Research Progress and Perspectives of the Reaction Kinetics of Fe-Based Oxygen Carriers in Chemical Looping Combustion
Abstract
1. Introduction
2. Reaction Kinetics of Fe-Based Oxygen Carriers
2.1. Kinetic Models of Fe-Based Oxygen Carrier Reaction
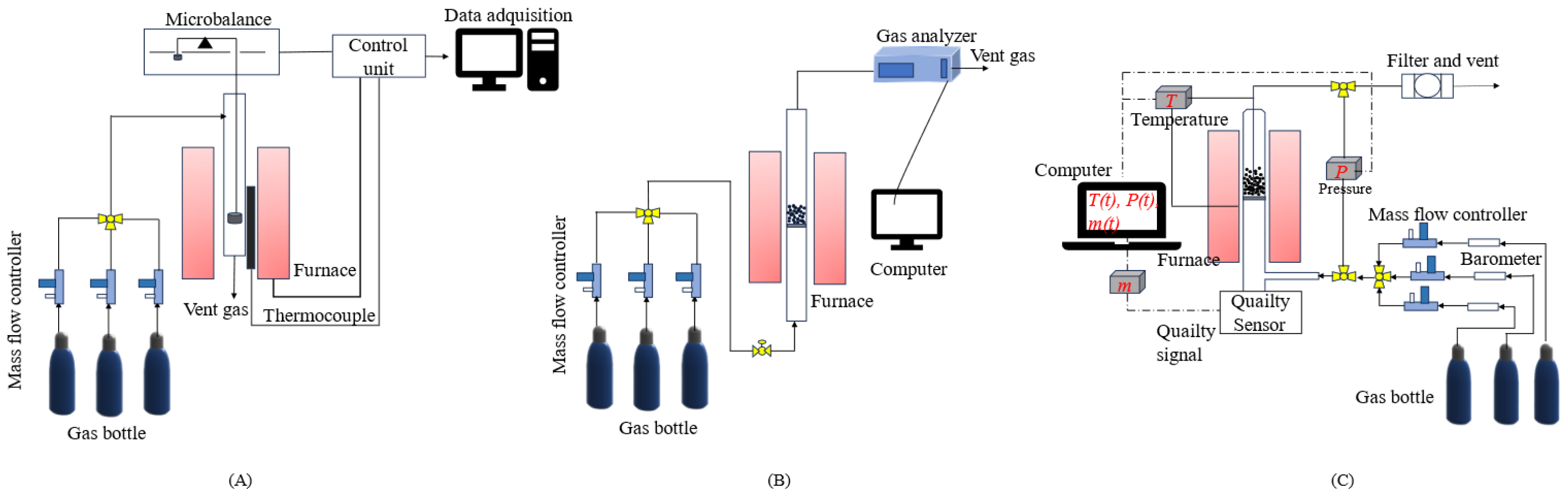
2.2. Reaction Kinetics of Fe-Based Oxygen Carriers in the TGA
2.3. Reaction Kinetics of Fe-Based Oxygen Carriers in Fluidized Bed Reactors
3. Effect of Different Reaction Conditions on the Reaction Kinetics of Fe-Based Oxygen Carriers
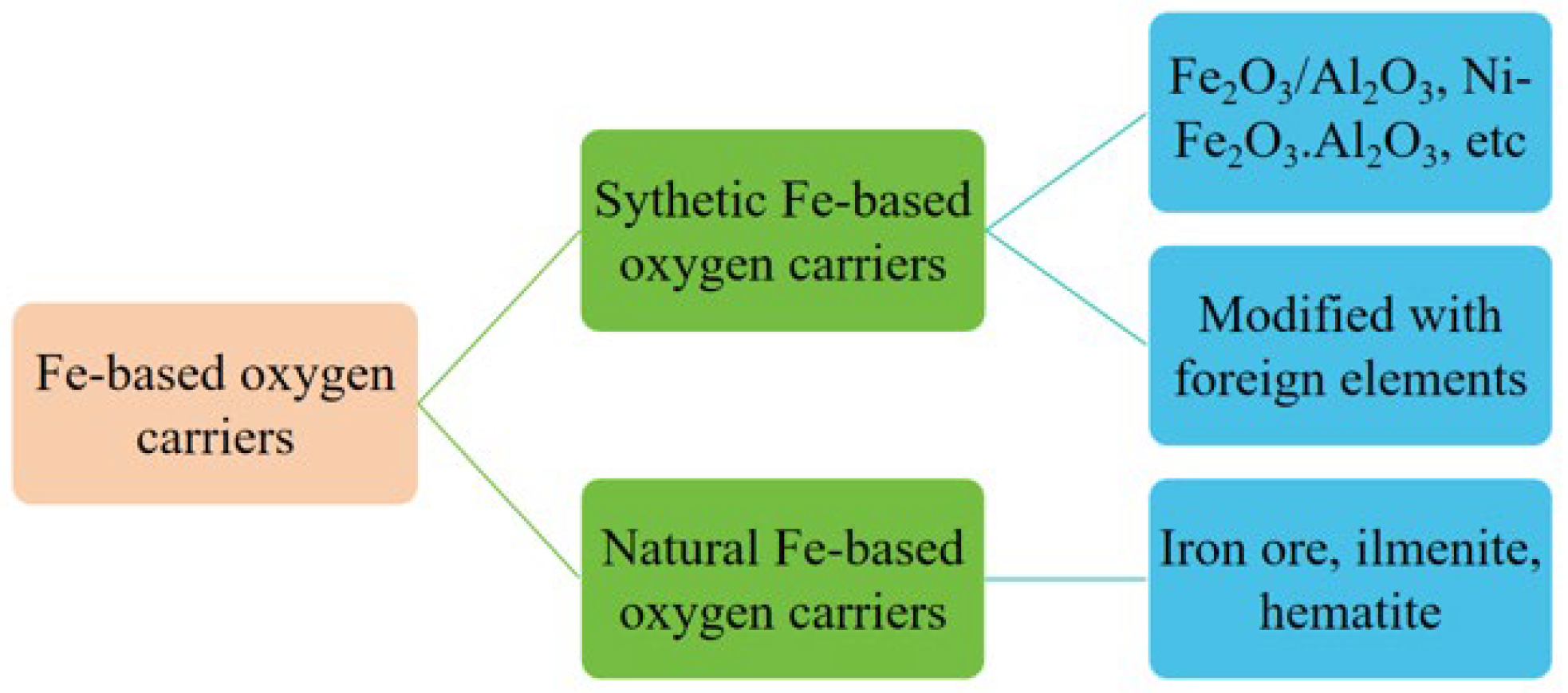
3.1. Reaction Kinetics of Natural Fe-Based Oxygen Carriers
3.2. Reaction Kinetics of Synthetic Fe-Based Oxygen Carriers
3.3. Effect of Temperature and Gas Concentration on Reaction Kinetics of Fe-Based Oxygen Carriers
3.4. Research on the Interaction and the Effect of Properties of Ash with Fe-Based Oxygen Carrier
4. Conclusions and Future Prospects
- (1)
- The measurement methods for characterizing the reaction kinetics of oxygen carriers primarily include TGA, FB reactors, and FB-TGA. For the Fe-based oxygen carriers, TGA determines kinetic parameters by tracking mass changes in real-time; however, it faces challenges such as low mass and heat transfer rates. In contrast, FB reactors evaluate the reaction process and derive kinetic parameters by measuring the concentration of gas components at the outlet. However, it is difficult to achieve real-time and accurate measurements compared with TGA. The activation energy derived from TGA could differ significantly from that obtained using fluidized bed reactors; hence, kinetic parameters obtained from TGA are not advisable for direct application in fluidized bed simulations.
- (2)
- A distinction exists between intrinsic and apparent reaction kinetics. Existing studies on intrinsic reaction kinetics primarily focus on pure Fe2O3, hematite, and ilmenite, and a broad investigation on the widely utilized materials is required.
- (3)
- Due to the relatively low mechanical stability of Fe-based oxygen carriers, a common strategy to enhance the reaction performance of Fe-based oxygen carriers is the incorporation of inert supports. The addition of inert supports can alleviate problems such as the sintering of Fe-based oxygen carriers at high temperatures and improve their cyclic stability. However, partially inert supports may impose a restrictive effect on the active component Fe2O3 in the Fe-based oxygen carrier, thereby influencing the amount of active components required for the reaction. Consequently, the apparent activation energy is generally higher than that of pure Fe2O3.
- (4)
- In FB reactors, attrition is a critical factor affecting the service life of Fe-based oxygen carriers. Specifically for ilmenite, during the reaction process, active iron components migrate to the surface, while particle collisions within the FB reactor may lead to attrition, resulting in a decrease in active component content and the reaction kinetics. However, research on the impact of attrition on the reaction kinetics of Fe-based oxygen carriers remains limited, necessitating the need for further investigation.
- (5)
- During the long-term operation of CLC, it is inevitable that solid fuel ash interacts with the oxygen carrier, leading to physical and chemical changes in the carrier. The interactions may affect the reaction kinetics of the oxygen carrier. Existing studies primarily focus on the mechanisms of ash–oxygen carrier interactions and their effects on the surface morphology of oxygen carriers, while investigations into their impact on reaction kinetics are required. Current studies on the interaction between ash and Fe-based oxygen carriers have primarily focused on two aspects: microstructural modifications after adding ash, which are analyzed using DFT calculations, and alterations in reaction mechanisms and reactivities after adding ash, which are evaluated using experimental studies. However, few studies have integrated the results based on DFT calculations with experimental results to explore the interactions of ash and oxygen carriers. It is recommended to combine experimental and DFT methodologies to systematically elucidate the influences of ash on the kinetics of Fe-based oxygen carriers in future work.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, A.; Fyfe, J.C.; Xie, S.-P.; Dai, X. Decadal modulation of global surface temperature by internal climate variability. Nat. Clim. Change 2015, 5, 555–559. [Google Scholar]
- Netz, B.; Davidson, O.R.; Bosch, P.R.; Dave, R.; Meyer, L.A. Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2007. [Google Scholar]
- Zhao, H.; Tian, X.; Ma, J.; Chen, X.; Su, M.; Zheng, C.; Wang, Y. Chemical looping combustion of coal in China: Comprehensive progress, remaining challenges, and potential opportunities. Energy Fuels 2020, 34, 6696–6734. [Google Scholar]
- Lyngfelt, A.; Brink, A.; Langørgen, Ø.; Mattisson, T.; Rydén, M.; Linderholm, C. 11,000 h of chemical-looping combustion operation—Where are we and where do we want to go? Int. J. Greenh. Gas Control 2019, 88, 38–56. [Google Scholar]
- Adánez, J.; Abad, A. Chemical-looping combustion: Status and research needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [Google Scholar]
- Richter, H.J.; Knoche, K.F. Reversibility of combustion processes. In Efficiency and Costing; ACS Publications: Washington, DC, USA, 1983. [Google Scholar]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar]
- Arjmand, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Investigation of different manganese ores as oxygen carriers in chemical-looping combustion (CLC) for solid fuels. Appl. Energy 2014, 113, 1883–1894. [Google Scholar]
- Ishida, M.; Jin, H. A new advanced power generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar]
- Lewis, W.; Gilliland, E.; Paxton, R. Low-temperature oxidation of carbon. Ind. Eng. Chem. 1954, 46, 1327–1331. [Google Scholar]
- Ishida, M.; Jin, H. A novel combustor based on chemicallooping reactions and its reaction kinetics. J. Chem. Eng. Jpn. 1994, 27, 296–301. [Google Scholar]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation;application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar]
- Lyngfelt, A.; Kronberger, B.; Adanez, J.; Morin, J.X.; Hurst, P. The grace project: Development of oxygen carrier particles for chemical-looping combustion. Design and operation of a 10 kW chemical-looping combustor. In Greenhouse Gas Control Technologies 7; Elsevier: Amsterdam, The Netherlands, 2005; pp. 115–123. [Google Scholar]
- Ryu, H.-J.; Jin, G.-T.; Yi, C.-K. Demonstration of inherent CO2 separation and no NOx emission in a 50kW chemical looping combustor: Continuous reduction and oxidation experiment. In Greenhouse Gas Control Technologies 7; Elsevier: Amsterdam, The Netherlands, 2005; Volume II, pp. 1907–1910. [Google Scholar]
- Berguerand, N.; Lyngfelt, A. Design and operation of a 10kWth chemical-looping combustor for solid fuels—Testing with South African coal. Fuel 2008, 87, 2713–2726. [Google Scholar]
- Berguerand, N.; Lyngfelt, A. The use of petroleum coke as fuel in a 10kWth chemical-looping combustor. Int. J. Greenh. Gas Control 2008, 2, 169–179. [Google Scholar]
- Shen, L.; Wu, J.; Xiao, J. Chemical-Looping Combustion of Biomass in a 10 kWth Reactor with Iron Oxide As an Oxygen Carrier. Energy Fuels 2009, 23, 2498–2505. [Google Scholar]
- Abdulally, I.; Beal, C.; Andrus, H.; Epple, B.; Lyngfelt, A.; Lani, B. Alstom’s chemical looping prototypes, program update. In Proceedings of the 37th International Technical Conference on Clean Coal & Fuel Systems, Clearwater, FL, USA, 31 May–4 June 2012. [Google Scholar]
- Ströhle, J.; Orth, M.; Epple, B. Design and operation of a 1 MWth chemical looping plant. Appl. Energy 2014, 113, 1490–1495. [Google Scholar]
- Li, Z. Demonstration of 5 MW chemical looping combustion for solid fuel. In Proceedings of the 14th International Conference on Circulating Fluidized Bed Technology (CFB-14), Taiyuan, China, 21–24 July 2024. [Google Scholar]
- Li, Z.; Li, W.; Liu, H.; Duan, Q.; Wang, Y.; Li, J.; Yang, Y.; Cai, N. Development and prospect of chemical looping combustion technology in china. Proc. CSEE 2024, 44, 7200–7220. [Google Scholar]
- Kronberger, B.; Johansson, E.; Löffler, G.; Mattisson, T.; Lyngfelt, A.; Hofbauer, H. A two-compartment fluidized bed reactor for CO2 capture by chemical-looping combustion. Chem. Eng. Technol. 2004, 27, 1318–1326. [Google Scholar]
- Adánez, J.; Gayán, P.; Celaya, J.; de Diego, L.F.; García-Labiano, F.; Abad, A. Chemical looping combustion in a 10 kWth prototype using a CuO/Al2O3 oxygen carrier: Effect of operating conditions on methane combustion. Ind. Eng. Chem. Res. 2006, 45, 6075–6080. [Google Scholar]
- Bolhàr-Nordenkampf, J.; Pröll, T.; Kolbitsch, P.; Hofbauer, H. Performance of a NiO-based oxygen carrier for chemical looping combustion and reforming in a 120 kW unit. Energy Procedia 2009, 1, 19–25. [Google Scholar]
- Lyngfelt, A. Chemical-looping combustion of solid fuels—Status of development. Appl. Energy 2014, 113, 1869–1873. [Google Scholar]
- Lyngfelt, A.; Linderholm, C. Chemical-Looping Combustion of solid fuels—Status and Recent Progress. Energy Procedia 2017, 114, 371–386. [Google Scholar]
- Shen, L.; Wu, J.; Xiao, J. Experiments on chemical looping combustion of coal with a NiO based oxygen carrier. Combust. Flame 2009, 156, 721–728. [Google Scholar]
- Thon, A.; Kramp, M.; Hartge, E.-U.; Heinrich, S.; Werther, J. Operational experience with a system of coupled fluidized beds for chemical looping combustion of solid fuels using ilmenite as oxygen carrier. Appl. Energy 2014, 118, 309–317. [Google Scholar]
- Linderholm, C.; Lyngfelt, A.; Cuadrat, A.; Jerndal, E. Chemical-looping combustion of solid fuels—Operation in a 10kW unit with two fuels, above-bed and in-bed fuel feed and two oxygen carriers, manganese ore and ilmenite. Fuel 2012, 102, 808–822. [Google Scholar]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Solid fuels in chemical-looping combustion. Int. J. Greenh. Gas Control 2008, 2, 180–193. [Google Scholar]
- Bayham, S.; McGiveron, O.; Tong, A.; Chung, E.; Kathe, M.; Wang, D.; Zeng, L.; Fan, L.-S. Parametric and dynamic studies of an iron-based 25-kWth coal direct chemical looping unit using sub-bituminous coal. Appl. Energy 2015, 145, 354–363. [Google Scholar]
- Kim, H.R.; Wang, D.; Zeng, L.; Bayham, S.; Tong, A.; Chung, E.; Kathe, M.V.; Luo, S.; McGiveron, O.; Wang, A.; et al. Coal direct chemical looping combustion process: Design and operation of a 25-kWth sub-pilot unit. Fuel 2013, 108, 370–384. [Google Scholar]
- Mayer, F.; Bidwe, A.R.; Schopf, A.; Taheri, K.; Zieba, M.; Scheffknecht, G. Comparison of a new micaceous iron oxide and ilmenite with respect to syngas conversion in a BFB reactor and adaptation of a 10 kWth DFB system for CLC to solid fuels. In Proceedings of the 2nd International Conference on Chemical Looping, Darmstadt, Germany, 25–27 September 2012. [Google Scholar]
- Lighty, J.; Whitty, K.; Smith, P.; Eyring, T. Chemical looping with oxygen uncoupling with coal. In Proceedings of the NETL CO2 Capture Technology Meeting, Pittsburgh, PA, USA, 9–12 July 2012. [Google Scholar]
- Thunman, H.; Lind, F.; Breitholtz, C.; Berguerand, N.; Seemann, M. Using an oxygen-carrier as bed material for combustion of biomass in a 12-MWth circulating fluidized-bed boiler. Fuel 2013, 113, 300–309. [Google Scholar]
- Wang, P.; Leion, H.; Yang, H. Oxygen-carrier-aided combustion in a bench-scale fluidized bed. Energy Fuels 2017, 31, 6463–6471. [Google Scholar]
- Garcia, E.; Liu, H. Ilmenite as alternative bed material for the combustion of coal and biomass blends in a fluidised bed combustor to improve combustion performance and reduce agglomeration tendency. Energy 2022, 239, 121913. [Google Scholar]
- Gyllén, A. Oxygen Carrier Aided Combustion: Implementation of Oxygen Carriers to Existing Industrial Settings. Ph.D. Thesis, Chalmers Tekniska Hogskola, Gothenburg, Sweden, 2019. [Google Scholar]
- Duan, L.; Li, L. Oxygen-Carrier-Aided Combustion Technology for Solid-Fuel Conversion in Fluidized Bed; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Moldenhauer, P.; Corcoran, A.; Thunman, H.; Lind, F. A Scale-Up Project for Operating a 115 MWth Biomass-Fired CFB boiler with Oxygen Carriers as Bed Material. In Proceedings of theProceedings of the 5th International Conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018. [Google Scholar]
- Zhang, T.; Song, T. Research progress of chemical looping combustion. Electr. Power Technol. Environ. Prot. 2024, 40, 582–590. [Google Scholar]
- Li, Z.; Han, H.; Cai, N. Research status and progress of chemical-looping combustion. J. Power Eng. 2006, 4, 538–543. [Google Scholar]
- Leion, H.; Lyngfelt, A.; Johansson, M.; Jerndal, E.; Mattisson, T. The use of ilmenite as an oxygen carrier in chemical-looping combustion. Chem. Eng. Res. Des. 2008, 86, 1017–1026. [Google Scholar]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T.; Chen, D. Iron ore as oxygen carrier improved with potassium for chemical looping combustion of anthracite coal. Combust. Flame 2012, 159, 2480–2490. [Google Scholar]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T. Chemical looping combustion of biomass/coal with natural iron ore as oxygen carrier in a continuous reactor. Energy Fuels 2010, 25, 446–455. [Google Scholar]
- Fraga-Cruz, G.S.; Pérez-Méndez, M.A.; Jiménez-García, G.; Huirache-Acuña, R.; Nápoles-Rivera, F.; Espino-Valencia, J.; Maya-Yescas, R. Integration of Chemical Looping Combustion to a Gasified Stream with Low Hydrogen Content. Processes 2024, 12, 1033. [Google Scholar] [CrossRef]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Johansson, M. The use of iron oxide as oxygen carrier in a chemical-looping reactor. Fuel 2007, 86, 1021–1035. [Google Scholar]
- Xuan, W.; Zhang, J. Reduction characterization of iron-based oxygen carriers. J. Eng. Therm. Energy Power, 2014; 29, 81–85+110–111. [Google Scholar]
- Wang, B.; Zhao, H.; Zheng, Y.; Liu, Z.; Yan, R.; Zheng, C. Chemical looping combustion of a Chinese anthracite with Fe2O3-based and CuO-based oxygen carriers. Fuel Process. Technol. 2012, 96, 104–115. [Google Scholar]
- Gu, H.M.; Shen, L.H.; Xiao, J.; Zhang, S.W.; Song, T. Cycle experiments on chemical looping combustion of coal using potassium-improved iron ore as oxygen carrier. J. Fuel Chem. Technol. 2012, 40, 927–934. [Google Scholar]
- Chen, D.-Q.; Shen, L.-H.; Xiao, J.; Song, T.; Gu, H.-M.; Zhang, S.-W. Experimental investigation of hematite oxygen carrier decorated with NiO for chemical looping combustion of coal. J. Fuel Chem. Technol. 2012, 40, 267–272. [Google Scholar]
- Bidwe, A.R.; Mayer, F.; Hawthorne, C.; Charitos, A.; Schuster, A.; Scheffknecht, G. Use of ilmenite as an oxygen carrier in chemical looping combustion-batch and continuous dual fluidized bed investigation. Energy Procedia 2011, 4, 433–440. [Google Scholar]
- Cuadrat, A.; Abad, A.; Adánez, J.; de Diego, L.; García-Labiano, F.; Gayán, P. Behavior of ilmenite as oxygen carrier in chemical-looping combustion. Fuel Process. Technol. 2012, 94, 101–112. [Google Scholar]
- Bao, J.; Li, Z.; Sun, H.; Cai, N. Continuous test of ilmenite-based oxygen carriers for chemical looping combustion in a dual fluidized bed reactor system. Ind. Eng. Chem. Res. 2013, 52, 14817–14827. [Google Scholar]
- Cabello, A.; Gayán, P.; García-Labiano, F.; de Diego, L.; Abad, A.; Adánez, J. On the attrition evaluation of oxygen carriers in Chemical Looping Combustion. Fuel Process. Technol. 2016, 148, 188–197. [Google Scholar]
- Li, H.; Sun, Z.; Tian, L.; Gao, L.; Xu, Y.; Cao, Y. The investigation on the attrition of hematite oxygen carrier particles in a fluidization-based chemical looping system. Fuel Process. Technol. 2022, 236, 107441. [Google Scholar]
- Hatanaka, T.; Yoda, Y. Attrition of ilmenite ore during consecutive redox cycles in chemical looping combustion. Powder Technol. 2019, 356, 974–979. [Google Scholar]
- Nelson, T.; van der Watt, J.G.; Laudal, D.; Feilen, H.; Mann, M.; Srinivasachar, S. Reactive jet and cyclonic attrition analysis of ilmenite in chemical looping combustion systems. Int. J. Greenh. Gas Control 2019, 91, 102837. [Google Scholar]
- Shen, T.; Qin, C.; Song, T.; Sun, D. Periodic abrasion and agglomeration development in hematite and ilmenite oxygen carriers: Long-Term redox cycling analysis via fluidized bed TGA. Fuel 2025, 381, 133400. [Google Scholar]
- Bao, J.; Li, Z.; Cai, N. Reduction kinetics of foreign-ion-promoted ilmenite using carbon monoxide (CO) for chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 10646–10655. [Google Scholar]
- Ei-Geassy, A.A.; Shehata, K.A.; Ezz, S.Y. Mechanism of iron oxide reduction with hydrogen/carbon monoxide mixtures. Trans. Iron Steel Inst. Jpn. 1977, 17, 629–635. [Google Scholar]
- Et-Tabirou, M.; Dupré, B.; Gleitzer, C. Hematite single crystal reduction into magnetite with CO-CO2. Metall. Trans. B 1988, 19, 311–317. [Google Scholar]
- Wang, B.; Lv, H.; Zhao, H.; Zheng, C. Experimental and Simulated Investigation of Chemical Looping Combustion of Coal with Fe2O3 based Oxygen Carrier. Procedia Eng. 2011, 16, 390–395. [Google Scholar]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Reduction of hematite (Fe2O3) to wüstite (FeO) by carbon monoxide (CO) for chemical looping combustion. Chem. Eng. J. 2014, 242, 204–210. [Google Scholar]
- Chen, H.; Zheng, Z.; Shi, W. Investigation on the Kinetics of iron ore fines reduction by CO in a micro-fluidized bed. Procedia Eng. 2015, 102, 1726–1735. [Google Scholar]
- Zhang, Z.; Yao, J.G.; Boot-Handford, M.E.; Fennell, P.S. Pressurised chemical-looping combustion of an iron-based oxygen carrier: Reduction kinetic measurements and modelling. Fuel Process. Technol. 2018, 171, 205–214. [Google Scholar]
- García-Labiano, F.; de Diego, L.F.; Adánez, J.; Abad, A.; Gayán, P. Reduction and oxidation kinetics of a copper-based oxygen carrier prepared by impregnation for chemical-looping combustion. Ind. Eng. Chem. Res. 2004, 43, 8168–8177. [Google Scholar]
- Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction kinetics of Cu-, Ni-, and Fe-based oxygen carriers using syngas (CO + H2) for chemical-looping combustion. Energy Fuels 2007, 21, 1843–1853. [Google Scholar]
- Su, M.; Zhao, H.; Tian, X.; Zhang, P.; Du, B.; Liu, Z. Intrinsic reduction kinetics investigation on a hematite oxygen carrier by CO in chemical looping combustion. Energy Fuels 2017, 31, 3010–3018. [Google Scholar]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar]
- Zafar, Q.; Abad, A.; Mattisson, T.; Gevert, B.; Strand, M. Reduction and oxidation kinetics of Mn3O4/Mg–ZrO2 oxygen carrier particles for chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 6556–6567. [Google Scholar]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P. Modeling of the chemical-looping combustion of methane using a Cu-based oxygen-carrier. Combust. Flame 2010, 157, 602–615. [Google Scholar]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar]
- Li, Z.; Cai, N. Rate equation theory for gas-solid reaction kinetics. J. Tsinghua Univ. Sci. Technol. 2022, 62, 704–721. [Google Scholar]
- Lee, D. An apparent kinetic model for the carbonation of calcium oxide by carbon dioxide. Chem. Eng. J. 2004, 100, 71–77. [Google Scholar]
- García-Labiano, F.; de Diego, L.F.; Adánez, J.; Abad, A.; Gayán, P. Temperature variations in the oxygen carrier particles during their reduction and oxidation in a chemical-looping combustion system. Chem. Eng. Sci. 2005, 60, 851–862. [Google Scholar]
- Habermann, A.; Winter, F.; Hofbauer, H.; Zirngast, J.; Schenk, J.L. An experimental study on the kinetics of fluidized bed iron ore reduction. ISIJ Int. 2000, 40, 935–942. [Google Scholar]
- Zhu, Q. Chemical-Looping Gasification of Sludge/Coal with Iron-Based Oxygen Carrier. Master’s Thesis, Ning Xia University, Ningxia, China, 2021. [Google Scholar]
- Mei, D. Experimental and Kinetics Investigation of Iron/Copper/Manganese Based Oxygen Carrier for Chemical Looping Combustion of Coal. Ph.D. Thesis, Huazhong University of Science and Technology, Hubei, China, 2015. [Google Scholar]
- Mei, D.; Zhao, H.; Yan, S. Kinetics model for the reduction of Fe2O3/Al2O3 by CO in Chemical Looping Combustion. Chem. Eng. Process. Process Intensif. 2018, 124, 137–146. [Google Scholar]
- Ksepko, E.; Sciazko, M.; Babinski, P. Studies on the redox reaction kinetics of Fe2O3–CuO/Al2O3 and Fe2O3/TiO2 oxygen carriers. Appl. Energy 2014, 115, 374–383. [Google Scholar]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R.; Richards, G.; Carpenter, S. Kinetics of the reduction of hematite (Fe2O3) by methane (CH4) during chemical looping combustion: A global mechanism. Chem. Eng. J. 2013, 232, 478–487. [Google Scholar]
- Su, M.; Ma, J.; Tian, X.; Zhao, H. Reduction kinetics of hematite as oxygen carrier in chemical looping combustion. Fuel Process. Technol. 2017, 155, 160–167. [Google Scholar]
- Piotrowski, K.; Mondal, K.; Lorethova, H.; Stonawski, L.; Szymanski, T.; Wiltowski, T. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process. Int. J. Hydrogen Energy 2005, 30, 1543–1554. [Google Scholar]
- Piotrowski, K.; Mondal, K.; Wiltowski, T.; Dydo, P.; Rizeg, G. Topochemical approach of kinetics of the reduction of hematite to wüstite. Chem. Eng. J. 2007, 131, 73–82. [Google Scholar]
- Li, Z.; Sun, H.; Cai, N. Rate Equation Theory for the Carbonation Reaction of CaO with CO2. Energy Fuels 2012, 26, 4607–4616. [Google Scholar]
- Li, Z. General rate equation theory for gas–solid reaction kinetics and its application to CaO carbonation. Chem. Eng. Sci. 2020, 227, 115902. [Google Scholar]
- Li, Z. First-principles-based microkinetic rate equation theory for oxygen carrier reduction in chemical looping. Chem. Eng. Sci. 2022, 247, 117042. [Google Scholar]
- Li, Z.; Cai, J.; Liu, L. A First-principles microkinetic rate equation theory for heterogeneous reactions: Application to reduction of Fe2O3 in chemical looping. Ind. Eng. Chem. Res. 2021, 60, 15514–15524. [Google Scholar]
- Wang, X.; Chen, Z.; Hu, M.; Tian, Y.; Jin, X.; Ma, S.; Xu, T.; Hu, Z.; Liu, S.; Guo, D.; et al. Chemical looping combustion of biomass using metal ferrites as oxygen carriers. Chem. Eng. J. 2017, 312, 252–262. [Google Scholar]
- Bao, J.; Li, Z.; Cai, N. Promoting the reduction reactivity of ilmenite by introducing foreign ions in chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 6119–6128. [Google Scholar]
- Zhou, Z.; Deng, G.; Li, L.; Liu, X.; Sun, Z.; Duan, L. Chemical looping co-conversion of CH4 and CO2 using Fe2O3/Al2O3 pellets as both oxygen carrier and catalyst in a fluidized bed reactor. Chem. Eng. J. 2022, 428, 132133. [Google Scholar]
- Shen, T.; Shen, L. Review of application of FB-TGA in chemical looping combustion reaction kinetics. J. Huazhong Univ. Sci. Technol. Nat. Sci. Ed. 2023, 51, 133–145. [Google Scholar]
- Li, Y.; Li, Z.; Liu, L.; Cai, N. Measuring the fast oxidation kinetics of a manganese oxygen carrier using microfluidized bed thermogravimetric analysis. Chem. Eng. J. 2020, 385, 123970. [Google Scholar]
- Wang, X. Experimental and Modeling Investigation of Coal-Fired Chemical-Looping Combustion Based on the High-Flux Circulating Fluidized Bed Technology. Master’s Thesis, Southeast University, Nanjing, China, 2017. [Google Scholar]
- Zhu, Q. Study on Oxygen Release Performance and Microscopic Mechanism of Iron—Based Oxygen Carrier. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2021. [Google Scholar]
- Zhao, X.; Bu, C.; Wang, X.; Zhang, X.; Cheng, X.; Wang, N.; Piao, G. Kinetics investigation on iron-based oxygen carrier aided oxy-fuel combustion of anthracite char. CIESC J. 2022, 73, 384–392. [Google Scholar]
- Liu, H. The Performance Research on Reaction of Fe-Based Oxygen Carrier and CO in Chemical-Looping Combustion Process. Master’s Thesis, North China Electric Power University, Beijing, China, 2012. [Google Scholar]
- Liu, F.; Zhang, Y.; Chen, L.; Qian, D.; Neathery, J.K.; Kozo, S.; Liu, K. Investigation of a canadian ilmenite as an oxygen carrier for chemical looping combustion. Energy Fuels 2013, 27, 5987–5995. [Google Scholar]
- Ksepko, E.; Babiński, P.; Nalbandian, L. The redox reaction kinetics of Sinai ore for chemical looping combustion applications. Appl. Energy 2017, 190, 1258–1274. [Google Scholar]
- Steiner, T.; Schulze, K.; Kienzl, N.; Pauritsch, M.; Hacker, V.; Bock, S.; Abad, A.; Scharler, R.; Anca-Couce, A. Chemical looping of synthetic ilmenite, Part I: Addressing challenges of kinetic TGA measurements with H2. Fuel 2024, 368, 131528. [Google Scholar]
- Khakpoor, N.; Mostafavi, E.; Mahinpey, N.; De la Hoz Siegler, H. Oxygen transport capacity and kinetic study of ilmenite ores for methane chemical-looping combustion. Energy 2019, 169, 329–337. [Google Scholar]
- Dilmaç, N. Isothermal and non-isothermal reduction kinetics of iron ore oxygen carrier by CO: Modelistic and model-free approaches. Fuel 2021, 296, 120707. [Google Scholar]
- Luo, M.; Wang, S.; Wang, L.; Lv, M. Reduction kinetics of iron-based oxygen carriers using methane for chemical-looping combustion. J. Power Sources 2014, 270, 434–440. [Google Scholar]
- Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Reduction and oxidation kinetics of Tierga iron ore for chemical looping combustion with diverse fuels. Chem. Eng. J. 2019, 359, 37–46. [Google Scholar]
- Nasr, S.; Plucknett, K.P. Kinetics of iron ore reduction by methane for chemical looping combustion. Energy Fuels 2014, 28, 1387–1395. [Google Scholar]
- Cabello, A.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled Chemical Looping Combustion. Chem. Eng. J. 2014, 258, 265–280. [Google Scholar]
- Li, Y.; Wang, Y.; Liu, K.; Zhao, H. Reduction Kinetics of Low-Cost Cu/Fe-Based Oxygen Carriers in Chemical Looping Mode. Energy Fuels 2023, 37, 16716–16728. [Google Scholar]
- Moed, N.M.; Chiang, M.-H.; Ku, Y.; Tseng, Y.-H. Kinetics for chemical looping process with fabricated Fe2O3-CuO/Al2O3 oxygen carriers. Chem. Eng. Sci. 2022, 258, 117730. [Google Scholar]
- Li, Q.; Qin, W.; Zhang, J.-J.; Cheng, W.-L.; Dong, C.-Q. Characteristics and kinetics of chemical looping combustion of Mn-doped Fe2O3 oxygen carrier with CO. J. Fuel Chem. Technol. 2014, 42, 932–937. [Google Scholar]
- Wang, Y.D.; Hua, X.N.; Zhao, C.C.; Fu, T.T.; Li, W.; Wang, W. Step-wise reduction kinetics of Fe2O3 by CO/CO2 mixtures for chemical looping hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 5667–5675. [Google Scholar]
- Choisez, L.; Hemke, K.; Özgün, Ö.; Pistidda, C.; Jeppesen, H.; Raabe, D.; Ma, Y. Hydrogen-based direct reduction of combusted iron powder: Deep pre-oxidation, reduction kinetics and microstructural analysis. Acta Mater. 2024, 268, 119752. [Google Scholar]
- Chai, Y.; Zhang, J.; Zhang, X.; Zhang, Z.; Luo, G.; An, S.; Bu, E. Non-isothermal kinetic study of pure-hydrogen reduction for various iron-containing raw materials. Int. J. Hydrogen Energy 2024, 81, 707–717. [Google Scholar]
- Abdel Halim, K.S.; Khedr, M.H.; Soliman, N.K. Reduction characteristics of iron oxide in nanoscale. Mater. Sci. Technol. 2010, 26, 445–452. [Google Scholar]
- Alonso, M.; Criado, Y.A.; Abanades, J.C.; Grasa, G. Undesired effects in the determination of CO2 carrying capacities of CaO during TG testing. Fuel 2014, 127, 52–61. [Google Scholar]
- Stanmore, B.; Gilot, P.; Prado, G. The influence of mass transfer in DTG combustion tests. Thermochim. Acta 1994, 240, 79–89. [Google Scholar]
- Cardona, M.; Boffito, D.C.; Patience, G.S. Thermogravimetric heat and mass transfer: Modeling of bitumen pyrolysis. Fuel 2015, 143, 253–261. [Google Scholar]
- Wu, L. Experimental Investigation on Reduction Reaction Kinetics and Hydrogen Generation Process of Iron Ore as Oxygen Carrier. Master’s Thesis, Southeast University, Nanjing, China, 2017. [Google Scholar]
- Schwebel, G.L.; Sundqvist, S.; Krumm, W.; Leion, H. Apparent kinetics derived from fluidized bed experiments for Norwegian ilmenite as oxygen carrier. J. Environ. Chem. Eng. 2014, 2, 1131–1141. [Google Scholar] [CrossRef]
- Perreault, P.; Patience, G.-S. Ilmenite–CO reduction kinetics. Fuel 2016, 165, 166–172. [Google Scholar]
- Yu, J.-W.; Han, Y.-X.; Gao, P.; Li, Y.-J. Reductive Transformation of Hematite to Magnetite with CO/CO2 under fluidized bed conditions. J. Northeast. Univ. Nat. Sci. 2019, 40, 261–266. [Google Scholar]
- Zhang, Z.; Hills, T.P.; Scott, S.A.; Fennell, P.S. Spouted bed reactor for kinetic measurements of reduction of Fe2O3 in a CO2/CO atmosphere Part I: Atmospheric pressure measurements and equipment commissioning. Chem. Eng. Res. Des. 2016, 114, 307–320. [Google Scholar]
- Alsabak, B.M.J.; Dilmaç, N. Ferronickel production via selective reduction of turkish laterite ore by h2 in fluidized bed reactor: Isothermal kinetic modeling. Metall. Mater. Trans. B 2024, 56, 33–45. [Google Scholar]
- Spreitzer, D.; Schenk, J. Iron ore reduction by hydrogen using a laboratory scale fluidized bed reactor: Kinetic investigation—Experimental setup and method for determination. Metall. Mater. Trans. B 2019, 50, 2471–2484. [Google Scholar]
- Purnomo, V.; Mei, D.; Staničić, I.; Mattisson, T.; Leion, H. Effect of the conversion degree on the apparent kinetics of iron-based oxygen carriers. Energy Fuels 2024, 38, 11824–11836. [Google Scholar]
- Chiron, F.-X.; Patience, G.S. Kinetics of mixed copper–iron based oxygen carriers for hydrogen production by chemical looping water splitting. Int. J. Hydrogen Energy 2012, 37, 10526–10538. [Google Scholar]
- Cheng, Y.; Liu, Y.; Tian, H.; Guo, Q. Chemical-looping gasification reaction characteristics and mechanism of coal and Fe-based composite oxygen carriers. CIESC J. 2013, 64, 2587–2595. [Google Scholar]
- Feilmayr, C.; Thurnhofer, A.; Winter, F.; Mali, H.; Schenk, J. reduction behavior of hematite to magnetite under fluidized bed conditions. ISIJ Int. 2004, 44, 1125–1133. [Google Scholar]
- Narindri Rara Winayu, B.; Li, J.-D.; Chu, H. Fe-based oxygen carrier for the chemical looping combustion of CO, H2, and CH4 syngas in fluidized bed reactor under interruption of H2S. Chem. Eng. Res. Des. 2023, 194, 514–528. [Google Scholar]
- Narindri Rara Winayu, B.; Li, C.-T.; Chu, H. Effective performance of ilmenite oxygen carrier for chemical looping combustion of carbon monoxide, hydrogen, and methane in a fluidized bed reactor. J. Clean. Prod. 2022, 379, 134881. [Google Scholar]
- Chen, H.; Zheng, Z.; Chen, Z.; Bi, X.T. Reduction of hematite (Fe2O3) to metallic iron (Fe) by CO in a micro fluidized bed reaction analyzer: A multistep kinetics study. Powder Technol. 2017, 316, 410–420. [Google Scholar]
- Zeng, D.; Li, Y.; Zhang, Z.; Luo, C.; Liu, T.; Kong, Q.; Xiao, R. Bulk oxygen conduction kinetics of iron oxides on the chemical looping combustion. Fuel Process. Technol. 2022, 237, 107442. [Google Scholar]
- Liu, X.; Zhang, H.; Hong, H. Reduction kinetics of fe-based oxygen carriers using syngas in a honeycomb fixed-bed reactor for chemical-looping combustion. J. Therm. Sci. 2019, 29, 13–24. [Google Scholar]
- Fennell, P.S.; Kadchha, S.; Lee, H.Y.; Dennis, J.S.; Hayhurst, A.N. The measurement of the rate of burning of different coal chars in an electrically heated fluidised bed of sand. Chem. Eng. Sci. 2007, 62, 608–618. [Google Scholar]
- Latifi, M.; Chaouki, J. A novel induction heating fluidized bed reactor: Its design and applications in high temperature screening tests with solid feedstocks and prediction of defluidization state. AIChE J. 2015, 61, 1507–1523. [Google Scholar]
- Samih, S.; Chaouki, J. Development of a fluidized bed thermogravimetric analyzer. AIChE J. 2014, 61, 84–89. [Google Scholar]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. Can. J. Chem. Eng. 2019, 98, 34–43. [Google Scholar]
- Liu, L.; Li, Z.; Li, Z.; Larring, Y.; Li, Y.; Cai, N. Fast redox kinetics of a perovskite oxygen carrier measured using micro-fluidized bed thermogravimetric analysis. Proc. Combust. Inst. 2021, 38, 5259–5269. [Google Scholar]
- Li, Y.; Wang, H.; Li, W.; Li, Z.; Cai, N. CO2 gasification of a lignite char in microfluidized bed thermogravimetric analysis for chemical looping combustion and chemical looping with oxygen uncoupling. Energy Fuels 2018, 33, 449–459. [Google Scholar]
- Shen, T.; Yan, J.; Zhu, X.; Wang, L.; Shen, L. Catalytic combustion behaviors of petroleum coke with hematite catalyst in a micro fluidized bed thermogravimetric analysis. Chem. Eng. J. 2021, 422, 130087. [Google Scholar]
- Yan, J.; Shen, T.; Wang, P.; Yin, X.; Zhu, X.; Jiang, S.; Shen, L. Redox performance of manganese ore in a fluidized bed thermogravimetric analyzer for chemical looping combustion. Fuel 2021, 295, 120564. [Google Scholar]
- Liu, L.; Li, Z.; Li, Z.; Larring, Y.; Cai, N. Heterogeneous reaction kinetics of a perovskite oxygen carrier for chemical looping combustion coupled with oxygen uncoupling. Chem. Eng. J. 2021, 417, 128054. [Google Scholar]
- Wang, Y.; Li, Z.; Cai, N. Redox reaction kinetics of a Fe–Cu-based oxygen carrier measured with microfluidized bed thermogravimetric analysis. Energy Fuels 2022, 36, 9672–9686. [Google Scholar]
- Shen, T.; Shen, L.; Song, T. Multiscale gas–solid reaction dynamics of hematite oxygen carrier in chemical looping combustion from fluidized bed thermogravimetric analysis. Energy Fuels 2024, 38, 8909–8927. [Google Scholar]
- Liu, X.; Li, Z.; Shen, L.; Ma, J.; Liu, X.; He, D.; Zhao, H. Quantitative evaluation of four oxygen carriers for natural gas chemical looping combustion. Proc. Combust. Inst. 2024, 40, 105641. [Google Scholar]
- Ma, J.; Tian, X.; Wang, C.; Chen, X.; Zhao, H. Performance of a 50 kWth coal-fuelled chemical looping combustor. Int. J. Greenh. Gas Control 2018, 75, 98–106. [Google Scholar]
- Huang, Z.; He, F.; Feng, Y.; Zhao, K.; Zheng, A.; Chang, S.; Li, H. Synthesis gas production through biomass direct chemical looping conversion with natural hematite as an oxygen carrier. Bioresour. Technol. 2013, 140, 138–145. [Google Scholar]
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Experimental investigation on biomass gasification using chemical looping in a batch reactor and a continuous dual reactor. Chem. Eng. J. 2016, 286, 689–700. [Google Scholar]
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Biomass gasification using chemical looping in a 25 kW th reactor with natural hematite as oxygen carrier. Chem. Eng. J. 2016, 286, 174–183. [Google Scholar]
- Liu, W.; Lim, J.Y.; Saucedo, M.A.; Hayhurst, A.N.; Scott, S.A.; Dennis, J.S. Kinetics of the reduction of wüstite by hydrogen and carbon monoxide for the chemical looping production of hydrogen. Chem. Eng. Sci. 2014, 120, 149–166. [Google Scholar]
- Khani, M.; Ale Ebrahim, H.; Habibzadeh, S. A comprehensive random pore model kinetic study of hematite to iron reduction by hydrogen. Chem. Eng. Sci. 2023, 281, 119116. [Google Scholar]
- Yang, W.; Zhao, H.; Wang, K.; Zheng, C. Synergistic effects of mixtures of iron ores and copper ores as oxygen carriers in chemical-looping combustion. Proc. Combust. Inst. 2015, 35, 2811–2818. [Google Scholar]
- Rydén, M.; Johansson, M.; Cleverstam, E.; Lyngfelt, A.; Mattisson, T. Ilmenite with addition of NiO as oxygen carrier for chemical-looping combustion. Fuel 2010, 89, 3523–3533. [Google Scholar]
- Mattisson, T.; Johansson, M.; Lyngfelt, A. Multicycle reduction and oxidation of different types of iron oxide particlesapplication to chemical-looping combustion. Energy Fuels 2004, 18, 628–637. [Google Scholar]
- Yang, W.; Zhao, H.; Ma, J.; Mei, D.; Zheng, C. Copper-decorated hematite as an oxygen carrier for in situ gasification chemical looping combustion of coal. Energy Fuels 2014, 28, 3970–3981. [Google Scholar]
- Jiang, S.; Shen, L.; Wu, J.; Yan, J.; Song, T. The investigations of hematite-CuO oxygen carrier in chemical looping combustion. Chem. Eng. J. 2017, 317, 132–142. [Google Scholar]
- Bao, J.; Liu, W.; Cleeton, J.P.E.; Scott, S.A.; Dennis, J.S.; Li, Z.; Cai, N. Interaction between Fe-based oxygen carriers and n-heptane during chemical looping combustion. Proc. Combust. Inst. 2013, 34, 2839–2846. [Google Scholar]
- Siriwardane, R.; Tian, H.; Simonyi, T.; Poston, J. Synergetic effects of mixed copper–iron oxides oxygen carriers in chemical looping combustion. Fuel 2013, 108, 319–333. [Google Scholar]
- Jin, B.; Fan, Y.; Lv, Y.; Li, G.; Zhao, H.; Liang, Z. Low concentration Ni doping to intensify redox kinetics of iron-based oxygen carriers for efficient chemical looping reverse water gas shift. Sep. Purif. Technol. 2025, 360, 131237. [Google Scholar]
- Li, Y.; Liu, M.; Liu, Y.; Shen, Y.; Pan, Y.; Jin, H. Reaction kinetics of Ni-Fe oxygen carriers with high performance for chemical looping hydrogen production. Chem. Eng. J. 2024, 499, 156067. [Google Scholar]
- Su, M.; Zhao, H.; Du, B.; Zhang, P.; Liu, Z. Experimental investigation for the intrinsic reduction kinetics of a hematite as oxygen carrier in chemical looping combustion. Shandong Sci. 2017, 30, 41–51. [Google Scholar]
- Bohn, C.D.; Cleeton, J.P.; Müller, C.R.; Davidson, J.F.; Hayhurst, A.N.; Scott, S.A.; Dennis, J.S. The kinetics of the reduction of iron oxide by carbon monoxide mixed with carbon dioxide. AIChE J. 2009, 56, 1016–1029. [Google Scholar]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2. Thermochim. Acta 2006, 447, 89–100. [Google Scholar]
- Siriwardane, R.; Tian, H.; Richards, G.; Simonyi, T.; Poston, J. Chemical-looping combustion of coal with metal oxide oxygen carriers. Energy Fuels 2009, 23, 3885–3892. [Google Scholar]
- Xiao, R.; Song, Q.; Song, M.; Lu, Z.; Zhang, S.; Shen, L. Pressurized chemical-looping combustion of coal with an iron ore-based oxygen carrier. Combust. Flame 2010, 157, 1140–1153. [Google Scholar]
- Bao, J.; Li, Z.; Cai, N. Interaction between iron-based oxygen carrier and four coal ashes during chemical looping combustion. Appl. Energy 2014, 115, 549–558. [Google Scholar]
- Rubel, A.; Zhang, Y.; Neathery, J.K.; Liu, K. Comparative study of the effect of different coal fly ashes on the performance of oxygen carriers for chemical looping combustion. Energy Fuels 2012, 26, 3156–3161. [Google Scholar]
- Azis, M.M.; Leion, H.; Jerndal, E.; Steenari, B.M.; Mattisson, T.; Lyngfelt, A. The effect of bituminous and lignite ash on the performance of ilmenite as oxygen carrier in chemical-looping combustion. Chem. Eng. Technol. 2013, 36, 1460–1468. [Google Scholar]
- Purnomo, V.; Hildor, F.; Knutsson, P.; Leion, H. Interactions between potassium ashes and oxygen carriers based on natural and waste materials at different initial oxidation states. Greenh. Gases Sci. Technol. 2023, 13, 520–534. [Google Scholar]
- Cheng, D.; Yong, Q.; Zhao, Y.; Gong, B.; Zhang, J. Study on the interaction of the Fe-based oxygen carrier with ashes. Energy Fuels 2020, 34, 9796–9809. [Google Scholar]
- Zhou, Y.; Shen, L.; Gu, H.; Niu, X. Effect of biomass ash on performance of iron ore as oxygen carrier in chemical looping combustion. J. Southeast Univ. Nat. Sci. Ed. 2015, 45, 503–508. [Google Scholar] [CrossRef]
- Ilyushechkin, A.Y.; Kochanek, M.; Lim, S. Interactions between oxygen carriers used for chemical looping combustion and ash from brown coals. Fuel Process. Technol. 2016, 147, 71–82. [Google Scholar]
- Yan, J.; Shen, L.; Ou, Z.; Wu, J.; Jiang, S.; Gu, H. Enhancing the performance of iron ore by introducing K and Na ions from biomass ashes in a CLC process. Energy 2019, 167, 168–180. [Google Scholar]
- Gu, H.; Shen, L.; Zhong, Z.; Zhou, Y.; Liu, W.; Niu, X.; Ge, H.; Jiang, S.; Wang, L. Interaction between biomass ash and iron ore oxygen carrier during chemical looping combustion. Chem. Eng. J. 2015, 277, 70–78. [Google Scholar]
- Feng, Y.; Wang, N.; Guo, X. Density functional theory study on improved reactivity of alkali-doped Fe2O3 oxygen carriers for chemical looping hydrogen production. Fuel 2019, 236, 1057–1064. [Google Scholar]
- Gu, H.; Shen, L.; Zhong, Z.; Niu, X.; Ge, H.; Zhou, Y.; Xiao, S. Potassium-Modified Iron Ore as Oxygen Carrier for Coal Chemical Looping Combustion: Continuous Test in 1 kW Reactor. Ind. Eng. Chem. Res. 2014, 53, 13006–13015. [Google Scholar]
- Yang, X.; Li, D.; Zhu, X.; Zhu, T.; Mun, T.-Y.; Wang, H.; Bao, G.; Shao, M.; Wang, X. Interaction of ilmenite oxygen carrier with wheat straw ash during chemical looping combustion: Mechanisms and performance variation. Fuel 2024, 374, 132434. [Google Scholar]
- Zhang, Z.; Wang, Y.; Zhu, L.; Li, J.; Wang, F.; Yu, G. Effects of coal ash on iron-based oxygen carrier in chemical-looping combustion using three different rank coals as fuel. Asia Pac. J. Chem. Eng. 2019, 14, e2313. [Google Scholar]
- Guo, X.; Li, Y.; Zhu, Q.; Hu, X.; Ma, J.; Guo, Q. Reactivity of iron-based oxygen carriers with coal ash in pressurized chemical looping gasification. Fuel Process. Technol. 2021, 219, 106890. [Google Scholar]
- Wang, K.; An, Z.; Wang, F.; Liang, W.; Wang, C.; Guo, Q.; Liu, Y.; Yue, G. Effect of ash on the performance of iron-based oxygen carrier in the chemical looping gasification of municipal sludge. Energy 2021, 231, 120939. [Google Scholar]
- Yilmaz, D.; Leion, H. Interaction of iron oxygen carriers and alkaline salts present in biomass-derived ash. Energy Fuels 2020, 34, 11143–11153. [Google Scholar]
- Yilmaz, D.; Steenari, B.-M.; Leion, H. Comparative study: Impacts of ca and mg salts on iron oxygen carriers in chemical looping combustion of biomass. ACS Omega 2021, 6, 16649–16660. [Google Scholar] [PubMed]


| Author/Organization | Year | Research Contents | Ref. |
|---|---|---|---|
| Lewis and Gilliland | 1954 | Reaction between metal oxides and carbonaceous fuels | [11] |
| Richter and Knoche | 1983 | Proposition of the concept of splitting the conventional combustion reaction into two cyclic redox reaction processes | [6] |
| Ishida and Jin | 1994 | Proposition of the concept of integrating CLC with a gas-turbine cycle for power generation while enabling CO2 capture | [10,12] |
| Lyngfelt et al. | 2001 | Proposition of the concept of dual fluidized beds for CLC | [13] |
| Lyngfelt et al. | 2004 | 10 kWth gas fuel CLC test rig | [14] |
| Korea Institute of Energy Research | 2005 | 50 kWth gas fuel CLC test rig | [15] |
| Lyngfelt et al. | 2008 | Experiments on CLC of solid fuels | [16,17] |
| Shen et al. | 2008 | [18] | |
| Alstom | 2012 | 3 MWth pilot-scale CLC units | [19] |
| Technical University of Darmstadt | 2014 | 1 MWth pilot-scale CLC units | [20] |
| Tsinghua University | 2024 | Auto-thermal continuous operation of a 5 MWth CLC system | [21] |
| Author/Organization | Fuel | CLC System Scale | Ref. |
|---|---|---|---|
| Berguerand and Lyngfelt | Coal | 10 kWth CLC unit | [16] |
| Shen et al. | Biomass | 10 kWth reactor | [18] |
| Linderholm et al. | Bituminous coal and coke | 10 kWth CLC unit | [30] |
| Leion et al. | Petroleum coke, etc. | [31] | |
| Bayham et al. | Sub-bituminous coal | 25 kWth CLC unit | [32,33] |
| University of Stuttgart University of Hamburg | Coal | 10 kWth CLC unit 25 kWth CLC unit | [34] |
| Utah State University | Coal | 200 kWth CLC unit | [35] |
| Technical University of Darmstadt | Coal | 1 MWth CLC unit | [20] |
| Alstom | Coal | 3MWth CLC unit | [19] |
| Author | Oxygen Carrier | Reaction Gas | Ref. |
|---|---|---|---|
| Wang | Low-grade iron ore | CO, CH4 | [96] |
| Zhu | Pure Fe2O3, Fe2O3/Al2O3 | CO | [97] |
| Zhao et al. | Pure Fe2O3, hematite, steel slag | O2/CO2 | [98] |
| Mei et al. | Fe2O3/Al2O3 | CO | [81] |
| Abad et al. | Ilmenite | H2, CO, CH4, O2 | [74] |
| Liu | Fe2O3/Al2O3 | CO | [99] |
| Kespko et al. | Fe2O3-CuO/Al2O3, Fe2O3/TiO2 | Air, H2 | [82] |
| Bao et al. | Modified ilmenite | CO | [61] |
| Liu et al. | Ilmenite | CO | [100] |
| Piotrowski et al. | Hematite | CO, H2 | [85,86] |
| Su et al. | Hematite | CO | [70,84] |
| Ksepko et al. | Sinai ore | CH4, O2 | [101] |
| Steiner et al. | Synthetic ilmenite | H2 | [102] |
| Khakpoor et al. | Ilmenite | CH4 | [103] |
| Dilmac | Iron ore | CO | [104] |
| Luo et al. | Pure Fe2O3, iron ore, Fe2O3/MgAl2O4 | CH4 | [105] |
| Mendiara et al. | Tierga iron ore | H2, CO, CH4, O2 | [106] |
| Nasr et al. | Iron ore | CH4 | [107] |
| Cabello et al. | Fe2O3/Al2O3 | CH4, H2, CO | [108] |
| Li et al. | Cu/Fe-based oxygen carrier | H2 | [109] |
| Monazam et al. | Hematite | CO | [65] |
| Abad et al. | Ilmenite | CH4, H2, CO, O2 | [74] |
| Moed et al. | Fe2O3-CuO/Al2O3 | H2, CO | [110] |
| Li et al. | Mn-Fe2O3 | CO | [111] |
| Wang et al. | Fe2O3 | CO/CO2 | [112] |
| Choisez et al. | Iron powder | H2 | [113] |
| Chai et al. | Iron powder | H2 | [114] |
| Halim et al. | Fe2O3 powder | H2 | [115] |
| Author | Reactor | Oxygen Carrier | Reaction Gas | Ref. |
|---|---|---|---|---|
| Wu | Fluidized bed | Iron ore | CO | [119] |
| Schwebel et al. | Fluidized bed | Ilmenite | CO, CH4, H2 | [120] |
| Su et al. | Fluidized bed | Hematite | CO | [70,84] |
| Perreault et al. | Micro-fixed bed | Ilmenite | CO | [121] |
| Yu et al. | Micro-fluidized bed | Hematite | CO/CO2 | [122] |
| Zhang et al. | Spouted bed | Fe2O3 | CO/CO2 | [123] |
| Alsabak et al. | Fluidized bed | Laterite ore | H2 | [124] |
| Spreitzer et.al | Fluidized bed | Iron ore | H2 | [125] |
| Purnomo et al. | Fluidized bed | Ilmenite, iron sand, LD slag | CO, H2, CH4 | [126] |
| Zhang et al. | Pressurized fluidized bed | Fe2O3, Fe2O3/Al2O3 | CO | [67] |
| Chiron et al. | Micro-fluidized bed | Fe-, Fe/Cu-based oxygen carriers | H2 | [127] |
| Cheng et al. | Fluidized bed | Fe2O3/Al2O3 | H2O, air | [128] |
| Feilmayr et al. | Fluidized bed | Hematite | H2, CO, H2O, CH4, CO2 | [129] |
| Winayu et al. | Fluidized bed | Fe2O3/Al2O3/SiO2 | H2, CO, CH4 | [130] |
| Habermann et al. | Fluidized bed | Iron ore | H2, H2O, CO, CO2 | [78] |
| Chen et al. | Micro-fluidized bed | Iron ore fines | CO | [66] |
| Winayu et al. | Fluidized bed | Ilmenite | CO, H2, CH4 | [131] |
| Chen et al. | Micro-fluidized bed | Fe2O3 | CO/CO2 | [132] |
| Zeng et al. | Homemade reactor | Fe2O3 | H2 | [133] |
| Liu et al. | Honeycomb fixed bed | Fe2O3/Al2O3 | H2/CO | [134] |
| Author | Oxygen Carrier | Reaction Gas | Reactor | Kinetic Parameters | Ref. | |
|---|---|---|---|---|---|---|
| Activation Energy (kJ/mol) | Preexponential Factor | |||||
| Wang | Low-grade iron ore | CO, CH4 | TGA | CO: 56 CH4: 62 | CO: 1.5 × 10−3 CH4: 2.7 × 10−4 | [96] |
| Schwebel et al. | Ilmenite | CO CH4 H2 | Batch fluidized bed | CO: 71.59–89.35 CH4: 146.90–186.75 H2: 16.02–17.52 | CO: 0.23–1.4 CH4: 476.84–27,563.78 H2: 0 | [120] |
| Piotrowski et al. | Hematite | CO | TGA | 58.13 | [86] | |
| Perreault et al. | Ilmenite | CO | Micro-fixed bed | 51 | [121] | |
| Yu et al. | Hematite | CO/CO2 | Micro-fluidized bed | 49.64 | 6.55 s−1 | [122] |
| Ksepko et al. | Sinai ore | CH4 O2 | TGA | CH4: 35.3 O2: 16.7 | CH4: 2.4 × 10−2 s−1 O2: 1.02 × 10−4 s−1 | [101] |
| Ilmenite | CH4 O2 | TGA | CH4: 62.4 O2: 125.7 | CH4: 2.21 × 10−3 s−1 O2: 129 s−1 | [70] | |
| Khakpoor et al. | Ilmenite | CH4 O2 | TGA | 106.7 ± 10.6 | 9.43 × 10−1 | [103] |
| Dilmac | Iron ore | CO | TGA | 40–65 | [104] | |
| Nasr et al. | Iron ore | CH4 | TGA | 215 | 2.87 × 108 min−1 | [107] |
| Liu et al. | Ilmenite | CO | TGA | 169.6 | 1328.4 L1.2 mol−1.2 s−1 | [100] |
| Luo et al. | Iron ore | CH4 | TGA | 183.63 | [105] | |
| Zhao et al. | Hematite | O2/CO2 | TGA | 78.43 | 1.04 × 104 | [98] |
| Wu | Iron ore | CO | Fluidized bed | 25.84–45.66 | 5.62 × 10−4–4.96 × 10−3 | [119] |
| Alsabak et al. | Laterite ore | H2 | Fluidized bed | 10.18–34.76 | [124] | |
| Spreitzer et al. | Iron ore | H2 | Fluidized bed | 11–55 | [125] | |
| Purnomo et al. | Ilmenite | CO H2 CH4 | Fluidized bed | CO: 91.6 H2: 251 CH4: 211 | CO: 0.003 H2: 1 × 105 CH4: 137 | [126] |
| Feilmayr et al. | Hematite | H2 CO H2O CH4 CO2 | Fluidized bed | [129] | ||
| Habermann et al. | Iron ore | H2 CO CO2 H2O | Fluidized bed | [78] | ||
| Chen et al. | Fire iron ore | CO | Micro-fluidized bed | 29.1–60.872 | 0.4053–18.681 | [66] |
| Winayu et al. | Ilmenite | CO H2 | Fluidized bed | CO: 25.54 H2: 63.11 | CO: 3.72 × 105 H2: 779 | [131] |
| Monazam | Hematite | CO | TGA | 19.0 ± 0.14 | [65] | |
| Abad et al. | Activated ilmenite | CO H2 CH4 O2 | TGA | CO: 80.7 ± 2.4 H2: 65.0 ± 2.7 CH4: 135.2 ± 6.6 O2: 25.5 ± 1.2 | CO: 1.0 × 10−1 mol1−n m3n−2 s−1 H2: 6.2 × 10−2 mol1−n m3n−2 s−1 CH4: 9.8 mol1−n m3n−2 s−1 O2: 1.9 × 10−3 mol1−n m3n−2 s−1 | [74] |
| Pre-oxidized ilmenite | CO H2 CH4 O2 | CO: 113.3 ± 3.0 H2: 109.32 ± 2.3 CH4: 165.2 ± 12.4 O2: Echr: 11.8 ± 0.1 Edif: 77.4 ± 0.3 | CO: 2.1 × 10−1 mol1−n m3n−2 s−1 H2: 5.1 × 10−1 mol1−n m3n−2 s−1 CH4: 8.8 mol1−n m3n−2 s−1 O2: ks0: 8.0 × 10−5 mol1−n m3n−2 s−1 De0: 1.37 × 10−5 mol1−n m3n−2 s−1 | |||
| Liu | Wüstite | H2 CO | Fluidized bed | H2: 62–79 CO: 66–83 | H2: 0.63–1.8 s−1 CO: 0.099–2.7 s−1 | [151] |
| Khani et al. | Hematite | H2 | TGA | 40.87 | [152] | |
| Author | Oxygen Carrier | Reaction Gas | Reactor | Kinetic Parameters | Ref. | |
|---|---|---|---|---|---|---|
| Activation Energy (kJ/mol) | Preexponential Factor | |||||
| Zhu | Fe2O3/Al2O3 | CO | TGA | 74.42 | 0.525 × 105 | [97] |
| Mei et.al | Fe2O3/Al2O3 | CO | TGA | 131–270 | 3.1 × 103–1.6 × 1012 | [81] |
| Liu | Fe2O3/Al2O3 | CO | TGA | [99] | ||
| Kespko et al. | Fe2O3-CuO/Al2O3 Fe2O3/TiO2 | Air, H2 | TGA | 41.318–42.594 33.08–35.379 | 0.027–0.255 0.056–0.069 | [82] |
| Bao et al. | Modified ilmenite | CO | TGA | [61] | ||
| Steiner et al. | Synthetic ilmenite | H2 | TGA | 15–80 | [102] | |
| Luo et al. | Fe2O3/MgAl2O4 | CH4 | TGA | 79.96 | [105] | |
| Cabello et al. | Fe2O3/Al2O3 | CH4 H2 CO O2 | TGA | CH4: 66 H2: 8 CO: 14 O2: 23 | CH4: 4.34 × 101 H2: 1.45 × 10−1 CO: 1.59 × 10−1 O2: 3.64 × 10−1 | [108] |
| Li et al. | Cu/Fe-based oxygen carrier | H2 | TGA | 59.07 | 6.69 × 102 | [109] |
| Moed et al. | Fe2O3-CuO/Al2O3 | H2 CO | TGA | H2: 32.2–54.1 CO: 6.6–17.1 | [110] | |
| Li et al. | Mn-Fe2O3 | CO | TGA | 315.4–907.8 | 9.65 × 1018–4.49 × 1048 | [111] |
| Zhang et al. | Fe2O3/Al2O3 | CO | Pressurized fluidized bed | 101 ± 14 | 1.8 × 10−3 | [67] |
| Chiron et al. | Cu-Fe oxygen carrier | H2 | Micro-fluidized bed | 46 51 | [127] | |
| Cheng et al. | Fe2O3/Al2O3 | H2O, Air | Fluidized bed reactor | 0.0026–0.0118 | [128] | |
| Winayu et al. | Fe2O3/Al2O3/SiO2 | H2 CO CH4 | Fluidized bed | H2: 41.4 CO: 79.2 CH4: 13.6 | H2: 27.7 CO: 712 CH4: 0.947 | [130] |
| Alberto et al. | Fe2O3/Al2O3 | CO | TGA | 20 | 2.5 × 10−4 mol1−n m3n−2 s−1 | [69] |
| Jin et al. | Ni-Fe-Zr oxygen carrier | H2 CO2 | TGA | H2: Fe2O3 → FeO:14.90–20.00 FeO → Fe:13.87–25.40 CO2: 46.22–55.28 | H2: Fe2O3 → FeO: 3.05–6.06 FeO → Fe: 0.45–1.55 CO2: 55.19–186.85 | [160] |
| Zhang et al. | Fe2O3/Al2O3 | CO | Pressurized fluidized bed | Cycle 2: 110 ± 12 Cycle 3: 115 ± 11 Cycle 4: 97 ± 13 Cycle 5: 101 ± 14 | Cycle 2: 104.6 (−11.7 + 113.2) m s−1 Cycle 3: 211.4 (−21.6 + 24.1) m s−1 Cycle 4: 17.8 (−2.2 + 2.5) m s−1 Cycle 5: 33.2 (−4.5 + 5.2) m s−1 | [67] |
| Li et al. | xwt%Ni-Fe2O3/Al2O3 (x = 0, 5, 10, 20, 50) | CH4 | TGA | x = 0: 75.40 x = 5: 71.25 x = 10: 65.70 x = 20: 53.93 x = 50: 62.03 | x = 0: 0.59 m3n mol−n s−1 x = 5: 0.75 m3n mol−n s−1 x = 10: 0.26 m3n mol−n s−1 x = 20: 0.11 m3n mol−n s−1 x = 50: 0.40 m3n mol−n s−1 | [161] |
| Liu et al. | Fe2O3/Al2O3 | H2/CO | Honeycomb fixed bed reactor | First stage: 3.5 Second stage:33.2 | First stage: 3.06 × 10−5 Second stage: 2.99 × 10−4 | [134] |
| Author | Oxygen Carrier | Gas Concentration | Temperature (℃) | Kinetic Parameters | Ref. | |
|---|---|---|---|---|---|---|
| Activation Energy (kJ/mol) | Preexponential Factor | |||||
| Wang | Low-grade iron ore | 10%, 20% | 850, 900, 950 | 56 | 1.5 × 10−3 mol1−n m3n−2 s−1 | [96] |
| Zhu | Fe2O3 | 20% | 750, 850, 950 | 52.46 | 0.7 × 106 min−1 | [97] |
| Abad et al. | Ilmenite | 5–50% | 800, 850, 900, 950 | 113.3 ± 3.0 | 2.1 × 10−1 mol1−n m3n−2 s−1 | [74] |
| Liu et al. | Ilmenite | 10%, 20%, 30% | 850, 950, 1050 | 169.6 | 1328.4 L1.2 mol−1.2 s−1 | [100] |
| Perreault et al. | Ilmenite | 15%, 20%, 25% | 800, 850, 900 | 51 | [121] | |
| Wu | Iron ore | 5%, 7.5%, 10%, 12.5% | 750–800 800–850 850–900 | 45.66 34.58 25.84 | 0.00496 s−1 0.00143 s−1 0.000562 s−1 | [119] |
| Su et al. | Hematite | 15–50% 5–20% 5%, 7.5%, 15% | 400–650 850, 900, 950, 1000 400–650 | 74.48 110.75 138.55 | 1.2 × 1012 s−1 88.55 m4.5 mol−1.5 s−1 6.8 × 1013 s−1 | [70,84,162] |
| Mei et al. | Fe2O3/Al2O3 | 50% | 1000 | 131–270 | 3.1 × 103–1.6 × 1012 s−1 | [81] |
| Bao et al. | Modified ilmenite | 11% | 900 | [61] | ||
| Dilmac | Iron ore | 10%, 20%, 25%, 50% | 750, 800, 850, 900 | 40–65 | [104] | |
| Wang et al. | Fe2O3 | 0–38%, 42–65%, 68–88% | 800–900 | 35–70 | [112] | |
| Mendiara et al. | Tierga iron ore | 5–60% | 800–1000 | 76.1 ± 6 139 ± 5 | 1.45 × 10−1 mol1−n m3n−2 s−1 9.16·10−5 | [106] |
| Cabello et al. | Fe2O3/Al2O3 | 5–60% | 700–1050 | 14 204 | 1.59 × 10−1 2.29 × 109 | [108] |
| Monazam et al. | Hematite | 5%, 10%, 20% | 700–950 | 19.0 ± 0.14 | [65] | |
| Moed et al. | Fe2O3-CuO/Al2O3 | 20% | 850, 875, 900, 925 | 6.6–13.8 | [110] | |
| Li et al. | Mn-Fe2O3 | 750, 800, 850 | 315.4–907.8 | 9.65 × 1018–4.49 × 1048 | [111] | |
| Purnomo et al. | Ilmenite iron sand LD slag | 50% | 850, 875, 900, 925, 950, 975 | 59.4 51.7 64.8 | 7.34 s−1 0.73 s−1 9.78 s−1 | [126] |
| Winayu et al. | Ilmenite Fe2O3/Al2O3/SiO2 | 25% | 900, 950, 1000 | 25.54 79.2 | 3.72 × 105 712 min−1 | [130,131] |
| Bohn et al. | Fe2O3 | 8.5–9.5% | 650–900 | 75.0–94.0 | [163] | |
| Chen et al. | Fe2O3 | 40%, 60%, 80%, 100% | 750–950 | Fe2O → Fe3O4: 30.60 ± 0.75 to 52.99 ± 0.78 Fe3O4 → FeO: 52.44 ± 0.10 to 80.83 ± 0.12 FeO → Fe: 45.74 ± 0.25–92.12 ± 0.27 | Fe2O3 → Fe3O4: 1.84 ± 0.69 s−1–13.71 ± 5 34 s−1 Fe3O4 → FeO: 17.57 ± 0.87 s−1–426.47 ± 25.92 s−1 FeO → Fe: 3.28 ± 0.41 s−1–628.46 ± 84.40 s−1 | [132] |
| Chen et al. | Iron ore fines | 50% | 700–850 | 29.1–60.872 | 0.4053–18.681 s−1 | [66] |
| Author | Oxygen Carrier | Ash | Conclusion | Ref. |
|---|---|---|---|---|
| Zhang et al. | Fe2O3/Al2O3 | Three types of coal ash | When the oxygen carrier was in contact with coal ash having a high Ca content, its reactivity was significantly enhanced after several cycles. Coal ash with a high Si content had a detrimental effect on the oxygen carrier. | [179] |
| Bao et al. | Fe2O3/Al2O3 | Four types of coal ash | Ash composed of CaSO4 enhanced the activity of oxygen carrier, and the effect of ash in decreasing carrier activity increased with the number of cycles. | [167] |
| Zhou et al. | iron ore | Biomass ash | The K-rich ash generated K3FeO2, which improved the reducing activity of the iron ore. | [172] |
| Guo et al. | Fe2O3/Al2O3 | Coal ash | The presence of ash exerted an inhibitory influence on the reduction reaction process from Fe2O3 to Fe3O4. | [180] |
| Ilyushechkin et al. | iron ore, ilmenite | Two types of coal ash (with Si, Fe, Mg) | The ash rich in Fe augmented the redox kinetics of iron ore while having no impact on ilmenite. The ash abundant in Si decreased the oxidation rate of iron ore. | [173] |
| Wang et al. | Fe2O3/Al2O3 | Sludge ash | When the number of cycles was fewer than 10, the activity of the oxygen carrier had the potential to increase. As the number of cycles increased, the ash decreased the reactivity of the oxygen carrier. | [181] |
| Purnomo et al. | Nature ores, waste materials | Three K salts | The initial oxidation state of the surface of the oxygen carrier had only a limited influence on its interaction with K salts. | [170] |
| Cheng et al. | Pure Fe2O3 | Coal ash, biomass ash | A small amount of ash promoted the reduction of Fe2O3. Moreover, Ca2+, K+, and Na+ in ash interacted with silica-aluminate to form low melting point compounds. | [171] |
| Gu et al. | Iron ore | Three types of biomass ash | Ash that was rich in K and had a low Si content served to enhance fuel conversion. | [175] |
| Yang et al. | Ilmenite | Biomass ash | The reactivity of ilmenite first increased and then decreased as the amount of biomass ash added increased, reaching its peak at 10% biomass ash addition. The addition of 15% biomass ash led to severe particle sintering. | [178] |
| Yilmaz et al. | Pure Fe2O3 | Synthetic biomass-derived ash (with K, Na) | The co-presence of K and Si in the ash increased agglomeration. | [182] |
| Synthetic biomass-derived ash (with Mg, Ca) | Under the same experimental conditions, it was observed that Mg led to more severe clumping phenomena than Ca. | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, J.; Quan, S.; Yang, H.; Zhang, M.; Zhou, T.; Yang, X.; Zhang, M.; Mun, T.-y.; Li, Z.; Kim, R.-G.; et al. Research Progress and Perspectives of the Reaction Kinetics of Fe-Based Oxygen Carriers in Chemical Looping Combustion. Energies 2025, 18, 2313. https://doi.org/10.3390/en18092313
Mei J, Quan S, Yang H, Zhang M, Zhou T, Yang X, Zhang M, Mun T-y, Li Z, Kim R-G, et al. Research Progress and Perspectives of the Reaction Kinetics of Fe-Based Oxygen Carriers in Chemical Looping Combustion. Energies. 2025; 18(9):2313. https://doi.org/10.3390/en18092313
Chicago/Turabian StyleMei, Jiakun, Shangkun Quan, Hairui Yang, Man Zhang, Tuo Zhou, Xi Yang, Mingyu Zhang, Tae-young Mun, Zhouhang Li, Ryang-Gyoon Kim, and et al. 2025. "Research Progress and Perspectives of the Reaction Kinetics of Fe-Based Oxygen Carriers in Chemical Looping Combustion" Energies 18, no. 9: 2313. https://doi.org/10.3390/en18092313
APA StyleMei, J., Quan, S., Yang, H., Zhang, M., Zhou, T., Yang, X., Zhang, M., Mun, T.-y., Li, Z., Kim, R.-G., Zhu, X., Wang, H., & Li, D. (2025). Research Progress and Perspectives of the Reaction Kinetics of Fe-Based Oxygen Carriers in Chemical Looping Combustion. Energies, 18(9), 2313. https://doi.org/10.3390/en18092313









