Effects of Impermeable Boundaries on Gas Production from Hydrate Accumulations in the Shenhu Area of the South China Sea
Abstract
:1. Introduction
1.1. Background
1.2. Hydrates in the Shenhu Area
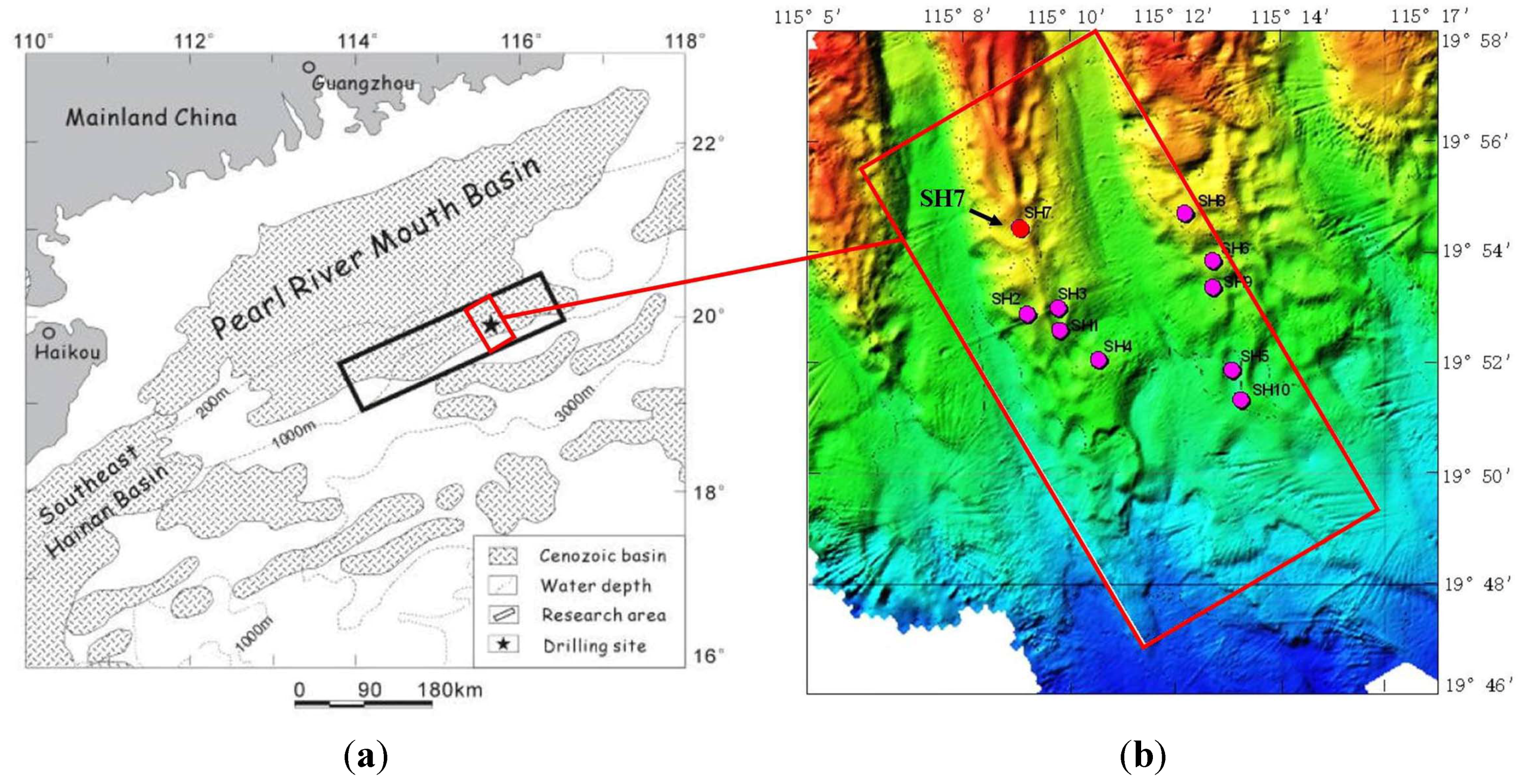
1.3. Objective and Approach
2. Production Strategies and Simulation Approach
2.1. Method of Production
2.2. Numerical Simulation Code
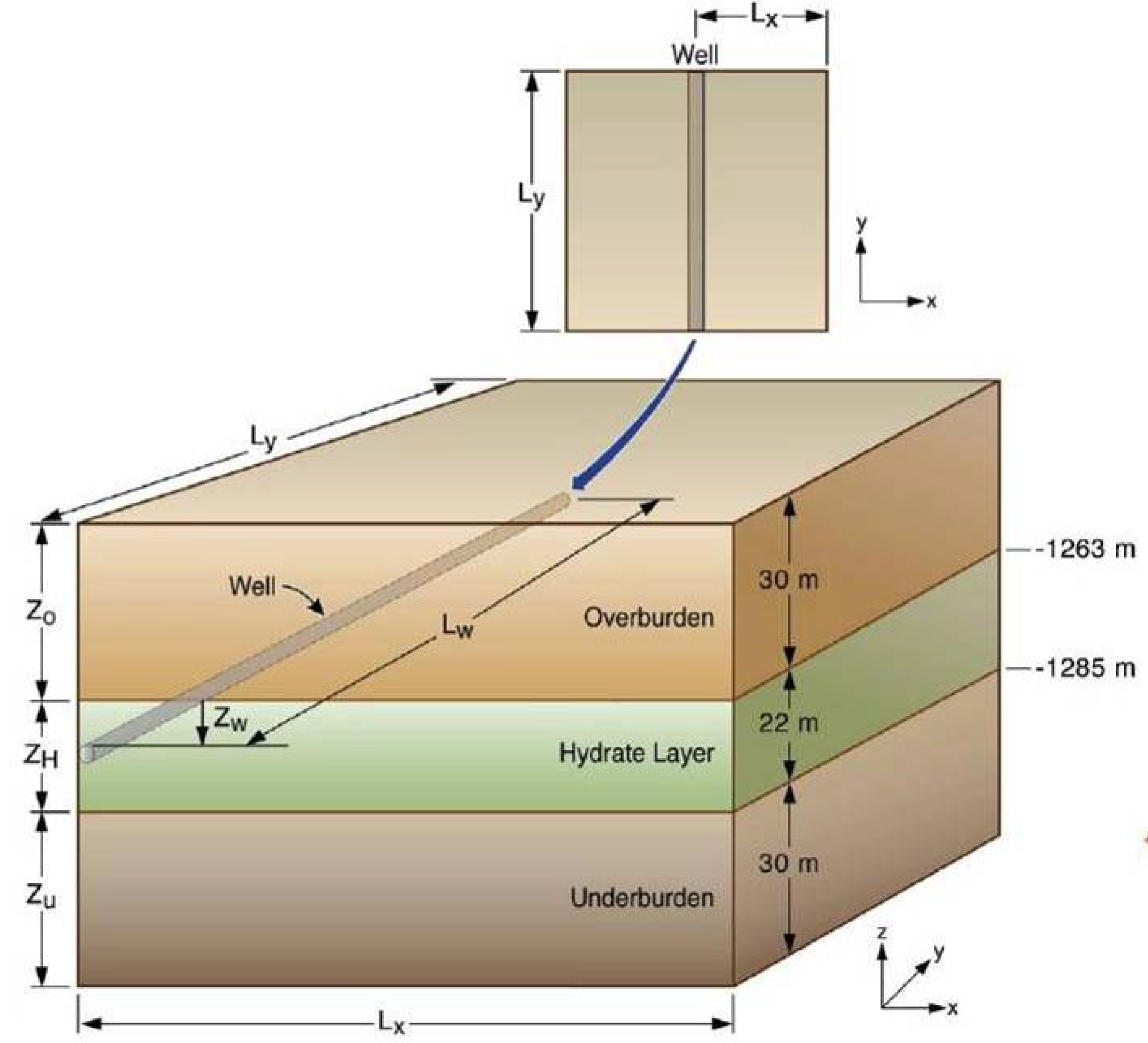
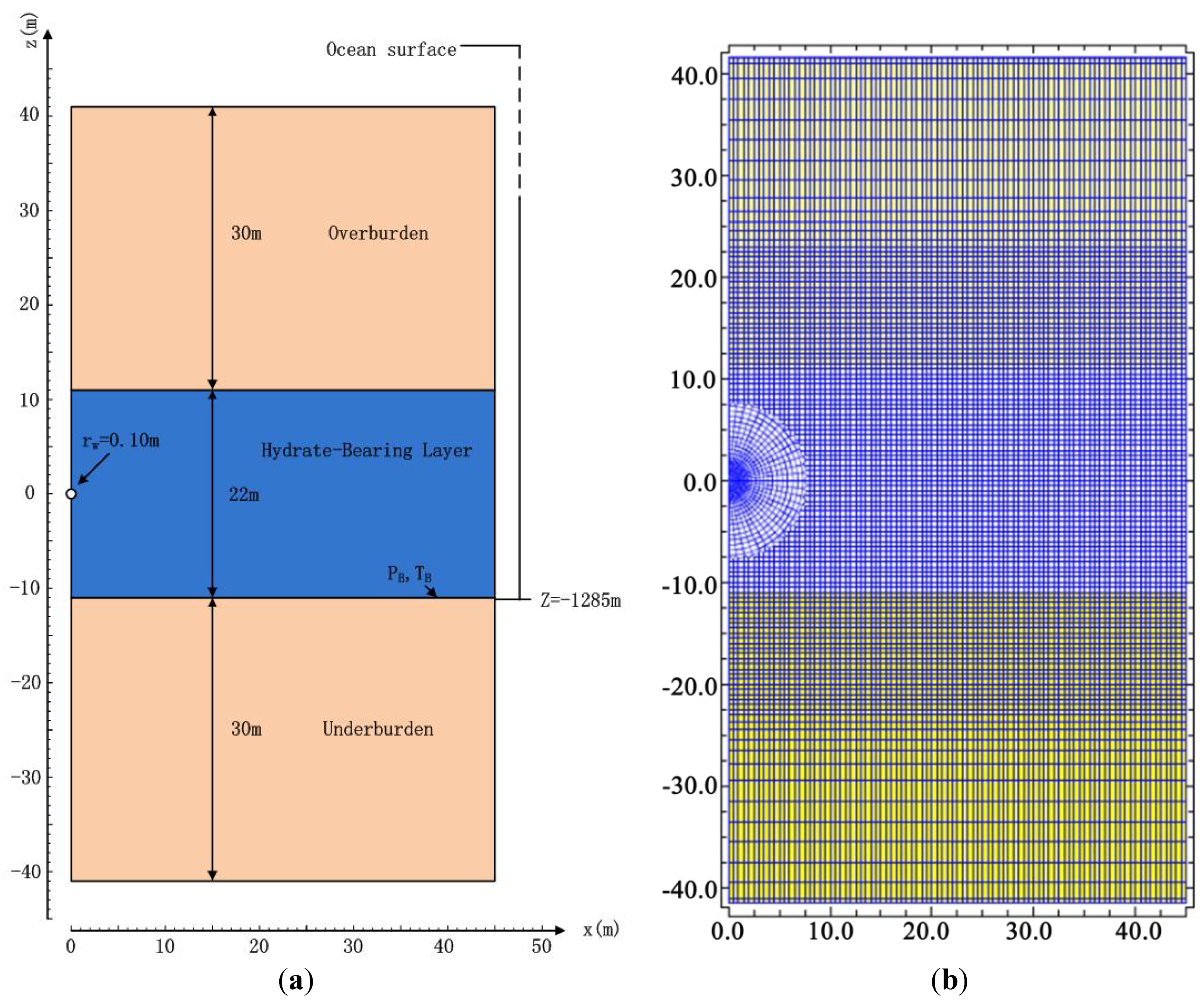
2.3. Geometry and Domain Discretization
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Overburden thickness ∆ZO | 30 m | Grain density ρR (all formations) | 2600 kg/m3 |
| HBL thickness ∆ZH | 20 m | Dry thermal conductivity kΘRD (all formations) | 1.0 W/m/K |
| Underburden thickness ∆ZU | 30 m | Wet thermal conductivity kΘRW (all formations) | 3.1 W/m/K |
| Well position above the HBL base ∆ZW | 11 m | Composite thermal conductivity model [34] | kΘ = kΘRD + (SA1/2 + SH1/2) (kΘRW − kΘRD) + φ SIkΘI |
| Initial pressure PB (at base of HBL) | 13.83 MPa | Capillary pressure model [35] | Pcap = P01 [(S*)−1/λ − 1]1 – λ S* = (SA − SirA)/(1 − SirA) |
| Initial temperature TB (at base of HBL) | 287.31 K (14.15 °C) | SirA | 0.29 |
| Initial saturation in the HBL | SH = 0.44, SA = 0.56 | λ | 0.45 |
| Gas composition | 100% CH4 | P01 | 105 Pa |
| Geothermal gradient | 0.0433 K/m | Relative permeability model [34] | krA = (SA − SirA)/(1 − SirA)n krG = (SG − SirG)/(1 − SirA)nG |
| Water salinity (mass fraction) | 0.0305 | n = nG | 3.572 |
| Intrinsic permeability of HBL kx = ky = kz | 7.5 × 10−14m2 (= 75 mD) | SirG | 0.05 |
| Porosity φ (all formations) | 0.41 | SirA | 0.30 |
3. Production from Site SH7 in Shenhu Area
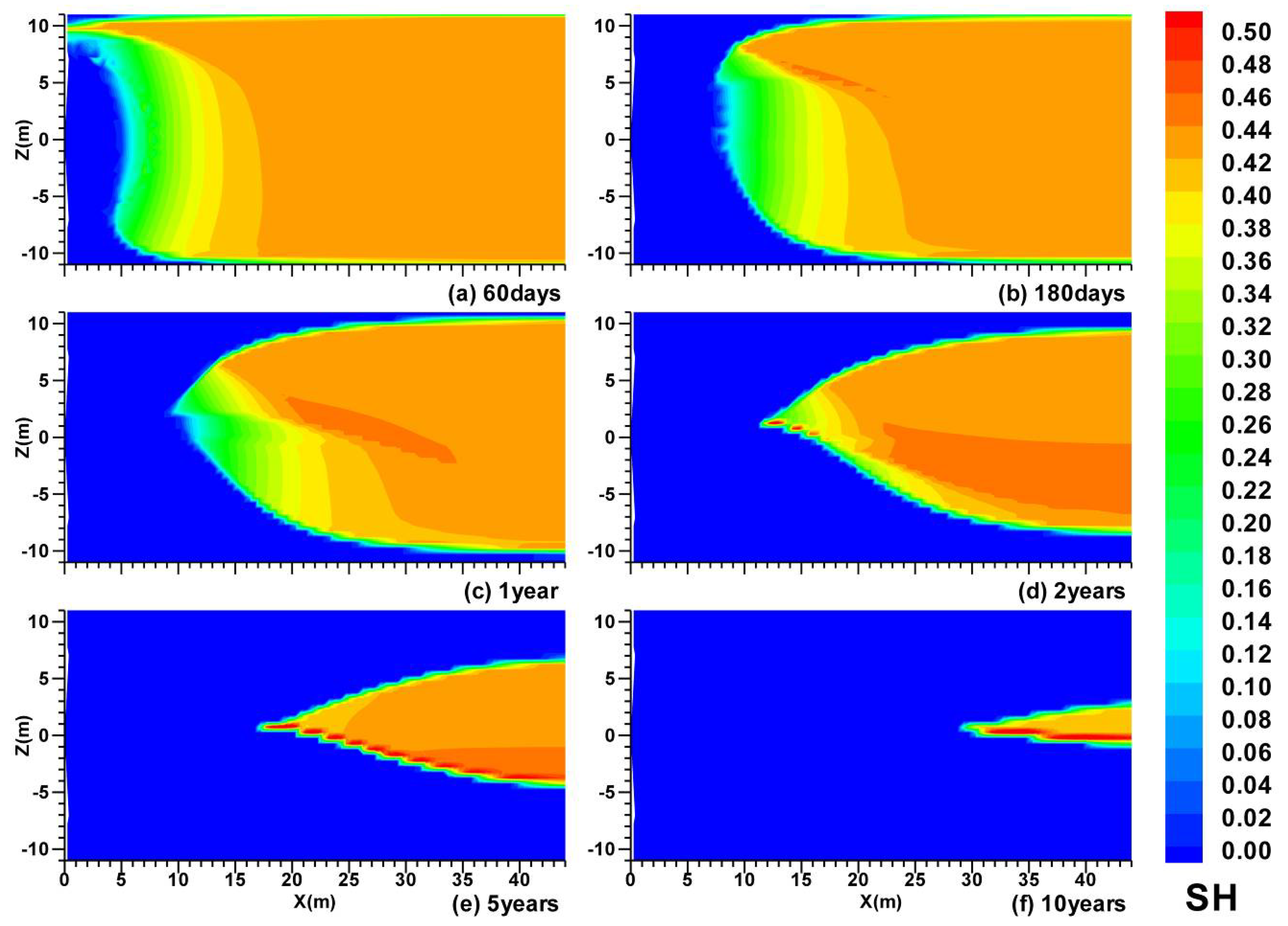
3.1. Spatial Distributions in Case (i)
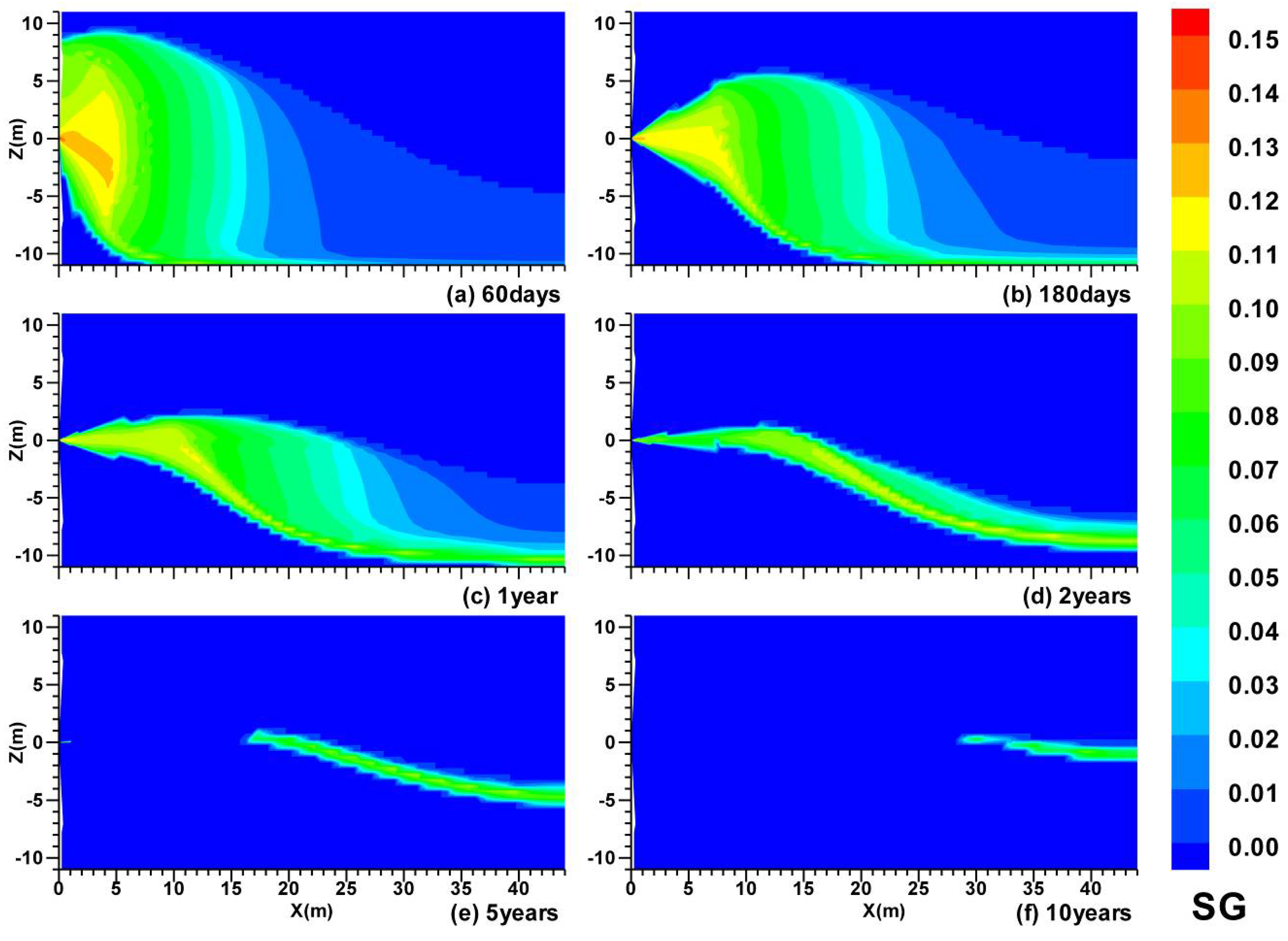
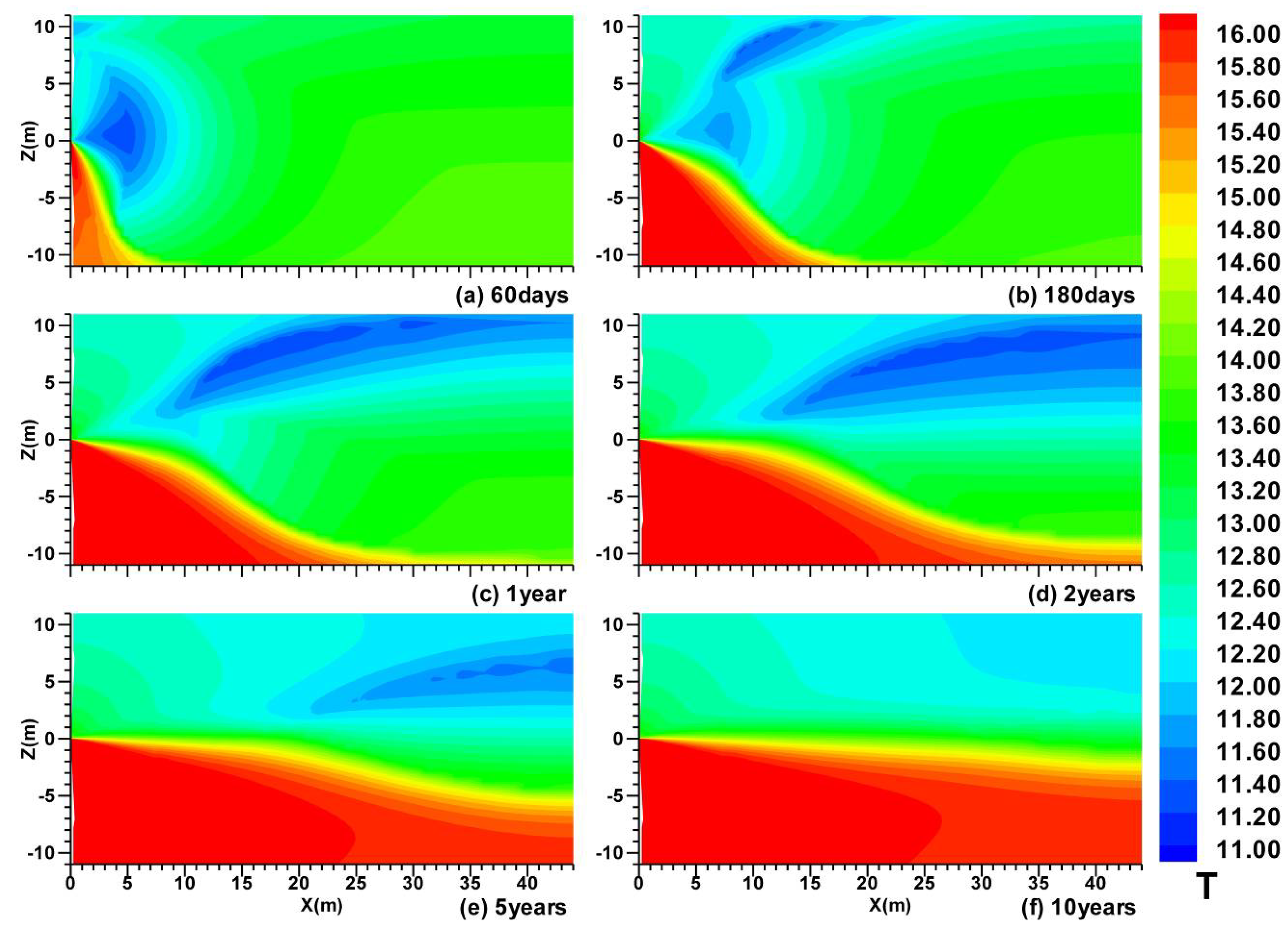

3.2. Spatial Distributions in Case (ii)
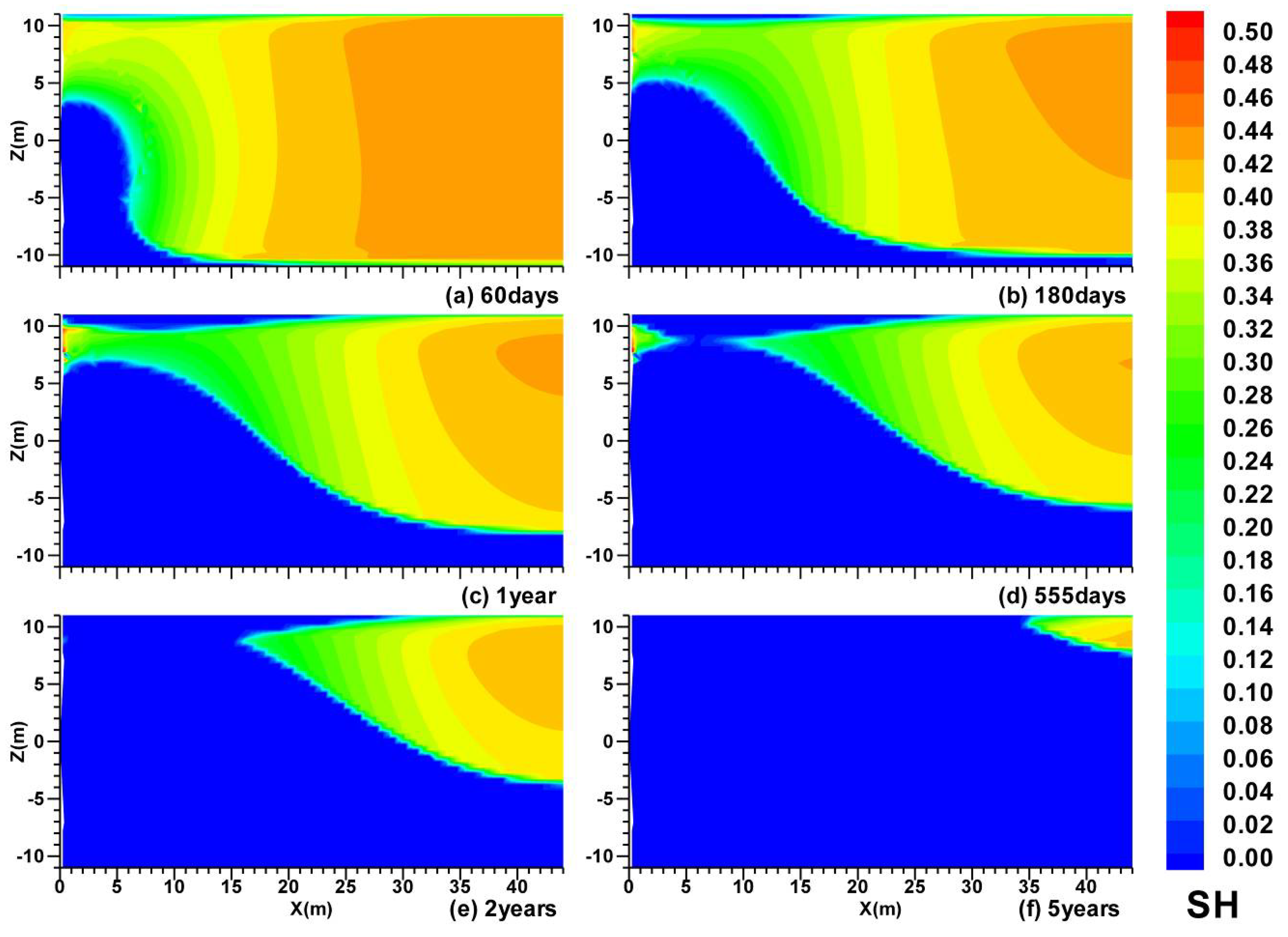
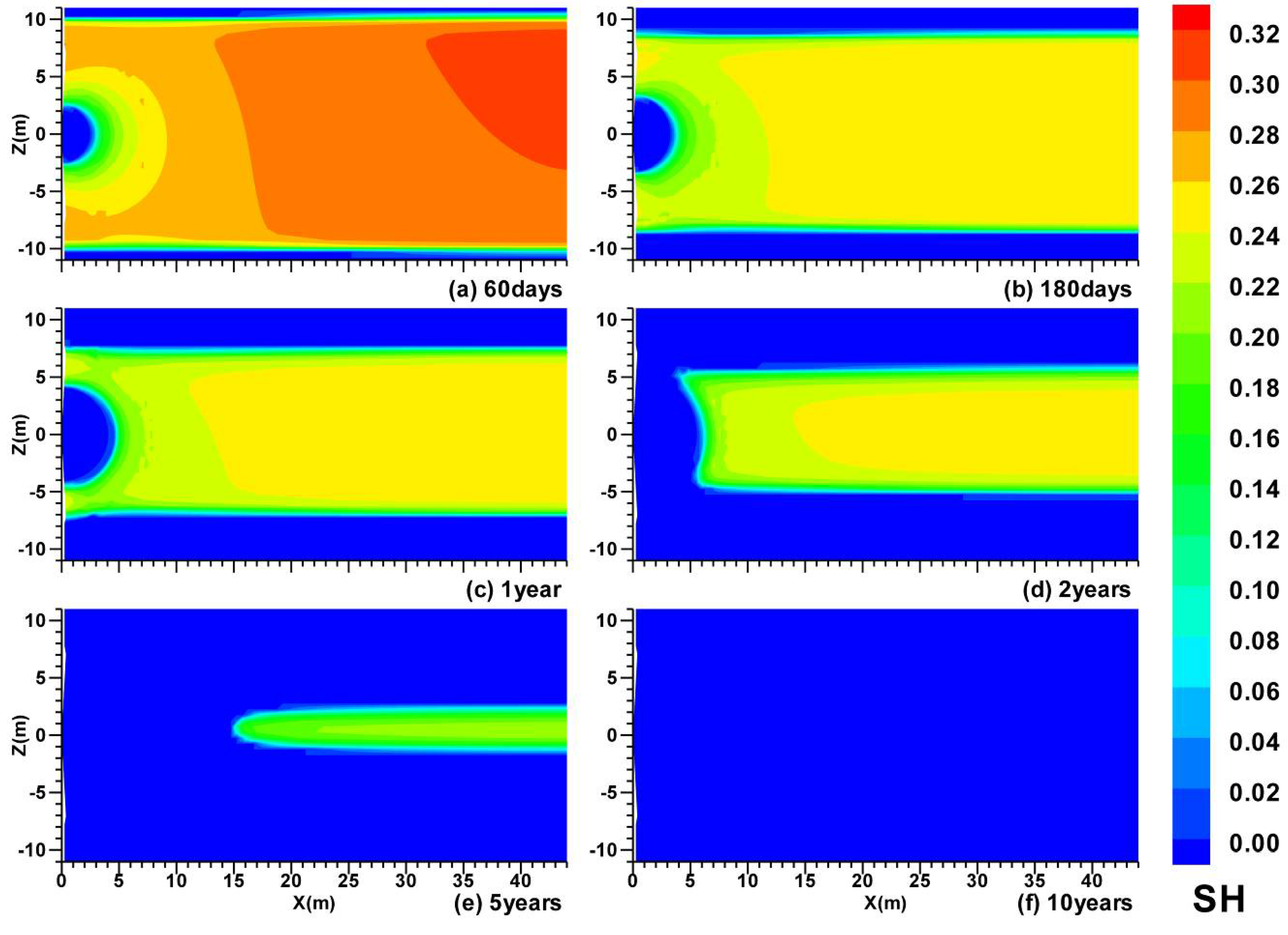
3.3. Spatial Distributions in Case (iii)
3.4. Gas and Water Production
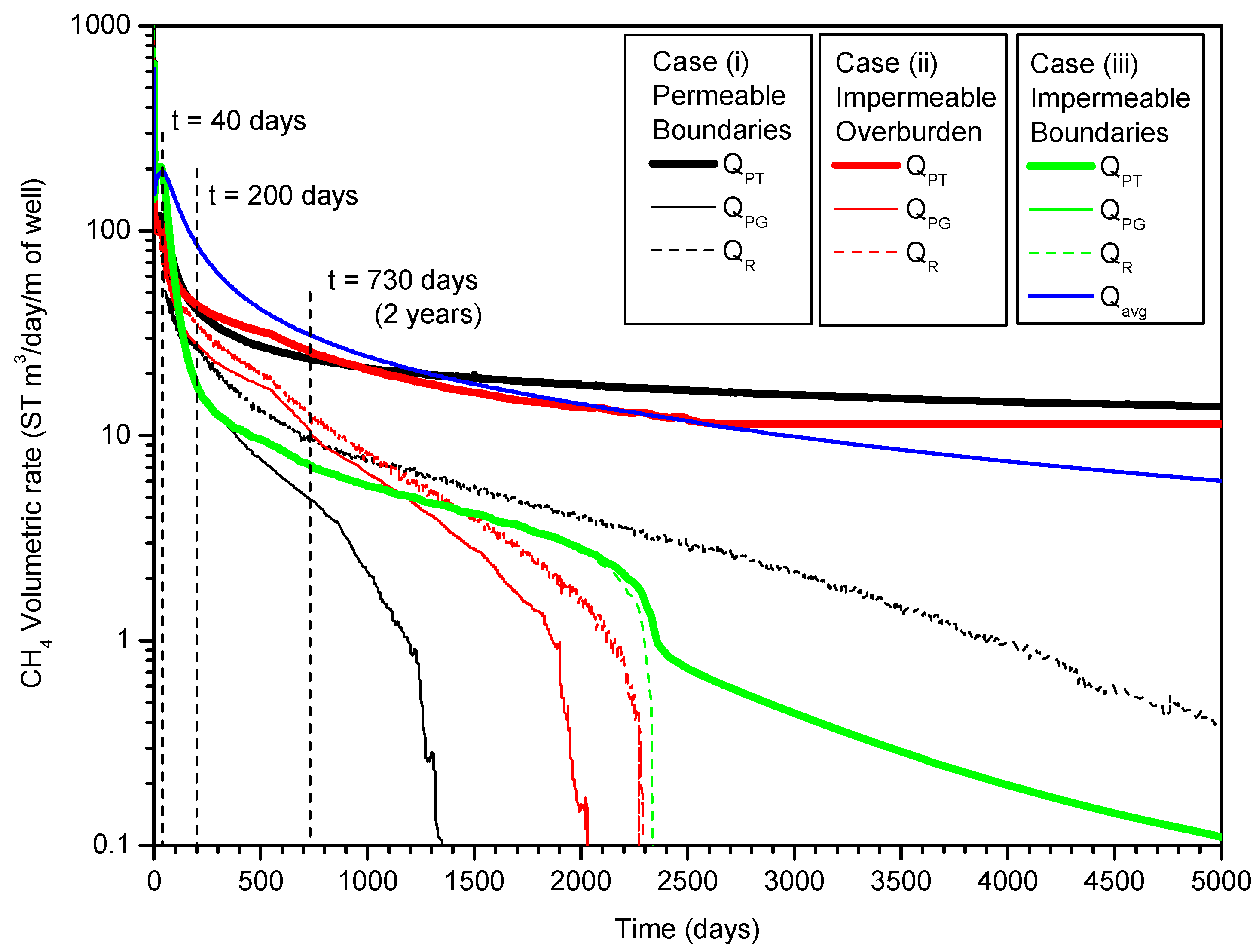
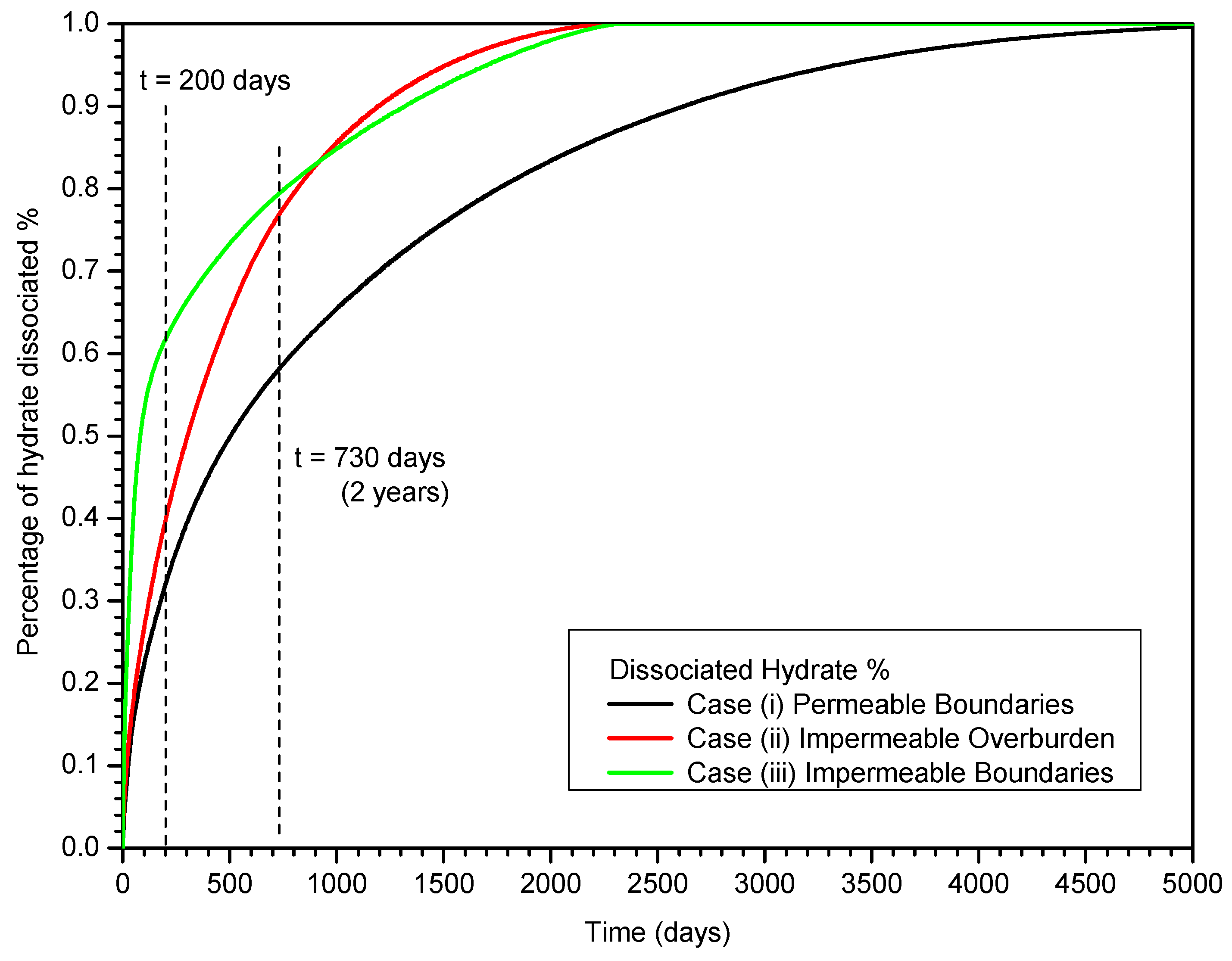
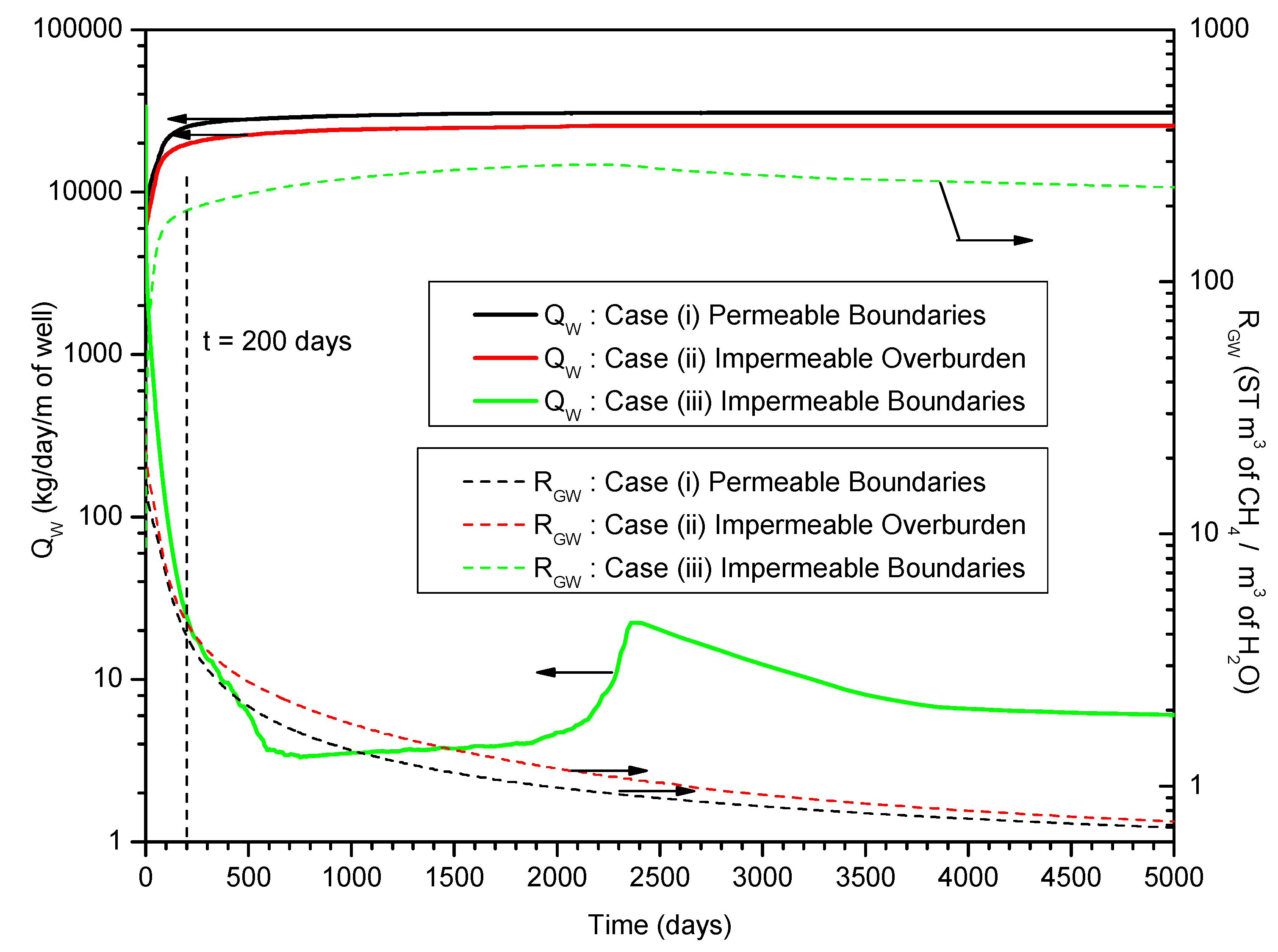
4. Summary and Conclusions
- In Case (i) with permeable upper and lower boundaries, the dissociation is characterized by the following features: the evolution of the hydrate dissociation interface around the well, the upper and the lower dissociation interfaces, the evolution of the secondary hydrate, and the accumulation of gas in the vicinity of the well and below the lower dissociation interface. The relative warmer water rises from the UB and is produced from the well;
- In Case (ii) with an impermeable upper boundary (OB) and permeable lower boundary (UB), with the enhancement of the effect of the depressurization in the semi-open system, the hydrate dissociation rate is much higher than that in Case (i);
- In both Case (i) and (ii), the water production rates QW increase rapidly after approximately t = 300 days, and the long-term RGW is prohibitively low, which indicates that the gas productions from the deposits are not economically profitable from the relative criterion point of view;
- In Case (iii) with impermeable upper and lower boundaries, the HBL is a closed system before gas production from hydrate, and the effect of the depressurization is much stronger than that in Case (i) and Case (ii). The depressurization affects the entire HBL obviously and the hydrate dissociation occurs in the entire HBL simultaneously;
- In Case (iii), the cumulative average gas production rate Qavg indicates that the large production potential of the hydrate deposits satisfies the absolute criterion. The RGW increases to larger than 190 ST m3 of CH4/m3 of H2O after approximately t = 200 days, which indicates that the relative criterion is satisfied;
- In summary, comparing with the open and semi-open system, the effect of the depressurization is much stronger in the hydrate accumulation with both the impermeable upper and lower boundaries, which shows the best effects on the gas production potential of the hydrate deposits.
Nomenclature:
| k | intrinsic permeability (m2) |
| keff | effective permeability (m2) |
| krA | aqueous relative permeability (m2) |
| krG | gas relative permeability (m2) |
| kΘ | thermal conductivity (W/m/K) |
| kΘRD | thermal conductivity of dry porous medium (W/m/K) |
| kΘRw | thermal conductivity of fully saturated porous medium (W/m/K) |
| kΘI | thermal conductivity of ice (W/m/K) |
| VW | cumulative volume of the produced water (m3 of H2O) |
| P | pressure (Pa) |
| P0 | initial pressure in the middle of HBL (Pa) |
| PW | pressure at the well (Pa) |
| PQ | pressure at the quadruple point (Pa) |
| Qavg | cumulative average production rate of total CH4 at time t (ST m3/day/m of well) |
| QW | mass rate of aqueous phase production at the well (kg/day/m of well) |
| QPG | volumetric rate of CH4 production at the well in the gas phase (ST m3/day/m of well) |
| QPT | volumetric rate of total CH4 production at the well (ST m3/day/m of well) |
| QR | volumetric rate of CH4 release from hydrate dissociation (ST m3/day/m of well) |
| R | radius (m) |
| RGW | the gas to water production ratio (ST m3 of CH4 /m3 of H2O) |
| S | phase saturation |
| t | times (days) |
| T | temperature (°C) |
| TB | initial temperature at base of HBL (°C) |
| T0F | temperature at the ocean floor (°C) |
| T0 | initial temperature in the middle of HBL (°C) |
| VP | cumulative volume of the produced CH4 (ST m3 of CH4) |
| x,y,z | Cartesian coordinates (m) |
| XS | salinity |
| ∆PW | driving force of depressurization, P0—PW (Pa) |
| ∆x | discretization along the x-axis (m) |
| ∆z | discretization along the z-axis (m) |
| ∆ZH | HBL thickness (m) |
| ∆ZO | Overburden thickness (m) |
| ∆ZU | Underburden thickness (m) |
| ∆ZW | well position above the HBL base (m) |
| ϕ | porosity |
| ρR | grain density (kg/m3) |
| λ | van Genuchten exponent—Table 1 |
Subcripts:
| 0 | denotes initial state |
| A | aqueous phase |
| B | base of HBL |
| cap | capillary |
| G | gas phase |
| H | solid hydrate phase |
| HBL | Hydrate-Bearing Layer |
| I | Ice phase |
| irA | irreducible aqueous phase |
| irG | irreducible gas |
| n | permeability reduction exponent—Table 1 |
| nG | gas permeability reduction exponent—Table 1 |
| OB | overburden |
| R | rock |
| S | salinity |
| UB | underburden |
| W | well |
Acknowledgement
Conflict of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: how much is really out there? Earth Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Collett, T.S. Gas hydrates as a future energy resource. Geotimes 2004, 49, 24–27. [Google Scholar]
- Klauda, J.B.; Sandler, S.I. Global distribution of methane hydrate in ocean sediment. Energy Fuels 2005, 19, 459–470. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st Century. J. Petrol. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Yousif, M.H.; Abass, H.H.; Selim, M.S.; Sloan, E.D. Experimental and theoretical investigation of methane-gas-hydrate dissociation in porous media. SPE Reserv. Eng. 1991, 6, 69–76. [Google Scholar] [CrossRef]
- Ahmadi, G.; Ji, C.A.; Smith, D.H. Production of natural gas from methane hydrate by a constant downhole pressure well. Energy Convers. Manag. 2007, 48, 2053–2068. [Google Scholar] [CrossRef]
- Moridis, G.J.; Kowalsky, M.B.; Pruess, K. Depressurization-induced gas production from class 1 hydrate deposits. SPE Reserv. Eval. Eng. 2007, 10, 458–481. [Google Scholar] [CrossRef]
- Li, G.; Li, X.S.; Chen, Q.; Chen, Z.Y. Numerical studies of gas production from gas hydrate zone in Shenhu Area, South China Sea. Acta Chim. Sin. 2010, 68, 1083–1092. [Google Scholar]
- Li, G.; Moridis, G.J.; Zhang, K.; Li, X.S. Evaluation of gas production potential from marine gas hydrate deposits in Shenhu Area of South China Sea. Energy Fuels 2010, 24, 6018–6033. [Google Scholar] [CrossRef]
- Li, G.; Tang, L.G.; Huang, C.; Feng, Z.P.; Fan, S.S. Thermodynamic evaluation of hot brine stimulation for natural gas hydrate dissociation. J. Chem. Ind. Eng. 2006, 57, 2033–2038. [Google Scholar]
- Li, G.; Li, X.S.; Tang, L.G.; Li, Q.P. Control Mechanisms for Methane Hydrate Production by Thermal Stimulation. In Proceedings of the Sixth International Conference on Gas Hydrates, Vancouver, Canada, 6–10 July 2008.
- Li, X.S.; Wan, L.H.; Li, G.; Li, Q.P.; Chen, Z.Y.; Yan, K.F. Experimental investigation into the production behavior of methane hydrate in porous sediment with hot brine stimulation. Ind. Eng. Chem. Res. 2008, 47, 9696–9702. [Google Scholar] [CrossRef]
- Li, G.; Moridis, G.J.; Zhang, K.; Li, X.S. The use of huff and puff method in a single horizontal well in gas production from marine gas hydrate deposits in the Shenhu area of the South China Sea. J. Petrol. Sci. Eng. 2011, 77, 49–68. [Google Scholar] [CrossRef]
- Sira, J.H.; Patil, S.L.; Kamath, V.A. Study of Hydrate Dissociation by Methanol and Glycol Injection. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 23–26 September 1990.
- Li, G.; Li, X.S.; Tang, L.G.; Zhang, Y. Experimental investigation of production behavior of methane hydrate under ethylene glycol injection in unconsolidated sediment. Energy Fuels 2007, 21, 3388–3393. [Google Scholar] [CrossRef]
- Moridis, G.J.; Reagan, M.T. Gas Production from oceanic Class 2 Hydrate Accumulations. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2007.
- Moridis, G.J.; Reagan, M.T. Strategies for Gas Production from Oceanic Class 3 Hydrate Accumulations. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2007.
- Moridis, G.J.; Reagan, M.T.; Zhang, K. The Use of Horizontal Wells in Gas Production from Hydrate Accumulations. In Proceedings of the Sixth International Conference on Gas Hydrates, Vancouver, Canada, 6–10 July 2008.
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Koh, C.; Sloan, E.D. Toward production from gas hydrates: Current status, assessment of resources, and simulation-based evaluation of technology and potential. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef]
- Moridis, G.J.; Reagan, M.T.; Kim, S.J.; Seol, Y.; Zhang, K. Evaluation of the gas production potential of marine hydrate deposits in the Ulleung basin of the Korean East Sea. SPE J. 2009, 14, 759–781. [Google Scholar] [CrossRef]
- Kurihara, M.; Funatsu, K.; Ouchi, H.; Masuda, Y.; Narita, H. Investigation on Applicability of Methane Hydrate Production Methods to Reservoirs with Diverse Characteristics. In Proceedings of the 5th International Conference on Gas Hydrates, Trondheim, Norway, 12–16 June 2005.
- Borowski, W.S. A review of methane and gas hydrates in the dynamic, stratified system of the Blake Ridge region, offshore southeastern North America. Chem. Geol. 2004, 205, 311–346. [Google Scholar] [CrossRef]
- Collett, T.S.; Ladd, J. Detection of gas hydrate with downhole logs and assessment of gas hydrate concentrations (saturations) and gas volumes on the Blake Ridge with electrical resistivity log data. Proc. Ocean Drill. Program Sci. Results 2000, 164, 179–191. [Google Scholar]
- Moridis, G.J.; Sloan, E.D. Gas production potential of disperse low-saturation hydrate accumulations in oceanic sediments. Energy Convers. Manag. 2007, 48, 1834–1849. [Google Scholar] [CrossRef]
- Su, Z.; Cao, Y.; Wu, N.Y. A model for predicting gas well performance of free gas zone beneath hydrate layer. J. Petrol. Sci. Eng. 2010, 71, 179–186. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Max, M.D.; Cherkis, N.Z.; Czarnecki, M.F. Tectono-sedimentary controls on the likelihood of gas hydrate occurrence near Taiwan. Mar. Petrol. Geol. 2000, 17, 929–936. [Google Scholar] [CrossRef]
- Wu, N.Y.; Zhang, H.Q.; Yang, S.X.; Liang, J.Q.; Wang, H.B. Preliminary discussion on natural gas hydrate (NGH) reservoir system of Shenhu area, north slope of South China Sea. Nat. Gas Ind. 2007, 27, 1–6. [Google Scholar]
- Wu, N.Y.; Zhang, H.Q.; Su, X.; Yang, S.X.; Zhang, G.; Liang, J.Q.; Lu, J.; Gong, J.; Scheltheiss, P.; Holland, M. High concentrations of hydrate in disseminated forms found in very fine-grained sediments of Shenhu area, South China Sea. Terra Nostra 2007, 1–2, 236–237. [Google Scholar]
- Wu, N.Y.; Yang, S.X.; Zhang, H.Q.; Liang, J.Q.; Wang, H.B.; Su, X.; Fu, S.Y. Preliminary Discussion on Gas Hydrate Reservoir System of Shenhu Area, North Slope of South China Sea. In Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, Canada, 6–10 July 2008.
- Yang, S.X.; Zhang, H.Q.; Wu, N.Y.; Su, X.; Scheltheiss, P.; Holland, M.; Zhang, G.X.; Liang, J.Q.; Lu, J.A.; Rose, K. High Concentration Hydrate in Disseminated Forms Obtained in Shenhu Area, North Slope of South China Sea. In Proceedings of the Sixth International Conference on Gas Hydrates, Vancouver, Canada, 6–10 July 2008.
- Moridis, G.J.; Kowalsky, M.B.; Pruess, K. TOUGH + HYDRATE v1.1 User’s Manual: A Code for the Simulation of System Behavior in Hydrate-Bearing Geologic Media; Lawrence Berkeley National laboratory: Berkeley, CA, USA, 2009. [Google Scholar]
- Zhang, K.; Moridis, G.J.; Wu, Y.S.; Pruess, K. A Domain Decomposition Approach for Large-Scale Simulations of Flow Processes in Hydrate-Bearing Geologic Media. In Proceedings of the Sixth International Conference on Gas Hydrates, Vancouver, Canada, 6–10 July 2008.
- Wu, N.Y.; Yang, S.X.; Zhang, H.Q.; Liang, J.Q.; Wang, H.B.; Lu, J.A. Gas Hydrate System of Shenhu Area, Northern South China Sea: Wire-line Logging, Geochemical Results and Preliminary Resources Estimate. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2007.
- Vanoudheusden, E.; Sultan, N.; Cochonat, P. Mechanical behaviour of unsaturated marine sediments: experimental and theoretical approaches. Mar. Geol. 2004, 213, 323–342. [Google Scholar] [CrossRef]
- Kowalsky, M.B.; Moridis, G.J. Comparison of kinetic and equilibrium reaction models in simulating gas hydrate behavior in porous media. Energy Convers. Manag. 2007, 48, 1850–1863. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, G.; Li, X.-S.; Zhang, K.; Li, B.; Zhang, Y. Effects of Impermeable Boundaries on Gas Production from Hydrate Accumulations in the Shenhu Area of the South China Sea. Energies 2013, 6, 4078-4096. https://doi.org/10.3390/en6084078
Li G, Li X-S, Zhang K, Li B, Zhang Y. Effects of Impermeable Boundaries on Gas Production from Hydrate Accumulations in the Shenhu Area of the South China Sea. Energies. 2013; 6(8):4078-4096. https://doi.org/10.3390/en6084078
Chicago/Turabian StyleLi, Gang, Xiao-Sen Li, Keni Zhang, Bo Li, and Yu Zhang. 2013. "Effects of Impermeable Boundaries on Gas Production from Hydrate Accumulations in the Shenhu Area of the South China Sea" Energies 6, no. 8: 4078-4096. https://doi.org/10.3390/en6084078
APA StyleLi, G., Li, X.-S., Zhang, K., Li, B., & Zhang, Y. (2013). Effects of Impermeable Boundaries on Gas Production from Hydrate Accumulations in the Shenhu Area of the South China Sea. Energies, 6(8), 4078-4096. https://doi.org/10.3390/en6084078




