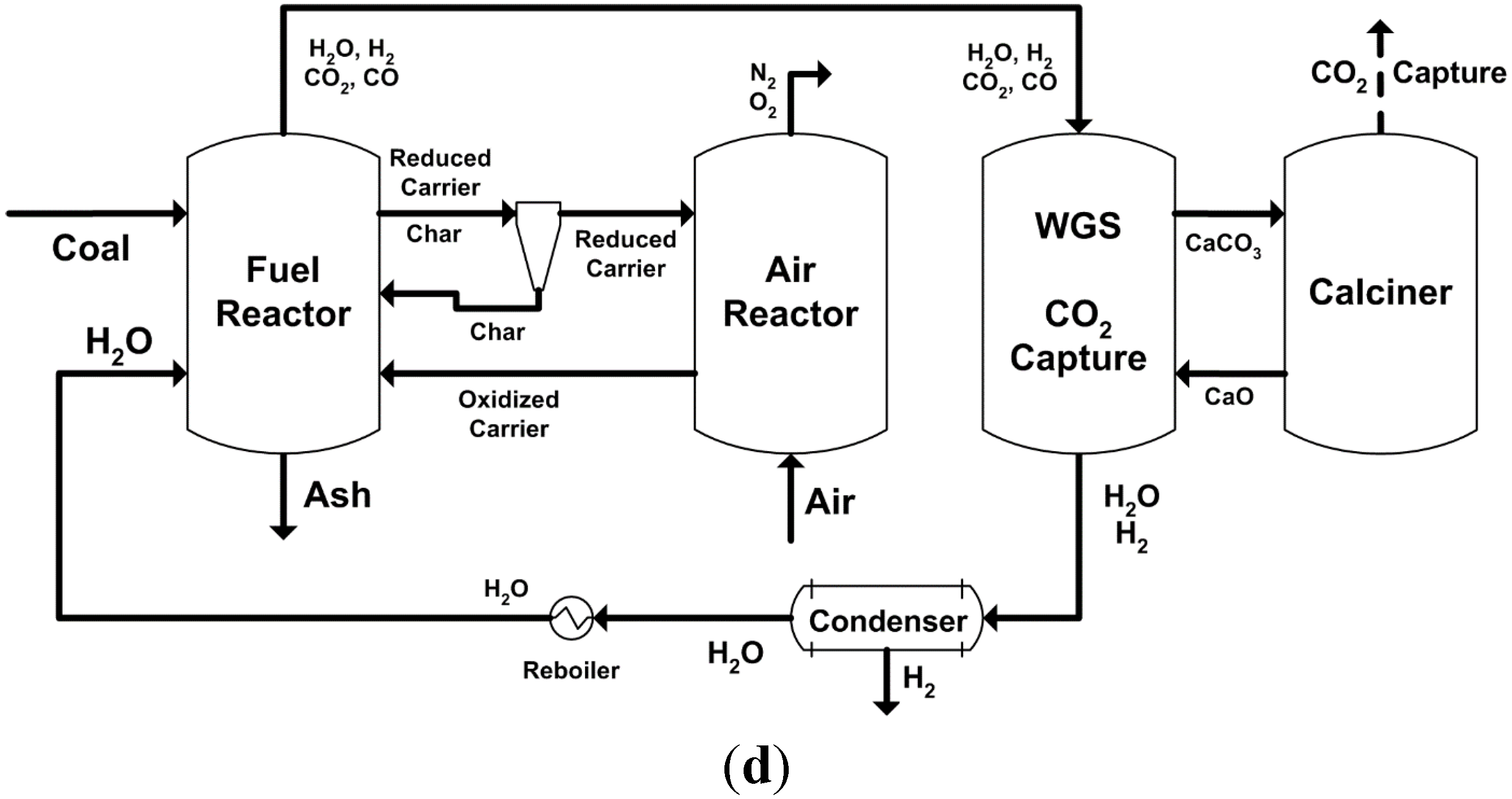
- devolatilization/pyrolysis;
- char gasification with steam and/or CO2;
- char and volatiles combustion;
- gaseous fuels (H2, CO, CH4, volatiles) react with oxygen carriers;
- oxidation of reduced oxygen carriers with air/O2 or steam.
3. Oxygen Carriers Development and Application of Thermogravimetric Analysis (TGA)
3.1. Important Oxygen Carrier Characteristics
3.2. Lab Scale TGA Studies for Reactivity and Recyclability of Oxygen Carriers
| Oxygen carrier (OC) | Process | Fuel | Sample wt. (mg) | Heating rate (°C/min) | TGA application | TGA test condition | Ref. |
|---|---|---|---|---|---|---|---|
| 60 wt.% CuO + MgAl2O4 (100–200 µm) | CLOU | NA | 50 | Not reported | Copper content determination | 15% H2/N2, 850 °C | [73] |
| Oxygen transport capacity | N2, 1000 °C | ||||||
| Fresh or used carrier reactivity, recyclability | Reduction N2, 1000 °C; oxidation air 1000 °C 5 cycles | ||||||
| Effect of temperatures on carrier reactivity | Reduction N2, 900 °C, 950 °C, 1000 °C; oxidation air 900 °C, 950 °C, 1000 °C | ||||||
| Effect of oxygen concentrations on carrier reactivity | Reduction N2, 1000 °C, 0, 1.5, 4, and 11% O2; oxidation air 900 °C, 4, 11, and 21% O2 | ||||||
| 60 wt.% NiO/NiAl2O4 (125–180 µm) | CLC | Coal char (<150 µm, prepared at 900 °C for 7 min) | 10 (OC/char: 50/2–3.5 by mass) | 15 | Reactivity | Reduction N2, 900 °C; oxidation air 1000 °C | [74] |
| Recyclability | Reduction 50% H2/N2, 900 °C; oxidation air 900 °C, 5 cycles | ||||||
| Iron ore | IG-CLC | NA | 50 | Not reported | Used carrier reactivity | 15% H2/N2, 950 °C | [29] |
| Pure metal oxide (CuO, Fe2O3, Co3O4, NiO, and Mn2O3) | CLC | Illinois #6 (100 µm) | 150 (OC/coal: 15–22.5 by mass) | 15 | Reactivity | Reduction N2 or CO2, 700–1000 °C; oxidation air 900 °C or 1000 °C | [75] |
| Recyclability | Fresh coal every cycles, 8 cycles | ||||||
| Pure metal oxide (CuO) | Solid fuel (PRB coal, wood, PE) (50–150 µm) | 10–50 (OC/coal: 75/25, OC/PE: 75/25, OC/wood: 90:10 by mass) | 30 | Reactivity | Reduction N2 or CO2, 850 °C | [76] |
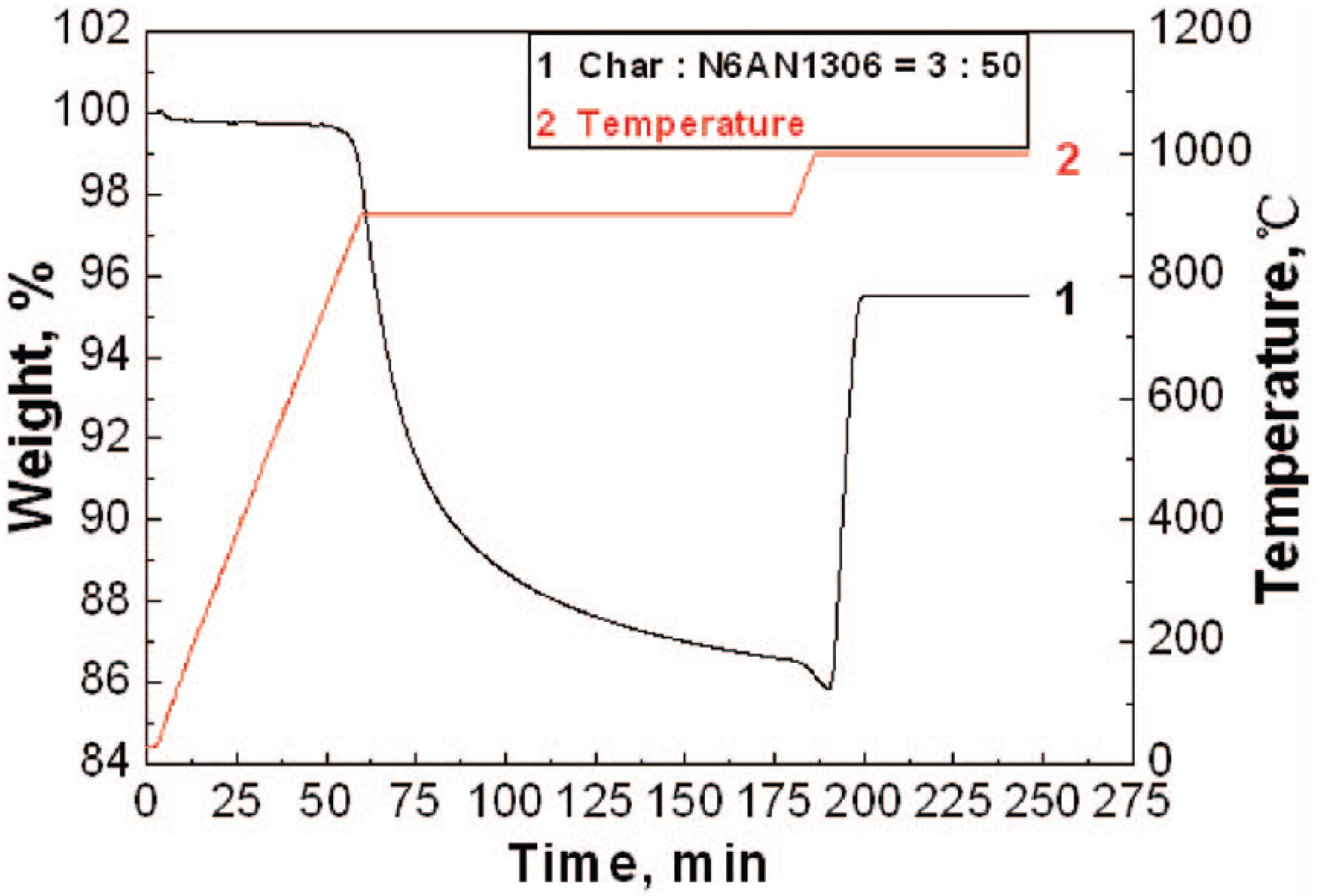
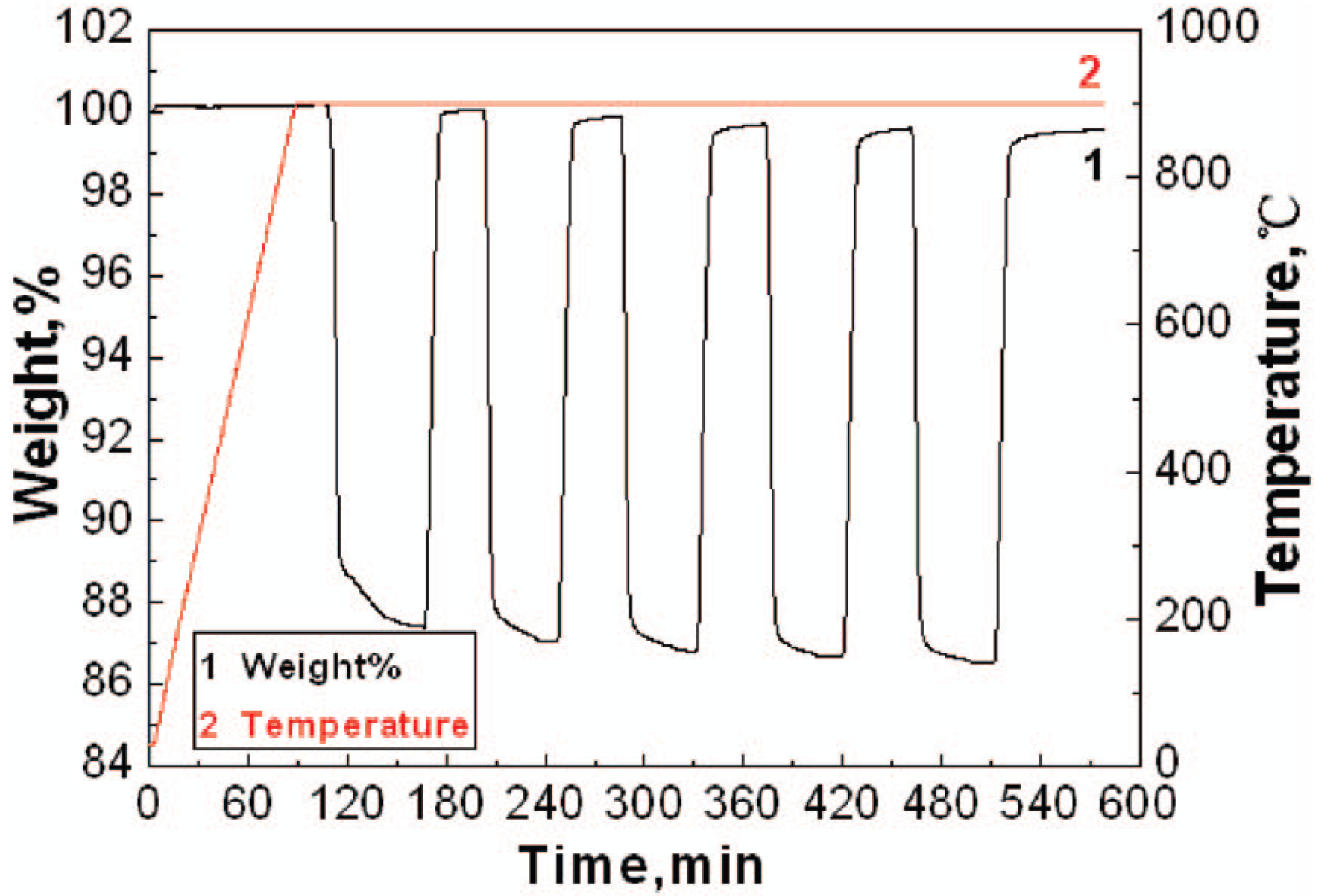
3.3. Comparison of Oxygen Carrier Reactivity Using TGA with Large Scale Fluidized Bed Studies
3.4. Kinetic Studies of Oxygen Carriers and Solid Fuels Using TGA
4. Challenges and Research on Direct Coals Chemical-Looping Combustion
4.1. Challenges and Recent Research on the iG-CLC Process
| Oxygen carrier | Fuel | Process | Size (kW) * | Temperature in fuel reactor | Reference |
|---|---|---|---|---|---|
| Ilmenite | Lignite bituminous, anthracite coal | iG-CLC | 0.5 | 870–950 °C | [6,39,50] |
| Bauxite waste (Fe-based) | 870–930 °C | [15,28] | |||
| Tiegra iorn ore | [29] | ||||
| Ilmenite, manganese | Bituminous coal, petcoke | iG-CLC | 10 | 950 °C | [9,33,53] |
| Ilmenite | Bituminous coal | iG-CLC | 100 | 940–980 °C | [51] |
| NiO/Al2O3 | Shenhua Bituminous coal | iG-CLC | 10 | 870–970 °C | [68,69] |
| Iron ore | Shenhua Bituminous coal and/or sawdust | iG-CLC | 1 | 900–980 °C for coal | [26,38,40,52] |
| 60%CuO with MgAl2O4 as support (Cu60MgAL) | Bituminous coal | CLOU | 1.5 | [43] |
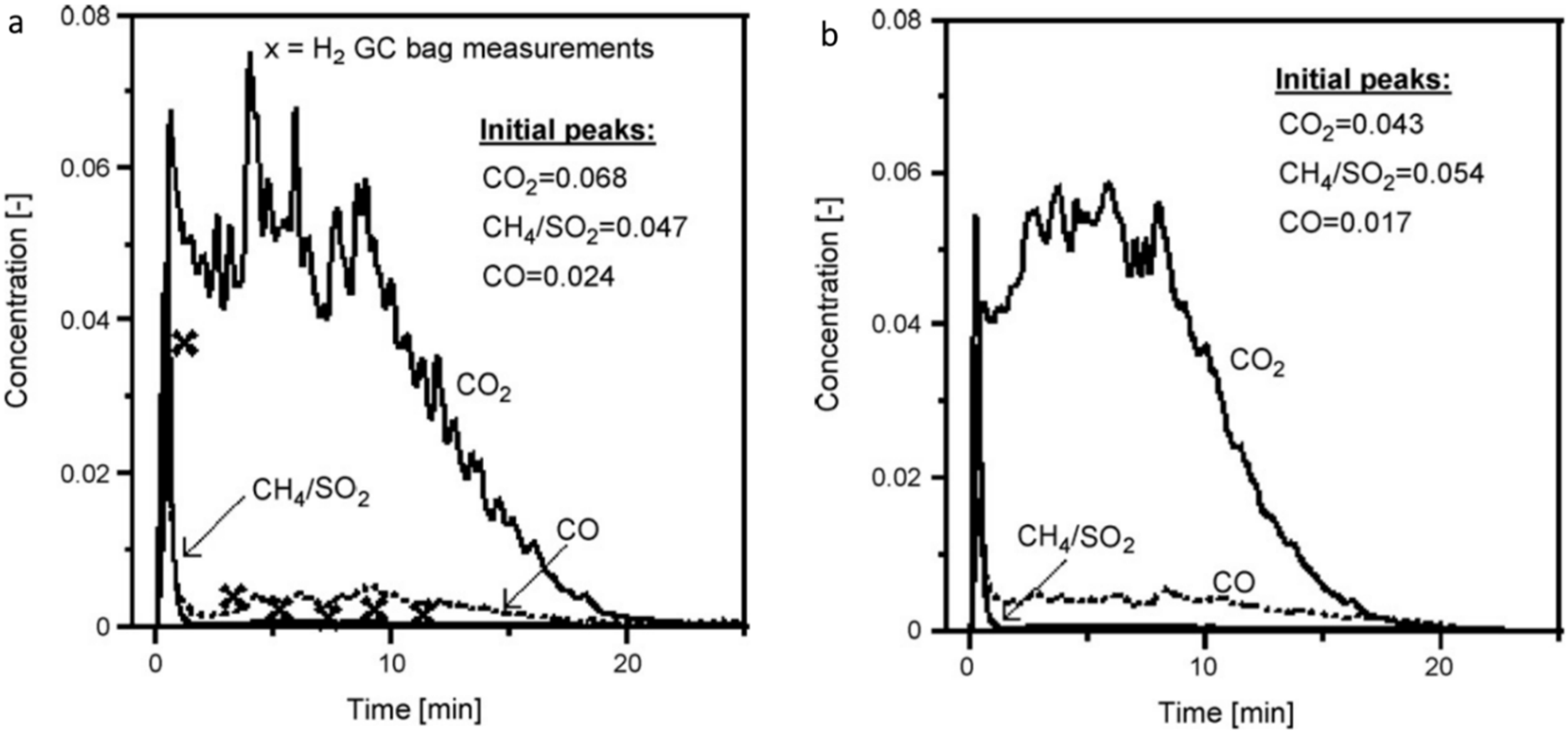
ΔH°298K = −74.8 kJ/mol
4.2. Challenges and Recent Research on the CLOU Process
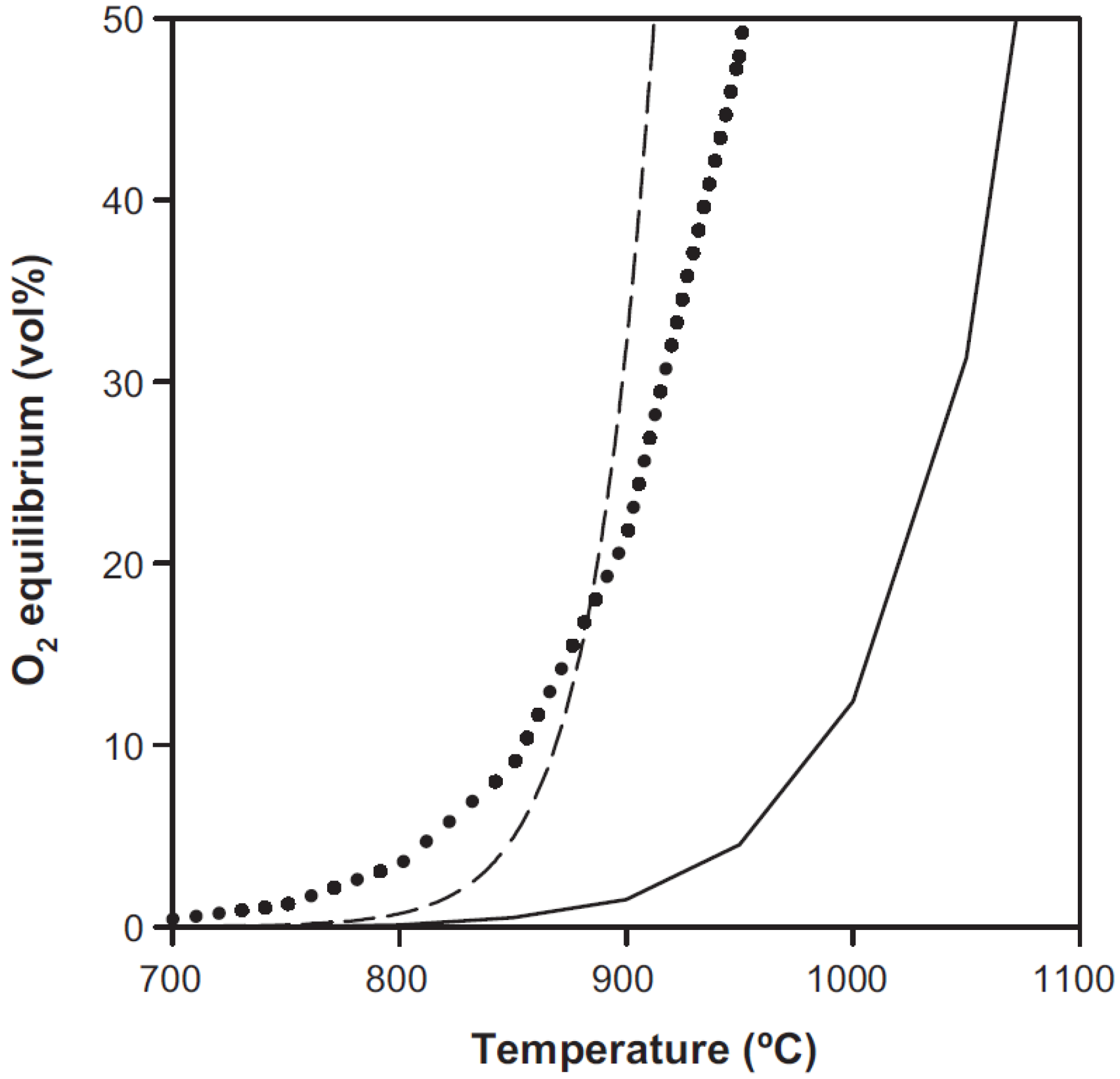
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Energy Outlook 2014; International Energy Agency: Paris, France, 2014.
- Velazquet-Vargas, L.G.; Devault, D.J.; Flynn, T.J.; Siengchum, T.; Zeng, L.; Tong, A.; Bayham, S.; Fan, L.S. Techno-economic analysis of a 550 MWe atmospheric iron-based coal-direct chemcial looping process. In Proceeding of 3rd International Conference on Chemical Looping, Goteborg, Sweden, 9–11 September 2014.
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Fan, L.S. Chemical Looping Systems for Fossil Energy Conversions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 420. [Google Scholar]
- Dieter, H.; Bidwe, A.R.; Varela-Duelli, G.; Charitos, A.; Hawthorne, C.; Scheffknecht, G. Development of the Calcium Looping CO2 Capture Technology from Lab to Pilot Scale at IFK, University of Stuttgart. Fuel 2014, 127, 23–37. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. Relevance of the coal rank on the performance of the in situ gasification chemical-looping combustion. Chem. Eng. J. 2012, 195, 91–102. [Google Scholar] [CrossRef]
- Saucedo, M.A.; Lim, J.Y.; Dennis, J.S.; Scott, S.A. CO2-gasification of a lignite coal in the presence of an iron-based oxygen carrier for chemical-looping combustion. Fuel 2014, 127, 186–201. [Google Scholar] [CrossRef]
- Hossain, M.M.; de lasa, H.I. Chemical-looping combustion (CLC) for inherent CO2 separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Berguerand, N.; Lyngfelt, A. Design and operation of a 10 kW(th) chemical-looping combustor for solid fuels—Testing with South African coal. Fuel 2008, 87, 2713–2726. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 1. Process analysis. Energy Fuels 2006, 20, 1836–1844. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The use of ilmenite as an oxygen carrier in chemical-looping combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Solid fuels in chemical-looping combustion. Int. J. Greenh. Gas Control 2008, 2, 180–193. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical-looping combustion of solid fuels—Status of development. Appl. Energy 2014, 113, 1869–1873. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Control 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Gayan, P.; Adanez, J. Use of an Fe-based residue from alumina production as an oxygen carrier in chemical-looping combustion. Energy Fuels 2012, 26, 1420–1431. [Google Scholar] [CrossRef]
- Mendiara, T.; Garcia-Labiano, F.; Gayan, P.; Abad, A.; de Diego, L.F.; Adanez, J. Evaluation of the use of different coals in chemical looping combustion using a bauxite waste as oxygen carrier. Fuel 2013, 106, 814–826. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Chemical-looping gasification of biomass for hydrogen-enriched gas production with in-process carbon dioxide capture. Energy Fuels 2009, 23, 5077–5083. [Google Scholar] [CrossRef]
- Dennis, J.S.; Scott, S.A. In situ gasification of a lignite coal and CO2 separation using chemical looping with a Cu-based oxygen carrier. Fuel 2010, 89, 1623–1640. [Google Scholar] [CrossRef]
- Thunman, H.; Lind, F.; Breitholtz, C.; Berguerand, N.; Seemann, M. Using an oxygen-carrier as bed material for combustion of biomass in a 12-MWth circulating fluidized-bed boiler. Fuel 2013, 113, 300–309. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. The use of petroleum coke as fuel in chemical-looping combustion. Fuel 2007, 86, 1947–1958. [Google Scholar] [CrossRef]
- Brown, T.A.; Dennis, J.S.; Scott, S.A.; Davidson, J.F.; Hayhurst, A.N. Gasification and chemical-looping combustion of a lignite char in a fluidized bed of iron oxide. Energy Fuels 2010, 24, 3034–3048. [Google Scholar] [CrossRef]
- Lyon, R.K.; Cole, J.A. Unmixed combustion: An alternative to fire. Combust. Flame 2000, 121, 249–261. [Google Scholar] [CrossRef]
- Miller, B.G. Coal Energy Systems; Elsevier: Burlington, MA, USA, 2005; p. 526. [Google Scholar]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Wang, P.; Massoudi, M. Slag behavior in gasifiers Part I: Influence of coal properties and gasification conditions. Energies 2013, 6, 784–806. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T.; Chen, D. Evaluation of the effect of sulfur on iron-ore oxygen carrier in chemical-looping combustion. Ind. Eng. Chem. Res. 2013, 52, 1795–1805. [Google Scholar] [CrossRef]
- Arjmand, M.; Leion, H.; Lyngfelt, A.; Mattisson, T. Use of manganese ore in chemical-looping combustion (CLC)-Effect on steam gasification. Int. J. Greenh. Gas Control 2012, 8, 56–60. [Google Scholar] [CrossRef]
- Mendiara, T.; de Diego, L.F.; Garcia-Labiano, F.; Gayan, P.; Abad, A.; Adanez, J. Behaviour of a bauxite waste material as oxygen carrier in a 500 Wth CLC unit with coal. Int. J. Greenh. Gas Control 2013, 17, 170–182. [Google Scholar] [CrossRef]
- Mendiara, T.; de Diego, L.F.; Garcia-Labiano, F.; Gayan, P.; Abad, A.; Adanez, J. On the use of a highly reactive iron ore in chemical looping combustion of different coals. Fuel 2014, 126, 239–249. [Google Scholar] [CrossRef]
- Wen, Y.-Y.; Li, Z.-S.; Xu, L.; Cai, N.-S. Experimental study of natural Cu ore particles as oxygen carriers in chemical looping with oxygen uncoupling (CLOU). Energy Fuels 2012, 26, 3919–3927. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Use of ores and industrial products as oxygen carriers in chemical-looping combustion. Energy Fuels 2009, 23, 2307–2315. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Ryden, M.; Lyngfelt, A. Testing of minerals and industrial by-products as oxygen carriers for chemical-looping combustion in a circulating fluidized-bed 300 W laboratory reactor. Fuel 2012, 93, 351–363. [Google Scholar] [CrossRef]
- Berguerand, N.; Lyngfelt, A. Batch testing of solid fuels with ilmenite in a 10 kW(th) chemical-looping combustor. Fuel 2010, 89, 1749–1762. [Google Scholar] [CrossRef]
- Frohn, P.; Arjmand, M.; Azimi, G.; Leion, H.; Mattisson, T.; Lyngfelt, A. On the high-gasification rate of brazilian manganese ore in chemical-looping combustion (CLC) for solid fuels. Aiche J. 2013, 59, 4346–4354. [Google Scholar] [CrossRef]
- Xu, L.; Edland, R.; Li, Z.; Leion, H.; Zhao, D.; Cai, N. Cu-modified manganese ore as an oxygen carrier for chemical looping combustion. Energy & Fuels 2014, 28, 7085–7092. [Google Scholar]
- Bao, J.; Li, Z.; Cai, N. Reduction kinetics of foreign-ion-promoted ilmenite using carbon monoxide (CO) for chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 10646–10655. [Google Scholar] [CrossRef]
- Song, T.; Shen, L.; Guo, W.; Chen, D.; Xiao, J. Enhanced reaction performance with hematite/Ca2Al2SiO7 oxygen carrier in chemical looping combustion of coal. Ind. Eng. Chem. Res. 2013, 52, 9573–9585. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T.; Chen, D. Iron ore as oxygen carrier improved with potassium for chemical looping combustion of anthracite coal. Combust. Flame 2012, 159, 2480–2490. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. Effect of operating conditions in chemical-looping combustion of coal in a 500 W-th unit. Int. J. Greenh. Gas Control 2012, 6, 153–163. [Google Scholar] [CrossRef]
- Song, T.; Wu, J.; Zhang, H.; Shen, L. Characterization of an Australia hematite oxygen carrier in chemical looping combustion with coal. Int. J. Greenh. Gas Control 2012, 11, 326–336. [Google Scholar] [CrossRef]
- Tong, A.; Bayham, S.; Kathe, M.V.; Zeng, L.; Luo, S.; Fan, L.-S. Iron-based syngas chemical looping process and coal-direct chemical looping process development at Ohio State University. Appl. Energy 2014, 113, 1836–1845. [Google Scholar] [CrossRef]
- de Diego, L.F.; Gayan, P.; Garcia-Labiano, F.; Celaya, J.; Abad, M.; Adanez, J. Impregnated CuO/Al2O3 oxygen carriers for chemical-looping combustion: Avoiding fluidized bed agglomeration. Energy Fuels 2005, 19, 1850–1856. [Google Scholar] [CrossRef]
- Abad, A.; Adanez-Rubio, I.; Gayan, P.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Demonstration of chemical-looping with oxygen uncoupling (CLOU) process in a 1.5 kW(th) continuously operating unit using a Cu-based oxygen-carrier. Int. J. Greenh. Gas Control 2012, 6, 189–200. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Gayan, P.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J.; Abad, A. Development of CuO-based oxygen-carrier materials suitable for chemical-looping with oxygen uncoupling (CLOU) process. Int. Conference on Greenh. Gas Control Technol. 2011, 4, 417–424. [Google Scholar] [CrossRef]
- Gayan, P.; Adanez-Rubio, I.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Development of Cu-based oxygen carriers for chemical-looping with oxygen uncoupling (CLOU) process. Fuel 2012, 96, 226–238. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Gayan, P.; Abad, A.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Kinetic analysis of a Cu-based oxygen carrier: Relevance of temperature and oxygen partial pressure on reduction and oxidation reactions rates in chemical looping with oxygen uncoupling (CLOU). Chem. Eng. J. 2014, 256, 69–84. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Ryden, M.; Mattisson, T.; Lyngfelt, A. Investigation of different Mn-Fe oxides as oxygen carrier for chemical-looping with oxygen uncoupling (CLOU). Energy Fuels 2013, 27, 367–377. [Google Scholar] [CrossRef]
- Ryden, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Combined oxides as oxygen-carrier material for chemical-looping with oxygen uncoupling. Applied Energy 2014, 113, 1924–1932. [Google Scholar] [CrossRef]
- Shulman, A.; Cleverstam, E.; Mattisson, T.; Lyngfelt, A. Manganese/iron, Manganese/nickel, and Manganese/silicon oxides used in chemical-looping with oxygen uncoupling (CLOU) for combustion of methane. Energy Fuels 2009, 23, 5269–5275. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. The use of ilmenite as oxygen-carrier in a 500 W-th chemical-looping coal combustion unit. Int. J. Greenh. Gas Control 2011, 5, 1630–1642. [Google Scholar] [CrossRef]
- Markstrom, P.; Linderholm, C.; Lyngfelt, A. Chemical-looping combustion of solid fuels—Design and operation of a 100 kW unit with bituminous coal. Int. J. Greenh. Gas Control 2013, 15, 150–162. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T. Chemical looping combustion of biomass/coal with natural iron ore as oxygen carrier in a continuous reactor. Energy Fuels 2011, 25, 446–455. [Google Scholar] [CrossRef]
- Cuadrat, A.; Linderholm, C.; Abad, A.; Lyngfelt, A.; Adanez, J. Influence of limestone addition in a 10 kW(th) chemical-looping combustion unit operated with petcoke. Energy Fuels 2011, 25, 4818–4828. [Google Scholar] [CrossRef]
- Jin, H.G.; Ishida, M. A new type of coal gas fueled chemical-looping combustion. Fuel 2004, 83, 2411–2417. [Google Scholar] [CrossRef]
- Soncini, R.M.; Means, N.C.; Weiland, N.T. Co-pyrolysis of low rank coals and biomass: Product distributions. Fuel 2013, 112, 74–82. [Google Scholar] [CrossRef]
- Weiland, N.T.; Means, N.C.; Morreale, B.D. Product distributions from isothermal co-pyrolysis of coal and biomass. Fuel 2012, 94, 563–570. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Mattisson, T.; Ryden, M.; Snijkers, F.; Lyngfelt, A. Mn-Fe oxides with support of MgAl2O4, CeO2, ZrO2 and Y2O3-ZrO2 for chemical-looping combustion and chemical-looping with oxygen uncoupling. Int. J. Greenh. Gas Control 2014, 53, 10358–10365. [Google Scholar]
- Pour, N.M.; Leion, H.; Ryden, M.; Mattisson, T. Combined Cu/Mn oxides as an oxygen carrier in chemical looping with oxygen uncoupling (CLOU). Energy Fuels 2013, 27, 6031–6039. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Arjmand, M.; Leion, H.; Gayan, P.; Abad, A.; Mattisson, T.; Lyngfelt, A. Investigation of combined supports for cu-based oxygen carriers for chemical-looping with oxygen uncoupling (CLOU). Energy Fuels 2013, 27, 3918–3927. [Google Scholar] [CrossRef]
- Shafiefarhood, A.; Stewart, A.; Li, F. Iron-containing mixed-oxide composites as oxygen carriers for chemical looping with oxygen uncoupling (CLOU). Fuel 2015, 139, 1–10. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Ramkumar, S. Utilization of chemical looping strategy in coal gasification processes. Particuology 2008, 6, 131–142. [Google Scholar] [CrossRef]
- Mehdizadeh, A.M.; Klausner, J.F.; Barde, A.; Mei, R. Enhancement of thermochemical hydrogen production using an iron-silica magnetically stabilized porous structure. Int. J. Hydrog. Energy 2012, 37, 8954–8963. [Google Scholar] [CrossRef]
- United States Department of Energy; National Energy Technology Laboratory. Advanced Combustion Systems: Chemical Looping Summary; United States Department of Energy National Energy Technology Laboratory: Pittsburgh, PA, USA, 2013.
- de Diego, L.F.; Garcia-Labiano, F.; Adanez, J.; Gayan, P.; Abad, A.; Corbella, B.M.; Palacios, J.M. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1749–1757. [Google Scholar] [CrossRef]
- Anhenden, M. Chemical looping combustion: Emerging technology for CO2 capture. In Proceedings of the Clearwater Clean Coal Conference, Clearwater, FL, USA, 6–10 June 2010.
- Linderholm, C.; Lyngfelt, A.; Cuadrat, A.; Jerndal, E. Chemical-looping combustion of solid fuels—Operation in a 10 kW unit with two fuels, above-bed and in-bed fuel feed and two oxygen carriers, manganese ore and ilmenite. Fuel 2012, 102, 808–822. [Google Scholar] [CrossRef]
- Leion, H.; Lyngfelt, A.; Mattisson, T. Solid fuels in chemical-looping combustion using a NiO-based oxygen carrier. Chem. Eng. Res. Des 2009, 87, 1543–1550. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Xiao, J. Experiments on chemical looping combustion of coal with a NiO based oxygen carrier. Combust. Flame 2009, 156, 721–728. [Google Scholar] [CrossRef]
- Shen, L.; Wu, J.; Gao, Z.; Xiao, J. Reactivity deterioration of NiO/Al2O3 oxygen carrier for chemical looping combustion of coal in a 10 kW(th) reactor. Combust. Flame 2009, 156, 1377–1385. [Google Scholar] [CrossRef]
- Johansson, M.; Mattisson, T.; Lyngfelt, A. Investigation of Mn3O4 with stabilized ZrO2 for chemical-looping combustion. Chem. Eng. Res. Des. 2006, 84, 807–818. [Google Scholar] [CrossRef]
- Leion, H.; Larring, Y.; Bakken, E.; Bredesen, R.; Mattisson, T.; Lyngfelt, A. Use of CaMn0.875Ti0.125O3 as oxygen carrier in chemical-looping with oxygen uncoupling. Energy Fuels 2009, 23, 5276–5283. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Using chemical-looping with oxygen uncoupling (CLOU) for combustion of six different solid fuels. Energy Procedia 2009, 1, 447–453. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Gayan, P.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Evaluation of a spray-dried CuO/MgAl2O4 oxygen carrier for the chemical looping with oxygen uncoupling process. Energy Fuels 2012, 26, 3069–3081. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Wang, B.; Xu, D.; Jiang, L.; Zheng, C. Sol-gel-derived NiO/NiAl2O4 oxygen carriers for chemical-looping combustion by coal char. Energy Fuels 2008, 22, 898–905. [Google Scholar] [CrossRef]
- Siriwardane, R.; Tian, H.; Richards, G.; Simonyi, T.; Poston, J. Chemical-looping combustion of coal with metal oxide oxygen carriers. Energy Fuels 2009, 23, 3885–3892. [Google Scholar] [CrossRef]
- Cao, Y.; Casenas, B.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 2. Redox reaction kinetics and product characterization with coal, biomass, and solid waste as solid fuels and CuO as an oxygen carrier. Energy Fuels 2006, 20, 1845–1854. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Abad, A.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Biomass combustion with CO2 capture by chemical looping with oxygen uncoupling (CLOU). Fuel Process. Technol. 2014, 124, 104–114. [Google Scholar] [CrossRef]
- An, H.; Song, T.; Shen, L.; Zhang, L.; Feng, B. Hydrogen production from a victorian brown coal with in Situ CO2 capture in a 1 kWth dual fluidized-bed gasification reactor. Ind. Eng. Chem. Res. 2012, 51, 13046–13053. [Google Scholar] [CrossRef]
- Song, T.; Shen, L.; Zhang, S.; Chen, D.; Xiao, J. Performance of hematite/Ca2Al2SiO7 oxygen carrier in chemical looping combustion of coal. Ind. Eng. Chem. Res. 2013, 52, 7350–7361. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Dennis, J.S.; Hayhurst, A.N.; Scott, S.A. Development and performance of Cu-based oxygen carriers for chemical-looping combustion. Combust. Flame 2008, 154, 109–121. [Google Scholar] [CrossRef]
- Xiao, R.; Song, Q.; Song, M.; Lu, Z.; Zhang, S.; Shen, L. Pressurized chemical-looping combustion of coal with an iron ore-based oxygen carrier. Combust. Flame 2010, 157, 1140–1153. [Google Scholar] [CrossRef]
- Adanez, J.; Cuadrat, A.; Abad, A.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F. Ilmenite activation during consecutive redox cycles in chemical-looping combustion. Energy Fuels 2010, 24, 1402–1413. [Google Scholar] [CrossRef]
- Lee, S. Gasification of coal. In Handbook of Alternative Fuel Technologies; Sunggyu Lee, J.G.S., Loyalka, S.K., Eds.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Liu, Z.L.; Zhu, H.H. Steam gasification of coal char using alkali and alkaline-earth metal-catalysts. Fuel 1986, 65, 1334–1338. [Google Scholar] [CrossRef]
- Teyssie, G.; Leion, H.; Schwebel, G.L.; Lyngfelt, A.; Mattisson, T. Influence of lime addition to ilmenite in chemical-looping combustion (CLC) with solid fuels. Energy Fuels 2011, 25, 3843–3853. [Google Scholar] [CrossRef]
- Yu, Z.; Li, C.; Jing, X.; Wang, Z.; Fang, Y.; Huang, J. Catalytic chemical looping combustion of carbon with an iron-based oxygen carrier modified by K2O3: Catlytic mechanism and multicycle tests. Fuel Process. Technol. 2015, 135, 119–124. [Google Scholar] [CrossRef]
- Arjmand, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Investigation of different manganese ores as oxygen carriers in chemical-looping combustion (CLC) for solid fuels. Appl. Energy 2014, 113, 1883–1894. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, K.; Fang, Y.; Ma, J.; Mei, D.; Zheng, C. Characterization of natural copper ore as oxygen carrier in chemical-looping with oxygen uncoupling of anthracite. Int. J. Greenh. Gas Control 2014, 22, 154–164. [Google Scholar] [CrossRef]
- Arjmand, M.; Frick, V.; Ryden, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Screening of combined Mn-Fe-Si oxygen carriers for chemical looping with oxygen uncoupling (CLOU). Energy Fuels 2015, 29, 1868–1880. [Google Scholar] [CrossRef]
- Shulman, A.; Cleverstam, E.; Mattisson, T.; Lyngfelt, A. Chemical—Looping with oxygen uncoupling using Mn/Mg-based oxygen carriers—Oxygen release and reactivity with methane. Fuel 2011, 90, 941–950. [Google Scholar] [CrossRef]
- Larring, Y.; Braley, C.; Pishahang, M.; Andreassen, K.A.; Bredesen, R. Evaluation of a mixed Fe-Mn oxide system for chemical looping combustion. Energy Fuels 2015, 29, 3438–3445. [Google Scholar] [CrossRef]
- Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. CO2 capture activities in ICB-CSIC: Chemical-looping combustion and oxy-fuel. In Proceedings of the 63rd IEA Fluidized Bed Conversion Meeting, Ponferrada, Spain, 29–30 November 2011.
- Smoot, D.L.; Smith, P.J. Coal Combustion and Gasification; Plenum Publishing Corporation: New York, NY, USA, 1985; p. 444. [Google Scholar]
- Wang, P.; Means, N.; Shekhawat, D.; Berry, D. The reactivity of coal char in chemical looping gasification and combustion. In Proceedings of the World of Coal Ash, Nashville, TN, USA, 5–7 May 2015.
- Wang, P.; Massoudi, M. Effect of Coal Properties and Operation Conditions on Flow Behavior of Coal Slag in Entrained Flow Gasifier: A Brief Review; United States Department of Energy National Energy Technology Laboratory: Pittsburgh, PA, USA, 2011.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Means, N.; Shekhawat, D.; Berry, D.; Massoudi, M. Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies 2015, 8, 10605-10635. https://doi.org/10.3390/en81010605
Wang P, Means N, Shekhawat D, Berry D, Massoudi M. Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies. 2015; 8(10):10605-10635. https://doi.org/10.3390/en81010605
Chicago/Turabian StyleWang, Ping, Nicholas Means, Dushyant Shekhawat, David Berry, and Mehrdad Massoudi. 2015. "Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review" Energies 8, no. 10: 10605-10635. https://doi.org/10.3390/en81010605
APA StyleWang, P., Means, N., Shekhawat, D., Berry, D., & Massoudi, M. (2015). Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies, 8(10), 10605-10635. https://doi.org/10.3390/en81010605





