Recovery of Waste-Activated Carbon for Synthesizing High-Efficiency ORR Electrocatalyst
Abstract
1. Introduction
2. Materials and Methods
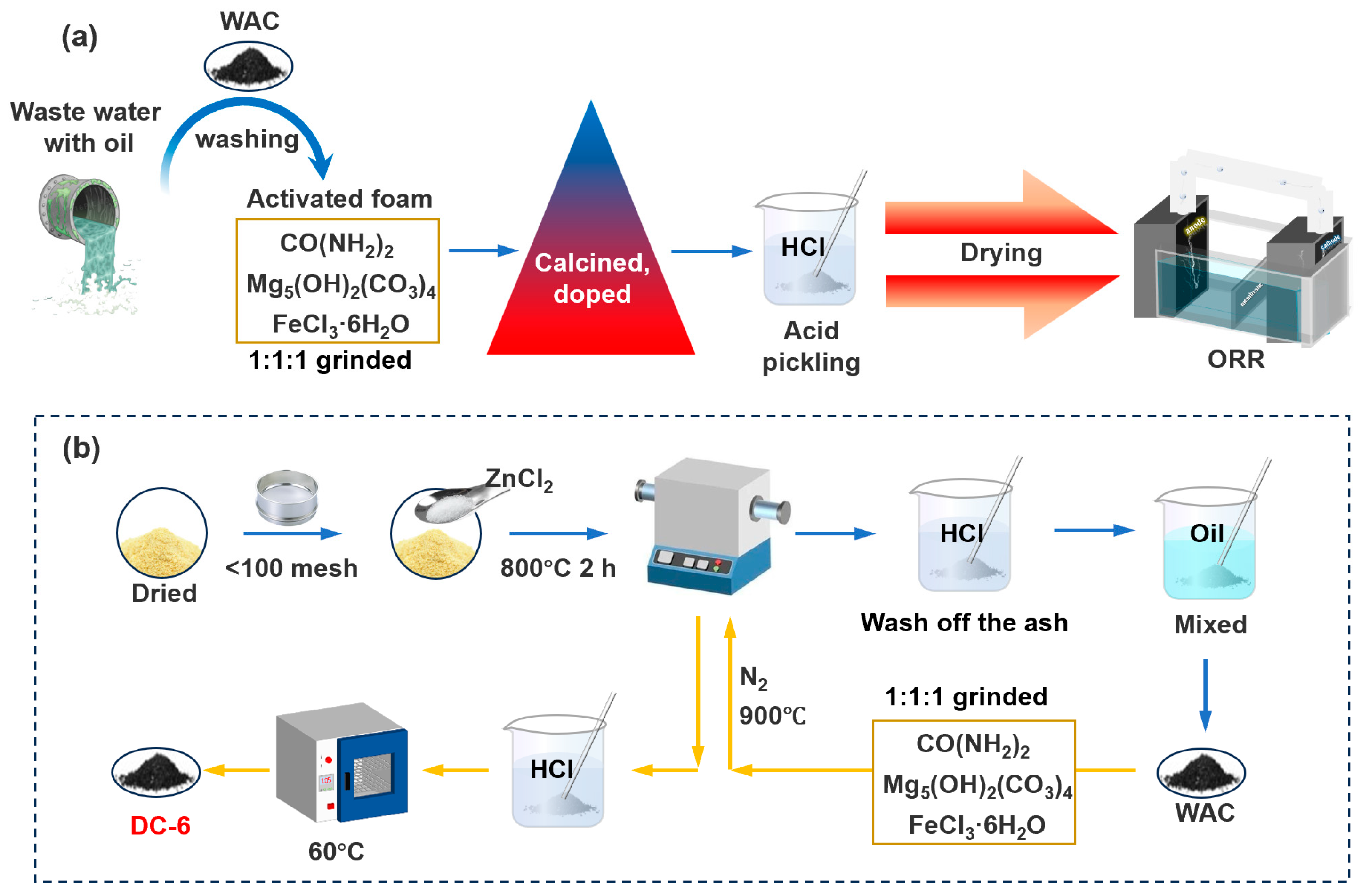
2.1. Synthesis of Activated Carbon
2.2. Electrochemical Tests
2.3. Catalyst Characterization
2.4. Simulation Details
3. Results and Discussion
3.1. Physicochemical Characterization
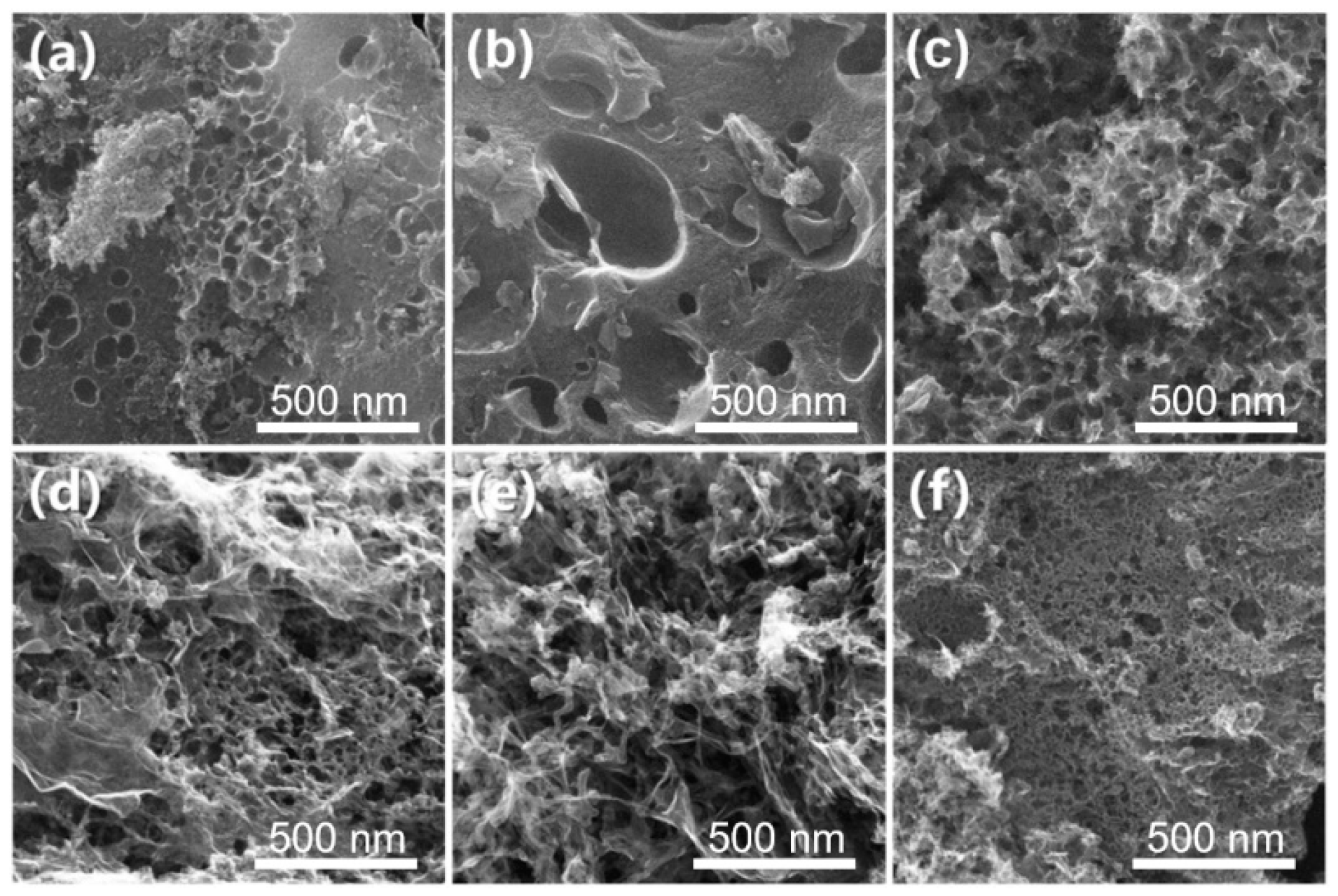
3.1.1. Morphology
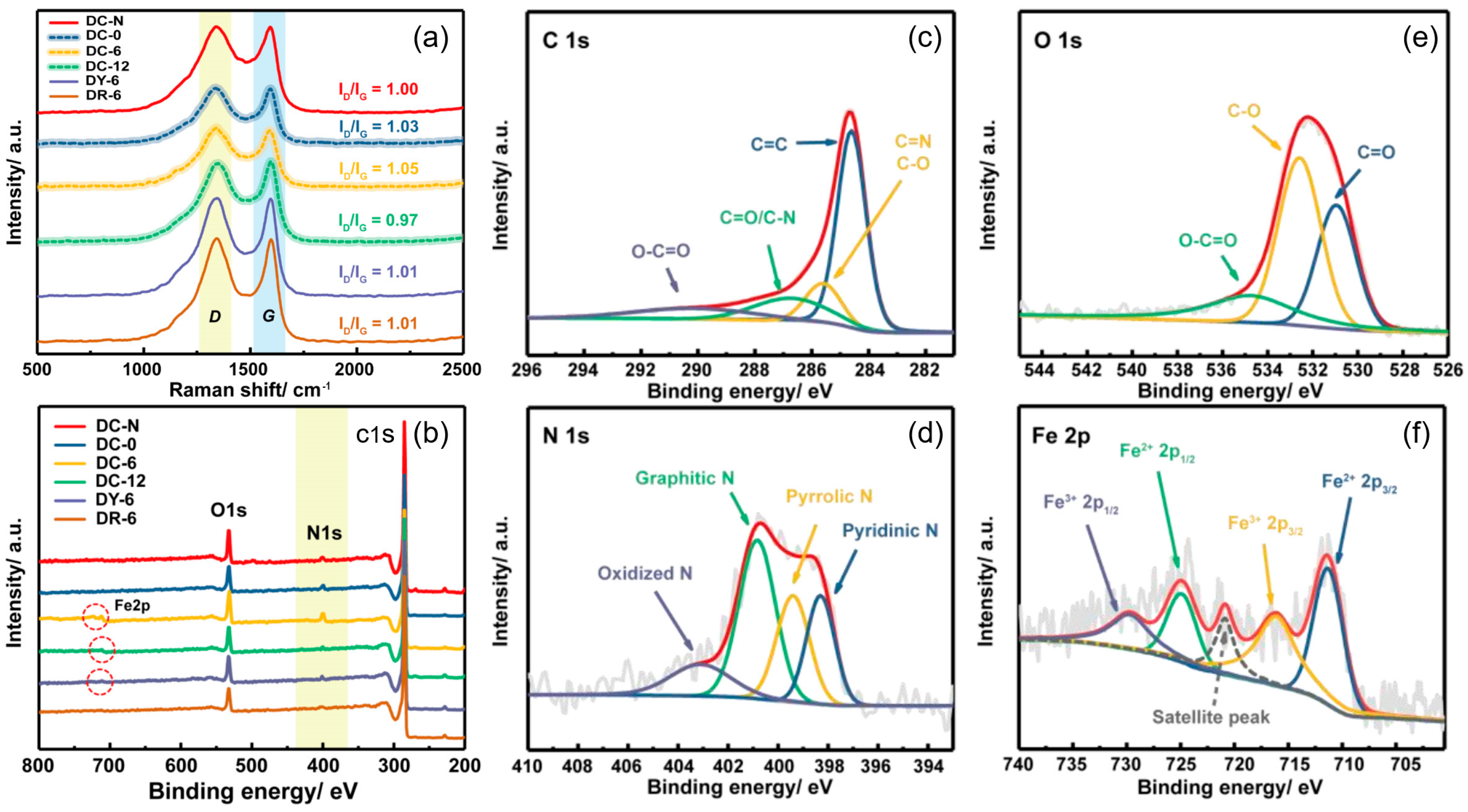
3.1.2. Structure Feature
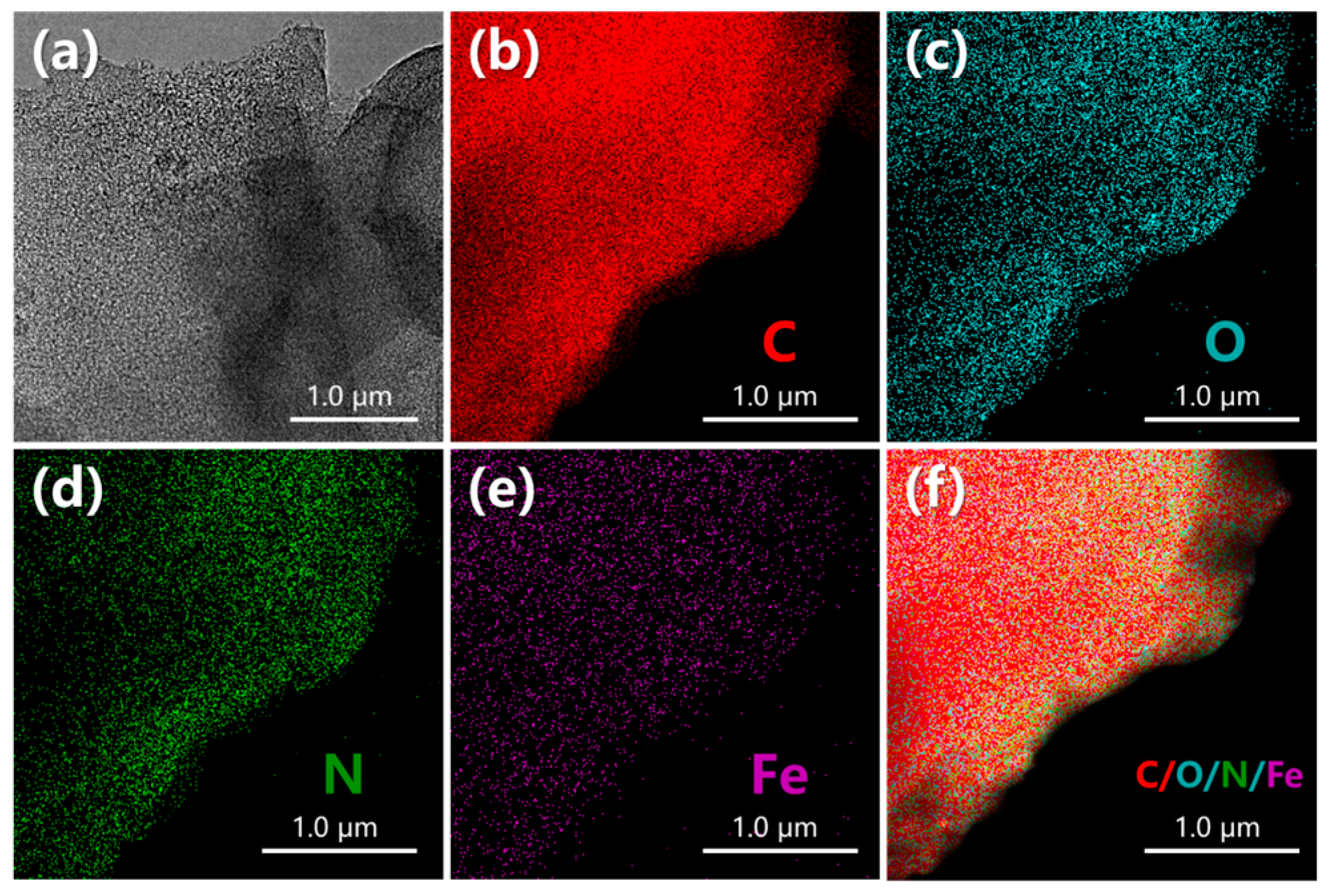
3.1.3. Composition Characteristics
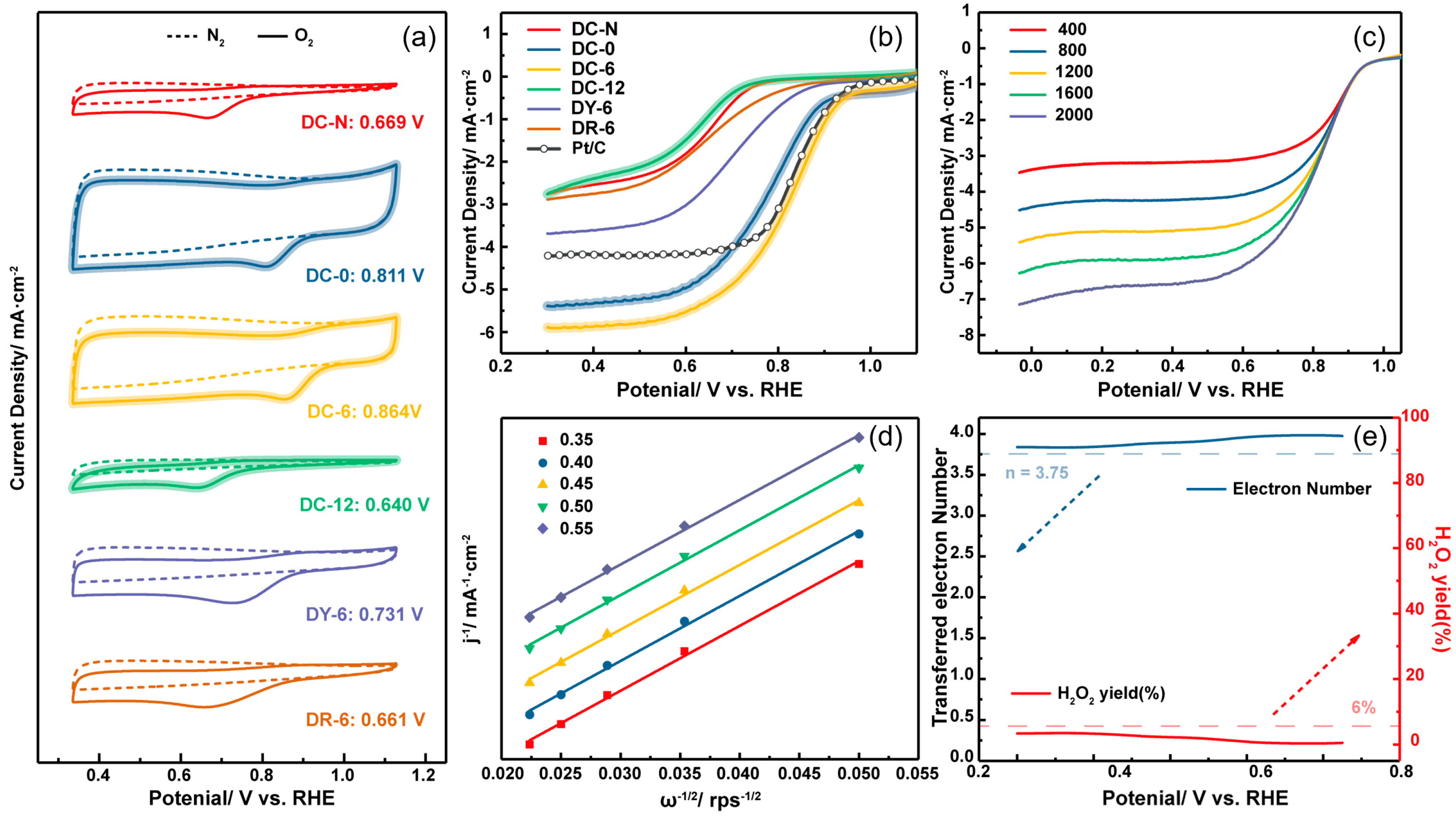
3.2. Electrochemical Characterization
3.2.1. Basic Electrochemical Performance
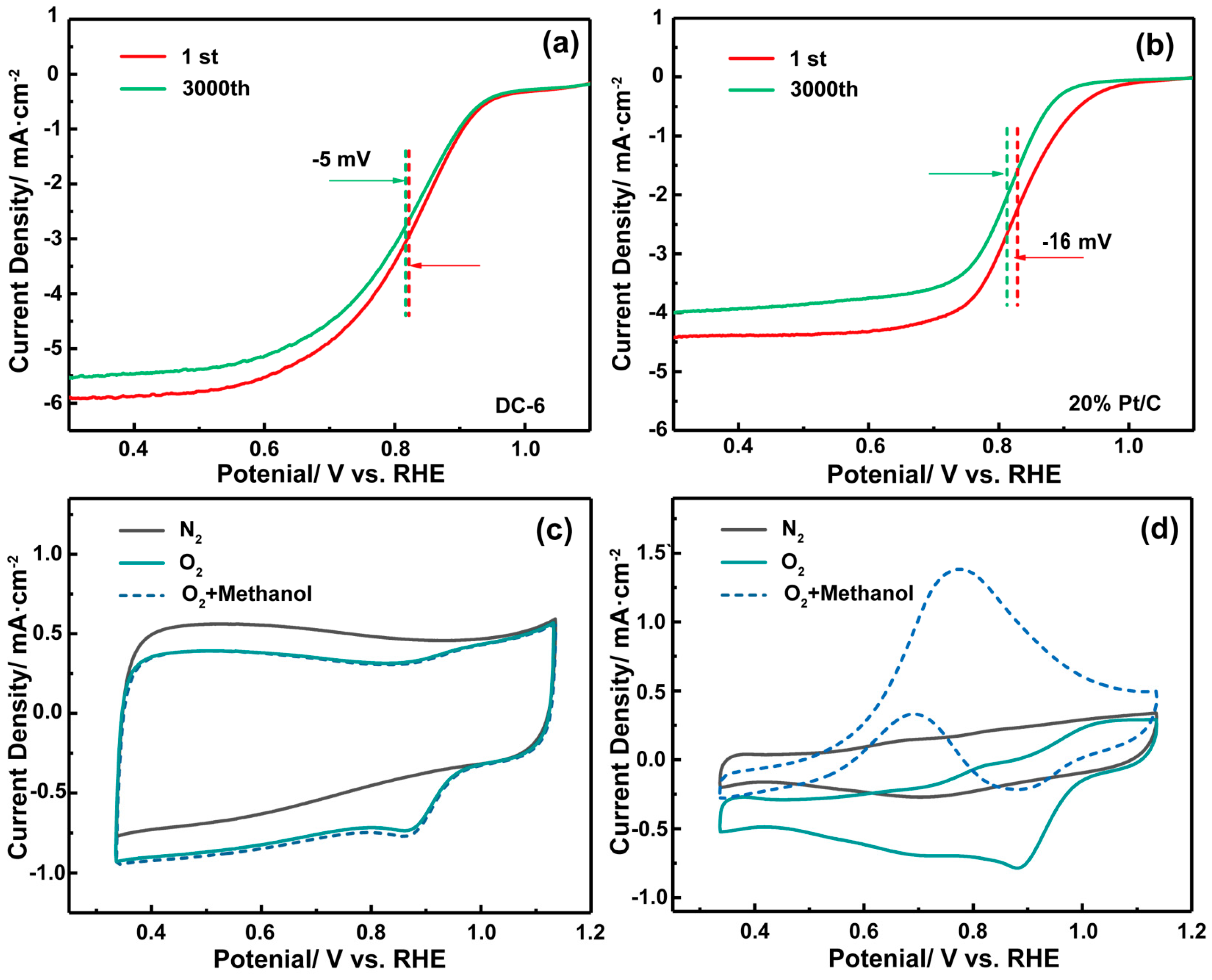
3.2.2. Methanol Resistance and Cycle Stability
3.3. Structure–Activity Relationships and Catalysis Mechanism
4. Conclusions
- (i)
- Reprocessed waste-activated carbon played a crosslinking role in the process of pyrolysis and activation of the honeycomb porous structure. In addition, it shows that Fe and well-doped N are evenly distributed on the surface of DC-6. This is very important for the structure of the microenvironment of the ORR area and for maintaining the continuity of the reaction. This is also proven by the DFT calculation.
- (ii)
- The carbon yield of DC-6 (24.3%) is significantly higher than that of DC-N (13.3%). The main reason is that the oil phase can take place in the pyrolysis process of cross-linking polymerization while retaining the original pore structure of coke products and increasing the carbon yield. When using waste-activated carbon, care should be taken to control the proportion of oil.
- (iii)
- It is found that DC-6 has an ORR pathway close to four electrons, and the yield of hydrogen peroxide produced in the reaction process is less than 3%, which is much lower than that of commercial-grade Pt/C (about 40%). In addition, DC-6 is superior to commercial-grade Pt/C in long-term cycle stability and methanol resistance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Q.; Wang, L.; Xue, Z.; Li, C.; Lv, D.; Zhang, N.; Zhu, K. Free-standing sulfur/carbon nanocomposite cathodes for lithium–sulfur rechargeable batteries. ACS Appl. Nano Mater. 2024, 8, 863–870. [Google Scholar] [CrossRef]
- Suresh, D.; Goh, P.S.; Kang, H.S.; Ahmad, M.N.; Ismail, A.F. Waste reutilization in pollution remediation: Paving new paths for wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 113570. [Google Scholar] [CrossRef]
- Ye, S.; Zhu, J. Application and Development Prospects of Activated Carbon in Heavy Metal Pollution Treatment. Highlights Sci. Eng. Technol. 2025, 125, 28–32. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Pahasup-anan, T.; Suwannahong, K.; Kampeerapappun, P.; Rangkupan, R.; Dechapanya, W. Conditional optimization on the photocatalytic degradation removal efficiency of formaldehyde using TiO2–nylon 6 electrospun composite membrane. Appl. Sci. Eng. Prog. 2025, 18, 7509. [Google Scholar] [CrossRef]
- Dupre, K.; Ryan, E.M.; Suleimenov, A.; Goldfarb, J.L. Experimental and computational demonstration of a low-temperature waste to by-product conversion of US oil shale semi-coke to a flue gas sorbent. Energies 2018, 11, 3195. [Google Scholar] [CrossRef]
- Reckien, W.; Janetzko, F.; Peintinger, M.; Bredow, T. Implementation of empirical dispersion corrections to density functional theory for periodic systems. J. Comput. Chem. 2012, 33, 23–31. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.J.; Feng, X.; Ma, Z.; Ma, Z.F.; Xue, Y. Progress of Pt and iron-group transition metal alloy catalysts with high ORR activity for PEMFCs. J. Electroanal. Chem. 2024, 959, 118165. [Google Scholar] [CrossRef]
- Tang, C.; Titirici, M.; Zhang, Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Zhang, G.; Zhang, C.; Sun, K.; Xiong, X.; Liu, J.; Sun, X. Pyrolysis -free formamide-derive d N-dope d carbon supporting atomically dispersed cobalt as high-performance bifunctional oxygen electrocatalyst. J. Energy Chem. 2020, 49, 283–290. [Google Scholar] [CrossRef]
- Gusiatin, M.Z. Advantages of Co-pyrolysis of sewage sludge with agricultural and forestry waste. Energies 2024, 17, 5736. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Zhang, Q. Nanostructured energy materials for electrochemical energy conversion and storage: A review. J. Energy Chem. 2016, 25, 967–984. [Google Scholar]
- Lu, Z.; Yao, S.; Dong, Y.; Wu, D.; Pan, H.; Huang, X.; Wang, T.; Sun, Z.; Chen, X. Earth-abundant coal-derived carbon nanotube/carbon composites as efficient bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. J. Energy Chem. 2021, 56, 87–97. [Google Scholar]
- Zhang, Y.; Wang, X.; Zheng, S.; Yang, B.; Li, Z.; Lu, J. Hierarchical cross-linked carbon aerogels with transition metal-nitrogen sites for highly efficient industrial-level CO2 Electroreduction. Adv. Funct. Mater. 2021, 31, 2104377. [Google Scholar] [CrossRef]
- Niu, H.; Lin, S.; Chen, Y.; Feng, J.; Zhang, Q.; Wang, A. Hydrogel derived FeCo/FeCoP embedded in N, P-codoped 3D porous carbon framework as a highly efficient electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2021, 536, 147950. [Google Scholar] [CrossRef]
- Han, Z.; Feng, J.; Yao, Y.; Wang, Z.; Zhang, L.; Wang, A. Mn, N, P-tridoped bamboo-like carbon nanotubes decorated with ultrafine Co2P/FeCo nanoparticles as bifunctional oxygen electrocatalyst for long-term rechargeable Zn-air battery. J. Colloid Interface Sci. 2021, 590, 330–340. [Google Scholar]
- Akula, S.; Mooste, M.; Kozlova, J.; Käärik, M.; Treshchalov, A.; Kikas, A. Transition metal (Fe, Co, Mn, Cu) containing nitrogen-doped porous carbon as efficient oxygen reduction electrocatalysts for anion exchange membrane fuel cells. Chem. Eng. J. 2023, 458, 141468. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J. Gaussian 16 Rev. C.01. Wallingford, CT2016. Available online: https://gaussian.com/g09citation/ (accessed on 19 March 2025).
- Zhao, H.; Tang, Z.; He, M.; Yang, X.; Lai, S.; An, K.; Han, S.; Qu, Z.; Zhou, W.; Wang, Z. Effect of oxygen functional groups on competitive adsorption of benzene and water on carbon materials: Density functional theory study. Sci. Total Environ. 2023, 863, 160772. [Google Scholar]
- Zhu, C.; Shi, Q.; Xu, B.; Fu, S.; Wan, G.; Yang, C. Hierarchically porous M-N-C (M = Co and Fe) single-atom electrocatalysts with robust MNx active moieties enable enhanced ORR Performance. Adv. Funct. Mater. 2018, 8, 1801956. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Taxi, D.; Shao, S.; Maimaitiyiming, X.; Sawut, A.; Tursun, M.; Kuerban, Z.; Lin, H. Biomass carbon with defective structures as effective ORR catalyst for DMFC. Sep. Purif. Technol. 2025, 356, 129775. [Google Scholar]
- Liu, X.; Wu, H.; Chen, S.; He, J.; Yang, Z.; Cheng, J.; Wu, J. Study on the alkali-free three-component flooding system in the Daqing oilfield. ACS Omega 2024, 9, 16006–16015. [Google Scholar]
- Xiong, Z.; Syed-Hassan, S.S.A.; Hu, X.; Guo, J.; Qiu, J.; Zhao, X.; Su, S.; Hu, S.; Wang, Y.; Xiang, J. Pyrolysis of the aromatic-poor and aromatic-rich fractions of bio-oil: Characterization of coke structure and elucidation of coke formation mechanism. Appl. Energy 2019, 239, 981–990. [Google Scholar]
- Yu, H.; Wang, W.; Lin, F.; Li, K.; Yan, B.; Song, Y.; Huang, C.; Chen, G. A facile and green strategy to synthesize N/P co-doped bio-porous carbon with high yield from fungi residue for efficient VOC adsorption. Sep. Purif. Technol. 2021, 276, 119291. [Google Scholar]
- Yusop MF, M.; Abdullah, A.Z.; Ahmad, M.A. Amoxicillin adsorption from aqueous solution by Cu (II) modified lemon peel based activated carbon: Mass transfer simulation, surface area prediction and F-test on isotherm and kinetic models. Powder Technol. 2024, 438, 119589. [Google Scholar]
- Sabitov, A.; Atamanov, M.; Doszhanov, O.; Saurykova, K.; Tazhu, K.; Kerimkulova, A.; Orazbayev, A.; Doszhanov, Y. Surface characteristics of activated carbon sorbents obtained from biomass for cleaning oil-contaminated soils. Molecules 2024, 29, 3786. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jia, Z.; Yu, B.; Zhang, W.; Zhang, L.; Chen, P.; Xu, L. Research progress of nanoparticles enhanced carbon dioxide foam stability and assisted carbon dioxide storage: A review. Chem. Eng. J. 2024, 495, 153177. [Google Scholar]
- Fu, X.; Zamani, P.; Choi, J.; Hassan, F.M.; Jiang, G.; Higgins, D.C.; Zhang, Y.; Hoque, A.; Chen, Z. In situ polymer graphenization ingrained with nanoporosity in a nitrogenous electrocatalyst boosting the performance of polymer-electrolyte-membrane fuel cells. Adv. Mater. 2017, 29, 1604456. [Google Scholar] [CrossRef]
- Chen, G.; Yu, H.; Lin, F.; Zhang, Z.; Yan, B.; Song, Y. Utilization of edible fungi residues towards synthesis of high-performance porous carbon for effective sorption of Cl-VOCs. Sci. Total Environ. 2020, 727, 138475. [Google Scholar]
- Yan, W.; Wu, Y.; Chen, Y.; Liu, Q.; Wang, K.; Cao, N.; Dai, F.; Li, X.; Jiang, J. Facile preparation of N-doped corncob-derived carbon nanofiber efficiently encapsulating Fe2O3 nanocrystals towards high ORR electrocatalytic activity. J. Energy Chem. 2020, 44, 121–130. [Google Scholar]
- Liang, B.; Su, M.; Zhao, Z.; Liang, S.X. Iron-involved zeolitic imidazolate framework-67 derived Co/Fe-NC as enhanced ORR catalyst in air–cathode microbial fuel cell. J. Electroanal. Chem. 2024, 962, 118260. [Google Scholar]

| Catalyst | SSA (m2·g−1) a | Vtotal (cm3·g−1) b | Vmicro (cm3·g−1) c | Vmeso (cm3·g−1) d | Production Rate of Carbon (%) |
|---|---|---|---|---|---|
| DC-N | 417.69 | 0.374 | 0.190 | 0.159 | 13.3 |
| DC-0 | 1242.55 | 0.730 | 0.566 | 0.156 | / |
| DC-6 | 1596.40 | 0.934 | 0.694 | 0.187 | 24.3 |
| DC-12 | 515.89 | 0.373 | 0.230 | 0.130 | 19.5 |
| DY-6 | 779.99 | 0.597 | 0.356 | 0.260 | 25.5 |
| DR-6 | 726.48 | 0.533 | 0.331 | 0.209 | 23.6 |
| Catalyst | Surface Chemical Composition a (Atomic Ratio at.%) | |||
|---|---|---|---|---|
| C | O | N | Fe | |
| DC-N | 89.49 | 7.72 | 2.04 | 0.38 |
| DC-0 | 89.16 | 7.30 | 2.24 | 0.28 |
| DC-6 | 85.17 | 8.24 | 5.24 | 0.41 |
| DC-12 | 90.98 | 6.91 | 1.65 | 0.47 |
| DY-6 | 91.31 | 6.28 | 1.98 | 0.43 |
| DR-6 | 91.58 | 4.19 | 1.94 | 0.33 |
| Catalyst | Epeaks a [2] | E1/2 b [2] | J0.3V b (mA·cm−2) |
|---|---|---|---|
| DC-N | 0.669 | 0.641 | 2.762 |
| DC-0 | 0.811 | 0.781 | 5.391 |
| DC-6 | 0.864 | 0.822 | 5.895 |
| DC-12 | 0.640 | 0.610 | 2.758 |
| DY-6 | 0.731 | 0.703 | 3.691 |
| DR-6 | 0.661 | 0.649 | 2.889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Li, H.; Jia, X.; Lin, F.; Li, K. Recovery of Waste-Activated Carbon for Synthesizing High-Efficiency ORR Electrocatalyst. Energies 2025, 18, 1666. https://doi.org/10.3390/en18071666
Tang Z, Li H, Jia X, Lin F, Li K. Recovery of Waste-Activated Carbon for Synthesizing High-Efficiency ORR Electrocatalyst. Energies. 2025; 18(7):1666. https://doi.org/10.3390/en18071666
Chicago/Turabian StyleTang, Ziyu, Haowen Li, Xiaojing Jia, Fawei Lin, and Kai Li. 2025. "Recovery of Waste-Activated Carbon for Synthesizing High-Efficiency ORR Electrocatalyst" Energies 18, no. 7: 1666. https://doi.org/10.3390/en18071666
APA StyleTang, Z., Li, H., Jia, X., Lin, F., & Li, K. (2025). Recovery of Waste-Activated Carbon for Synthesizing High-Efficiency ORR Electrocatalyst. Energies, 18(7), 1666. https://doi.org/10.3390/en18071666





