Pyrolysis Characterization of Simulated Radioactive Solid Waste: Pyrolysis Behavior, Kinetics, and Product Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Thermogravimetric Analysis
2.3. TG-FTIR-MS Analysis
2.4. Py-GC/MS Analysis
3. Results and Discussion
3.1. Proximate and Ultimate Analysis
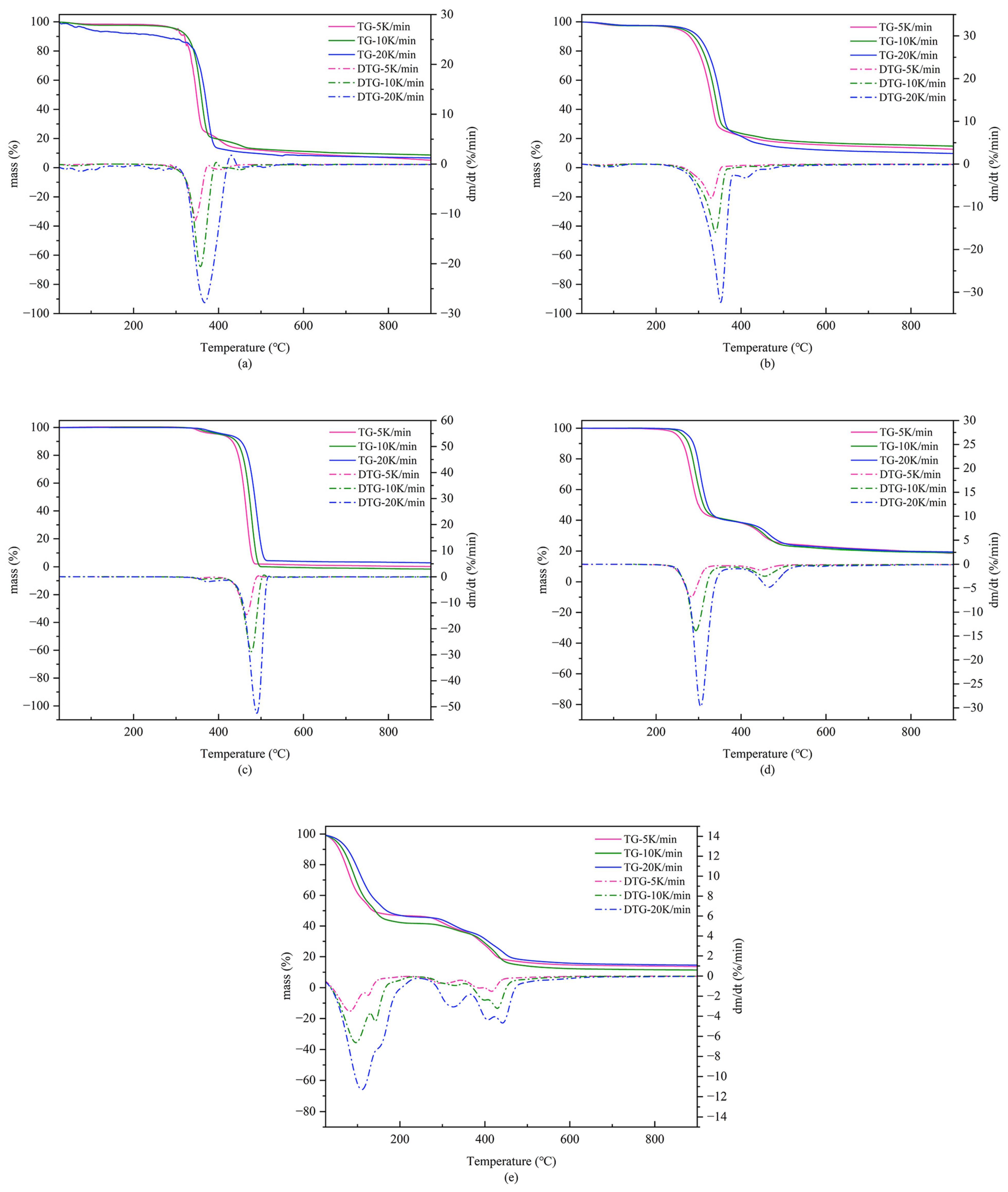
3.2. TG-DTG Analysis
3.3. Pyrolysis Kinetics Analysis
3.4. TG-FTIR-MS Analysis
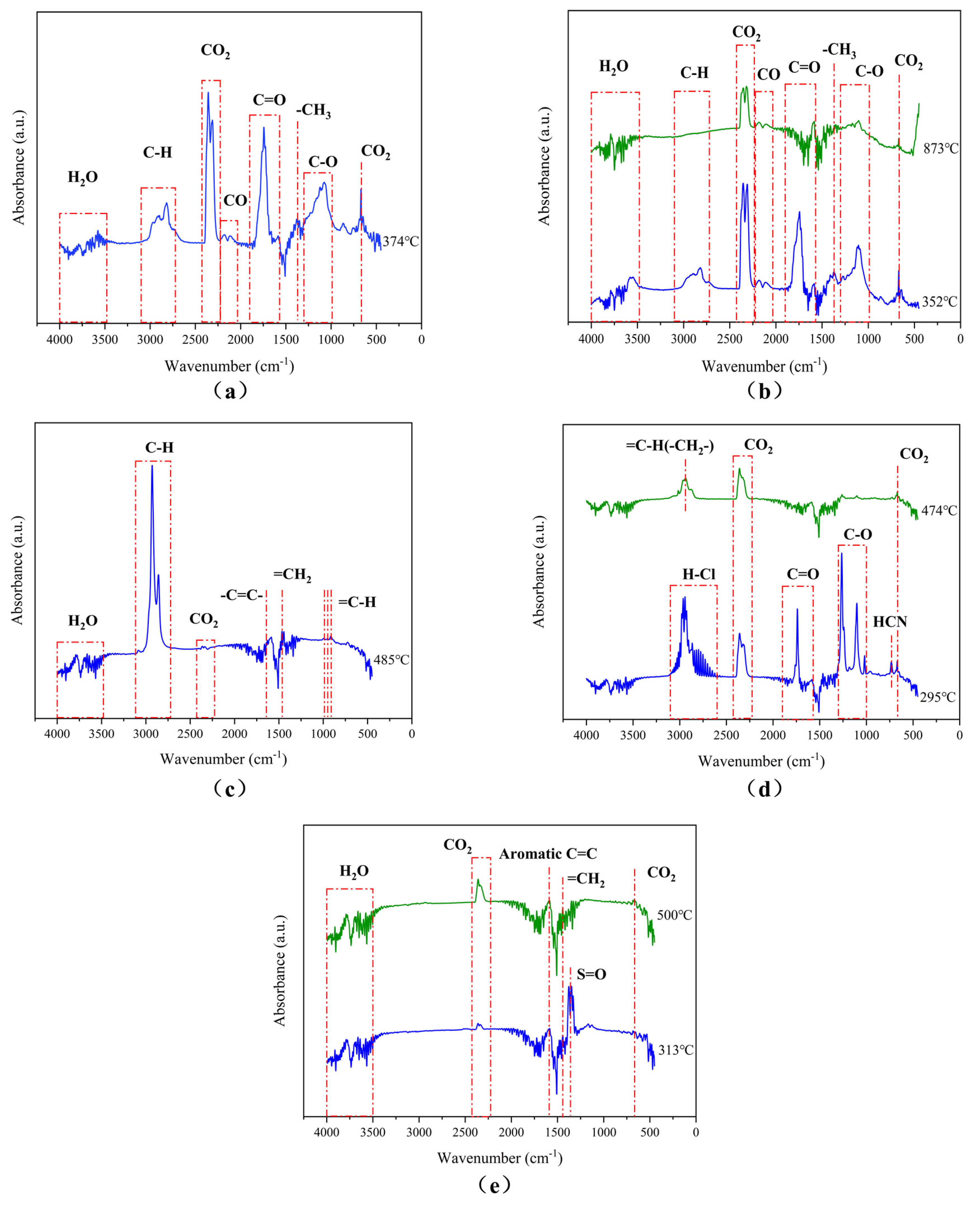
3.4.1. FTIR Analysis
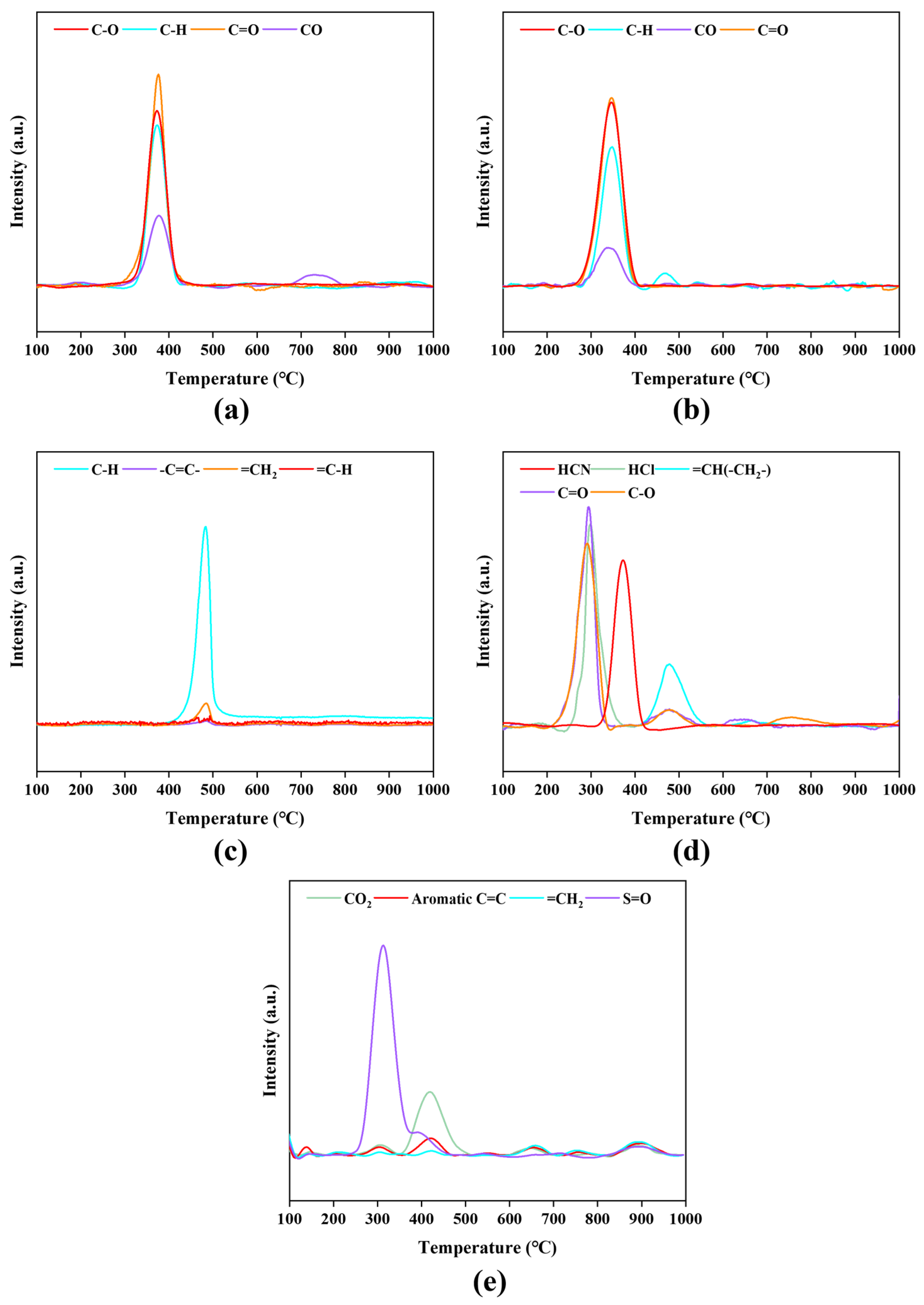
3.4.2. MS Analysis
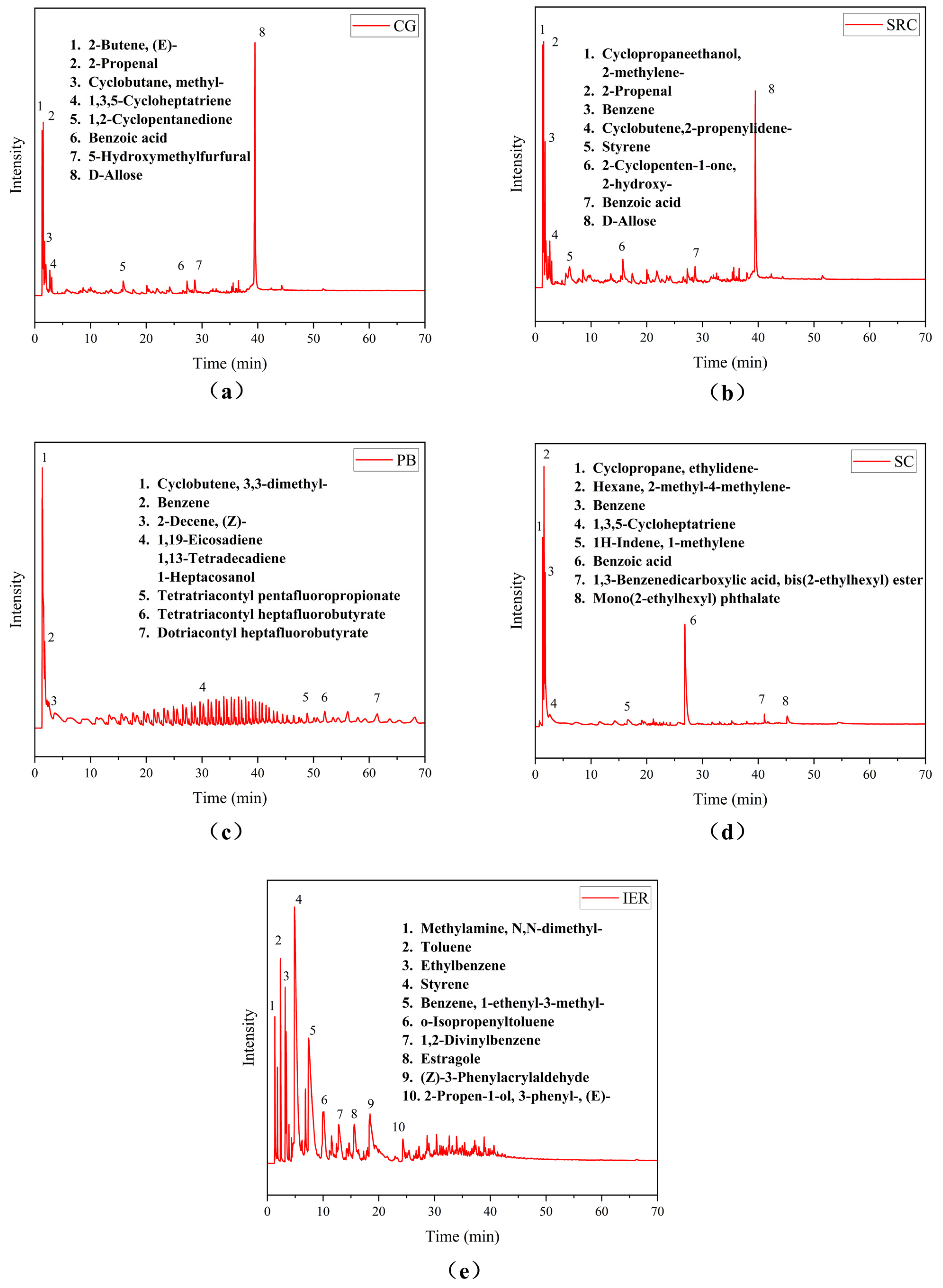
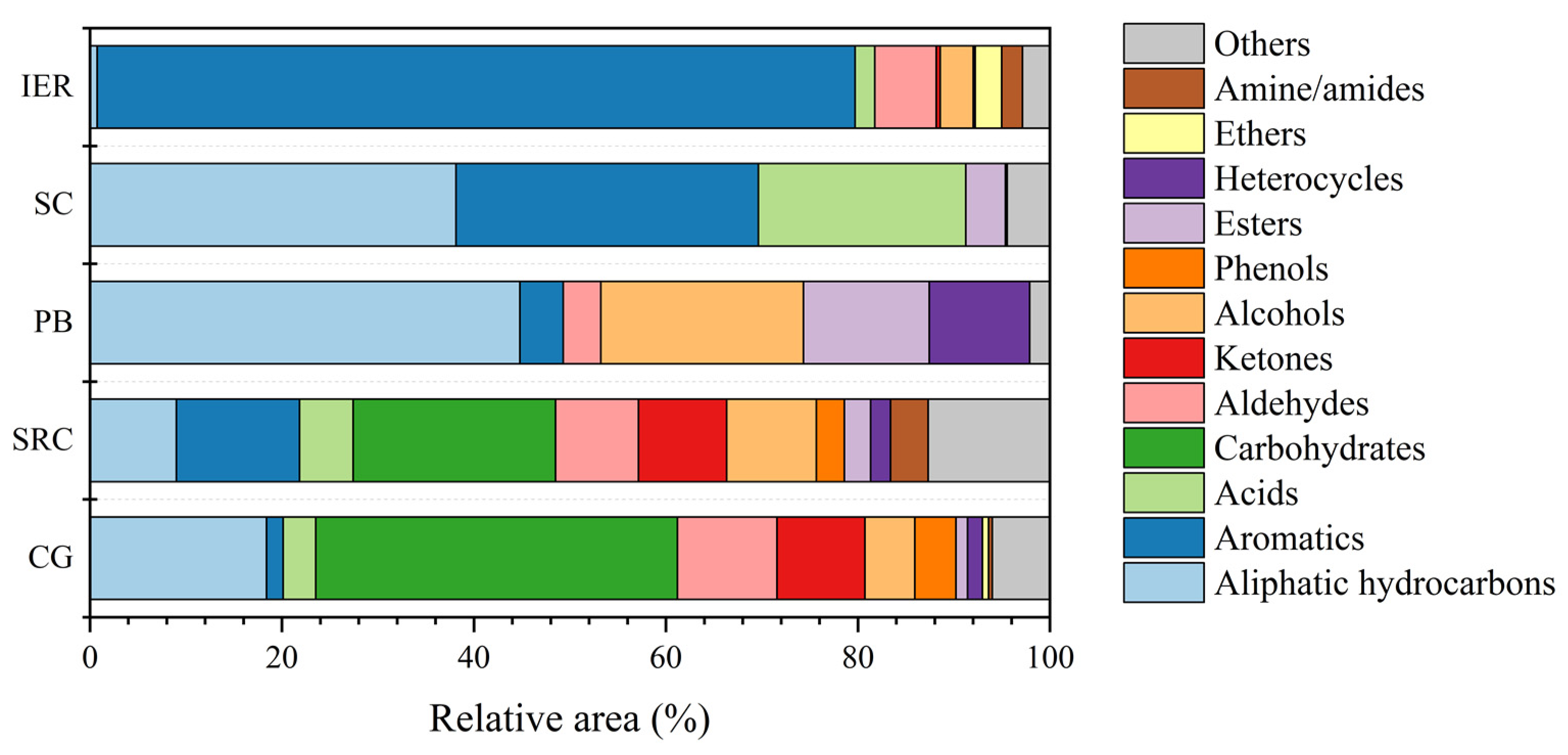
3.5. Py-GC/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, S.; Park, J.; Suh, H.; Kim, G.; Lim, J.; Seok, S.; Bae, S. Effects of ultrasound-assisted sulfuric acid treatment on the decontamination of Co nuclides in cement paste simulating radioactive concrete waste from dismantled nuclear power plants. Constr. Build. Mater. 2023, 373, 130878. [Google Scholar] [CrossRef]
- Cai, X.; Yang, H.; Yang, X.; He, Y.; Shan, J. Research on a novel γ-ray spectrum analysis method for low- and intermediate-level radioactive solid waste in nuclear power plants. Nucl. Eng. Technol. 2024, 56, 4688–4697. [Google Scholar] [CrossRef]
- Pancholi, K.C.; Mishra, R.; Kolay, S.; Singh, P.J.; Vincent, T.; Kaushik, C.P. Thermal decomposition study for effective management of low level radioactive combustible solids. J. Radioanal. Nucl. Chem. 2022, 331, 1309–1322. [Google Scholar] [CrossRef]
- Xu, W.; Chu, H.; Zheng, B.; Tang, C.; Yu, E.; Xue, P.; Sheng, J.; Li, X. Application and improvement of low & medium-level radioactive waste pyrolysis incineration technology in China. Radiat. Prot. 2020, 40, 387–393. [Google Scholar]
- Wang, H.W.; Chen, M.Q.; Fu, K.; Wei, S.H.; Zhong, X.B. Evaluation on migration and transformation of trace nuclides in thermal degradation for low-level radioactive waste. J. Anal. Appl. Pyrolysis 2022, 161, 105420. [Google Scholar] [CrossRef]
- Matsuda, M.; Funabashi, K.; Yusa, H.; Kikuchi, M. Influence of Functional Sulfonic Acid Group on Pyrolysis Characteristics for Cation Exchange Resin. J. Nucl. Sci. Technol. 1987, 24, 124–128. [Google Scholar] [CrossRef]
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Womack, R.; Shuey, M. Development and Use of the Dual-Mode Plasma Torch. In Proceedings of the Conference: Waste Management 2002 Symposium, Tucson, AZ, USA, 24–28 February 2002. [Google Scholar]
- Nakashima, M.; Fukui, T.; Nakashio, N.; Isobe, M.; Ohtake, A.; Wakui, T.; Hirabayashi, T. Characterization of Solidified Products Yielded by Plasma Melting Treatment of Simulated Non-Metallic Radioactive Wastes. J. Nucl. Sci. Technol. 2002, 39, 687–694. [Google Scholar] [CrossRef]
- Polyakov, N.; Chulkin, S. Application of plasma-pyrolytic processing of solid radioactive waste on a floating nuclear power plant. IOP Conf. Ser. Earth Environ. Sci. 2020, 578, 012027. [Google Scholar] [CrossRef]
- Sampathraju, R.; Mansuri, M.A. Assessment of air pollutants in the occupational environment of plasma biomedical waste disposal system in Ahmedabad, India. J. Environ. Saf. 2019, 10, 89–93. [Google Scholar] [CrossRef]
- Prado, E.S.P.; Miranda, F.S.; de Araujo, L.G.; Petraconi, G.; Baldan, M.R. Thermal plasma technology for radioactive waste treatment: A review. J. Radioanal. Nucl. Chem. 2020, 325, 331–342. [Google Scholar] [CrossRef]
- Ma, Y.; Chu, H.; Zheng, B. Research progress of plasma melting technology in radioactive waste treatment of nuclear power plants. Ann. Nucl. Energy 2024, 198, 110307. [Google Scholar] [CrossRef]
- Tzeng, C.-C.; Kuo, Y.-Y.; Huang, T.-F.; Lin, D.-L.; Yu, Y.-J. Treatment of radioactive wastes by plasma incineration and vitrification for final disposal. J. Hazard. Mater. 1998, 58, 207–220. [Google Scholar] [CrossRef]
- Tee, M.Y.; Woon, K.S.; Wong, S.L.; Nyakuma, B.B.; Tan, J.P.; Chong, W.W.F.; Mong, G.R. Unveiling the dynamics of solid waste co-pyrolysis through thermogravimetric analysis and kinetic analysis for technological upscaling (2001–2022). J. Anal. Appl. Pyrolysis 2024, 183, 106806. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Reprint of: Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2015, 37, 116–136. [Google Scholar] [CrossRef]
- Prajapati, S.B.; Gautam, A.; Gautam, S. Co-pyrolysis of printed circuit boards (PCBs): A review on current research and future prospects. Mater. Today Proc. 2024, 111, 44–52. [Google Scholar] [CrossRef]
- Bianasari, A.A.; Khaled, M.S.; Hoang, T.-D.; Reza, M.S.; Bakar, M.S.A.; Azad, A.K. Influence of combined catalysts on the catalytic pyrolysis process of biomass: A systematic literature review. Energy Convers. Manag. 2024, 309, 118437. [Google Scholar] [CrossRef]
- Bassyouni, M.; Nasser, R.; El-Bagoury, M.; Shaker, I.; Attia, A.M.; Elhenawy, Y.; Aboelela, D. Integrating medical plastic waste pyrolysis and circular economy for environmental sustainability. Renew. Sustain. Energy Rev. 2025, 209, 115062. [Google Scholar] [CrossRef]
- Huang, Y.; Sekyere, D.T.; Zhang, J.; Tian, Y. Fast pyrolysis behaviors of biomass with high contents of ash and nitrogen using TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2023, 170, 105922. [Google Scholar] [CrossRef]
- Das, P. Pyrolysis study of a waste plastic mixture through different kinetic models using isothermal and nonisothermal mechanism††Electronic supplementary information (ESI) available. RSC Adv. 2024, 14, 25599–25618. [Google Scholar] [CrossRef] [PubMed]
- Athparia, M.; Saikia, N.; Namdeo, A.; Garg, A.; Sarangi, P.K.; Kataki, R. Kinetic analysis and pyrolysis behavior of organic fraction of municipal solid waste towards the production of renewable fuel: A sustainable approach to resource recovery for circular economy. Sustain. Chem. Pharm. 2024, 42, 101822. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Chen, A.; Mao, W.; Zhu, Z.; Zhao, Y.; Zhang, L. Experimental and kinetic study on pyrolysis and combustion characteristics of pesticide waste liquid. J. Environ. Chem. Eng. 2024, 12, 111994. [Google Scholar] [CrossRef]
- Gulshan, S.; Shafaghat, H.; Wang, S.; Dai, L.; Tang, C.; Fu, W.; Wen, Y.; Wang, C.-H.; Evangelopoulos, P.; Yang, W. Kinetic investigation on the catalytic pyrolysis of plastic fractions of waste electrical and electronic equipment (WEEE): A mathematical deconvolution approach. Waste Manag. 2024, 187, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Aboulkas, A.; Elharfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis Kinet. mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Ullah, F.; Ji, G.; Zhang, L.; Irfan, M.; Fu, Z.; Manzoor, Z.; Li, A. Assessing pyrolysis performance and product evolution of various medical wastes based on model-free and TG-FTIR-MS methods. Chem. Eng. J. 2023, 473, 145300. [Google Scholar] [CrossRef]
- Zhang, Q.; Dor, L.; Fenigshtein, D.; Yang, W.; Blasiak, W. Gasification of municipal solid waste in the Plasma Gasification Melting process. Appl. Energy 2012, 90, 106–112. [Google Scholar] [CrossRef]
- Pei, S.-L.; Chen, T.-L.; Pan, S.-Y.; Yang, Y.-L.; Sun, Z.-H.; Li, Y.-J. Addressing environmental sustainability of plasma vitrification technology for stabilization of municipal solid waste incineration fly ash. J. Hazard. Mater. 2020, 398, 122959. [Google Scholar] [CrossRef]
- Cai, X.; Du, C. Thermal Plasma Treatment of Medical Waste. Plasma Chem. Plasma Process. 2021, 41, 1–46. [Google Scholar] [CrossRef]
- Byun, Y.; Cho, M.; Chung, J.W.; Namkung, W.; Lee, H.D.; Jang, S.D.; Kim, Y.-S.; Lee, J.-H.; Lee, C.-R.; Hwang, S.-M. Hydrogen recovery from the thermal plasma gasification of solid waste. J. Hazard. Mater. 2011, 190, 317–323. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, C.; Lan, W.; Zhang, X.; Liu, D.; Yang, S.-Z. Experimental Study of Thermal Plasma Processing Waste Circuit Boards. Adv. Mater. Res. 2013, 652–654, 1553–1561. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Alfonso, J.E.M.; Blanco, J.D. Kinetic models based in biomass components for the combustion and pyrolysis of sewage sludge and its compost. J. Anal. Appl. Pyrolysis 2009, 86, 108–114. [Google Scholar] [CrossRef]
- Yu, Z.; Ahmad, M.S.; Shen, B.; Li, Y.; Ibrahim, M.; Bokhari, A.; Klemeš, J.J. Activated waste cotton cellulose as renewable fuel and value-added chemicals: Thermokinetic analysis, coupled pyrolysis with gas chromatography and mass spectrometry. Energy 2023, 283, 128341. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M. Thermogravimetric kinetic study of poly(vinyl chloride) pyrolysis. Polym. Degrad. Stab. 1995, 48, 219–229. [Google Scholar] [CrossRef]
- Kuttiyathil, M.S.; Ali, L.; Teoh, W.Y.; Altarawneh, M. Dechlorination of waste polyvinyl chloride (PVC) via its co-pyrolysis with Ca(OH)2: A TG-IR-GCMS investigation. Case Stud. Chem. Environ. Eng. 2025, 11, 101162. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog. Nucl. Energy 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Castro, H.A.; Luca, V.; Bianchi, H.L. Study of plasma off-gas treatment from spent ion exchange resin pyrolysis. Environ. Sci. Pollut. Res. 2018, 25, 21403–21410. [Google Scholar] [CrossRef]
- Luo, J.; Hu, W.; Suo, Z.; Wang, Y.; Zhang, Y. Co-pyrolysis of spent radioactive ion exchange resin and manganese dioxide: Decrease the decomposition temperatures of functional groups. J. Hazard. Mater. 2021, 418, 126275. [Google Scholar] [CrossRef]
- Huang, X.; Yin, H.; Zhang, H.; Mei, N.; Mu, L. Pyrolysis characteristics, gas products, volatiles, and thermo–kinetics of industrial lignin via TG/DTG–FTIR/MS and in–situ Py–PI–TOF/MS. Energy 2022, 259, 125062. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Al Ayed, O.S.; Ye, G.; Luo, H.; Ibrahim, M.; Rashid, U.; Arbi Nehdi, I.; Qadir, G. Kinetic analyses and pyrolytic behavior of Para grass (Urochloa mutica) for its bioenergy potential. Bioresour. Technol. 2017, 224, 708–713. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Ozawa, T. Estimation of activation energy by isoconversion methods. Thermochim. Acta 1992, 203, 159–165. [Google Scholar] [CrossRef]
- Sharara, M.; Sadaka, S. Thermogravimetric Analysis of Swine Manure Solids Obtained from Farrowing, and Growing-Finishing Farms. J. Sustain. Bioenergy Syst. 2014, 4, 75–86. [Google Scholar] [CrossRef]
- Li, F.; Tao, J.; Kumar, A.; Zhang, J.; Sun, Y.; Guo, W.; Cheng, Z.; Yan, B.; Chen, G. Pyrolysis characteristics and kinetics of waste photovoltaic module: A TG-MS-FTIR study. J. Clean. Prod. 2024, 444, 141267. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour. Technol. 2018, 250, 422–428. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Yin, F.; Liang, D. Pyrolysis kinetics and reaction mechanism of waste medical masks by sectional heating process. Therm. Sci. Eng. Prog. 2024, 56, 103065. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Li, L.; Liu, C.; Cheng, K.; Li, Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py–GC/MS. J. Anal. Appl. Pyrolysis 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, J.; Li, J.; Li, H.; Li, F.; Cheng, Z.; Yan, B.; Chen, G. Pyrolysis of combustible fractions in excavated waste: Effect of landfill time on pyrolysis characteristics analyzed by TG-FTIR-MS. J. Anal. Appl. Pyrolysis 2024, 177, 106298. [Google Scholar] [CrossRef]
- Bi, H.; Ni, Z.; Tian, J.; Wang, C.; Jiang, C.; Zhou, W.; Bao, L.; Sun, H.; Lin, Q. The effect of biomass addition on pyrolysis characteristics and gas emission of coal gangue by multi-component reaction model and TG-FTIR-MS. Sci. Total Environ. 2021, 798, 149290. [Google Scholar] [CrossRef]
- Zhou, A.; Deng, N.; Deng, S.; Hu, Z.; Magdziarz, A.; Tan, H.; Wang, X. Study on pyrolysis behavior of municipal sludge based on TG-FTIR-MS. J. Energy Inst. 2024, 114, 101643. [Google Scholar] [CrossRef]
- Volli, V.; Gollakota, A.R.K.; Shu, C.-M. Comparative studies on thermochemical behavior and kinetics of lignocellulosic biomass residues using TG-FTIR and Py-GC/MS. Sci. Total Environ. 2021, 792, 148392. [Google Scholar] [CrossRef]
- Pang, Y.; Zhu, X.; Li, N.; Wang, Z. Study on CO2 co-gasification of cellulose and high-density polyethylene via TG-FTIR and ReaxFF MD. Process Saf. Environ. Protect. 2024, 186, 1471–1480. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. J. Energy Inst. 2020, 93, 2362–2370. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Li, J.; Yu, Q. Kinetic modeling of thermal degradation of TDI/MDI-based flexible polyurethane foam under nitrogen and air atmospheres with Shuffled Complex Evolution algorithm: Insights from TG-FTIR analysis. J. Anal. Appl. Pyrolysis 2024, 177, 106279. [Google Scholar] [CrossRef]
- Luo, X.T.; Wang, Z.Q.; Wu, J.L.; Wu, J.H. Study on the pyrolysis mechanism of polyethylene, polystyrene, and polyvinyl chloride by TGA-FTIR. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2012, 40, 1147–1152. [Google Scholar]
- Castro, H.A.; Rodríguez, R.A.; Luca, V.; Bianchi, H.L. Pyrolysis and High Performance Plasma Treatment Applied to Spent Ion Exchange Resins. J. Nucl. Eng. Radiat. Sci. 2019, 5, 020901. [Google Scholar] [CrossRef]
- Ni, Z.; Bi, H.; Jiang, C.; Sun, H.; Zhou, W.; Qiu, Z.; Lin, Q. Research on the co-pyrolysis of coal slime and cellulose based on TG-FTIR-MS, artificial neural network, and principal component analysis. Fuel 2022, 320, 123960. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, W.; Bi, H.; Ni, Z.; Sun, H.; Lin, Q. Co-pyrolysis of coal slime and cattle manure by TG–FTIR–MS and artificial neural network modeling: Pyrolysis behavior, kinetics, gas emission characteristics. Energy 2022, 247, 123203. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-pyrolysis of sewage sludge and rice husk by TG–FTIR–MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Pappa, A.; Mikedi, K.; Agapiou, A.; Karma, S.; Pallis, G.C.; Statheropoulos, M.; Burke, M. TG–MS analysis of nitrile butadiene rubber blends (NBR/PVC). J. Anal. Appl. Pyrolysis 2011, 92, 106–110. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Z.; Chen, G.; Zhang, M.; Sun, T.; Wang, Q.; Zhu, H.; Guo, S.; Chen, Y.; Zhu, Y.; et al. Synergistic effects and products distribution during Co-pyrolysis of biomass and plastics. J. Energy Inst. 2023, 111, 101392. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Huang, W.; Peng, X.; Yuan, B.; Hu, Y. TG-FTIR-MS and Py-GC/MS study on the pyrolysis characteristics and gas evolution behavior of forest duff. J. Anal. Appl. Pyrolysis 2023, 176, 106233. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Wang, W.; Ji, G.; Zhao, M.; Li, A. Kinetics, Product Evolution, and Mechanism for the Pyrolysis of Typical Plastic Waste. ACS Sustain. Chem. Eng. 2022, 10, 91–103. [Google Scholar] [CrossRef]
- Idris, I.A.; Nisamaneenate, J.; Atong, D.; Sricharoenchaikul, V. A comprehensive study of facemasks pyrolysis using Py-GC/MS, kinetic analysis and ANN modeling. Arab. J. Chem. 2024, 17, 105605. [Google Scholar] [CrossRef]

| Material | Proximate Analysis | Ultimate Analysis | Cl | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | ashad | VMad | FCad,diff | Cad | Had | Nad | Sad | Oad, diff | ||
| CG | 4.26 | 1.23 | 88.21 | 6.30 | 44.13 | 6.04 | 0.32 | 0.46 | 43.56 | 0.00 |
| SRC | 8.36 | 1.03 | 81.31 | 9.30 | 41.33 | 5.49 | 0.78 | 0.45 | 42.56 | 0.04 |
| PB | 0.10 | 2.39 | 96.47 | 1.04 | 80.52 | 15.02 | 0.48 | 0.87 | 0.62 | 0.01 |
| SC | 0.24 | 8.38 | 79.85 | 11.53 | 47.43 | 6.08 | 0.82 | 0.52 | 36.53 | 24.46 |
| IER | 19.38 | 0.01 | 64.01 | 16.60 | 51.13 | 7.10 | 2.07 | 4.74 | 15.57 | 0.02 |
| Material | Degrees of Conversion (α) | FWO Method | KAS Method | Friedman Method | |||
|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ea (kJ/mol) | Ea (kJ/mol) | R2 | Ea (kJ/mol) | ||
| CG | 0.1 | 152.93 | 0.9963 | 150.97 | 0.9958 | 153.92 | 0.9981 |
| 0.2 | 153.56 | 0.9984 | 151.37 | 0.9982 | 181.74 | 0.9995 | |
| 0.3 | 184.26 | 0.9999 | 178.10 | 0.9995 | 202.08 | 0.9974 | |
| 0.4 | 168.62 | 0.9995 | 179.33 | 0.9997 | 184.49 | 0.9949 | |
| 0.5 | 182.75 | 0.9999 | 181.75 | 0.9998 | 187.03 | 0.9999 | |
| 0.6 | 184.53 | 0.9999 | 183.56 | 0.9999 | 187.62 | 0.9999 | |
| 0.7 | 185.98 | 0.9999 | 185.02 | 0.9999 | 190.80 | 0.9999 | |
| 0.8 | 188.33 | 0.9999 | 198.48 | 0.9983 | 224.57 | 0.9936 | |
| 0.9 | 256.83 | 0.9654 | 248.27 | 0.9766 | 390.87 | 0.9067 | |
| Average | 184.20 | 184.09 | 211.46 | ||||
| SRC | 0.1 | 153.82 | 0.9617 | 152.52 | 0.9570 | 164.97 | 0.9837 |
| 0.2 | 164.27 | 0.9939 | 163.17 | 0.9931 | 175.47 | 0.9982 | |
| 0.3 | 170.41 | 0.9955 | 169.42 | 0.9949 | 181.70 | 0.9964 | |
| 0.4 | 174.62 | 0.9934 | 173.70 | 0.9926 | 184.36 | 0.9924 | |
| 0.5 | 176.72 | 0.9888 | 176.32 | 0.9906 | 185.68 | 0.9890 | |
| 0.6 | 180.95 | 0.9990 | 184.64 | 0.9915 | 187.27 | 0.9874 | |
| 0.7 | 181.72 | 0.9899 | 180.86 | 0.9887 | 184.20 | 0.9810 | |
| 0.8 | 179.10 | 0.9888 | 177.97 | 0.9873 | 183.13 | 0.9892 | |
| Average | 172.70 | 172.33 | 180.85 | ||||
| PB | 0.1 | 285.93 | 0.9999 | 288.90 | 0.9999 | 277.81 | 0.9998 |
| 0.2 | 271.64 | 0.9999 | 273.63 | 0.9999 | 270.90 | 0.9999 | |
| 0.3 | 269.74 | 0.9999 | 271.51 | 0.9999 | 297.56 | 0.9966 | |
| 0.4 | 268.69 | 0.9999 | 270.33 | 0.9999 | 269.44 | 0.9999 | |
| 0.5 | 266.74 | 0.9999 | 268.20 | 0.9999 | 263.08 | 0.9999 | |
| 0.6 | 263.84 | 0.9999 | 265.09 | 0.9999 | 239.11 | 0.9972 | |
| 0.7 | 260.28 | 0.9999 | 259.86 | 0.9945 | 253.96 | 0.9997 | |
| 0.8 | 258.21 | 0.9999 | 259.04 | 0.9999 | 256.12 | 0.9994 | |
| 0.9 | 254.92 | 0.9999 | 255.51 | 0.9999 | 253.67 | 0.9992 | |
| Average | 266.67 | 268.01 | 264.63 | ||||
| SC | 0.1 | 65.70 | 0.9509 | 60.21 | 0.9370 | 118.02 | 0.9955 |
| 0.2 | 107.62 | 0.9913 | 104.00 | 0.9898 | 166.04 | 0.9987 | |
| 0.3 | 126.08 | 0.9981 | 123.29 | 0.9979 | 165.76 | 0.9982 | |
| 0.4 | 135.99 | 0.9990 | 133.62 | 0.9989 | 163.64 | 0.9991 | |
| 0.5 | 145.38 | 0.9999 | 143.39 | 0.9999 | 169.11 | 0.9971 | |
| 0.6 | 152.95 | 0.9997 | 151.24 | 0.9996 | 169.21 | 0.9991 | |
| 0.7 | 153.67 | 0.9992 | 151.78 | 0.9991 | 162.01 | 0.9899 | |
| 0.8 | 209.47 | 0.9270 | 208.75 | 0.9194 | 235.66 | 0.9485 | |
| 0.9 | 276.15 | 0.9941 | 278.17 | 0.9936 | 309.83 | 0.9999 | |
| Average | 152.56 | 150.49 | 184.36 | ||||
| IER | 0.1 | 65.80 | 0.9920 | 63.63 | 0.9904 | 55.91 | 0.9929 |
| 0.2 | 59.53 | 0.9948 | 56.76 | 0.9935 | 51.79 | 0.9937 | |
| 0.3 | 57.10 | 0.9932 | 53.99 | 0.9914 | 51.32 | 0.9887 | |
| 0.4 | 56.34 | 0.9893 | 52.96 | 0.9864 | 54.66 | 0.9792 | |
| 0.5 | 63.01 | 0.9751 | 59.02 | 0.9833 | 71.57 | 0.9528 | |
| 0.6 | 86.45 | 0.8391 | 82.61 | 0.8140 | 108.38 | 0.7947 | |
| 0.7 | 390.28 | 0.8692 | 400.64 | 0.8635 | 426.57 | 0.8954 | |
| 0.8 | 340.74 | 0.9999 | 347.39 | 0.9999 | 325.99 | 0.9996 | |
| 0.9 | 238.74 | 0.9999 | 239.46 | 0.9999 | 218.76 | 0.9997 | |
| Average | 150.89 | 150.72 | 151.66 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Dong, L.; Wang, W.; Ding, P.; Jiang, W.; Zuo, C.; Li, L.; Tang, M. Pyrolysis Characterization of Simulated Radioactive Solid Waste: Pyrolysis Behavior, Kinetics, and Product Distribution. Energies 2025, 18, 2341. https://doi.org/10.3390/en18092341
Wei Z, Dong L, Wang W, Ding P, Jiang W, Zuo C, Li L, Tang M. Pyrolysis Characterization of Simulated Radioactive Solid Waste: Pyrolysis Behavior, Kinetics, and Product Distribution. Energies. 2025; 18(9):2341. https://doi.org/10.3390/en18092341
Chicago/Turabian StyleWei, Zhigang, Lulu Dong, Wei Wang, Pan Ding, Wenqian Jiang, Chi Zuo, Lei Li, and Minghui Tang. 2025. "Pyrolysis Characterization of Simulated Radioactive Solid Waste: Pyrolysis Behavior, Kinetics, and Product Distribution" Energies 18, no. 9: 2341. https://doi.org/10.3390/en18092341
APA StyleWei, Z., Dong, L., Wang, W., Ding, P., Jiang, W., Zuo, C., Li, L., & Tang, M. (2025). Pyrolysis Characterization of Simulated Radioactive Solid Waste: Pyrolysis Behavior, Kinetics, and Product Distribution. Energies, 18(9), 2341. https://doi.org/10.3390/en18092341






