Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study
Abstract
:1. Introduction
2. Experimental
2.1. Materials
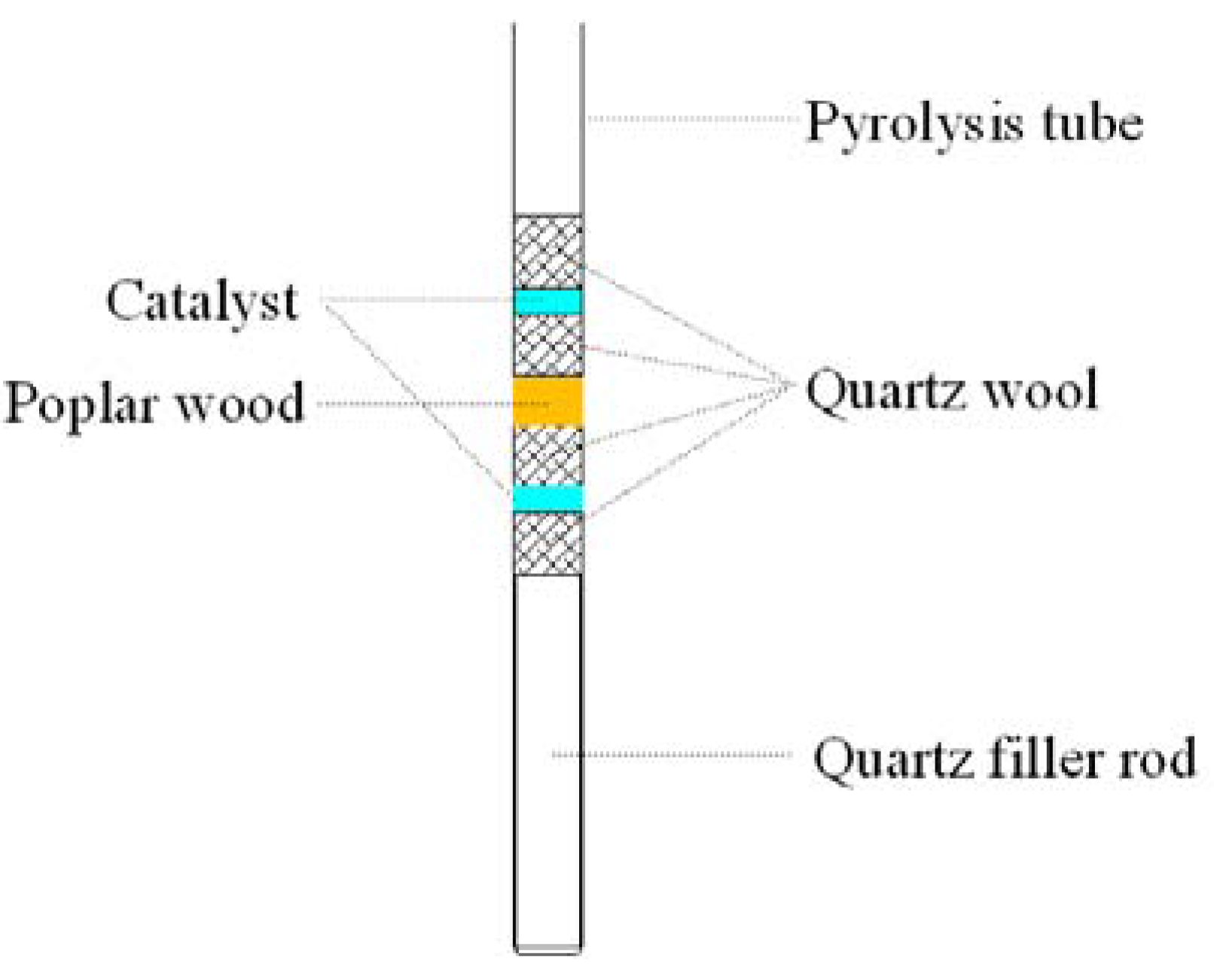
2.2. Analytical Py-GC/MS Experiments
3. Results and Discussion
3.1. Catalytic Effects on the Distribution of the Pyrolytic Products
| No. | RT | Compound | No. | RT | Compound |
|---|---|---|---|---|---|
| 1 | 2.22 | methanol | 44 | 18.23 | 1-(2-furanyl)-2-hydroxyethanone |
| 2 | 2.30 | acetaldehyde | 45 | 18.30 | 2-methoxyphenol |
| 3 | 2.54 | 2-propenal | 46 | 18.36 | 2,5-dimethyl-4-hydroxy-3(2H)-furanone |
| 4 | 2.58 | acetone | 47 | 19.32 | maltol |
| 5 | 2.61 | furan | 48 | 19.43 | 3-ethyl-2-hydroxy-2-cyclopentenone |
| 6 | 2.79 | 1,3-cyclopentadiene | 49 | 19.50 | levoglucosenone |
| 7 | 2.85 | 2-propen-1-ol | 50 | 20.44 | 3-methyl-2,4(3H,5H)-furandione |
| 8 | 3.06 | hydroxyacetaldehyde | 51 | 20.52 | 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one |
| 9 | 3.22 | 2,3-butanedione | 52 | 20.60 | 2,4-dimethylphenol |
| 10 | 3.35 | 2-methylfuran | 53 | 21.43 | 2,3-dihydrobenzaldehyde |
| 11 | 3.50 | acetic acid | 54 | 21.93 | 3,5-dihydroxy-2-methyl-4-pyrone |
| 12 | 4.12 | 2-butenal | 55 | 22.13 | 2-methoxy-4-methylphenol |
| 13 | 4.32 | 1-hydroxy-2-propanone | 56 | 23.28 | 2,3-dihydrobenzofruan |
| 14 | 4.99 | 1,2-ethanediol | 57 | 23.39 | 1,4:3,6-dianhydro-D-glucopyranose |
| 15 | 5.08 | 2,5-dimethylfuran | 58 | 23.74 | 5-(hydroxymethyl)-2-furaldehyde |
| 16 | 5.55 | vinylfuran | 59 | 23.85 | 3,4-anhydro-D-galactosan |
| 17 | 6.62 | toluene | 60 | 24.61 | 3-methoxy-1,2-benzenediol |
| 18 | 6.73 | 1-hydroxy-2-butanone | 61 | 25.12 | 4-ethyl-2-methoxyphenol |
| 19 | 6.83 | acetoxyacetic acid | 62 | 25.70 | 4-methyl-1,2-benzenediol |
| 20 | 7.28 | butanedial | 63 | 26.53 | 2-methoxy-4-vinylphenol |
| 21 | 7.39 | methyl pyruvate | 64 | 26.85 | 3-methoxy-5-methylphenol |
| 22 | 8.74 | furfural | 65 | 27.76 | 2,6-dimethoxyphenol |
| 23 | 9.39 | 2-furanmethanol | 66 | 28.10 | 2-methoxy-4-propylphenol |
| 24 | 9.91 | 1-(acetyloxy)-2-propanone | 67 | 29.54 | 4-hydroxy-3-methoxybenzaldehyde |
| 25 | 9.97 | 5-methyl-2(3H)-furanone | 68 | 30.79 | 1,2,4-trimethoxybenzene |
| 26 | 10.19 | 4-hydroxydihydro-2(3H)-furanone | 69 | 30.99 | 2-methoxy-4-propenylphenol |
| 27 | 10.58 | 2-cyclopentene-1,4-dione | 70 | 31.31 | 2-methoxy-4-propylphenol |
| 28 | 11.07 | 1,3-dihydroxy-2-propanone | 71 | 32.02 | 6-methoxy-3-methylbenzofuran |
| 29 | 11.14 | 5-(hydroxymethyl)dihydro-2(3H)-furanone | 72 | 32.22 | 1-(4-hydroxy-3-methoxyphenyl)-ethanone |
| 30 | 11.31 | 2-methyl-2-cyclopentenone | 73 | 33.14 | levoglucosan |
| 31 | 11.46 | 1-(2-furanyl)-ethanone | 74 | 33.42 | 1-(4-hydroxy-3-methoxyphenyl)-2-propanone |
| 32 | 12.10 | 1,2-cyclopentanedione | 75 | 34.46 | 3,5-dimethoxyacetophenone |
| 33 | 12.69 | 5-methyl-2(5H)-furanone | 76 | 34.86 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 34 | 13.01 | 3-methyl-2,5-furandione | 77 | 35.14 | 1-(2,4-dihydroxy-3-methylphenyl)-1-propanone |
| 35 | 13.51 | 5-methylfurfural | 78 | 35.22 | 3-hydroxy-1-(4-hydroxy-3-methoxy-phenyl)-1-propanone |
| 36 | 13.99 | 5-acetyldihydro-2(3H)-furanone | 79 | 35.72 | 1,6-anhydro-D-galactofuranose |
| 37 | 14.15 | phenol | 80 | 37.37 | 4-hydroxy-3,5-dimethoxybenzaldehyde |
| 38 | 14.79 | 2H-pyran-2,6-3(H)-dione | 81 | 38.41 | 4-allyl-2,6-dimethoxyphenol |
| 39 | 14.88 | 3-hydroxydihydro-2(3H)-furanone | 82 | 39.31 | 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone |
| 40 | 15.93 | 2-hydroxy-3-methyl-2-cyclopentenone | 83 | 39.55 | 4-hydroxy-2-methoxycinnamaldehyde |
| 41 | 16.35 | 2,3-dimethyl-2-cyclopentenone | 84 | 40.18 | 1-(2,6-dihydroxy-4-methoxyphenyl)-1-butanone |
| 42 | 17.00 | 2-methylphenol | 85 | 41.73 | 4-biphenyl ethyl ketone |
| 43 | 17.83 | 4-methylphenol | 86 | 45.85 | 3,5-dimethoxy-4-hydroxycinnamaldehyde |
| No. | RT | Compound | MgO | CaO | TiO2 | Fe2O3 | NiO | ZnO |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.72 | methyl acetate | * | * | * | * | * | * |
| 2 | 2.96 | 2-methylpropanal | * | |||||
| 3 | 3.27 | 2-butanone | * | * | * | * | * | * |
| 4 | 3.70 | methyl propionate | * | |||||
| 5 | 3.88 | 4-methylene-1-cyclopentene | * | * | * | * | ||
| 6 | 4.31 | benzene | * | * | ||||
| 7 | 4.70 | 2-pentanone | * | |||||
| 8 | 4.94 | 3-pentanone | * | |||||
| 9 | 4.98 | propanoic acid | * | * | ||||
| 10 | 5.29 | 3-hydroxy-2-butanone | * | * | * | |||
| 11 | 5.91 | 1-methylcyclohexa-1,3-diene | * | * | * | * | ||
| 12 | 5.97 | 1-methyl-1,4-cyclohexadiene | * | |||||
| 13 | 5.98 | (E)-3-penten-2-one | * | * | * | |||
| 14 | 6.07 | 1,2-dimethyl-1,3-cyclopentadiene | * | |||||
| 15 | 7.06 | 3-hexanone | * | |||||
| 16 | 7.19 | 2-hexanone | * | |||||
| 17 | 7.40 | cyclopentanone | * | * | ||||
| 18 | 7.61 | 4-hydroxy-3-hexanone | * | |||||
| 19 | 8.76 | 2-cyclopentenone | * | * | * | * | * | * |
| 20 | 8.98 | 2-methylcyclopentanone | * | * | * | |||
| 21 | 9.29 | 3-methylcyclopentanone | * | |||||
| 22 | 9.51 | ethylbenzene | * | * | ||||
| 23 | 9.52 | 1,5-dimethyl-1,4-cyclohexadiene | * | |||||
| 24 | 9.86 | p-xylene | * | |||||
| 25 | 9.95 | 2-ethylfuran | * | |||||
| 26 | 10.33 | 2,3-dimethyl-1,3-pentadiene | * | |||||
| 27 | 10.68 | o-xylene | * | |||||
| 28 | 10.8 | 2,5-dimethylcyclopentanone | * | |||||
| 29 | 10.96 | 4-methyl-cyclohexanone | * | |||||
| 30 | 11.02 | 3-methyl-cyclohexanone | * | |||||
| 31 | 11.67 | methoxybenzene | * | |||||
| 32 | 11.91 | 3-methyl-3-cyclohexenone | * | |||||
| 33 | 12.44 | 3,4-dimethyl-2-cyclopentenone | * | * | ||||
| 34 | 12.59 | 2-ethylcyclopentanone | * | |||||
| 35 | 13.41 | 3-ethylcyclopentanone | * | |||||
| 36 | 14.57 | 1,3,5-trimethylbenzene | * | |||||
| 37 | 15.77 | 3-methyl-3-cyclohexenone | * | |||||
| 38 | 16.68 | indene | * | * | * | * | * | |
| 39 | 17.64 | acetophenone | * | |||||
| 40 | 18.29 | 2,3,4-trimethyl-2-cyclopentenone | * | |||||
| 41 | 18.64 | methyl benzoate | * | * | * | |||
| 42 | 19.13 | 2-methylbenzofuran | * | |||||
| 43 | 20.13 | 4-ethylphenol | * | |||||
| 44 | 20.94 | 1-methyl-4-(1-propynyl)-benzene | * | |||||
| 45 | 23.11 | 2,3,4,5-tetramethyl-2-cyclopentenone | * | |||||
| 46 | 23.13 | 4,7-dimethylbenzofuran | * | |||||
| 47 | 23.83 | 2,6-dimethoxytoluene | * | |||||
| 48 | 24.08 | 2(3H)-benzofuranone | * | * | ||||
| 49 | 24.57 | 3-propyl-phenol | * | |||||
| 50 | 24.68 | 4,7-dimethyl-1H-indene | * | |||||
| 51 | 24.90 | 3,5-dimethoxytoluene | * | * | ||||
| 52 | 25.67 | 1-indanone | * | * | ||||
| 53 | 26.11 | 1-methylnaphthalene | * | * | ||||
| 54 | 26.34 | 1,2,3-trimethoxybenzene | * | |||||
| 55 | 26.64 | 2-methylnaphthalene | * | |||||
| 56 | 27.47 | 3-methoxy-2,4,5-trimethylphenol | * | |||||
| 57 | 27.97 | 7-methyl-1-indanone | * | |||||
| 58 | 28.69 | methyl 3-methoxybenzoate | * | * | * | |||
| 59 | 28.90 | biphenyl | * | |||||
| 60 | 31.32 | 4-hydroxybenzamide | * | |||||
| 61 | 33.12 | 3,4,5-trimethoxytoluene | * | * | ||||
| 62 | 32.01 | 6-methoxy-3-methylbenzofuran | * | * | * | |||
| 63 | 32.44 | 1,2-dimethoxy-4-propenylbenzene | * | |||||
| 64 | 36.83 | 1,2,3-trimethoxy-5-[(1E)-1-propenyl]benzene | * | |||||
| 65 | 41.04 | phenanthrene | * | |||||
| 66 | 41.34 | anthracene | * | |||||
| 67 | 43.43 | 3,5-dimethoxy-4-hydroxyphenylacetic acid | * | * | * | * | * | |
| 68 | 47.92 | 5-hydroxy-3,4’-dimethoxy-1,1’-biphenyl | * | * | * | * | * | |
| 69 | 48.09 | pyrene | * |
| Catalyst | — | MgO | CaO | TiO2 | Fe2O3 | NiO | ZnO |
|---|---|---|---|---|---|---|---|
| Anhydrosugars | 10.1 | 8.4 | 1.2 | 4.0 | 7.5 | 13.6 | 7.2 |
| Furans | 7.0 | 7.4 | 5.2 | 9.2 | 6.8 | 6.4 | 8.2 |
| Aldehydes | 14.5 | 8.6 | 15.1 | 12.0 | 9.4 | 9.0 | 12.6 |
| Ketones | 3.8 | 8.5 | 20.9 | 5.3 | 7.4 | 4.8 | 4.5 |
| Phenols | 26.5 | 30.2 | 13.0 | 27.3 | 28.1 | 32.6 | 28.3 |
| Acids | 11.0 | 10.2 | 0.0 | 11.5 | 12.9 | 9.3 | 11.4 |
| Alcohols | 2.5 | 3.4 | 8.6 | 2.1 | 2.0 | 2.2 | 2.8 |
| Hydrocarbons | 0.2 | 0.8 | 4.2 | 0.9 | 3.1 | 0.3 | 0.3 |
| Cyclopentenones | 2.4 | 4.7 | 16.7 | 6.9 | 4.6 | 3.3 | 3.6 |
| Others | 3.3 | 3.5 | 1.6 | 3.9 | 3.2 | 2.7 | 2.9 |

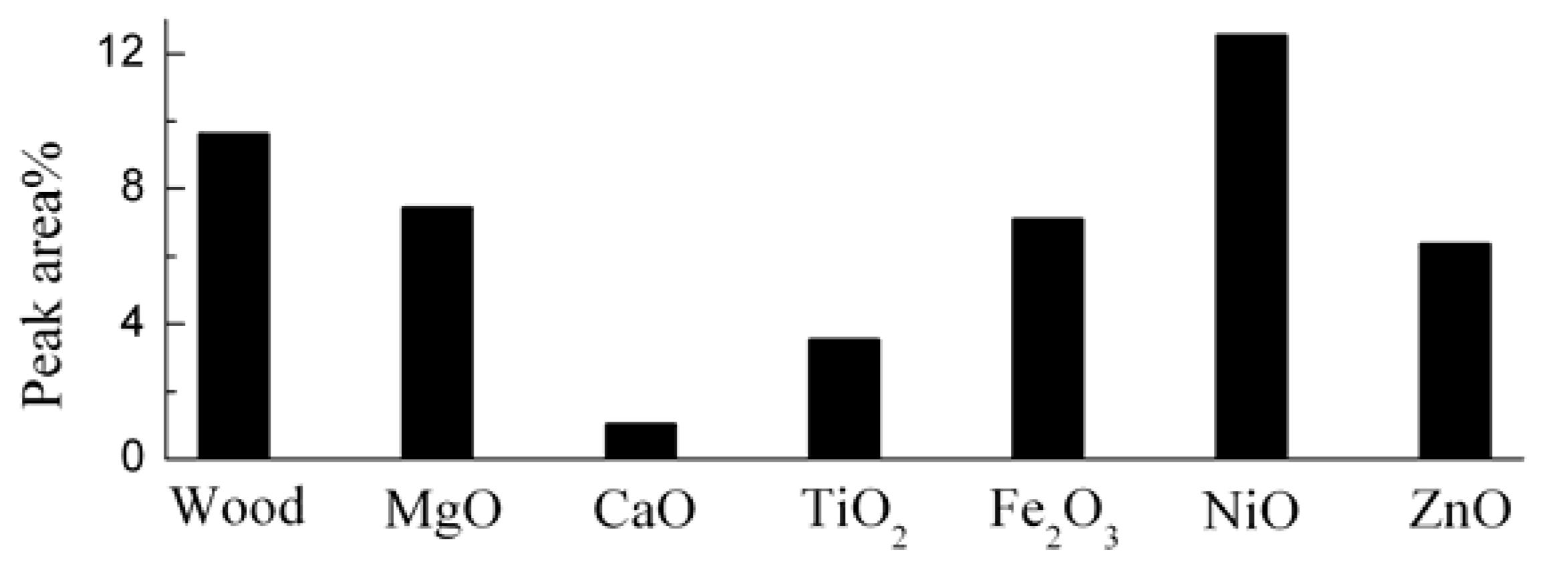
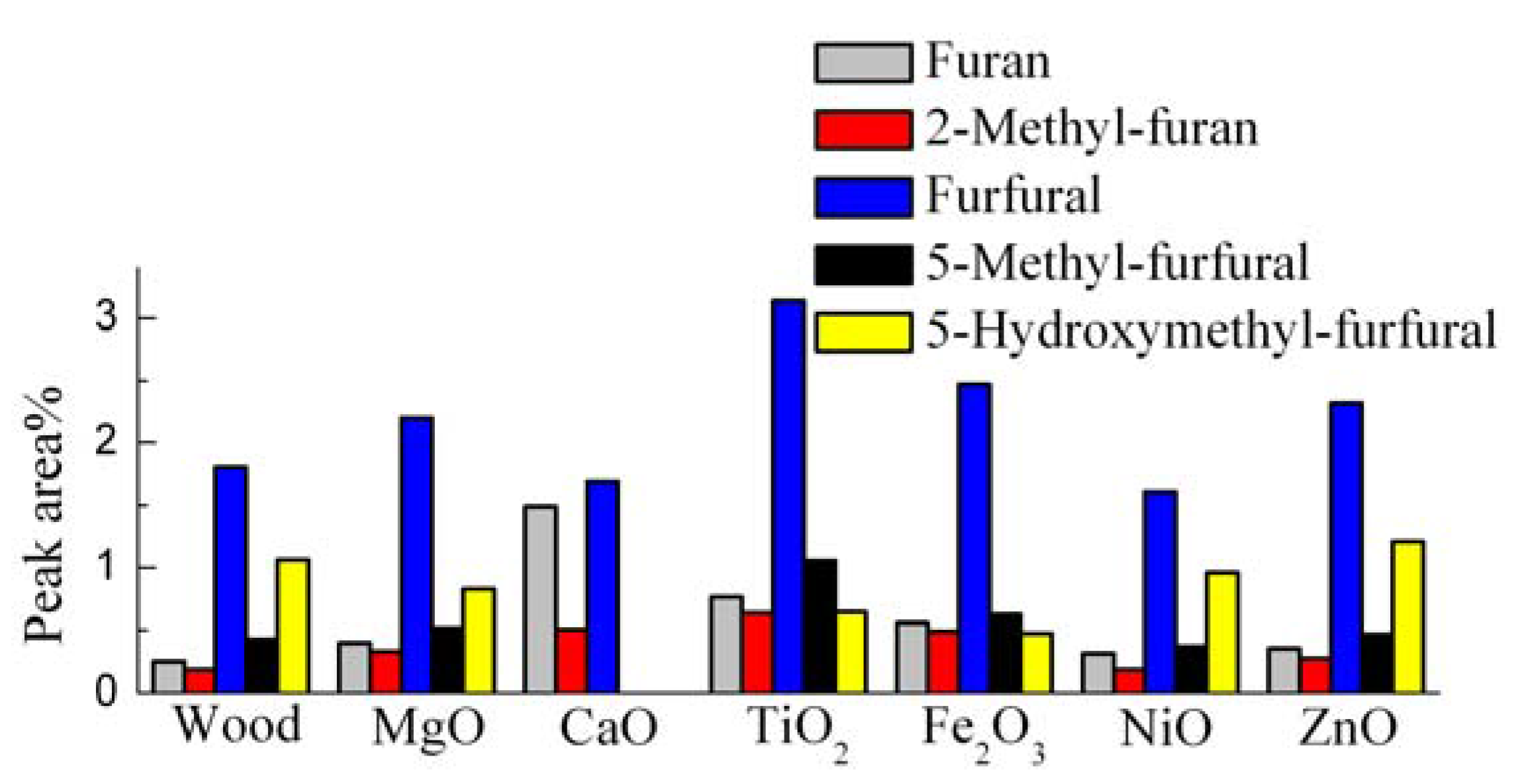
3.2. Catalytic Effects on the Levoglucosan and Furan Compounds
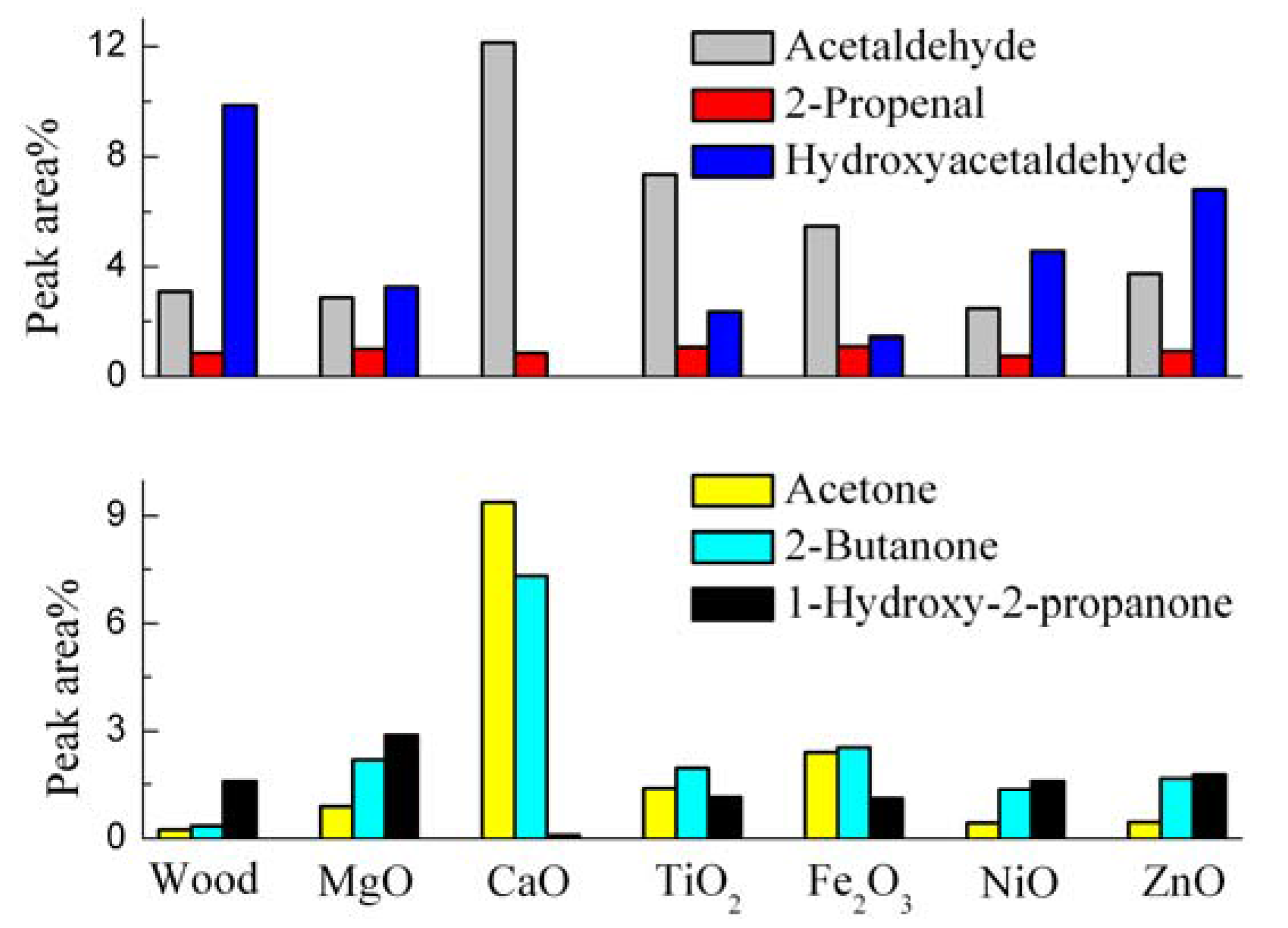
3.3. Catalytic Effects on the Linear Carbonyl Compounds
3.4. Catalytic Effects on the Phenolic Compounds
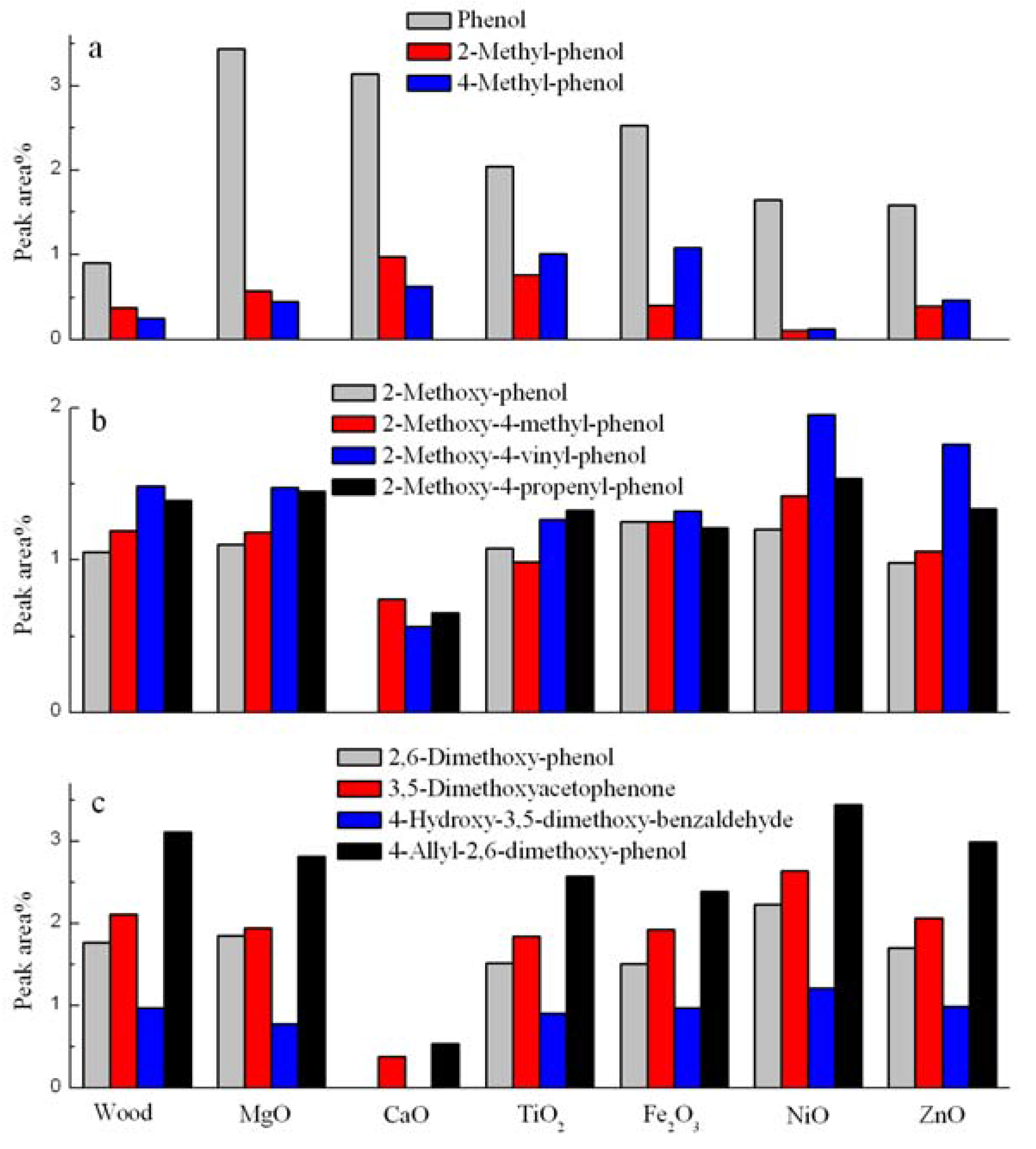
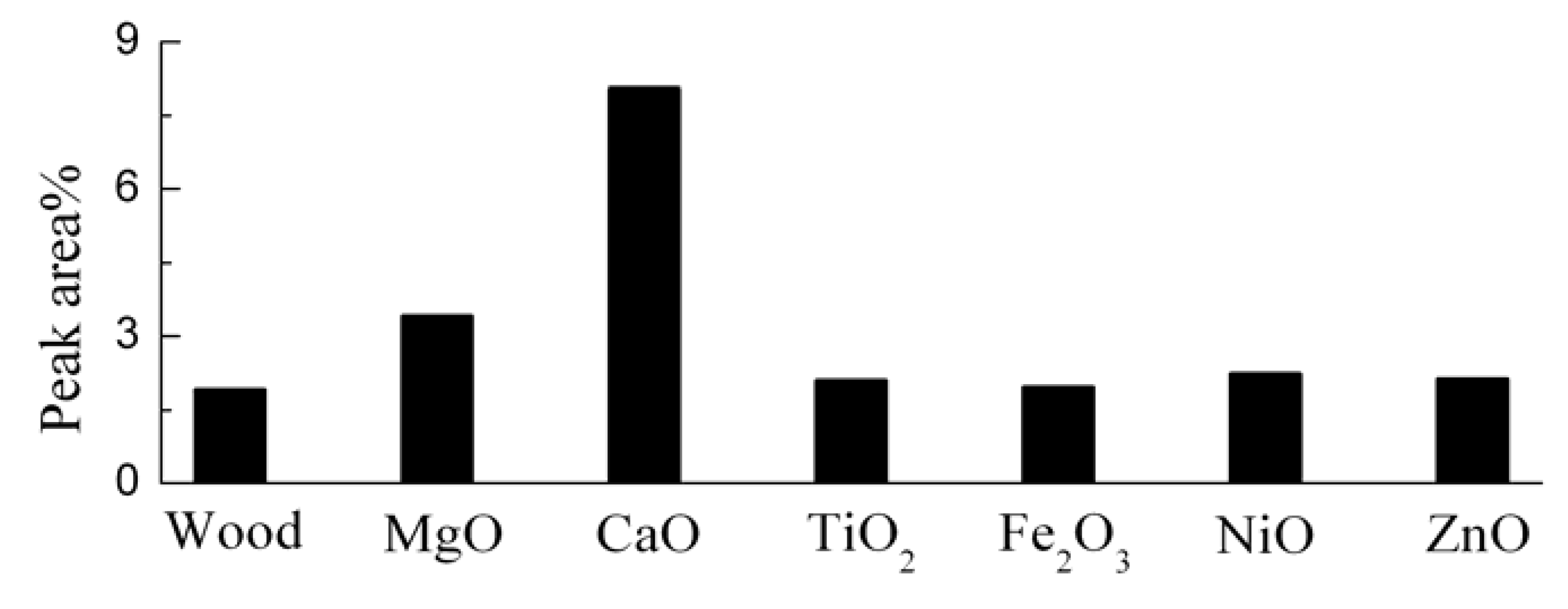
3.5. Catalytic Effects on the Acid and Alcohol Compounds

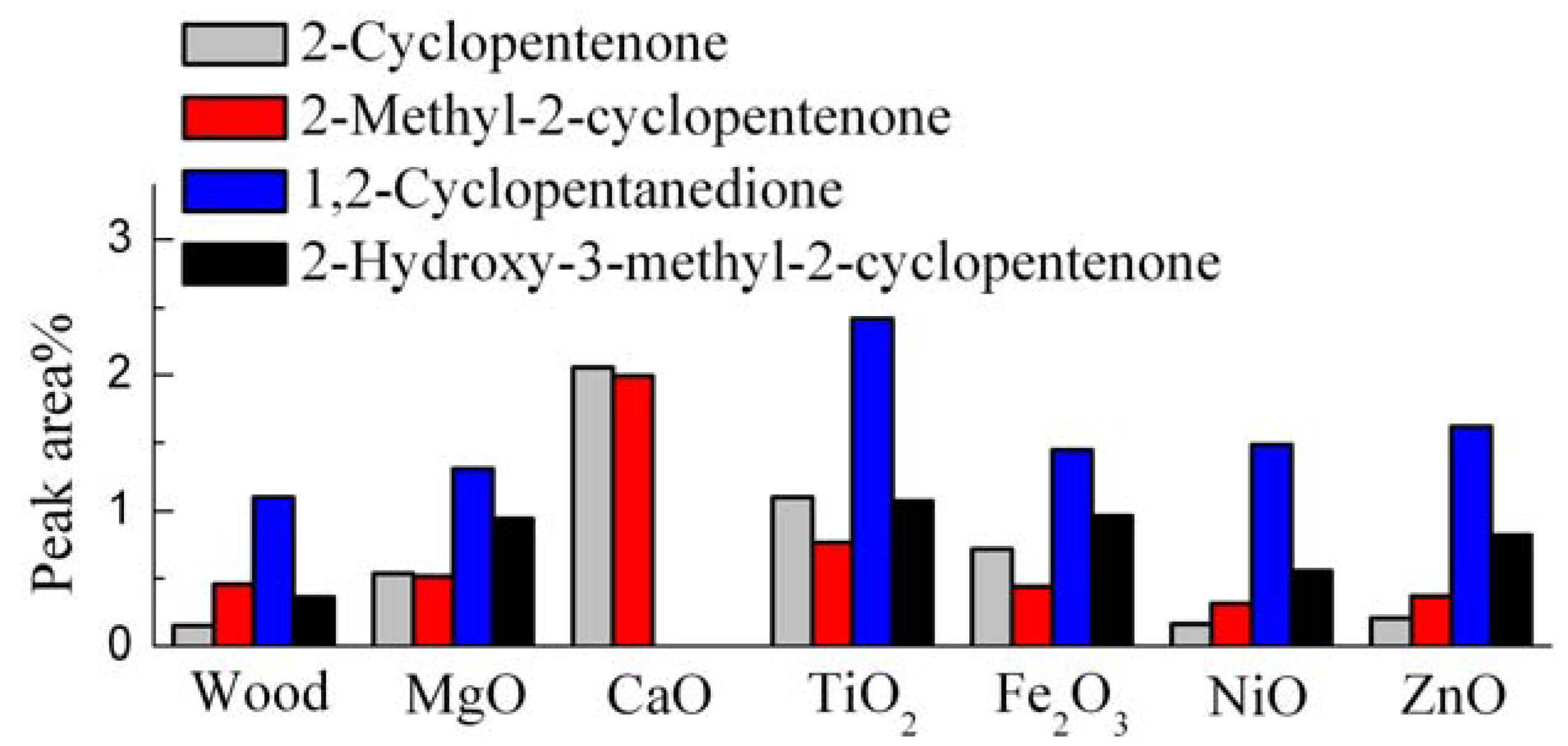
3.6. Catalytic Effects on the Hydrocarbons and Cyclopentanones
4. Conclusions
Acknowledgements
References
- Bridgwater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Oasmaa, A.; Czernik, S. Fuel oil quality of biomass pyrolysis oils—State of the art for the end user. Energy Fuels 1999, 13, 914–921. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.Z.; Zhu, X.F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manage. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Oasmaa, A.; Solantausta, Y. Power generation using fast pyrolysis liquids from biomass. Renewable Sustainable Energy Rev. 2007, 11, 1056–1086. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Bakhshi, N.N. Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Part II: Comparative catalyst performance and reaction pathways. Fuel Process. Technol. 1995, 45, 185–202. [Google Scholar]
- Vitolo, S.; Bresci, B.; Seggiani, M.; Gallo, M.G. Catalytic upgrading of pyrolytic oils over HZSM-5 zeolite: Behaviour of the catalyst when used in repeated upgrading-regenerating cycles. Fuel 2001, 80, 17–26. [Google Scholar] [CrossRef]
- Adam, J.; Blazso, M.; Meszaros, E.; Stocker, M.; Nilsen, M.H.; Bouzga, A.; Hustad, J.E.; Gronli, M.; Oye, G. Pyrolysis of biomass in the presence of Al-MCM-41 type catalysts. Fuel 2005, 84, 1494–1502. [Google Scholar]
- Adam, J.; Antonakou, E.; Lappas, A.; Stocker, M.; Nilsen, M.H.; Bouzga, A.; Hustad, J.E.; Oye, G. In situ catalytic upgrading of biomass derived fast pyrolysis vapours in a fixed bed reactor using mesoporous materials. Microporous Mesoporous Mater. 2006, 96, 93–101. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Iliopoulou, E.F.; Antonakou, E.V.; Lappas, A.A.; Wang, H.; Pinnavaia, T.J. Hydrothermally stable mesoporous aluminosilicates (MSU-S) assembled from zeolite seeds as catalysts for biomass pyrolysis. Microporous Mesoporous Mater. 2007, 99, 132–139. [Google Scholar] [CrossRef]
- Pattiya, A.; Titiloye, J.O.; Bridgwater, A.V. Fast pyrolysis of cassava rhizome in the presence of catalysts. J. Anal. Appl. Pyrolysis 2008, 81, 72–79. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.Z.; Zhang, D.; Zhu, X.F. Analytical pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) of sawdust with Al/SBA-15 catalysts. J. Anal. Appl. Pyrolysis 2009, 84, 131–138. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, Z.; Zhang, Y.; Zhu, X.F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Pd/SBA-15 Catalysts. Ind. Eng. Chem. Res. 2010, 49, 2573–2580. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Dobele, G.; Dizhbite, T.; Rossinskaja, G.; Telysheva, G.; Mier, D.; Radtke, S.; Faix, O. Pre-treatment of biomass with phosphoric acid prior to fast pyrolysis—A promising method for obtaining 1,6-anhydrosaccharides in high yields. J. Anal. Appl. Pyrolysis 2003, 68–69, 197–211. [Google Scholar]
- Fabbri, D.; Torri, C.; Mancini, I. Pyrolysis of cellulose catalysed by nanopowder metal oxides: Production and characterisation of a chiral hydroxylactone and its role as building block. Green Chem. 2007, 9, 1374–1379. [Google Scholar] [CrossRef]
- Chen, M.Q.; Wang, J.; Zhang, M.X.; Chen, M.G.; Zhu, X.F.; Min, F.F.; Tan, Z.C. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Lu, Q.; Xiong, W.M.; Li, W.Z.; Guo, Q.X.; Zhu, X.F. Catalytic pyrolysis of cellulose with sulfated metal oxides: A promising method for obtaining high yield of light furan compounds. Bioresour. Technol. 2009, 100, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Q.; Chen, P.; Zhang, B.; Yang, C.Y.; Liu, Y.H.; Lin, X.Y.; Ruan, R. Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Li, J.F.; Yan, R.; Xiao, B.; Liang, D.T.; Lee, D.H. Preparation of nano-NiO particles and evaluation of their catalytic activity in pyrolyzing biomass components. Energy Fuels 2008, 22, 16–23. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Tang, Z.; Li, W.; Zhu, X. Catalytic upgrading of biomass fast pyrolysis vapors with titania and zirconia/titania based catalysts. Fuel 2010, 89, 2096–2103. [Google Scholar] [CrossRef]
- Ranganathan, S.; Macdonald, D.G.; Bakhshi, N.N. Kinetic studies of wheat straw hydrolysis using sulphuric acid. Can. J. Chem. Eng. 1985, 63, 840–844. [Google Scholar] [CrossRef]
- Branca, C.; Giudicianni, P.; Di Blasi, C. GC/MS characterization of liquids generated from low-temperature pyrolysis of wood. Ind. Eng. Chem. Res. 2003, 42, 3190–3202. [Google Scholar] [CrossRef]
- Marsman, J.H.; Wildschut, J.; Mahfud, F.; Heeres, H.J. Identification of components in fast pyrolysis oil and upgraded products by comprehensive two-dimensional gas chromatography and flame ionisation detection. J. Chromatogr. A 2007, 1150, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.A.; Boateng, A.A. Chemical composition of bio-oils produced by fast pyrolysis of two energy crops. Energy Fuels 2008, 22, 2104–2109. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, X.F.; Li, W.Z.; Zhang, Y.; Chen, D.Y. On-line catalytic upgrading of biomass fast pyrolysis products. Chin. Sci. Bull. 2009, 54, 1941–1948. [Google Scholar] [CrossRef]
- Nokkosmaki, M.I.; Kuoppala, E.T.; Leppamaki, E.A.; Krause, A.O.I. Catalytic conversion of biomass pyrolysis vapours with zinc oxide. J. Anal. Appl. Pyrolysis 2000, 55, 119–131. [Google Scholar] [CrossRef]
- Piskorz, J.; Radlein, D.; Scott, D.S. On the mechanism of the rapid pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 1986, 9, 121–137. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Lai, Y.Z. Thermal degradation of 1,6-anhydro-b-D-glucopyranose. J. Org. Chem. 1972, 37, 278–284. [Google Scholar] [CrossRef]
- Scholze, B.; Meier, D. Characterization of the water-insoluble fraction from pyrolysis oil (pyrolytic lignin). Part I: PY-GC/MS, FTIR, and functional groups. J. Anal. Appl. Pyrolysis 2001, 60, 41–54. [Google Scholar] [CrossRef]
- Scholze, B.; Hanser, C.; Meier, D. Characterization of the water-insoluble fraction from fast pyrolysis liquids (pyrolytic lignin). Part II: GPC, carbonyl goups, and C-13-NMR. J. Anal. Appl. Pyrolysis 2009, 85, 98–107. [Google Scholar]
- Bayerbach, R.; Nguyen, V.D.; Schurr, U.; Meier, D. Characterization of the water-insoluble fraction from fast pyrolysis liquids (pyrolytic lignin). Part III: Molar mass characteristics by SEC, MALDI-TOF-MS, LDI-TOF-MS, and Py-FIMS. J. Anal. Appl. Pyrolysis 2006, 77, 95–101. [Google Scholar]
- Bayerbach, R.; Meier, D. Characterization of the water-insoluble fraction from fast pyrolysis liquids (pyrolytic lignin). Part IV: Structure elucidation of oligomeric molecules. J. Anal. Appl. Pyrolysis 2009, 85, 98–107. [Google Scholar] [CrossRef]
- Himmelblau, A. Method and apparatus for producing water-soluble resin and resin product made by that method. U.S. Patent 5034498, 23 July 1991. [Google Scholar]
- Roy, C.; Lu, X.; Pakdel, H. Process for the production of phenolic-rich pyrolysis oils for use in making phenol-formaldehyde resole resins. U.S. Patent 6143856, 7 November 2000. [Google Scholar]
- Oasmaa, A.; Kuoppala, E.; Selin, J.F.; Gust, S.; Solantausta, Y. Fast pyrolysis of forestry residue and pine. 4. Improvement of the product quality by solvent addition. Energy Fuels 2004, 18, 1578–1583. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, Q.; Zhang, Z.-F.; Dong, C.-Q.; Zhu, X.-F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study. Energies 2010, 3, 1805-1820. https://doi.org/10.3390/en3111805
Lu Q, Zhang Z-F, Dong C-Q, Zhu X-F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study. Energies. 2010; 3(11):1805-1820. https://doi.org/10.3390/en3111805
Chicago/Turabian StyleLu, Qiang, Zhi-Fei Zhang, Chang-Qing Dong, and Xi-Feng Zhu. 2010. "Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study" Energies 3, no. 11: 1805-1820. https://doi.org/10.3390/en3111805
APA StyleLu, Q., Zhang, Z.-F., Dong, C.-Q., & Zhu, X.-F. (2010). Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study. Energies, 3(11), 1805-1820. https://doi.org/10.3390/en3111805





