Simulation and Optimization of Biomass Gasification Process in Fluidized Bed Coupled with Entrained-Flow Bed
Abstract
1. Introduction
- To model the complex gasification process, the well-established Restricted Chemical Equilibrium approach was employed using the RGIBBS reactors in Aspen Plus V12. This method is particularly useful for approximating kinetically limited systems. In this work, it was calibrated and applied to the novel coupled-bed configuration to enable a systematic analysis of parameter interactions. The novelty of the study, therefore, stems from the new insights into the operation and optimization of this specific process, rather than from the thermodynamic method itself.
- System-Specific Parameter Interaction and Matching: While the concepts of staging and equilibrium modeling are established, this work aims to provide the first model-based, quantitative answers to the following open questions for the FB-EFB configuration: (1) What is the optimal distribution of oxygen between the beds? (2) At what temperatures do further increases yield negligible benefits? This study thereby delivers novel, actionable insights for the design and operation of this specific advanced gasification system. The core novelty is the investigation of the “matching relationship” and interaction between the two reactors. The manuscript systematically analyzes how operating parameters (like the oxygen distribution ratio between the beds) uniquely affect the overall system performance, which is a critical design and operational consideration not explored in conventional single-reactor or similar dual-bed systems. The finding that the entrained-flow bed is significantly more sensitive to the oxygen distribution ratio than the fluidized bed provides crucial new insights for optimizing this specific configuration. This study aims to establish an accurate process model using Aspen Plus to systematically investigate the effects of pressure, equivalence ratio (ER), CO2/biomass ratio, H2O/biomass ratio, oxygen distribution ratio, fluidized bed gasification temperature, and entrained-flow bed gasification temperature, tar conversion efficiency on gas composition, gas yield, gas low heating value (LHV), and cold gas efficiency. This will reveal the intrinsic mechanisms, determine the optimal parameter range, and thereby provide direct guidance for the efficient design and operational control strategies of industrial-scale gasification units.
2. Experiment and Model
2.1. Experiment
2.2. Model Establishment
2.2.1. Model Assumptions
- (1)
- The process operates under steady-state conditions, with the system maintaining constant temperature and pressure.
- (2)
- The assumption of instantaneous pyrolysis, while a simplification, is adopted as it is computationally efficient and appropriate for a steady-state equilibrium model focused on the final gasification products (H2, CO, CO2, CH4, and H2O) rather than transient pyrolysis kinetics. Given the high operating temperatures (≥800 °C) and the turbulent environment of the fluidized bed, the characteristic devolatilization time for the biomass particles is considered short compared to their residence time, making this a reasonable first-order approximation Tar is generated in the fluidized bed via the RYield block but does not react, with its yield proportional to the carbon content of the fed biomass. Tar is modeled to be partly cracked (ηtar) into H2, O2, N2, and Cin the high-temperature entrained-flow bed simulated by the RYield reactor. These effects were further conducted in the support information. This represents an ideal scenario and is justified by the design purpose of this stage, which is to achieve near-complete tar conversion at temperatures exceeding 1200 °C. This assumption allows the model to focus on the gas-phase equilibrium and provides an optimistic benchmark for syngas quality, though it may lead to a slight over-prediction of light gas yields compared to an actual system with trace residual tar.
- (3)
- Most elements entering into the gas phase, including C, H, O, N, S, and Cl, some unconverted (e.g., C) ones were entered into solid (slag) or tar. Ash is considered inert and does not participate in reactions, neglecting the catalytic effects of alkali and alkaline earth metals in ash.
- (4)
- The heat loss of the gasifier is set to 5% of the lower heating value (LHV) of the biomass, an estimate based on values reported for similar industrial-scale, insulated fluidized-bed gasifiers [5,13]. While this value is fixed for simplicity, it provides a realistic and consistent basis for evaluating the energy balance across all simulated cases. These values were further modified according to the real operation data.
- (5)
- Key reactions involved in the gasification process (including the steam gasification reaction C + H2O→CO + H2, the carbon dioxide reduction reaction C + CO2 → 2CO, and the water-gas shift reaction CO + H2O ⇌ CO2 + H2) all reach thermodynamic equilibrium;
- (6)
- The particle size is assumed constant, which is a simplification that neglects fragmentation and attrition with particle size distribution. This is deemed acceptable because the core of the model relies on thermodynamic equilibrium in the RGibbs blocks, which are not directly dependent on particle size. This assumption allows the model to focus on chemical equilibrium without the added complexity of evolving particle properties. The neglect of internal and external diffusion limitations aligns with the equilibrium modeling framework, which prioritizes thermodynamic limits over kinetic constraints. This is a standard approach for preliminary screening and optimization studies. It is acknowledged that this may lead to optimistic predictions of carbon conversion, particularly for larger biomass particles, but it is considered acceptable for a system-level analysis aimed at identifying overarching trends.
2.2.2. Aspen Model
2.2.3. Chemical Reactions
- Core Gasification Reactions (R1–R4): These reactions (Boudouard, steam gasification, combustion, and methanation) are essential for modeling the main conversion of carbon, hydrogen, and oxygen.
- Trace-Element Constraints (R5–R10): Reactions involving NH3, H2S, Cl2, HCN, and COS are included not as dominant pathways, but to ensure proper elemental balance of N, S, and Cl from the biomass feedstock into realistic gaseous species (e.g., NH3, H2S, HCl). Their high equilibrium temperatures (set at 1000 °C with a +100 °C approach) intentionally limit their forward progress, which is consistent with their minor yields under high-temperature gasification conditions.
- The tar component was complex, which made it difficult to consider in the model. Its decomposition component was simplified into H2, CO, CO2, CH4, H2O and char. The methane was mainly formed from the pyrolysis. After many continuous attempts, the methane generated by C-H2 was considered.
2.2.4. Rationale, Impact, and Limitations of Key Assumptions
- (1)
- Instantaneous pyrolysis
- (2)
- Total neglect of hydrodynamics and kinetics
- (3)
- Fixed heat losses
3. Results and Discussion
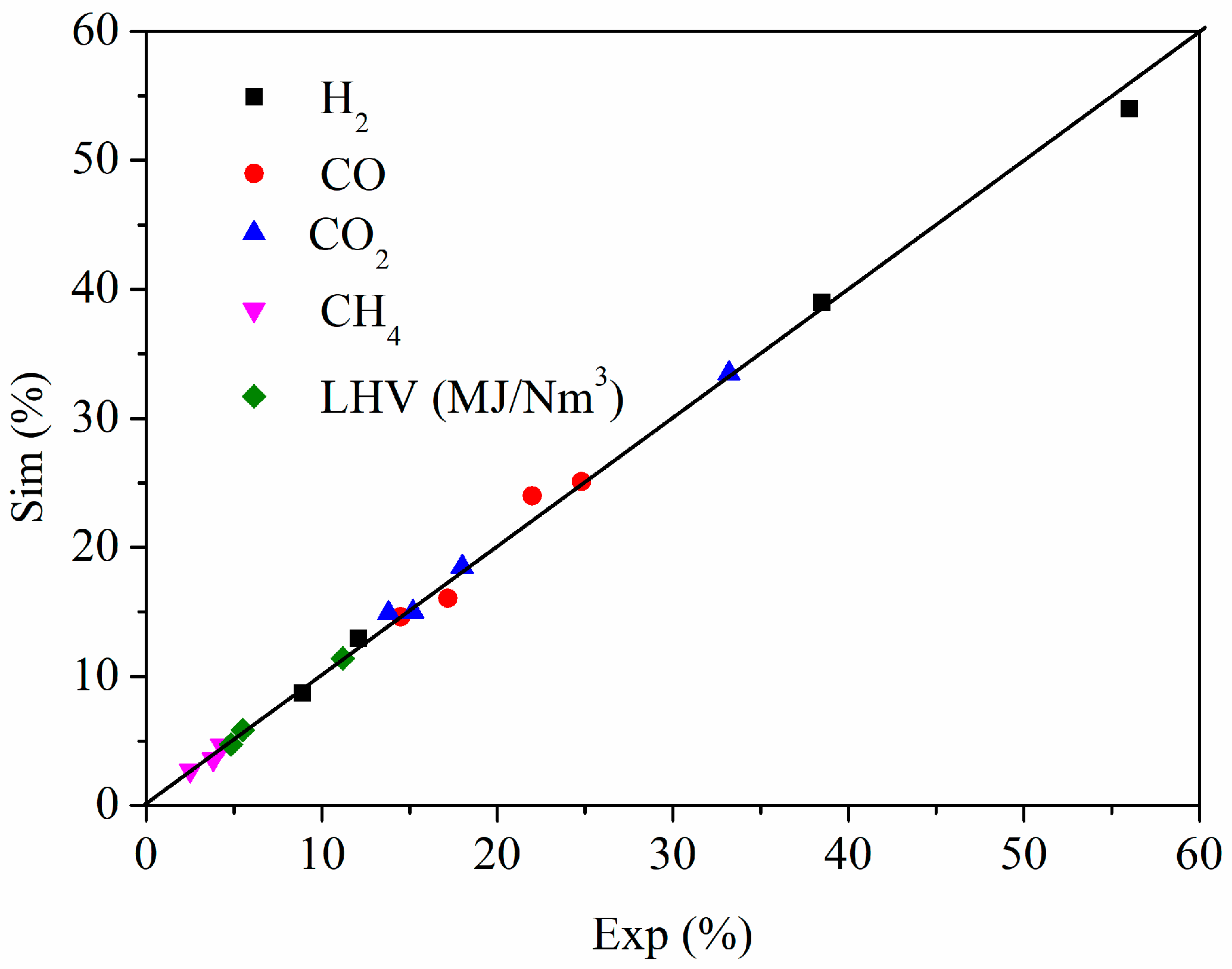
3.1. Model Validation
3.2. Model Predictions
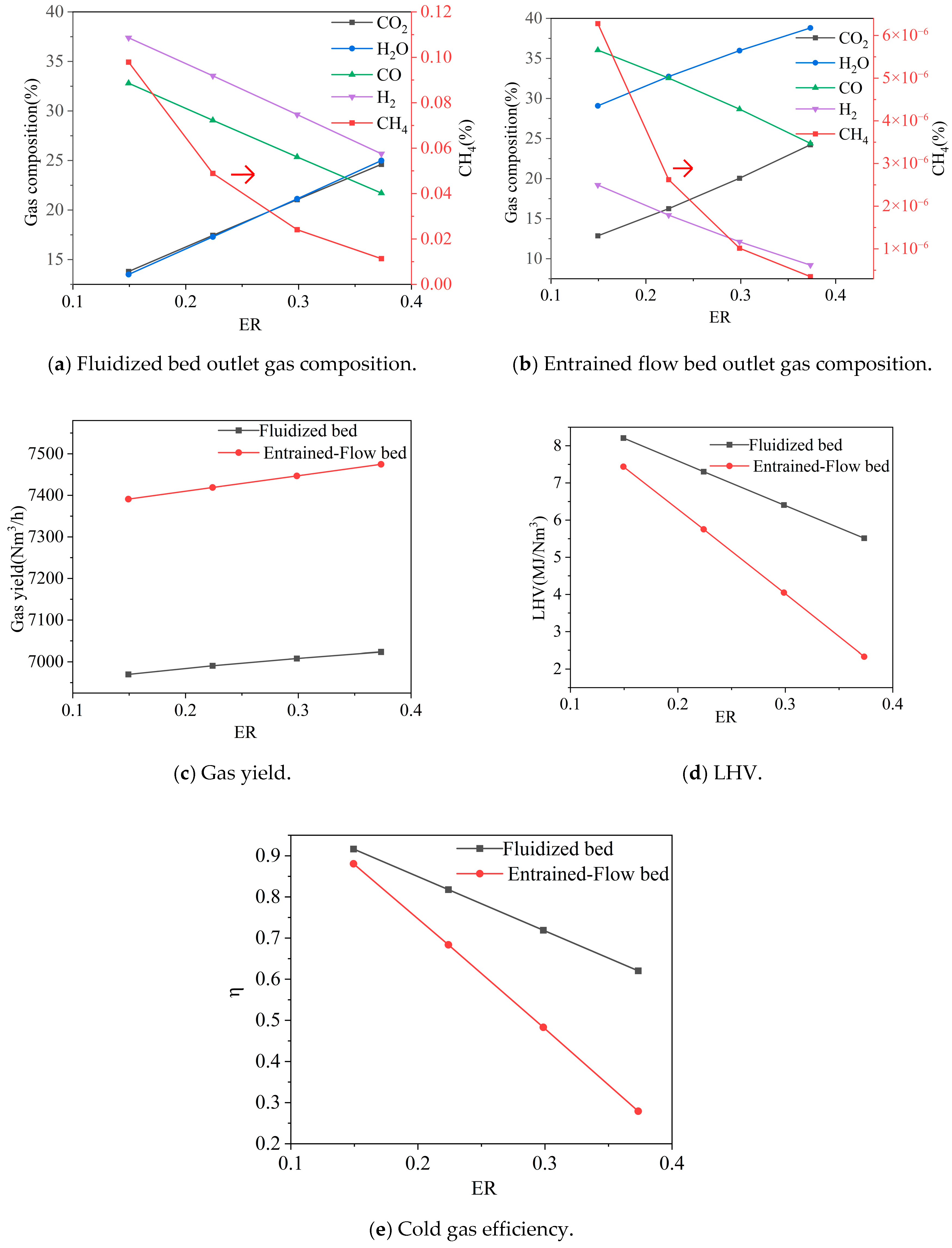
3.2.1. Equivalence Ratio (ER)
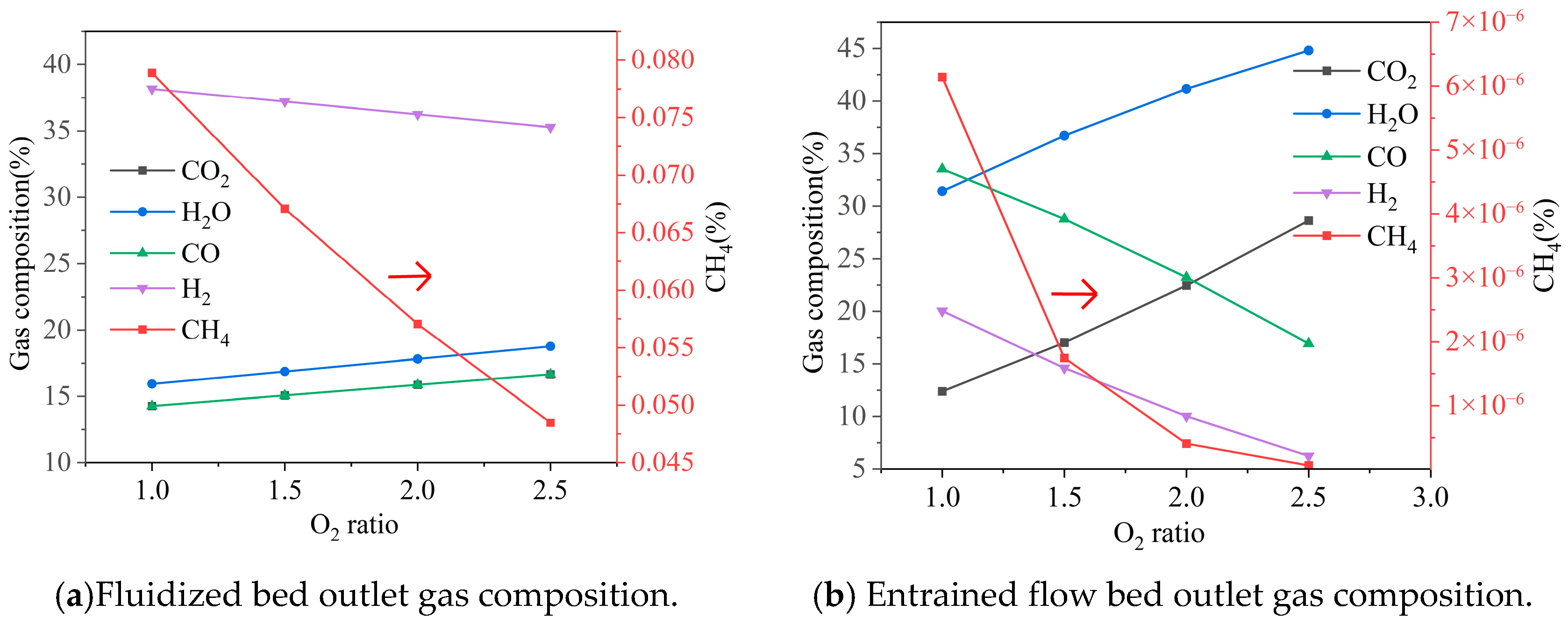
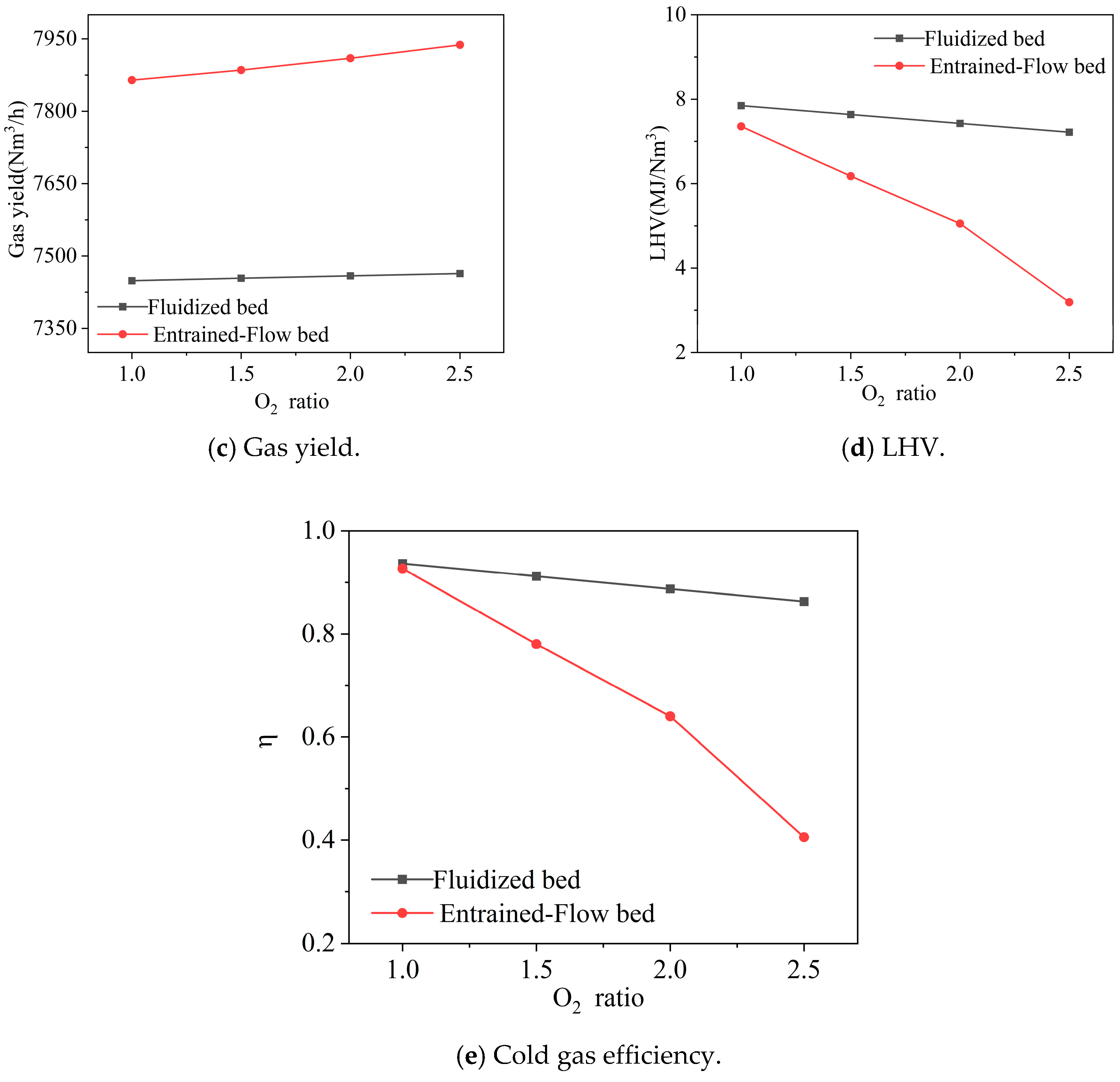
3.2.2. Oxygen Distribution Ratio
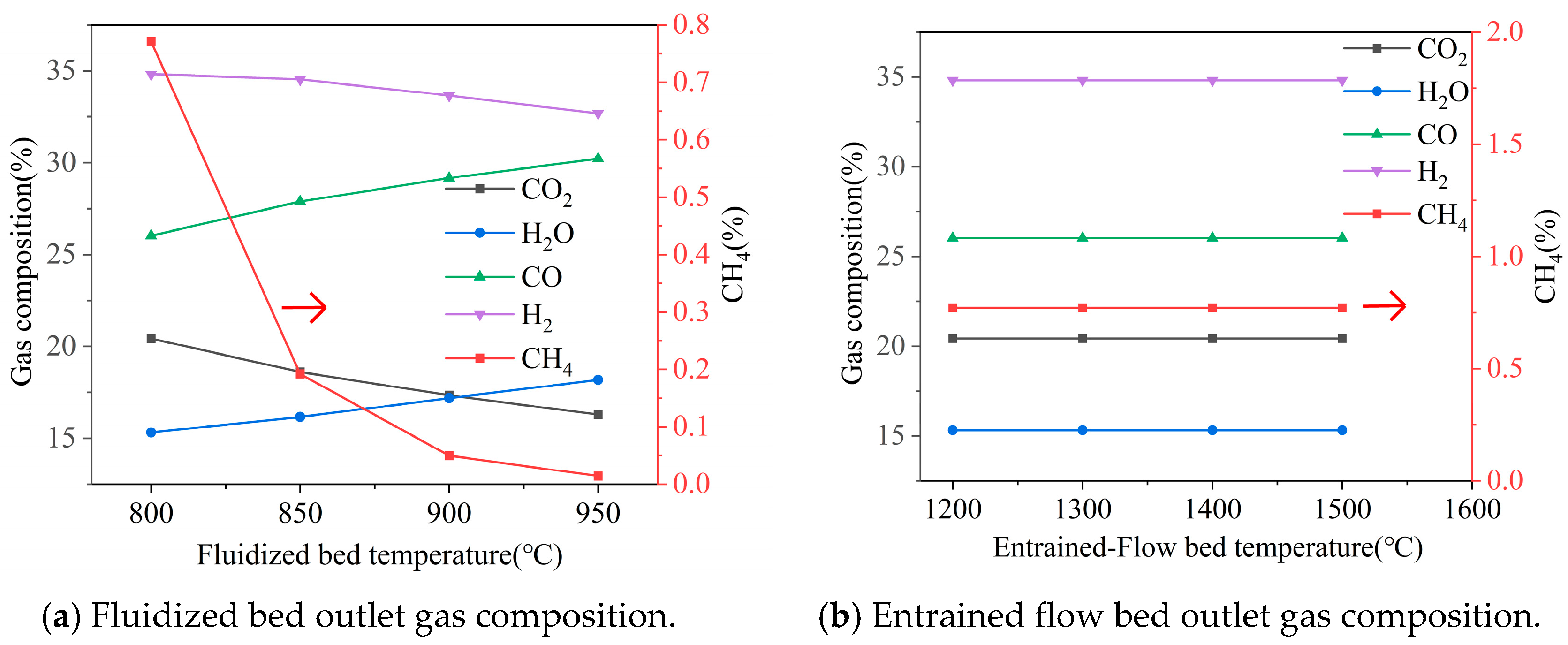
3.2.3. Effect of Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kartal, F.; Ozveren, U. A comparative study for biomass gasification in bubbling bed gasifier using Aspen HYSYS. Bioresour. Technol. Rep. 2021, 13, 100615. [Google Scholar] [CrossRef]
- Kaushal, P.; Tyagi, R. Advanced simulation of biomass gasification in a fluidized bed reactor using ASPEN PLUS. Renew. Energy 2017, 101, 629–636. [Google Scholar] [CrossRef]
- Hoo, K.K.; Said, M.S.M. Air gasification of empty fruit bunch: An Aspen Plus model. Bioresour. Technol. Rep. 2021, 16, 100848. [Google Scholar] [CrossRef]
- Jadoon, U.K.; Díaz, I.; Rodrígue, M. Comparative analysis of aspen plus simulation strategies for woody biomass air gasification processes. Biomass Bioenergy 2025, 194, 107626. [Google Scholar] [CrossRef]
- Mahapatro, A.; Mahanta, P. Gasification studies of low-grade Indian coal and biomass in a lab scale pressurized circulating fluidized bed. Renew. Energy 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Gao, N.B.; Chen, C.X.; Magdziarz, A.; Zhang, L.H.; Quan, C. Modeling and simulation of pine sawdust gasification considering gas mixture reflux. J. Anal. Appl. Pyrolysis 2021, 155, 105094. [Google Scholar] [CrossRef]
- Fan, H.D.; Zhang, H.; Li, G.; Zhang, X.; Luo, Z.X.; Huang, L.; Zhang, S.H. Modeling of industrial biomass fluidized bed gasification system and optimization of operating conditions. Biomass Bioenergy 2026, 204, 108403. [Google Scholar] [CrossRef]
- Shi, H.; Huang, Y.J.; Qiu, Y.Z.; Zhang, J.; Li, Z.Y.; Song, H.K.; Tang, T.H.; Xiao, Y.X.; Liu, H. Modelling of biomass gasification for fluidized bed in Aspen Plus: Using machine learning for fast pyrolysis prediction. Energy Convers. Manag. 2025, 332, 119695. [Google Scholar] [CrossRef]
- Castro, J.; Leaver, J.; Pang, S. Modelling of enhanced dual fluidized bed steam gasification with integration of biomass-specific devolatilization. Biomass Bioenergy 2026, 204, 108445. [Google Scholar] [CrossRef]
- De, S.; Yadav, S.; Singh, D.; Sistla, Y.S. Simplified approach of modeling air gasification of biomass in a bubbling fluidized bed gasifier using Aspen Plus as a simulation tool. J. Environ. Chem. Eng. 2025, 13, 115402. [Google Scholar] [CrossRef]
- Hoo, K.K.; Said, M.S.M. Simulation of air gasification of Napier grass using Aspen Plus. Sustain. Energy Technol. Assess. 2022, 50, 101837. [Google Scholar] [CrossRef]
- Pauls, J.H.; Mahinpey, N.; Mostafavi, E. Simulation of air-steam gasification of woody biomass in a bubbling fluidized bed using Aspen Plus: A comprehensive model including pyrolysis, hydrodynamics and tar production. Biomass Bioenergy 2016, 95, 157–166. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Pio, D.T.; Tarelho, L.A.C.; Sanchez, P.; Sanchez-Silva, L. Simulation of biomass gasification in bubbling fluidized bed reactor using aspen plus®. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpey, N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Yang, S.G.; Zhao, J.L.; Zhang, C.H.; Liu, J.; Zhang, Z.X.; Lu, Q. Process simulation of biomass gasification for hydrogen production with different agents. Clean Coal Technol. 2025, 31, 50–60. [Google Scholar]
- Liu, L.Q. Experimental Research and Simulation Based on Aspen Plus of Biomass Gasification in Fluidized Bed. Master’s Thesis, Southeast University, Nanjing, China, 2016. [Google Scholar]
- Si, W.F. Research on Biomass Staged Gasification Characteristics in a Dual Circulated Fluidized Bed. Master’s Thesis, Zhejiang University, Hangzhou, China, 2018. [Google Scholar]
- Wang, Z.Q. Experiment and Numerical Simulation on Gasification Behavior of Biomass in Quadruple Fluidized Bed. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2021. [Google Scholar]
- Zhou, X.P. Research on Gasification/Co-Gasification Characteristics and Mechanism of Coal, Waste and Other Carbonaceous Materials. Ph.D. Thesis, East China University of Science and Technology, Shanghai, China, 2009. [Google Scholar]
- Sun, H.Y. Influence of Gasification Parameters Research on Active Air Distribution Fixed-Bed Biomass Gasifier. Ph.D. Thesis, Shandong University, Jinan, China, 2011. [Google Scholar]
- Kuttin, K.W.; Salem, A.M.; Kuttin, A.W.; Ding, L.; Yu, G.S. Nonlinear numerical modelling assessment of pressurized biomass downdraft fixed bed gasification process with experimental validation. Fuel 2025, 397, 135364. [Google Scholar] [CrossRef]
- Berrueco, C.; Recari, J.; Güell, B.M.; del Alamo, G. Pressurized gasification of torrefied woody biomass in a lab scale fluidized bed. Energy 2014, 70, 68–78. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.H.; Long, Z.Y.; Rong, N.; Deng, G.Y. H2 rich gas production via pressurized fluidized bed gasification of sawdust with in situ CO2 capture. Appl. Energy 2013, 109, 36–43. [Google Scholar] [CrossRef]
- Fermoso, J.; Arias, B.; Gil, M.V.; Plaza, M.G.; Pevida, C.; Pis, J.J.; Rubiera, F. Co-gasification of different rank coals with biomass and petroleum coke in a high-pressure reactor for H2-rich gas production. Bioresour. Technol. 2010, 101, 3230–3235. [Google Scholar] [CrossRef]
- Nikitin, A.D.; Abaimov, N.A.; Ershov, M.I.; Tuponogov, V.G.; Simbiryatin, L.V.; Ryzhkov, A.F.; Alekseenko, S.V. Investigation of air-blown biomass gasification in a pilot setup. Thermophys. Aeromech. 2025, 32, 171–178. [Google Scholar] [CrossRef]
- Matsumoto, K. An entrained flow biomass gasification technology with the fluidized bed concept for low-carbon fuel production. Appl. Energy Combust. Sci. 2024, 20, 100292. [Google Scholar] [CrossRef]
- Yan, L.B.; Lim, C.J.; Yue, G.X.; He, B.; Grace, J.R. One-dimensional modeling of a dual fluidized bed for biomass steam. Gasif. Energy Convers. Manag. 2016, 127, 612–622. [Google Scholar] [CrossRef]
- Hashemisohi, A.; Wang, L.; Shahbazi, A. Numerical analysis of biomass gasification in a fluidized bed reactor using a computational fluid dynamics model integrated with reduced-order reaction kinetics. Chem. Eng. Res. Des. 2024, 206, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Hu, C.; Zheng, Y.Z.; Jin, H.; Wu, Z. Predictive control of reactor network model using machine learning for hydrogen-rich gas and biochar poly-generation by biomass waste gasification in supercritical water. Energy 2023, 282, 128441. [Google Scholar] [CrossRef]
- Kuttin, K.W.; An, F.B.; Richter, A.; Yu, G.S.; Wang, Y.F.; Wang, F.C.; Ding, L. CFD modelling of hydrothermal carbonized biomass pellets gasification: Synergistic effects of pellets size and moisture content on gasification efficiency. Int. J. Hydrogen Energy 2024, 84, 420–434. [Google Scholar] [CrossRef]



| Proximate Analysis (wt%, Dry) | Ultimate Analysis (wt%, daf) | |||||||
|---|---|---|---|---|---|---|---|---|
| FC | A | VM | C | H | O | N | S | Cl |
| 17.57 | 7.88 | 74.55 | 45.83 | 5.43 | 39.2 | 0.6 | 0.06 | 1 |
| Item | Value |
|---|---|
| Fluidized bed temperature (T, °C) | 800~1000 |
| Fluidized bed pressure (P, MPa) | 0.1~3 |
| Entrained flow bed temperature (T, °C) | 1200~1500 |
| Entrained flow bed pressure (P, MPa) | 0.1~3 |
| Feed amount of biomass (t/h) | 4 |
| Average particle size (mm) | 5–10 |
| ER | 0.2–0.6 |
| CO2/biomass | 0.1–0.5 |
| H2O/biomass | 0.5–0.8 |
| Chemical Reaction | Temperature Difference (°C) | |
|---|---|---|
| R1 | C + O2 → CO2 | −141 |
| R2 | 2C + O2 → 2CO | −195 |
| R3 | C + H2O → CO + H2 | −40 |
| R4 | C + 2H2 → CH4 | −200 |
| R5 | N2 + 3H2 → 2NH3 | 100 |
| R6 | CO + H2S → COS + H2 | 100 |
| R7 | C + NH3 → HCN + H2 | 100 |
| R8 | 2CH4 → C2H6 + H2 | 100 |
| R9 | H2 + Cl2 → 2HCl | 100 |
| R10 | S + H2 → H2S | 100 |
| Models | Description | Input Data | Equations | Numerical Methods |
|---|---|---|---|---|
| RSTOIC | Simulates the drying of the raw biomass feedstock. | T = 280 °C, P = 0.1–3 bar Conversion = 100% | Outlet(i) = Inlet(i) + Stoich Coeff(i) × Extent | Direct material balance calculation; no iteration required |
| RYIELD1 | Models the instantaneous, non-equilibrium pyrolysis/devolatilization of dried biomass into conventional components and a lumped tar pseudo-component. | T = 280 °C, P = 0.1–3 bar, specified via a component yield matrix calculated to satisfy the ultimate and proximate analysis. | Mass_Flow_Out(i) = Yield_Fraction(i) × Mass_Flow_In (Biomass) | Embedded Fortran calculator block that ensures atomic closure for C, H, O, N, S, Cl based on the input analyses |
| MIXER1 | Mixes the pyrolysis products from RYIELD1 with the primary gasifying agents (O2, CO2, H2O, N2) and the recycled gas stream GAS1. | Flow rates, composition, T, P of all inlet streams. | Σ(Ėnthalpy_In) = Ėnthalpy_Out and Σ(Mass_In) = Mass_Out for each component | Linear solving of material and energy balance equations |
| RGIBBS1 | Simulates the restricted chemical equilibrium in the fluidized-bed gasifier. | T = 800–1000 °C, P = 0.1–3 MPa. The equilibrium of the reactions listed in Table 3 is constrained. | Reduced Gradient Method, convergence tolerance of 1 × 10−4 | |
| SEP1 | Separates the RGIBBS1 effluent into a raw gas stream and a solid stream (SLAG1) containing unreacted carbon, ash, and tar. | All solid-phase and gas components. Recovery fraction of 1.0. | M_{out, i} = SF_i × M_{in, i} | Direct scalar multiplication |
| SEP2 | separates the tar (TAR) from the raw gas. | TAR Recovery fraction of 1.0. | Identical to SEP1 | Identical to SEP1 |
| RYIELD2 | Models the complete cracking of the separated tar in the entrained-flow section. | TAR → 1.0 × C(s) + 0.75 × H2 + 0.1 × O2. | Identical to RYIELD1 | Identical to RYIELD1 |
| MIXER2 | Mixes the cracked products from RYIELD2 with a secondary oxygen stream for the entrained-flow bed. | Similar to MIXER1 | Identical to MIXER1 | Identical to MIXER1 |
| RGIBBS2 | Simulates the final high-temperature equilibrium in the entrained-flow bed gasifier. | T = 1200–1500 °C, P = 0.1–3 MPa | Similar to RGIBBS1 | Identical to RGIBBS1 |
| SSPLIT | Final product separation. Splits the RGIBBS2 outlet into clean syngas and solid ash/residual carbon. | All gaseous components to SYNGAS, all solid components (C(s), Ash) to BOTTOM_ASH. | Identical to SEP1 | Identical to SEP1 |
| MIXER3 | Mixes a split fraction of the SLAG1 stream (semi-coke) from SEP1 with a tertiary gasifying agent (CO2, H2O) for bottom slag gasification. | A fixed 30% of the SLAG1 mass flow is recycled. The remainder is purged. | Identical to MIXER1 | Identical to MIXER1 |
| RGGIBBS3 | Simulates the gasification of the recycled semi-coke to improve carbon conversion. | T = 1200–1500 °C, P = 0.1–3 MPa. | Similar to RGIBBS1 | Identical to RGIBBS1 |
| SEP3 | Separates the RGIBBS3 effluent into the recycled gas GAS1 and the final waste slag SLAG2. | Perfect separation. All gases to GAS1, all solids to SLAG2. | Identical to SEP1 | Identical to SEP1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, J.; Liu, Z.; Zhang, H.; Huang, X.; Peng, B.; Chang, L.; Yang, R.; Li, W. Simulation and Optimization of Biomass Gasification Process in Fluidized Bed Coupled with Entrained-Flow Bed. Energies 2026, 19, 37. https://doi.org/10.3390/en19010037
Wang J, Liu Z, Zhang H, Huang X, Peng B, Chang L, Yang R, Li W. Simulation and Optimization of Biomass Gasification Process in Fluidized Bed Coupled with Entrained-Flow Bed. Energies. 2026; 19(1):37. https://doi.org/10.3390/en19010037
Chicago/Turabian StyleWang, Jingjing, Zhen Liu, Huimin Zhang, Xin Huang, Baozai Peng, Liang Chang, Ruihan Yang, and Weiwei Li. 2026. "Simulation and Optimization of Biomass Gasification Process in Fluidized Bed Coupled with Entrained-Flow Bed" Energies 19, no. 1: 37. https://doi.org/10.3390/en19010037
APA StyleWang, J., Liu, Z., Zhang, H., Huang, X., Peng, B., Chang, L., Yang, R., & Li, W. (2026). Simulation and Optimization of Biomass Gasification Process in Fluidized Bed Coupled with Entrained-Flow Bed. Energies, 19(1), 37. https://doi.org/10.3390/en19010037