Accidental Continuous Releases from Coal Processing in Semi-Confined Environment
Abstract
:1. Introduction
| Input | Output | ||
|---|---|---|---|
| Energy | 42.7 GJ/t coke | Energy loss | 8.65% |
| Coal | 91.44% | Coke | 69.63% |
| Electric power | 0.37% | Coke oven gas (COG) | 17.92% |
| Fuel gas (firing gas) | 7.61% | Tar | 2.77% |
| Steam | 0.58% | Benzene, toluene, xylenes (BTX) | 0.98% |
| - | - | Sulphur | 0.05% |
2. Theoretical Model
2.1. Allowed Gas Build-up Considering Overpressure
- completely sealed enclosure: na = nr; α = 1;
- well ventilated building, small quantity released: α ≈ 0;
- condensed phase release: α ≈ 0.
| Compound | ya* | yL | ya*/yL | n*a/V |
|---|---|---|---|---|
| H2 | 0.00248 | 0.040 | 0.062 | 0.1034 |
| CH4 | 0.00075 | 0.051 | 0.015 | 0.0311 |
| C2H2 | 0.00048 | 0.025 | 0.019 | 0.0199 |
| C2H4 | 0.00045 | 0.031 | 0.015 | 0.0189 |
| CO | 0.00212 | 0.155 | 0.014 | 0.0883 |
| C2H6 | 0.00042 | 0.030 | 0.014 | 0.01750 |
| C3H6 | 0.00031 | 0.024 | 0.013 | 0.01298 |
| C3H8 | 0.00029 | 0.022 | 0.013 | 0.01224 |
| C4H8 | 0.00024 | 0.016 | 0.015 | 0.00984 |
| C4H10 | 0.00023 | 0.019 | 0.012 | 0.00941 |
| C5H10 | 0.00019 | 0.015 | 0.013 | 0.00799 |
| C5H12 | 0.00018 | 0.015 | 0.012 | 0.00770 |
| C6H6 | 0.00019 | 0.014 | 0.014 | 0.00797 |
| C6H12 | 0.00016 | 0.013 | 0.012 | 0.00669 |
| C6H14 | 0.00016 | 0.012 | 0.013 | 0.00648 |
2.2. Allowed Gas Build-up Considering Thermal Radiating Exposure
- build-up of a flammable gas/air cloud resulting from a finite duration subsonic leak and stratification due to density difference between gas and air;
- dimensions and shape of the cloud constrained by the geometric characteristics of the room;
- flammable cloud ignition → flame development → thermal radiation from a plane radiating surface of area Ar.
3. Results and Discussion
| Compound | −∆Hc (J mol−1) | fw = (1 + d2/Ar)−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | ||
| n*a/Ar (mol m−2) | ||||||||
| H2 | 241,800 | 1.68204 | 1.93574 | 2.26491 | 2.70628 | 3.32380 | 4.23848 | 5.70721 |
| CH4 | 802,600 | 0.46972 | 0.54057 | 0.63249 | 0.75574 | 0.92819 | 1.18362 | 1.59377 |
| C2H2 | 1,257,000 | 0.22360 | 0.25732 | 0.30108 | 0.35975 | 0.44184 | 0.56342 | 0.75866 |
| C2H4 | 1,323,000 | 0.24503 | 0.28198 | 0.32993 | 0.39423 | 0.48419 | 0.61743 | 0.83138 |
| CO | 283,000 | 1.19235 | 1.37219 | 1.60553 | 1.91840 | 2.35615 | 3.00454 | 4.04567 |
| C2H6 | 1,428,600 | 0.24615 | 0.28327 | 0.33144 | 0.39603 | 0.48640 | 0.62025 | 0.83518 |
| C3H6 | 1,925,700 | 0.16997 | 0.19561 | 0.22887 | 0.27347 | 0.33587 | 0.42830 | 0.57671 |
| C3H8 | 2,043,100 | 0.16841 | 0.19381 | 0.22677 | 0.27096 | 0.33278 | 0.42436 | 0.57141 |
| C4H8 | 2,540,800 | 0.12871 | 0.14812 | 0.17331 | 0.20709 | 0.25434 | 0.32433 | 0.43672 |
| C4H10 | 2,657,300 | 0.12797 | 0.14727 | 0.17232 | 0.20590 | 0.25288 | 0.32247 | 0.43421 |
| C5H10 | 3,129,600 | 0.10549 | 0.12141 | 0.14205 | 0.16973 | 0.20846 | 0.26583 | 0.35794 |
| C5H12 | 3,244,900 | 0.10505 | 0.12089 | 0.14145 | 0.16901 | 0.20758 | 0.26470 | 0.35642 |
| C6H6 | 3,136,000 | 0.10502 | 0.12086 | 0.14141 | 0.16897 | 0.20753 | 0.26464 | 0.35634 |
| C6H12 | 3,739,400 | 0.08826 | 0.10158 | 0.11885 | 0.14201 | 0.17442 | 0.22241 | 0.29948 |
| C6H14 | 3,855,100 | 0.08794 | 0.10121 | 0.11842 | 0.14149 | 0.17378 | 0.22160 | 0.29839 |
| Compound | Mi (g mol−1) | V/Ar |
|---|---|---|
| H2 | 2 | 16.3 |
| CH4 | 16 | 15.1 |
| C2H2 | 26 | 11.2 |
| C2H4 | 28 | 13.0 |
| CO | 28 | 13.5 |
| C2H6 | 30 | 14.1 |
| C3H6 | 42 | 13.1 |
| C3H8 | 44 | 13.8 |
| C4H8 | 56 | 13.1 |
| C4H10 | 58 | 13.6 |
| C5H10 | 70 | 13.2 |
| C5H12 | 72 | 13.6 |
| C6H6 | 78 | 13.2 |
| C6H12 | 84 | 13.2 |
| C6H14 | 86 | 13.6 |
- exposure time to the flame;
- height of the flammable layer;
- thermal flux density;
- protective clothes worn by workers.
| Compound | fw | |||||
|---|---|---|---|---|---|---|
| 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | |
| H2 | 18.7 | 21.9 | 26.2 | 32.1 | 41.0 | 55.2 |
| CH4 | 17.4 | 20.3 | 24.3 | 29.8 | 38.0 | 51.2 |
| C2H2 | 12.9 | 15.1 | 18.1 | 22.2 | 28.3 | 38.1 |
| C2H4 | 14.9 | 17.5 | 20.9 | 25.6 | 32.7 | 44.0 |
| CO | 15.5 | 18.2 | 21.7 | 26.7 | 34.0 | 45.8 |
4. Case-Study Application

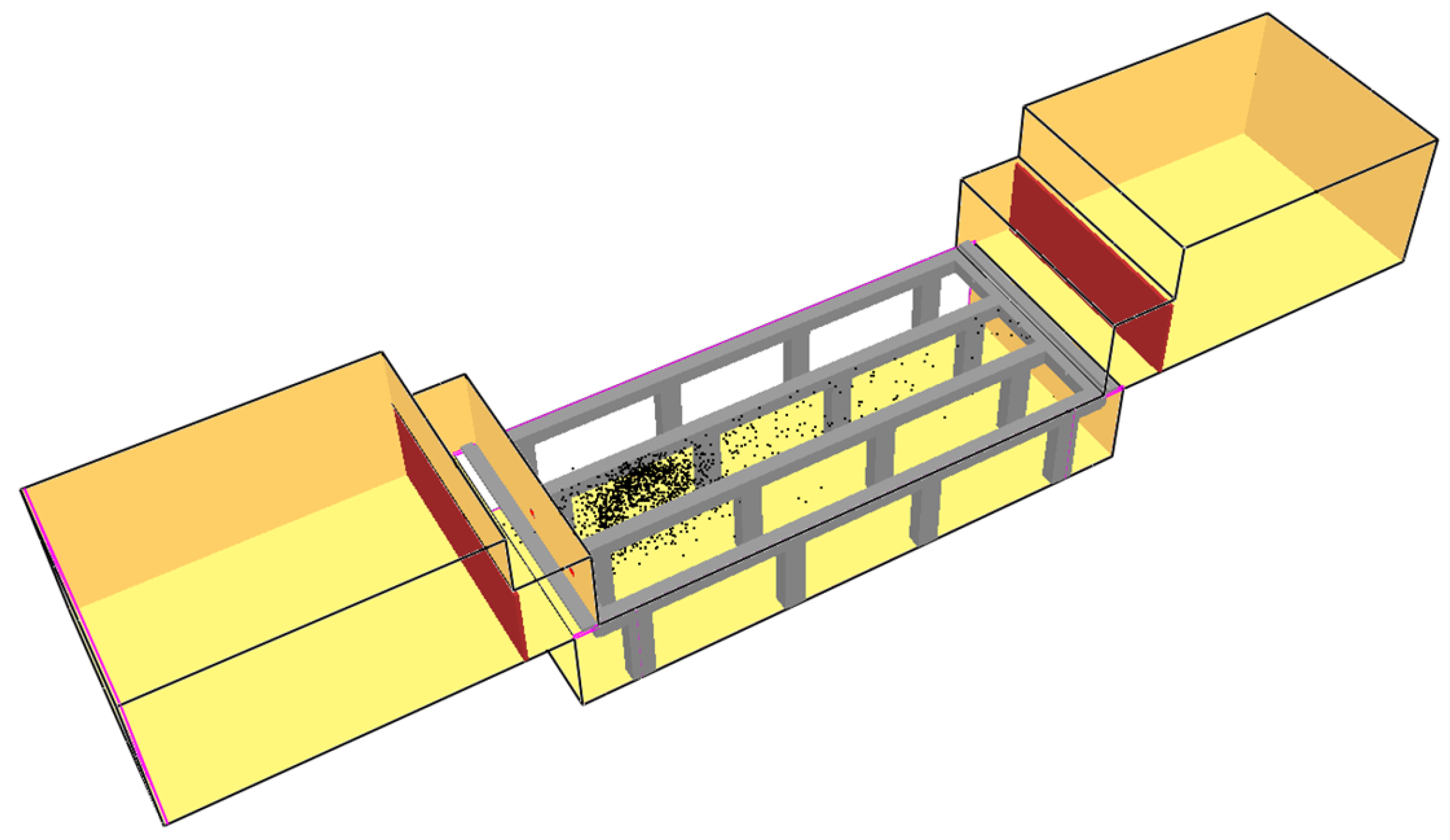
| Compound | ωi (% w/w) | xi (% v/v) |
|---|---|---|
| O2 | 0.79 | 0.29 |
| N2 | 26.22 | 11.17 |
| CH4 | 30.95 | 23.07 |
| CO | 10.76 | 4.58 |
| CO2 | 8.32 | 2.07 |
| C2H4 | 4.85 | 2.07 |
| C2H6 | 2.38 | 0.94 |
| H2 | 9.00 | 53.65 |
| H2O | 2.31 | 1.53 |
| CnHm | 4.44 | 0.63 |
| Compound | nir (mol) | nig (mol) | nia (mol) | nim (mol) | nismoke (mol) | mim (g) | mir (g) |
|---|---|---|---|---|---|---|---|
| O2 | 2.9 | −921.1 | 918.2 | 921.1 | 0.0 | 29,476 | 93 |
| N2 | 111.7 | 0.0 | 3,454.3 | 3,566.0 | 3,566.0 | 99,847 | 3,128 |
| CO2 | 20.7 | 376.5 | - | 20.7 | 397.2 | 911 | 911 |
| H2O | 15.3 | 1,089.1 | - | 15.3 | 1,104.4 | 275 | 275 |
| CO | 45.8 | −45.8 | - | 45.8 | 0.0 | 1,282 | 1,282 |
| H2 | 536.5 | −536.5 | - | 536.5 | 0.0 | 1,073 | 1,073 |
| CH4 | 230.7 | −230.7 | - | 230.7 | 0.0 | 3,691 | 3,691 |
| C2H4 | 20.7 | −20.7 | - | 20.7 | 0.0 | 580 | 580 |
| C2H6 | 9.4 | −9.4 | - | 9.4 | 0.0 | 282 | 282 |
| C6H6 | 4.41 | −4.41 | - | 4.41 | 0.0 | 344 | 344 |
| C7H8 | 1.30 | −1.30 | - | 1.30 | 0.0 | 120 | 120 |
| C8H10 | 0.39 | −0.39 | - | 0.39 | 0.0 | 42 | 42 |
| C5H12 | 0.04 | −0.04 | - | 0.04 | 0.0 | 3 | 3 |
| C6H14 | 0.06 | −0.06 | - | 0.06 | 0.0 | 5 | 5 |
| C7H16 | 0.03 | −0.03 | - | 0.03 | 0.0 | 3 | 3 |
| C5H10 | 0.03 | −0.03 | - | 0.03 | 0.0 | 2 | 2 |
| C6H12 | 0.04 | −0.04 | - | 0.04 | 0.0 | 3 | 3 |
| Mixture | 1,000 | −304.8 | 4,372.5 | 5,372.5 | 5,067.7 | 137,939 | 11,836 |
| Compound | nir (mol) | nig (mol) | nia (mol) | nim (mol) | nismoke (mol) | mim (g) | mir (g) |
|---|---|---|---|---|---|---|---|
| O2 | 2.9 | 2,625 | 2,628 | −921.1 | 1,707 | 84,093 | 93 |
| N2 | 111.7 | 9,875 | 9,987 | 0.00 | 9,987 | 279,628 | 3,128 |
| CO2 | 20.7 | - | 20.7 | 376.5 | 397.2 | 911 | 911 |
| H2O | 15.3 | - | 15.3 | 1,089 | 1,104 | 275 | 275 |
| CO | 45.8 | - | 45.8 | −45.80 | 0 | 1,282 | 1,282 |
| H2 | 536.5 | - | 536.5 | −536.5 | 0 | 1,073 | 1,073 |
| CH4 | 230.7 | - | 230.7 | −230.70 | 0 | 3,691 | 3,691 |
| C2H4 | 20.7 | - | 20.7 | −20.70 | 0 | 580 | 580 |
| C2H6 | 9.4 | - | 9.4 | −9.40 | 0 | 282 | 282 |
| C6H6 | 4.41 | - | 4.41 | −4.41 | 0 | 344 | 344 |
| C7H8 | 1.30 | - | 1.30 | −1.30 | 0 | 120 | 120 |
| C8H10 | 0.39 | - | 0.39 | −0.39 | 0 | 42 | 42 |
| C5H12 | 0.04 | - | 0.04 | −0.04 | 0 | 3 | 3 |
| C6H14 | 0.06 | - | 0.06 | −0.06 | 0 | 5 | 5 |
| C7H16 | 0.03 | - | 0.03 | −0.03 | 0 | 3 | 3 |
| C5H10 | 0.03 | - | 0.03 | −0.03 | 0 | 2 | 2 |
| C6H12 | 0.04 | - | 0.04 | −0.04 | 0 | 3 | 3 |
| Mixture | 1,000 | 12,500 | 13,500 | −305 | 13,195 | 372,336 | 11,836 |
| Battery | Gas mixture | Ar (m2) | Q (kJ) | nr.amm (mol) | xr | nm.amm (mol) | Vamm (m3) | hamm (m) |
|---|---|---|---|---|---|---|---|---|
| A | Stoichiometric | 22 | 9,797 | 22.4 | 0.19 | 120 | 3.0 | 0.14 |
| LFL | 22 | 21,564 | 49.3 | 0.08 | 616 | 15.6 | 0.72 | |
| B, C, D | Stoichiometric | 23 | 10,334 | 23.6 | 0.19 | 127 | 3.2 | 0.14 |
| LFL | 23 | 22,747 | 52.0 | 0.08 | 650 | 16.4 | 0.72 |
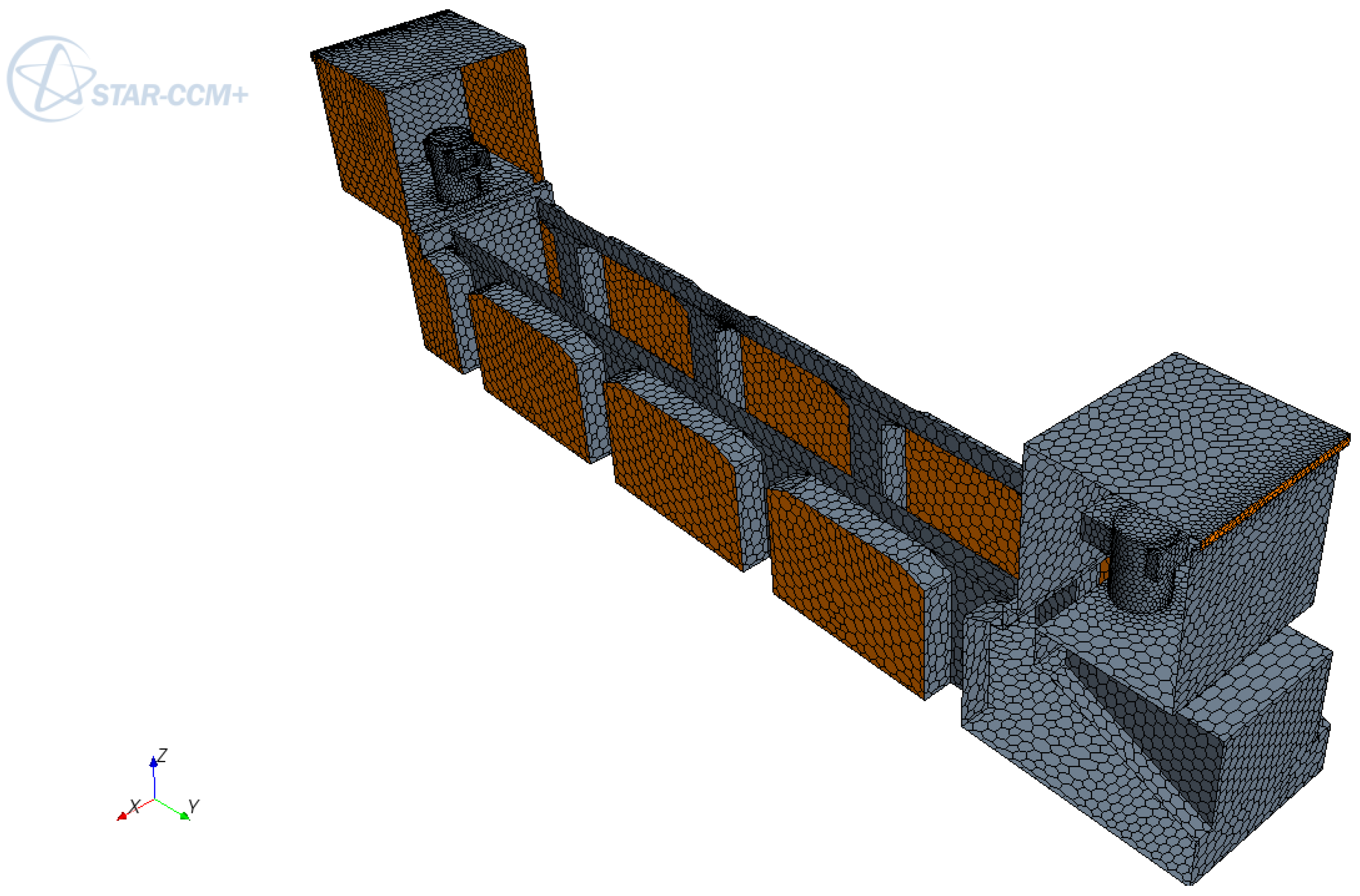
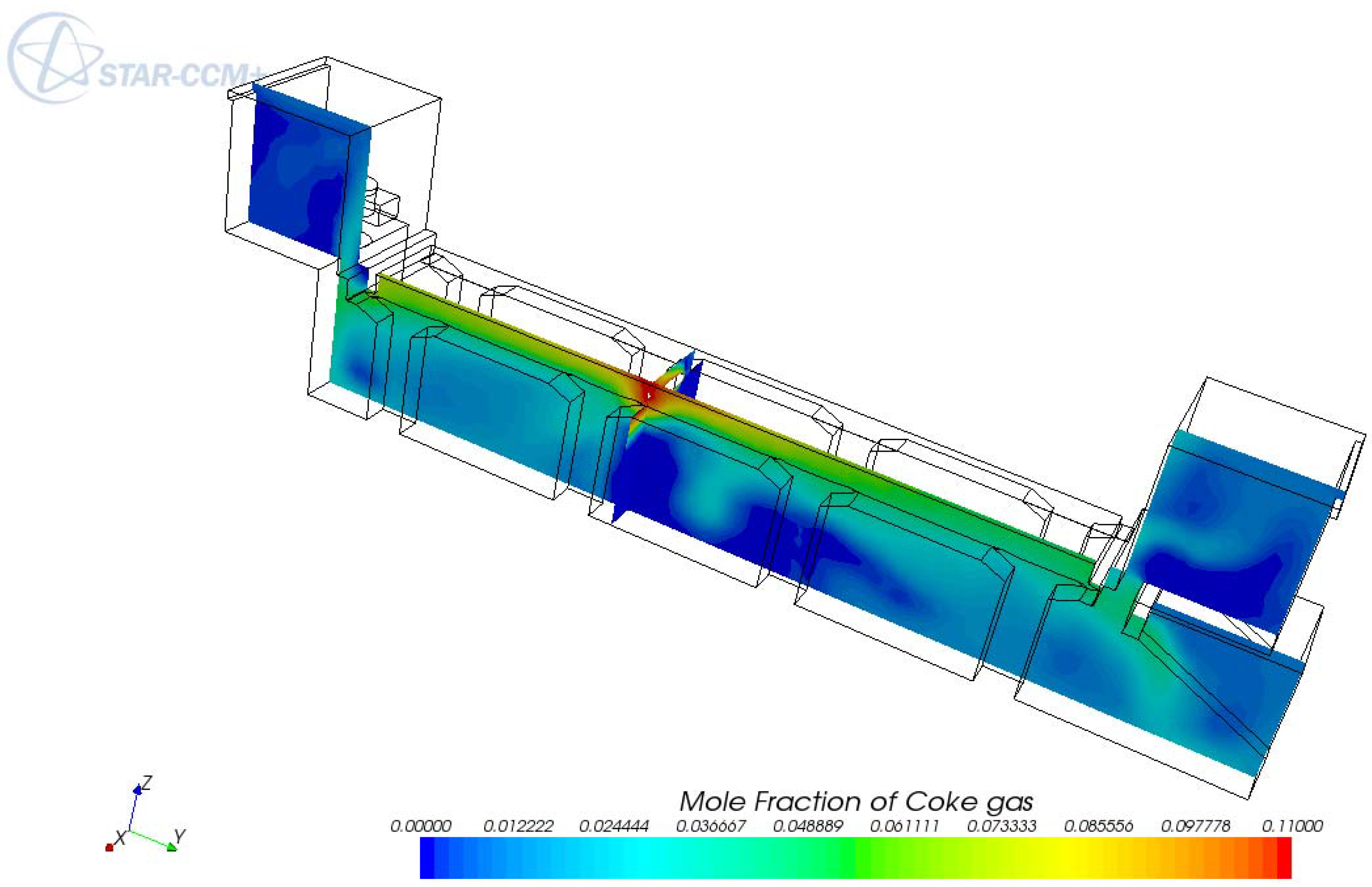
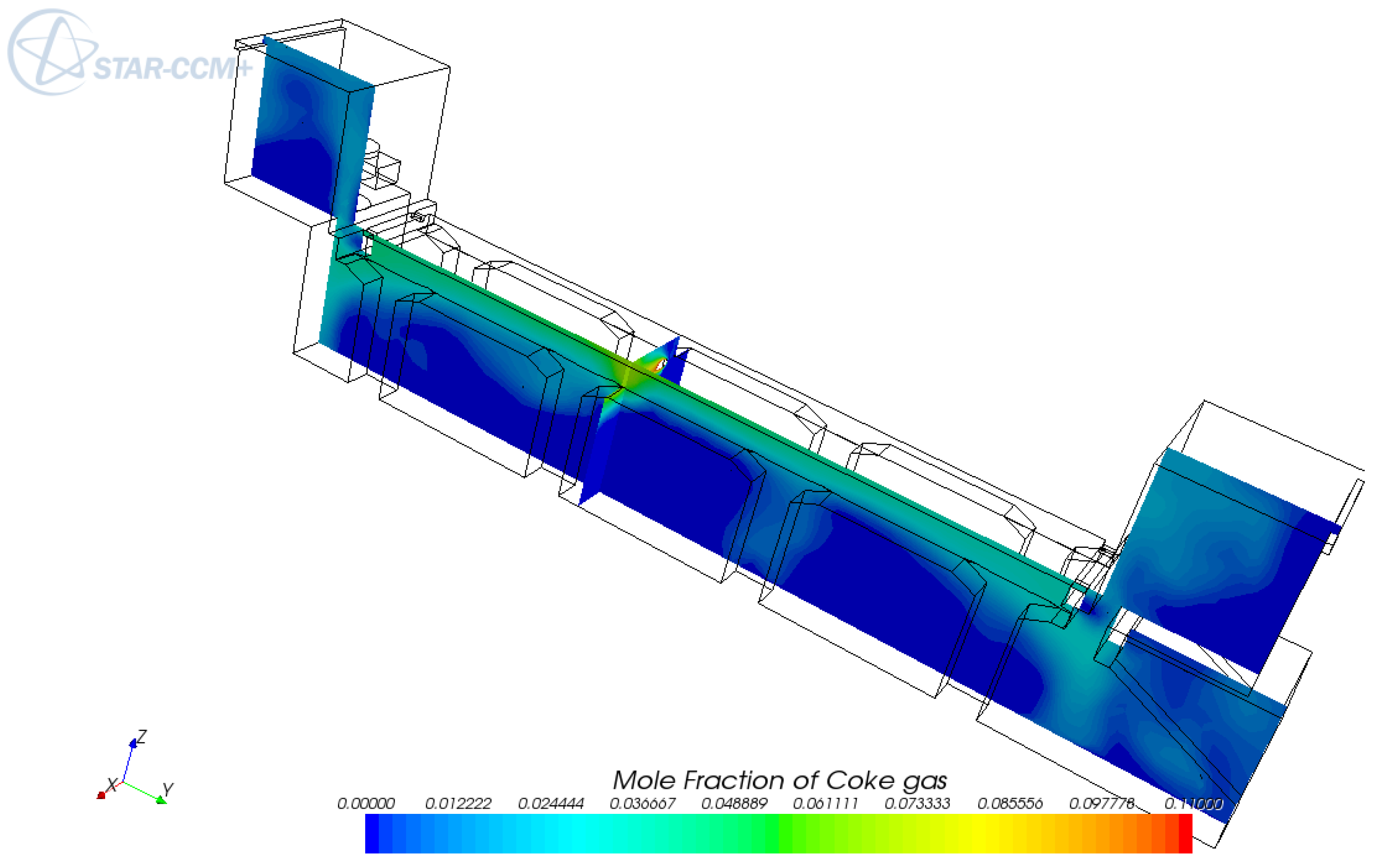


5. Conclusions
Nomenclature:
| Ar | radiating surface (m2) |
mean molar heat of the explosive mixture, in the temperature interval T0–Te (kJ kmol−1 K−1) | |
mean molar heat of smokes in the temperature range T0–Tf (kJ kmol−1 K−1) | |
| d | distance between source of radiation and target (m) |
| fw | view factor (-) |
| h | height of the enclosure (m) |
| k | coefficient defined by Equation (5) (-) |
| n0 | air quantity inside the building before the release (mol) |
| na | flammable compound quantity (mol) |
maximum allowed build-up of flammable gas (mol) | |
maximum allowed gas accumulation considering overpressure (mol) | |
maximum allowed gas accumulation considering radiation (mol) | |
| ne | gas quantity inside enclosure after the explosion (mol) |
| nf | smoke moles in the cloud at the end of complete combustion (mol) |
| nia | quantity of the compound i entrained by the release (mol) |
| nig | quantity of the compound i consumed or produced by combustion (mol) |
| ni,smoke | quantity of the compound i in the combustion smokes (mol) |
| pe | pressure inside the enclosure after the explosion (Pa) |
| p0 | internal pressure before the release (Pa) |
| Qr | total energy radiated (kW) |
radiated thermal flux density (kW·m−2) | |
| R | radius of radiating surface (m) |
| t | exposure duration (s) |
| T0 | ambient temperature before the release (K) |
| Te | temperature after the combustion (K) |
| Tf | flame temperature in the cloud (K) |
| xi | volume fraction of i in the mixture (-) |
| ya | molar fraction of flammable gas (-) |
| yas | molar ratio of flammable gas to the total gas in the cloud (-) |
| yL | lower flammable limit (-) |
| α | coefficient defined in Equation (4) (-) |
| β | coefficient defined by Equation (16) (kW4/3 m−8/3) |
| γ | fraction of the total combustion energy converted to radiation for hydrocarbons (HC) (-) |
| ΔHc | standard molar hydrocarbon enthalpy of combustion at 298 K (J mol−1) |
| ε | flame emissivity (-) |
| vi | stoichiometric coefficient (-) |
| ρa | air density (kg·m−3) |
| ρm | gaseous mixture density (kg·m−3) |
| σ | 5.67 × 10−11 kW·m−2·K−4 |
| ωi | mass fraction of i in the mixture (-) |
| * | limiting value |
Conflicts of Interest
References
- Smith, K.L.; Smoot, L.D.; Fletcher, T.H.; Pugmire, R.J. The Structure and Reaction Processes of Coal; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Joseck, F.; Wang, M.; Wu, Y. Potential energy and greenhouse gas emission effects of hydrogen production from coke oven gas in U.S. steel mills. Int. J. Hydrog. Energy 2008, 33, 1445–1454. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Diemer, P.; Killich, H.J.; Knop, K.; Lüngen, H.B.; Reinke, M.; Schmöle, P. Potentials for Utilization of Coke Oven Gas in Integrated Iron and Steel Works. In Proceedings of the 2nd International Meeting on Ironmaking/1st International Symposium on Iron Ore, Vitoria, Brazil, 12–15 September 2004; Volume 124, pp. 7–21.
- Fabiano, B.; Reverberi, A.P.; Del Borghi, A.; Dovì, V.G. Biodiesel production via transesterification: Process safety insights from kinetic modeling. Theor. Found. Chem. Eng. 2012, 46, 673–680. [Google Scholar] [CrossRef]
- Gomez, G.E.; Ramos, M.A.; Cadena, J.E.; Gomez, J.; Munoz, F.G. Inherently safer design applied to the biodiesel production. Chem. Eng. Trans. 2013, 31, 619–624. [Google Scholar]
- Wang, C.; Larsson, M.; Ryman, C.; Grip, C.E.; Wikström, J.O.; Johnsson, A.; Engdahl, J. A model on CO2 emission reduction in integrated steelmaking by optimization methods. Int. J. Energy Res. 2008, 32, 1092–1106. [Google Scholar] [CrossRef]
- Lisi, R.; Milazzo, M.F.; Maschio, G. A Quantitative Methodology for Risk Assessment of Explosive Atmospheres According to the ATEX Directive. In Proceedings of the ESREL European Safety and Reliability Conference and 17th SRA-Europe Conference, Valencia, Spain, 22–25 September 2008; Volume 2, pp. 1019–1025.
- Yang, Z.; Ding, W.; Zhang, Y.; Lu, X.; Zhang, Y.; Shen, P. Catalytic partial oxidation of coke oven to syngas in an oxygen permeation membrane reactor combined with NiO/MgO catalyst. Int. J. Hydrog. Energy 2010, 35, 6239–6247. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhang, Y. Effect of CH4-air ratios on gas explosion flame microstructure and propagation behaviors. Energies 2012, 5, 4132–4145. [Google Scholar] [CrossRef]
- Fabiano, B.; Currò, F.; Reverberi, A.P.; Palazzi, E. Coal dust emissions: From environmental control to risk minimization by underground transport. An applicative case-study. Process Saf. Environ. Prot. 2013. [Google Scholar] [CrossRef]
- Fabiano, B.; Currò, F. From a survey on accidents in the downstream oil industry to the development of a detailed near-miss reporting system. Process Saf. Environ. Prot. 2012, 90, 357–367. [Google Scholar] [CrossRef]
- Fabiano, B.; Currò, F.; Pastorino, R. Occupational injuries in Italy: Risk factors and long term trend (1951–98). Occup. Environ. Med. 2001, 58, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Mazzoldi, A.; Hill, T.; Colls, J.J. CFD and Gaussian atmospheric dispersion models: A comparison for leak from carbon dioxide transportation and storage facilities. Atmos. Environ. 2008, 42, 8046–8054. [Google Scholar] [CrossRef]
- Palazzi, E.; Currò, F.; Pastorino, R.; Fabiano, B. Safety and environmental impact reduction. A case-study applied to coal dry distillation industry. Chem. Eng. Trans. 2013, 31, 163–168. [Google Scholar]
- Milazzo, M.F.; Aven, T. An extended risk assessment approach for chemical plants applied to a study related to pipe ruptures. Reliab. Eng. Sys. Saf. 2012, 99, 183–192. [Google Scholar] [CrossRef]
- Lees, F.P. Loss Prevention in the Process Industries, 2nd ed.; Butterworth-Heineman: Oxford, UK, 1996. [Google Scholar]
- Dell’Ambiente, M. Decreto Ministeriale 9/5/2001 n. 151; Official Journal of Italian Republic (Gazzetta Ufficiale): Rome, Italy, 2001. [Google Scholar]
- TNO—The Netherlands Organisation of Applied Scientific Research. Methods for the Determination of Possible Damage to People and Objects Resulting from Releases of Hazardous Materials; The Directorate General of Labour: The Hague, The Netherlands, 1992. [Google Scholar]
- Henrych, J. The Dynamics of Explosion and Its Use. In Developments in Civil Engineering; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1979; Volume 27. [Google Scholar]
- Gugan, K. Unconfined Vapour Cloud Explosions; The Institution of Chemical Engineers: Rugby, UK, 1979. [Google Scholar]
- Mudan, S. Thermal radiation hazards from hydrocarbon pool fires. Prog. Energy Combust. Sci. 1984, 10, 59–80. [Google Scholar] [CrossRef]
- API Recommended Practice 521. In Guide for Pressure—Relieving and Depressuring System, 4th ed.; American Petroleum Institute: Washington, DC, USA, 1997.
- Palazzi, E.; Fabiano, B. Analytical modelling of hydrocarbon pool fires: Conservative evaluation of flame temperature and thermal power. Process Saf. Environ. Prot. 2012, 90, 121–128. [Google Scholar] [CrossRef]
- MacDiarmid, J.A.; North, G.J.T. Lessons learned from a hydrogen explosion in a process unit. Plant/Oper. Prog. 1989, 8, 96–99. [Google Scholar] [CrossRef]
- Mazzoldi, A.; Picard, D.; Sriram, P.G.; Oldenburg, C.M. Simulation-based estimates of safety distances for pipeline transportation of carbon dioxide. Greenh. Gases Sci. Technol. 2013, 3, 66–83. [Google Scholar] [CrossRef]
- Palazzi, E.; Currò, F.; Pastorino, R.; Fabiano, B. Evaluation and mitigation of risk connected to lighter than air gaseous releases in confined environment. Chem. Eng. Trans. 2011, 24, 1333–1338. [Google Scholar]
- Le, H.; Nayak, S.; Mannan, M.S. Upper flammability limits of hydrogen and light hydrocarbons in air at sub atmospheric pressures. Ind. Eng. Chem. Res. 2012, 51, 9396–9402. [Google Scholar] [CrossRef]
- Milazzo, M.F.; Maschio, G.; Uguccioni, G. The influence of risk prevention measures on the frequency of failure of piping. Int. J. Perform. Eng. 2010, 6, 19–33. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Palazzi, E.; Currò, F.; Fabiano, B. Accidental Continuous Releases from Coal Processing in Semi-Confined Environment. Energies 2013, 6, 5003-5022. https://doi.org/10.3390/en6105003
Palazzi E, Currò F, Fabiano B. Accidental Continuous Releases from Coal Processing in Semi-Confined Environment. Energies. 2013; 6(10):5003-5022. https://doi.org/10.3390/en6105003
Chicago/Turabian StylePalazzi, Emilio, Fabio Currò, and Bruno Fabiano. 2013. "Accidental Continuous Releases from Coal Processing in Semi-Confined Environment" Energies 6, no. 10: 5003-5022. https://doi.org/10.3390/en6105003
APA StylePalazzi, E., Currò, F., & Fabiano, B. (2013). Accidental Continuous Releases from Coal Processing in Semi-Confined Environment. Energies, 6(10), 5003-5022. https://doi.org/10.3390/en6105003





