Evolution of Hydrate Dissociation by Warm Brine Stimulation Combined Depressurization in the South China Sea
Abstract
:1. Introduction
1.1. Background
1.2. Oceanic Hydrates and the Hydrates in the South China Sea
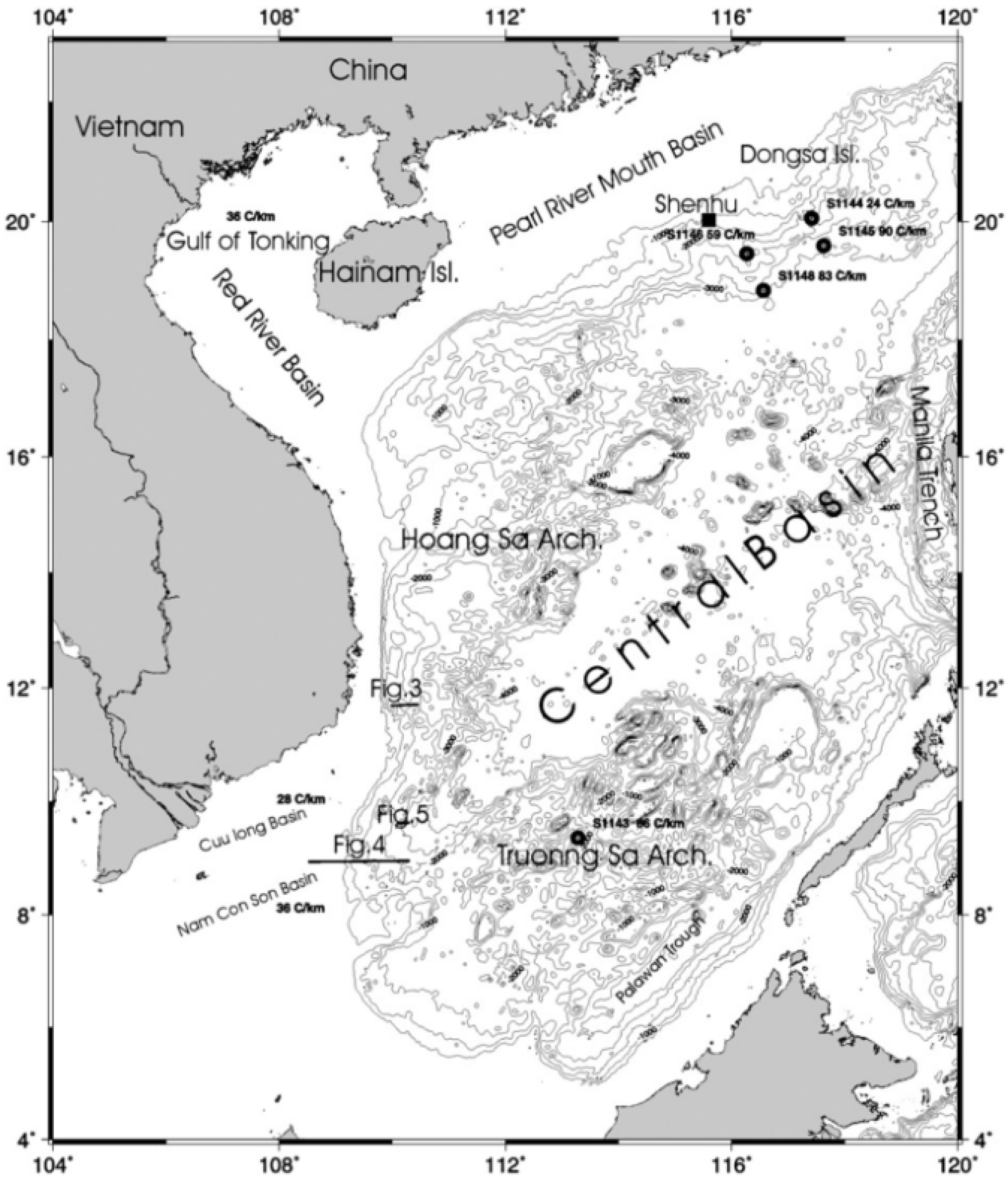
1.3. Objectives
2. Production Schemes and Well Design
2.1. Dissociation Method
2.2. Well Configuration
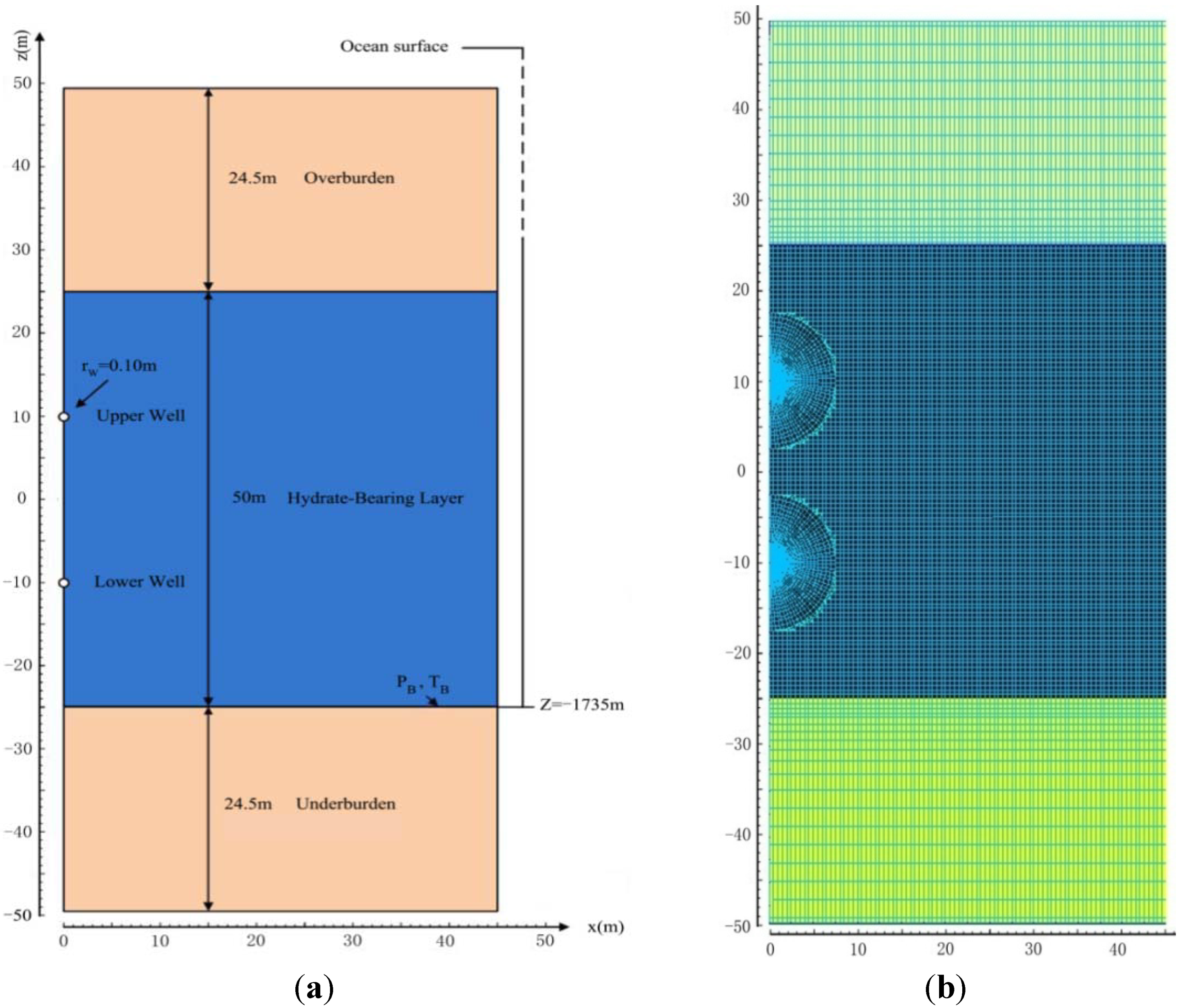
3. Numerical Models and Simulation Method
3.1. Geometry, Domain Discretization, and System Properties
3.2. Initial Conditions.
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Thickness of HBL | 50 m | Capillary pressure model [34] | Pcap = − P01 [(S*)−1/λ − 1] 1−λ S* = (SA − SirA)/(SmxA − SirA) |
| Thickness of OB and UB | 24.5 m | SirA | 0.29 |
| Position of HBL below the ground | 160.5 m | λ | 0.45 |
| distance between two wells | 20 m | P01 | 105 Pa |
| Initial PB and TB (at base of HBL) | 18.25 MPa, 288.28 K | Relative permeability Model | krA = (SA*)n krG = (SG*)nG SA* = (SA − SirA)/(1 − SirA) SG* = (SG − SirG)/(1 − SirA) |
| Gas composition | 100% CH4 | P01 | 105 Pa |
| Composite thermalconductivity model [29,35] | kΘC = kΘRD + (SA1/2 + SH1/2 ) (kΘRW − kΘRD) + ϕSIkΘI | Relative permeability Model | krA = (SA*)n krG = (SG*)nG SA* = (SA − SirA)/(1 − SirA) SG* = (SG − SirG)/(1 − SirA) |
| n | 3.572 | ||
| nG | 3.572 | ||
| Sea floor temperature T0 | 277.15 K | Relative permeability Model | krA = (SA*)n krG = (SG*)nG SA* = (SA − SirA)/(1 − SirA) SG*= (SG − SirG)/(1 − SirA) |
| Geothermal gradient G | 0.045 K/m | ||
| Water salinity (mass fraction) | 3.50% | ||
| Intrinsic permeability kx = ky= kz (HBL, OB & UB) | 7.5 × 10−14 m2 (= 0.075 mD) | n | 3.572 |
| nG | 3.572 | ||
| Porosity ϕ (all formations) | 0.40 | SirG | 0.05 |
| Initial hydrate saturation | SH = 0.451 | SirA | 0.30 |
4. Results and Discussion
4.1. Dual Horizontal Wells Design with Warm Brine Injection
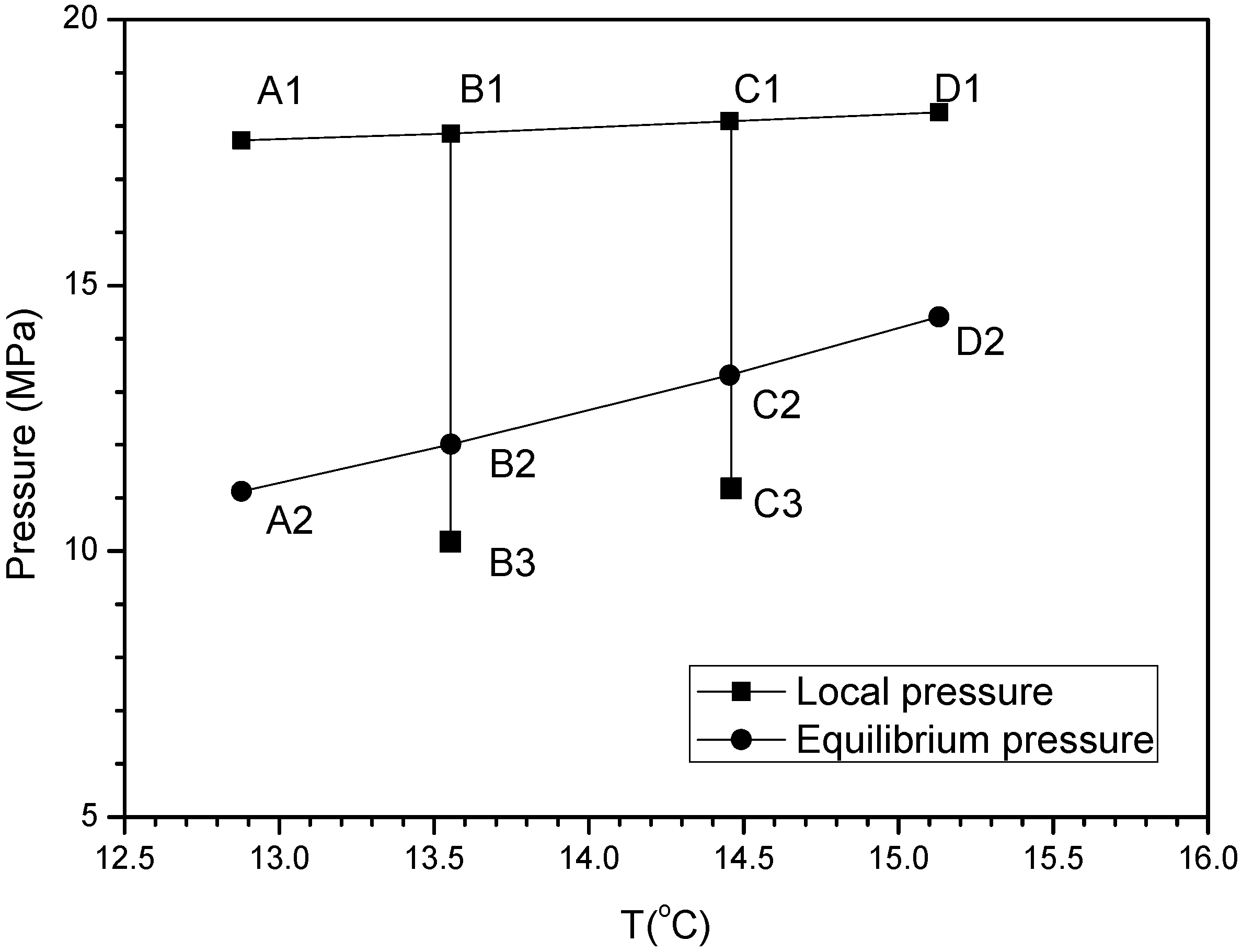
4.2. The Reference Case and the Gas and Water Production
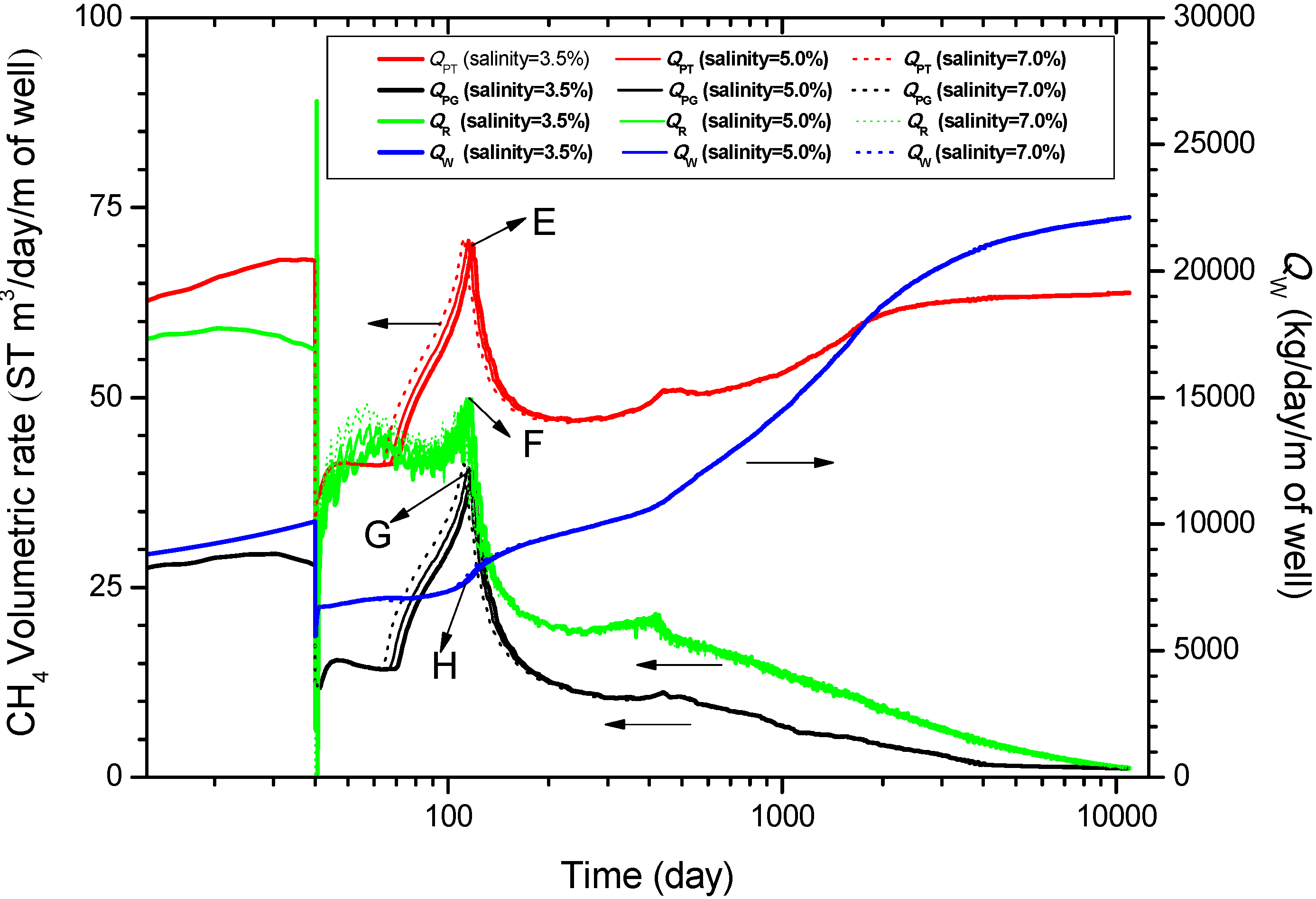
4.3. Energy Efficiency and Gas-to-Water Ratio

4.4. Spatial Distribution of SH, SG and XS
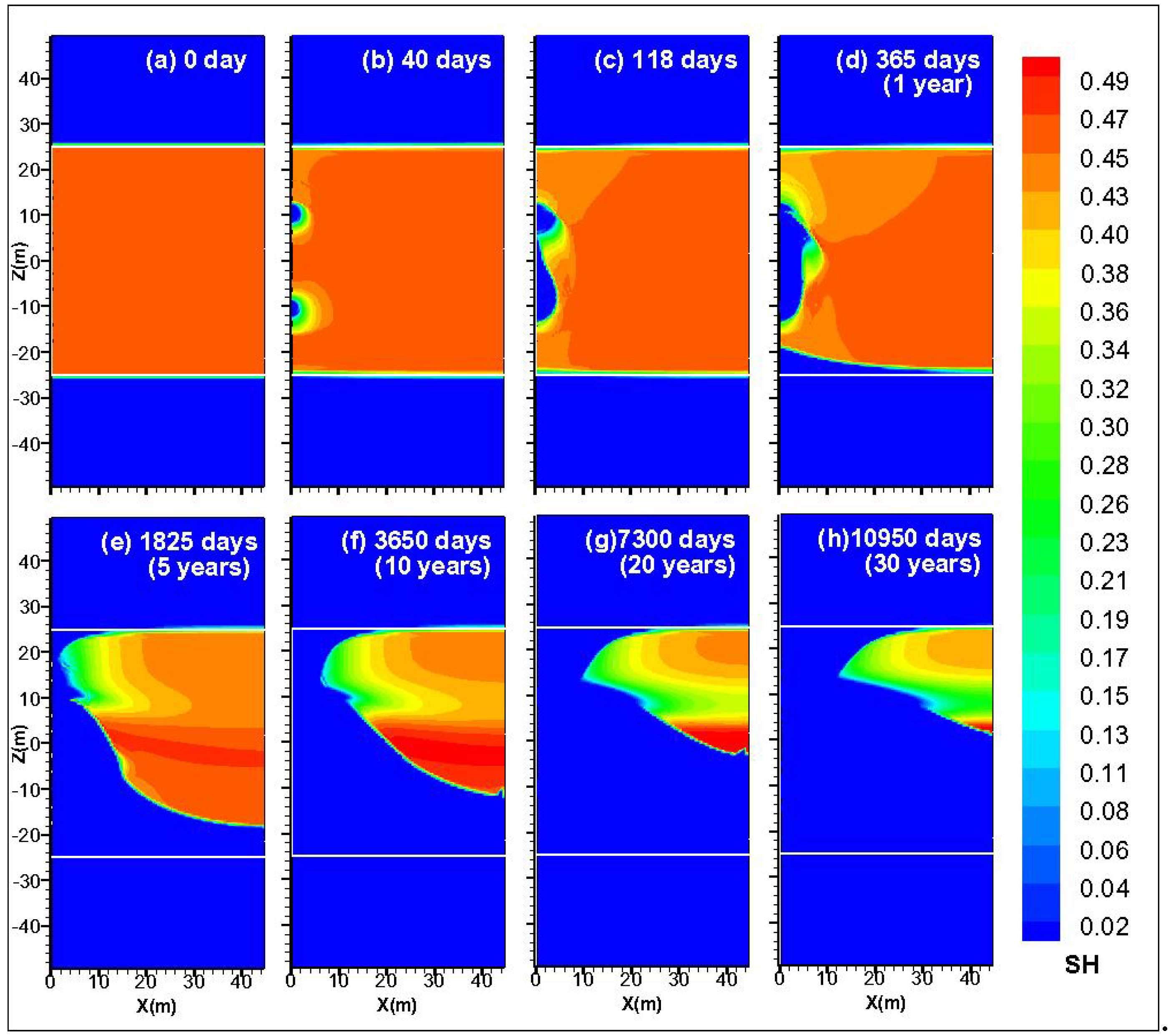

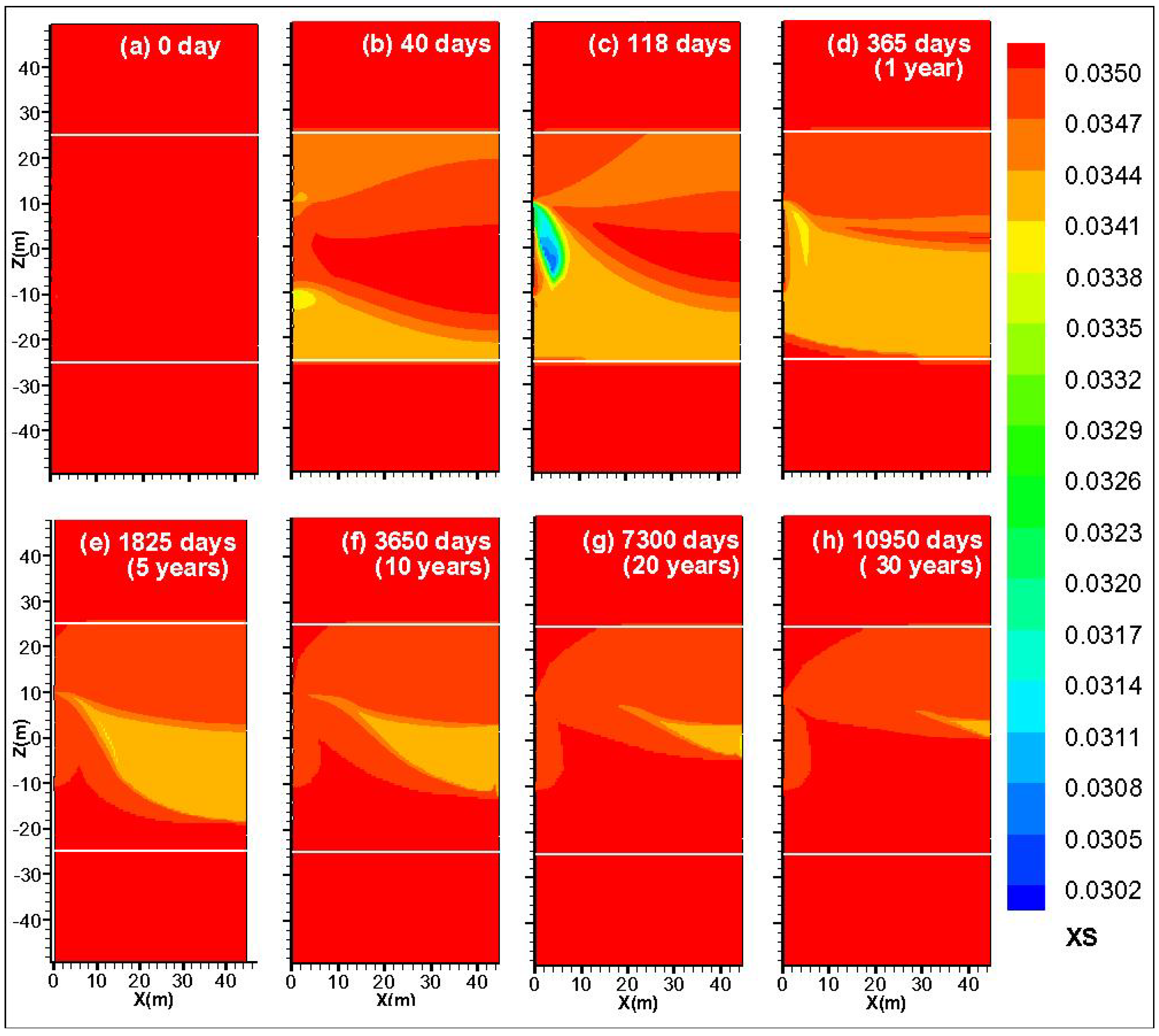
4.5. Spatial Distribution of P and T


4.6. Sensitivity Analyses
4.6.1. Sensitivity to the Temperature of the Injected Water TW

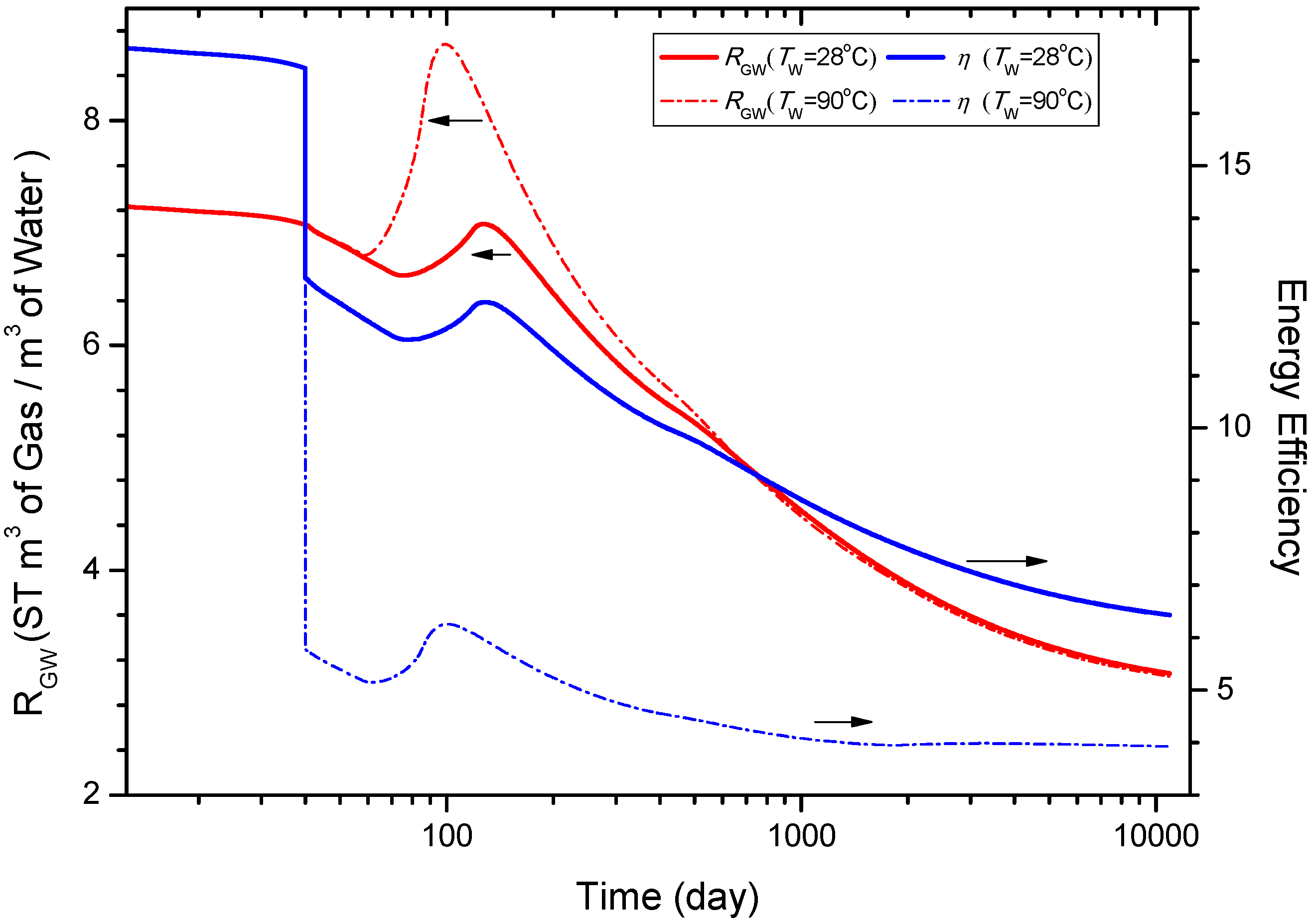
4.6.2. Sensitivity to the Salinity of the Injected Water
4.6.3. Sensitivity to the Intrinsic Permeability k of the Hydrate Reservoir

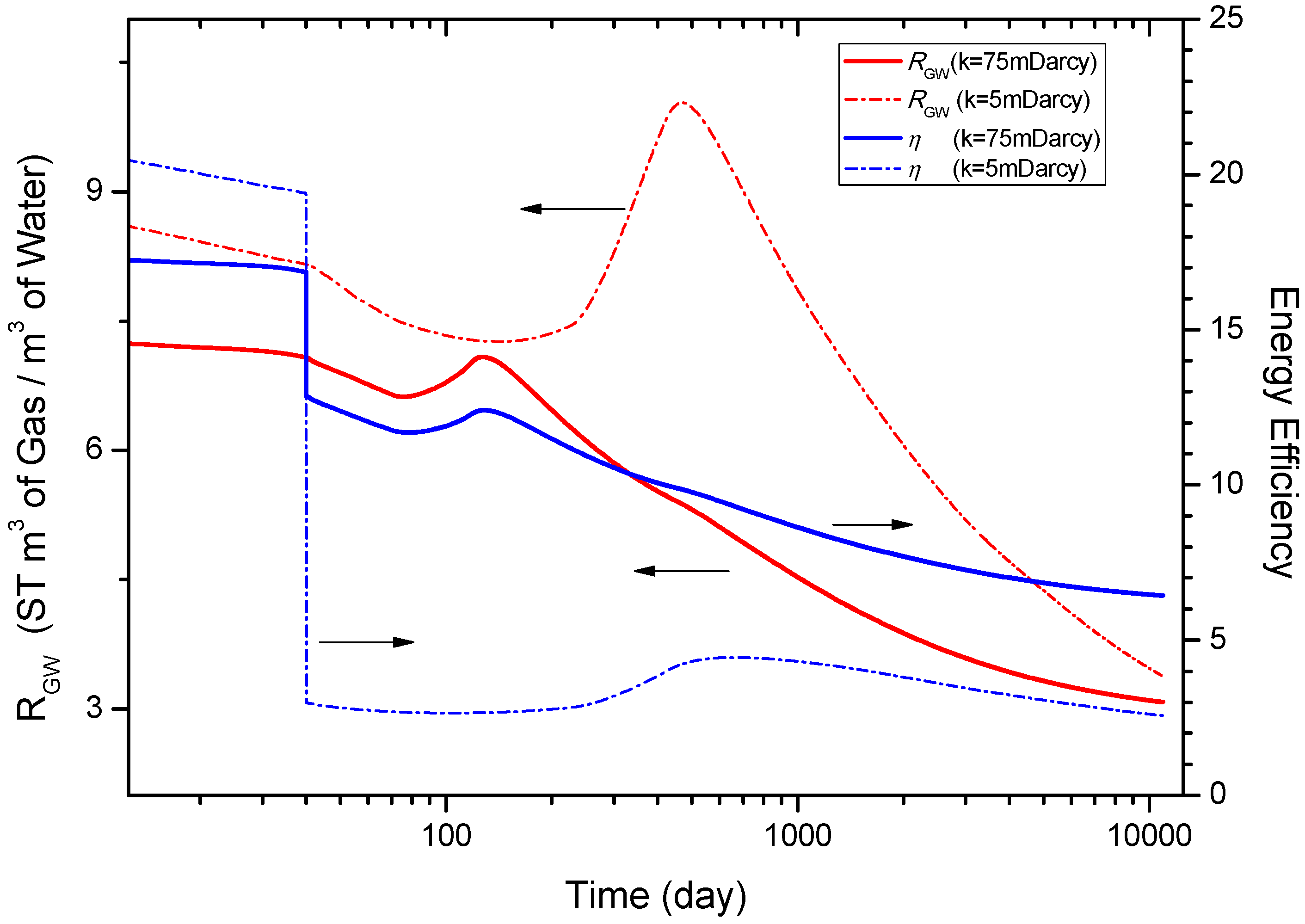
5. Conclusions
- (1)
- A dual horizontal well layout has been considered as the well configuration for this work. During the exploitation process, the upper well is regarded as the production well. A constant flow rate of warm brine is simultaneously injected into the hydrate reservoir through the lower well. For avoiding the pressure of the area around the lower well increase to an extremely high level by continuous injection at the start of the production process, a period of constant-pressure production in the lower well to decompose the hydrate and free the pores around the well is needed.
- (2)
- During the depressurization production stage, two cylindrical dissociation fronts exist around the two wells. As the injection process starts, the dissociation fronts enlarge with the injection of warm brine. The gas production rate increases when the hydrate between the two wells is gradually dissociated. Meanwhile, QPT and QR reach the peak value when the two wells are connected completely. A dissociation interface emerges between the HBL and the underburden, and then gradually merges with the cylindrical dissociation interface around the lower well. A similar dissociation interface occurs in the upper well and the overburden lately.
- (3)
- In the reference case, QPT exceeds QR and QPG in the whole production of 30 years, indicating that most of the produced gas comes from the CH4 dissolved in the water rather than from the free gas. A maximum value (17.3 ST m3/day/m) of QPT is obtained before a decline starts at the 118th day. The variation of QR is similar to that of QPT. The average gas production rate Qavg is about 1.23 × 105 ST m3/day for a 1000 m-long well with spacing of 90 m, which can obtain the same order of magnitude compared with the commercially viable production rate. The corresponding water production rate QW is about 3.98 × 107 kg/day which is massive. Furthermore, it causes a relative low level of RGW (from 3.08 to 7.35). The other criterion η gets the range of 6.43 to 17.5. The simulation results provide economical feasibility of the warm brine stimulation combined depressurization method from the point of high level of energy efficiency.
- (4)
- The analysis of sensitivity to the temperature of the injected brine indicates that increasing TW can enhance QPT, QR, and RGW remarkably when the injection process starts. However, the advantage gradually disappears as the hydrate blocking the channel of the two wells is dissociated totally. The analysis of sensitivity to the salinity of the injected brine comes to the conclusion that the larger the XS is, the faster the gas production rate is. But this advantage gradually fades away after the two wells are connected. Consequently, some practical methods such as enlarging the distance between the two wells may be taken into account to make the warm brine injected deep into the hydrate reservoir.
- (5)
- Sensitivity analysis shows that the gas and water production are strongly dependent on the intrinsic permeability of the hydrate reservoir. The gas and water production rates decrease when the intrinsic permeability decline from 75 mD to 5 mD. The energy efficiency η also gets an evident decrease with the reduction of k, on the other hand, an obvious enhancement of the gas-to-water ratio RGW can be observed. When k decreases to 5 mD, QR is larger than QPT in the initial 1475 days, suggesting that the produced gas mainly comes from the released gas.
Acknowledgments
Conflicts of Interest
Nomenclature
| G | thermal gradient within the sea (°C/m) |
| H | depth of the sea water |
| k | intrinsic permeability (m2) |
| keff | effective permeability (m2) |
| krA | aqueous relative permeability (m2) |
| krG | gas relative permeability (m2) |
| kΘC | thermal conductivity (W/m/K) |
| kΘRD | thermal conductivity of dry porous medium (W/m/K) |
| kΘRW | thermal conductivity of fully saturated porous medium (W/m/K) |
| kΘI | thermal conductivity of ice (W/m/K) |
| MW | cumulative mass of produced water (kg) |
| P | pressure (MPa) |
| PB | initial pressure at base of HBL (MPa) |
| P0 | atmosphere pressure (MPa) |
| PW | pressure at the well (MPa) |
| PW0 | initial pressure at the well (MPa) |
| Q | injected heat (J) |
| Qavg | average gas production rate (ST m3/day/m of well) |
| QW | average water production rate (kg/day/m of well) |
| Qinj | heat injection rate (W/m of well) |
| r | radius (m) |
| RGW | the gas to water production ratio (ST m3 of CH4 / m3 of H2O) |
| S | phase saturation |
| t | time (days) |
| T | temperature (°C) |
| T0 | the temperature of the sea floor |
| TW | injected warm water temperature (°C) |
| TB | initial temperature at the base of HBL (°C) |
| TT | initial temperature at the top of HBL (°C) |
| W | pump work (J) |
| x,y,z | Cartesian coordinates (m) |
| SH | hydrate saturation |
| SG | gas saturation |
| XS | the mass fraction of salt in the aqueous phase |
| mole fraction of the inhibitor in the aqueous phase | |
| reference mole fraction of the inhibitor in the aqueous phase (K) | |
| inhibitor-induced temperature depression (K) | |
| temperature depression at the reference mole fraction | |
| Δ Hc | combustion enthalpy of produced methane (J) |
| Δ PW | driving force of depressurization, PW0 − PW (MPa) |
| Δ x | discretization along the x-axis (m) |
| Δ y | discretization along the y-axis (m) |
| Δ z | discretization along the z-axis (m) |
| ϕ | porosity |
| η | energy efficiency |
| λ | van Genuchten exponent ( Table 1) |
| Subscripts and Superscripts | |
| 0 | denotes initial state |
| A | aqueous phase |
| B | base of HBL |
| cap | capillary |
| G | gas phase |
| H | solid hydrate phase |
References
- Shiraki, M. Physical characterization of TRK-720 hydrate, the very late Antigen-4 (VLA-4) inhibitor, as a solid form for inhalation: Preparation of the hydrate by solvent exchange among its solvates and mechanistical considerations. J. Pharm. Sci. 2010, 99, 3986–4004. [Google Scholar] [PubMed]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Moridis, G.J. Challenges, uncertainties and issues facing gas production from gas hydrate deposits. SPE Reserv. Eval. Eng. 2011, 14, 76–112. [Google Scholar] [CrossRef]
- Li, G.; Li, B.; Li, X.S.; Zhang, Y.; Wang, Y. Experimental and numerical studies on gas production from methane hydrate in porous media by depressurization in pilot-scale hydrate simulator. Energy Fuels 2012, 26, 6300–6310. [Google Scholar] [CrossRef]
- Zhao, J.F.; Yu, T.; Song, Y.C.; Liu, D.; Liu, W.G.; Liu, Y.; Yang, M.J.; Ruan, X.K.; Li, Y.H. Numerical simulation of gas production from hydrate deposits using a single vertical well by depressurization in the Qilian Mountain permafrost, Qinghai-Tibet Plateau, China. Energy 2013, 52, 308–319. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Y.; Duan, L.P.; Li, G.; Zhang, Y.; Huang, N.S.; Chen, D.F. Experimental investigation into methane hydrate production during three-dimensional thermal huff and puff. Appl. Energy 2012, 94, 48–57. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Phase equilibria of hydrogen sulfide and carbon dioxide simple hydrates in the presence of methanol, (methanol plus NaCl) and (ethylene glycol plus NaCl) aqueous solutions. J. Chem. Thermodyn. 2012, 44, 26–30. [Google Scholar] [CrossRef]
- Moridis, G.J.; Collett, T.S.; Dallimore, S.R.; Satoh, T.; Hancock, S.; Weatherill, B. Numerical studies of gas production from several CH4 hydrate zones at the Mallik site, Mackenzie Delta, Canada. J. Petrol. Sci. Eng. 2004, 43, 219–238. [Google Scholar] [CrossRef]
- Li, G.; Tang, L.; Huang, C.; Feng, Z.; Fan, S. Thermodynamic evaluation of hot brine stimulation for natural gas hydrate dissociation. J. Chem. Ind. Eng. (China) 2006, 57, 2033–2038. [Google Scholar]
- Li, X.S.; Wan, L.H.; Li, G.; Li, Q.P.; Chen, Z.Y.; Yan, K.F. Experimental investigation into the production behavior of methane hydrate in porous sediment with hot brine stimulation. Ind. Eng. Chem. Res. 2008, 47, 9696–9702. [Google Scholar] [CrossRef]
- Moridis, G.J.; Reagan, M.T.; Boyle, K.L.; Zhang, K. Evaluation of the gas production potential of some particularly challenging types of oceanic hydrate deposits. Transp. Porous Media 2011, 90, 269–299. [Google Scholar] [CrossRef]
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Koh, C.; Sloan, E.D. Toward production from gas hydrates: current status, assessment of resources, and simulation-based evaluation of technology and potential. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef]
- Japan Taps “Fiery Ice” Fuel from Seabed. Available online: http://www.newscientist.com/blogs/shortsharpscience/2013/03/japan-taps-methane-hydrate-fro.html (accessed on 13 March 2013).
- Boswell, R.; Frye, M.; Shelander, D.; Shedd, W.; McConnelle, D.R.; Cook, A. Architecture of gas-hydrate-bearing sands from Walker Ridge 313, Green Canyon 955, and Alaminos Canyon 21: Northern deepwater Gulf of Mexico. Mar. Pet. Geol. 2012, 34, 134–149. [Google Scholar] [CrossRef]
- Myshakin, E.M.; Gaddipati, M.; Rose, K.; Anderson, B.J. Numerical simulations of depressurization-induced gas production from gas hydrate reservoirs at the Walker Ridge 313 site, northern Gulf of Mexico. Mar. Petrol. Geol. 2012, 34, 169–185. [Google Scholar] [CrossRef]
- Trung, N.N. The gas hydrate potential in the South China Sea. J. Petrol. Sci. Eng. 2012, 88–89, 41–47. [Google Scholar]
- Liu, H.L.; Yao, Y.J.; Deng, H. Geological and geophysical conditions for potential natural gas hydrate resources in southern South China Sea waters. J. Earth Sci. (China) 2011, 22, 718–725. [Google Scholar] [CrossRef]
- Wang, C.J.; Du, D.W.; Zhu, Z.W.; Liu, Y.G.; Yan, S.J.; Yang, G. Estimation of potential distribution of gas hydrate in the northern South China Sea. Chin. J. Oceanol. Limn. 2010, 28, 693–699. [Google Scholar] [CrossRef]
- Li, G.; Moridis, G.J.; Zhang, K.; Li, X.S. The use of huff and puff method in a single horizontal well in gas production from marine gas hydrate deposits in the Shenhu Area of South China Sea. J. Petrol Sci. Eng. 2011, 77, 49–68. [Google Scholar] [CrossRef]
- Su, Z.; Moridis, G.J.; Zhang, K.; Wu, N. A huff-and-puff production of gas hydrate deposits in Shenhu area of South China Sea through a vertical well. J. Petrol. Sci. Eng. 2012, 86–87, 54–61. [Google Scholar]
- Yao, B.C. The gas hydrate in the South China Sea. J. Trop. Oceanogr. 2001, 20, 20–28. [Google Scholar]
- Wang, S.; Yan, W.; Song, H. Mapping the thickness of the gas hydrate stability zone in the South China Sea. Terr. Atmos. Ocean Sci. 2006, 17, 815–828. [Google Scholar]
- Moridis, G.J.; Reagan, M.T. Strategies for Gas Production From Oceanic Class 3 Hydrate Accumulations. In Proceedings of the OTC 18865, 2007 Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2007.
- Moridis, G.J.; Reagan, M.T.; Boyle, K.L.; Zhang, K. Evaluation of the Gas Production Potential of Challenging Hydrate Deposits. In TOUGH Symposium; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 14–16 September 2009. [Google Scholar]
- Li, X.S.; Zhang, Y.; Li, G.; Chen, Z.Y.; Wu, H.J. Experimental investigation into the production behavior of methane hydrate in porous sediment by depressurization with a novel three-dimensional cubic hydrate simulator. Energy Fuels 2011, 25, 4497–4505. [Google Scholar] [CrossRef]
- Konno, Y.; Masuda, Y.; Hariguchi, Y.; Kurihara, M.; Ouchi, H. Key factors for depressurization-induced gas production from oceanic methane hydrates. Energy Fuels 2010, 24, 1736–1744. [Google Scholar] [CrossRef]
- Moridis, G.J.; Reagan, M.T.; Zhang, K. The Use of Horizontal Wells in Gas Production from Hydrate Accumulations. In Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008), Vancouver, BC, Canada, 6–10 July 2008.
- Li, X.S.; Li, B.; Li, G.; Yang, B. Numerical simulation of gas production potential from permafrost hydrate deposits by huff and puff method in a single horizontal well in Qilian Mountain, Qinghai province. Energy 2012, 40, 59–75. [Google Scholar] [CrossRef]
- Moridis, G.J.; Kowalsky, M.B.; Pruess, K. Tough+Hydrate v1.1 User’s Manual: A Code for the Simulation of System Behavior in Hydrate-Bearing Geologic Media; Lawrence Berkeley National laboratory: Berkeley, CA, USA, 2009. [Google Scholar]
- Wang, D.; Li, D.L.; Zhang, H.L.; Fan, S.S.; Zhao, H.B. Laboratory measurement of longitudinal wave velocity of artificial gas hydrate under different temperatures and pressures. Sci. China Ser. G Phy. Mech. Astron. 2008, 51, 1905–1913. [Google Scholar] [CrossRef]
- Gamwo, I.K.; Liu, Y. Mathematical modeling and numerical simulation of methane production in a hydrate reservoir. Ind. Eng. Chem. Res. 2010, 49, 5231–5245. [Google Scholar] [CrossRef]
- Liu, Y.; Strumendo, M.; Arastoopour, H. Simulation of methane production from hydrates by depressurization and thermal stimulation. Ind. Eng. Chem. Res. 2009, 48, 2451–2464. [Google Scholar] [CrossRef]
- Yao, B.C.; Yang, M.Y.; Wu, S.G.; Wang, H.B. The gas hydrate resources in the China seas. Geoscience (China) 2008, 22, 333–341. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Moridis, G.J.; Seol, Y.; Kneafsey, T.J. Studies of Reaction Kinetics of Methane Hydrate Dissociation in Porous Media. In Proceedings of the 5th International Conference on Gas Hydrate, Trondheim, Norway, 3–5 June 2005; pp. 1004–1014.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feng, J.-C.; Li, G.; Li, X.-S.; Li, B.; Chen, Z.-Y. Evolution of Hydrate Dissociation by Warm Brine Stimulation Combined Depressurization in the South China Sea. Energies 2013, 6, 5402-5425. https://doi.org/10.3390/en6105402
Feng J-C, Li G, Li X-S, Li B, Chen Z-Y. Evolution of Hydrate Dissociation by Warm Brine Stimulation Combined Depressurization in the South China Sea. Energies. 2013; 6(10):5402-5425. https://doi.org/10.3390/en6105402
Chicago/Turabian StyleFeng, Jing-Chun, Gang Li, Xiao-Sen Li, Bo Li, and Zhao-Yang Chen. 2013. "Evolution of Hydrate Dissociation by Warm Brine Stimulation Combined Depressurization in the South China Sea" Energies 6, no. 10: 5402-5425. https://doi.org/10.3390/en6105402
APA StyleFeng, J.-C., Li, G., Li, X.-S., Li, B., & Chen, Z.-Y. (2013). Evolution of Hydrate Dissociation by Warm Brine Stimulation Combined Depressurization in the South China Sea. Energies, 6(10), 5402-5425. https://doi.org/10.3390/en6105402






