Exploring Options for the Application of Azobenzene for Molecular Solar Thermal Energy Storage: Integration with Parabolic Trough Solar Systems
Abstract
1. Introduction
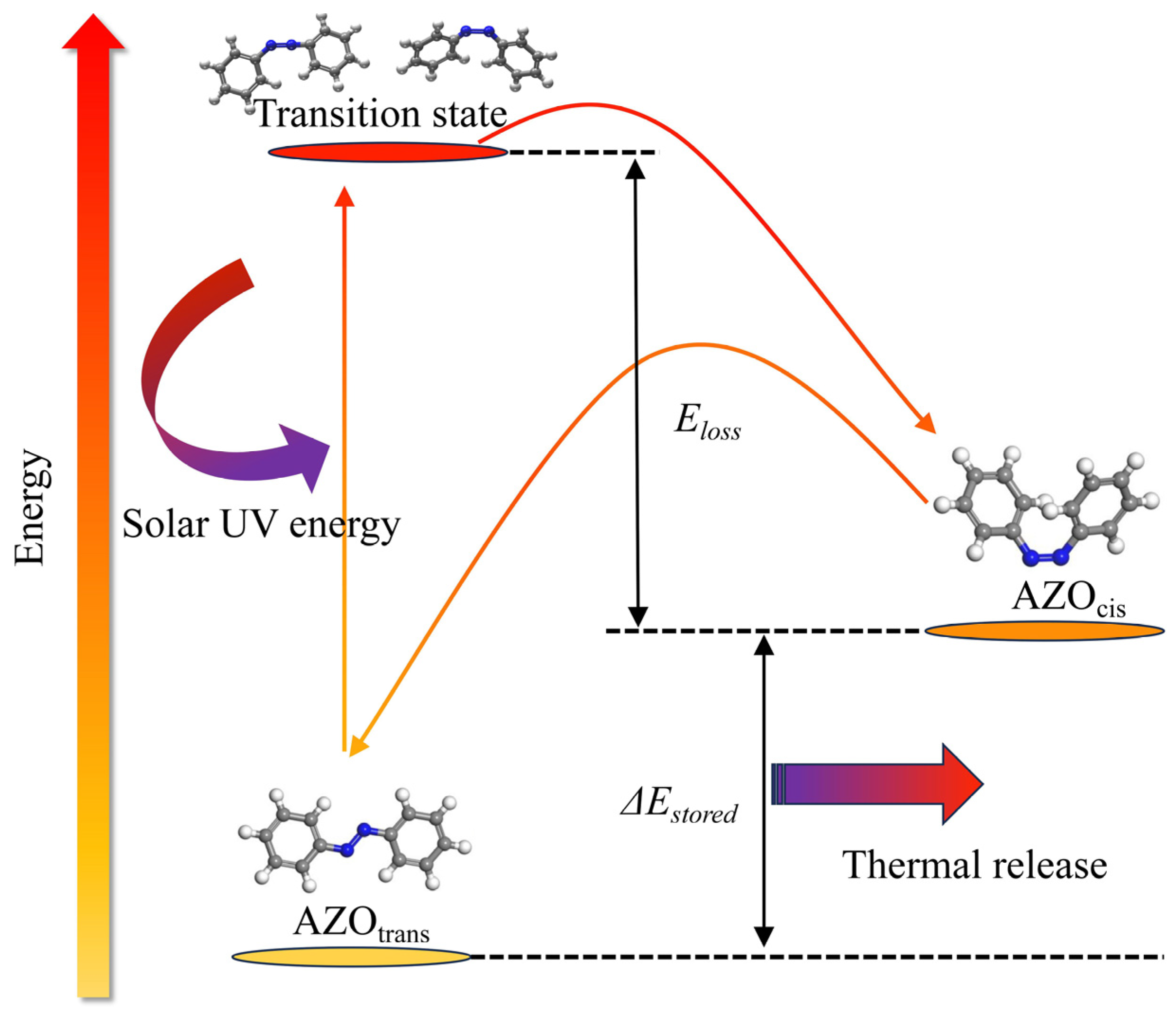
2. The Molecular Solar Thermal Energy Storage and Release System and Thermal Conductivity Model of the MOST Device
2.1. Parabolic Trough Solar
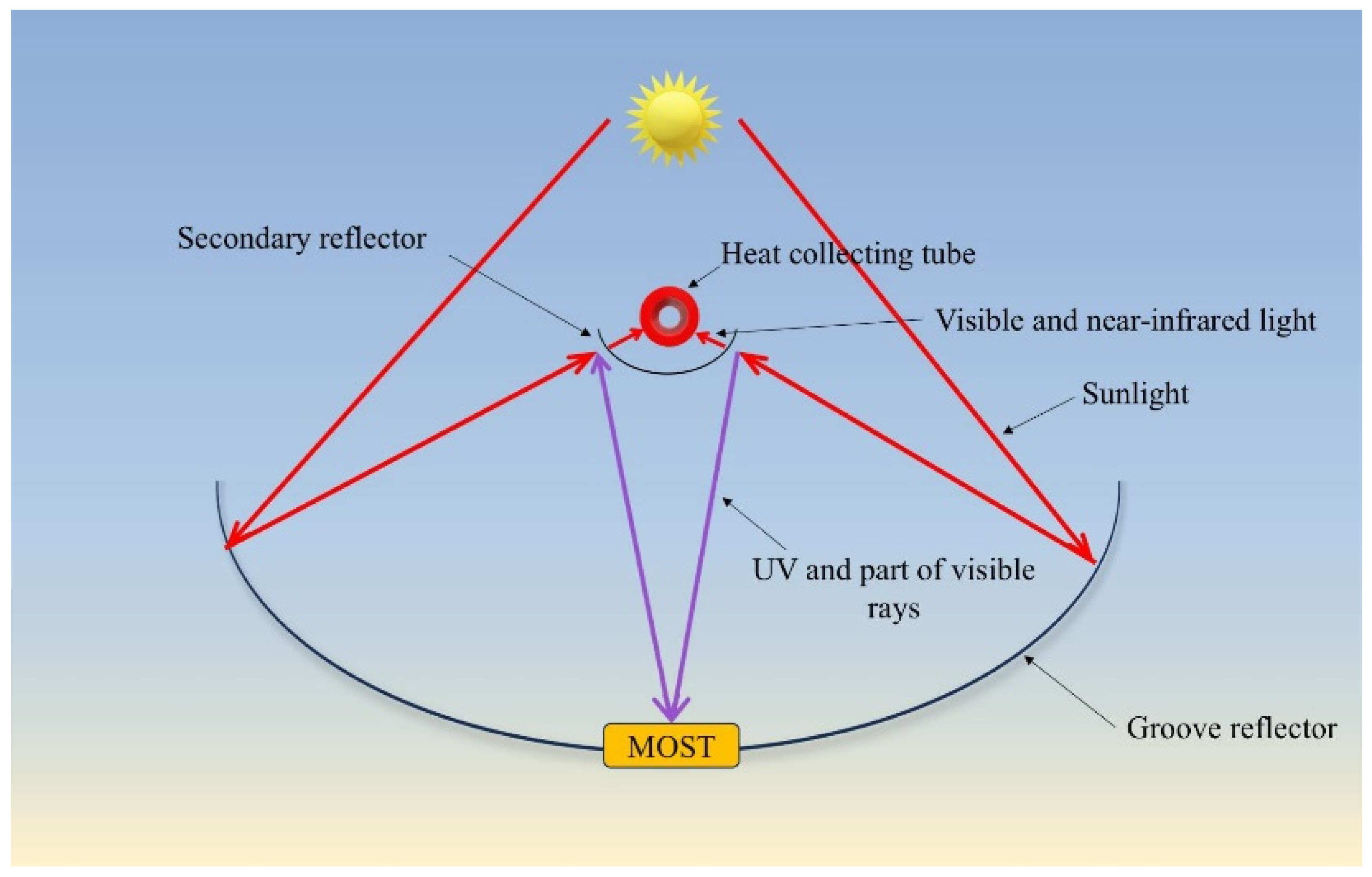
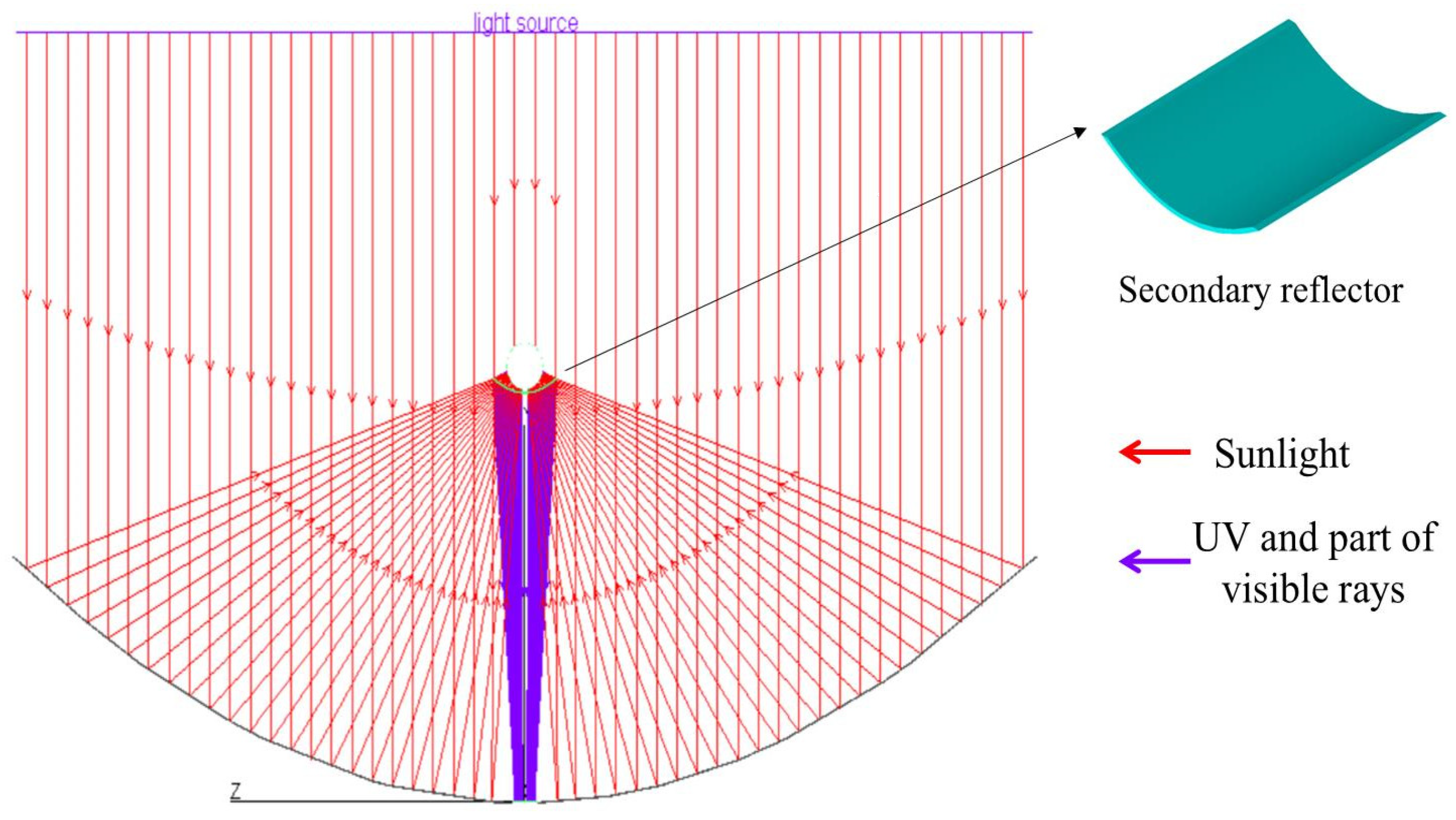
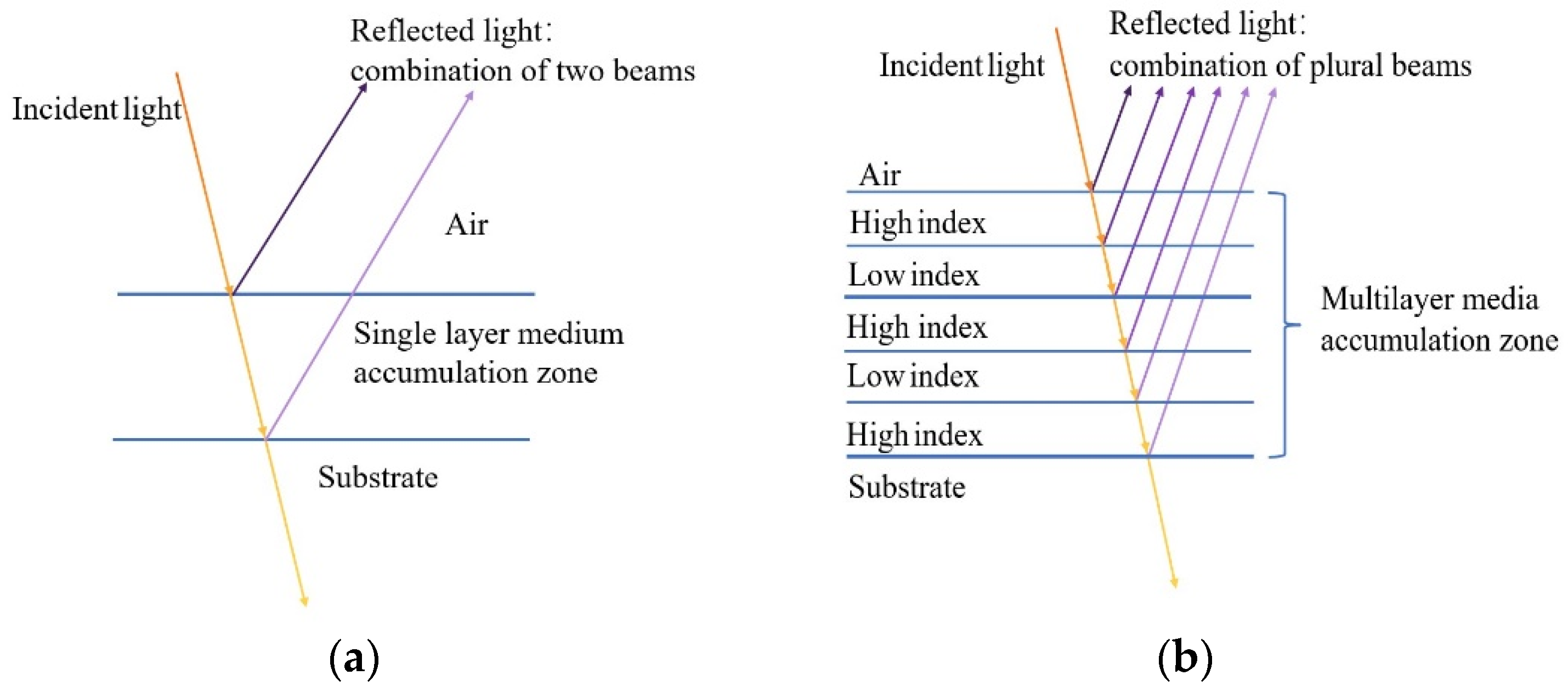
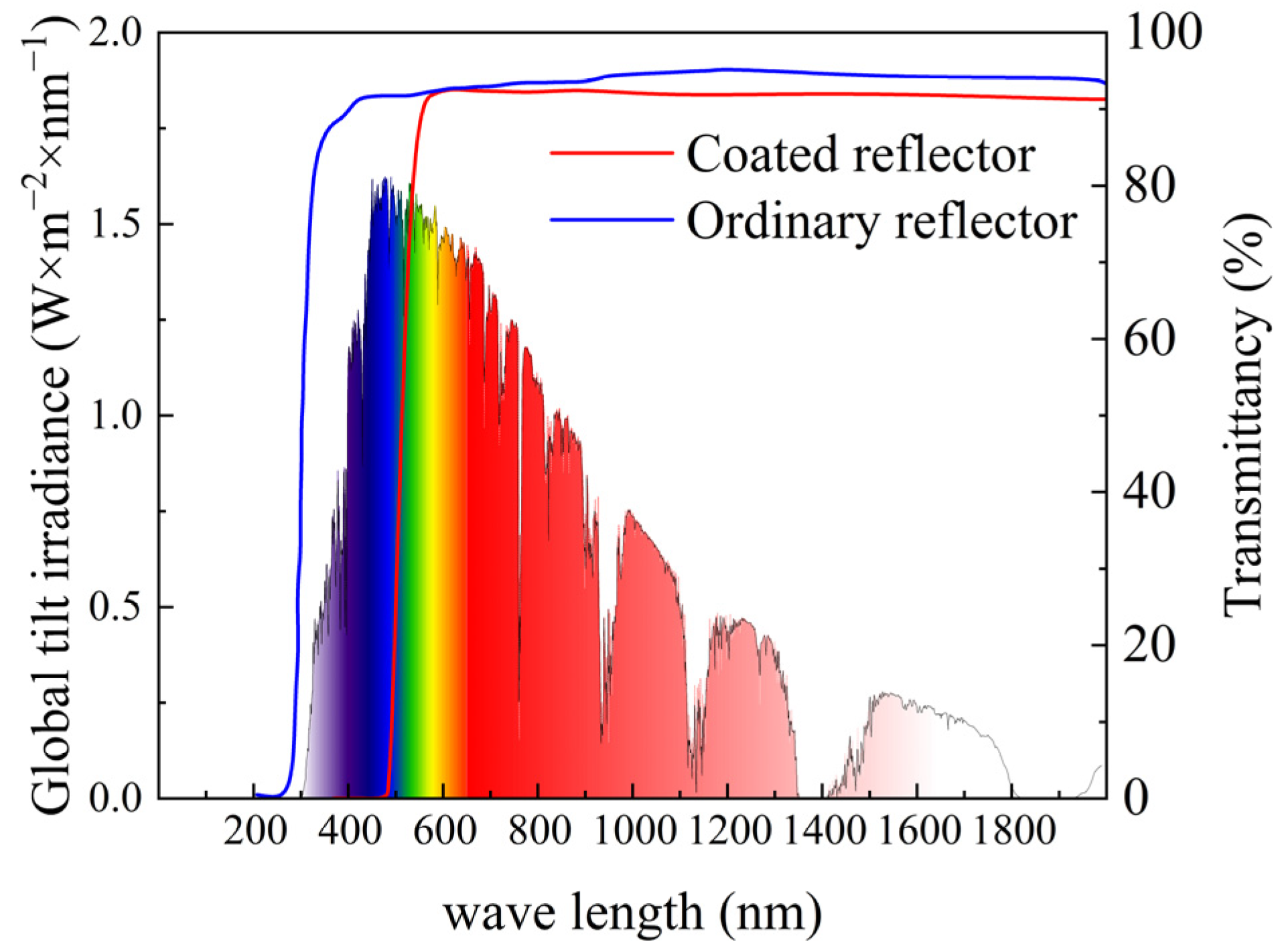
2.2. Design of the Secondary Reflector and Principle of Light Interference
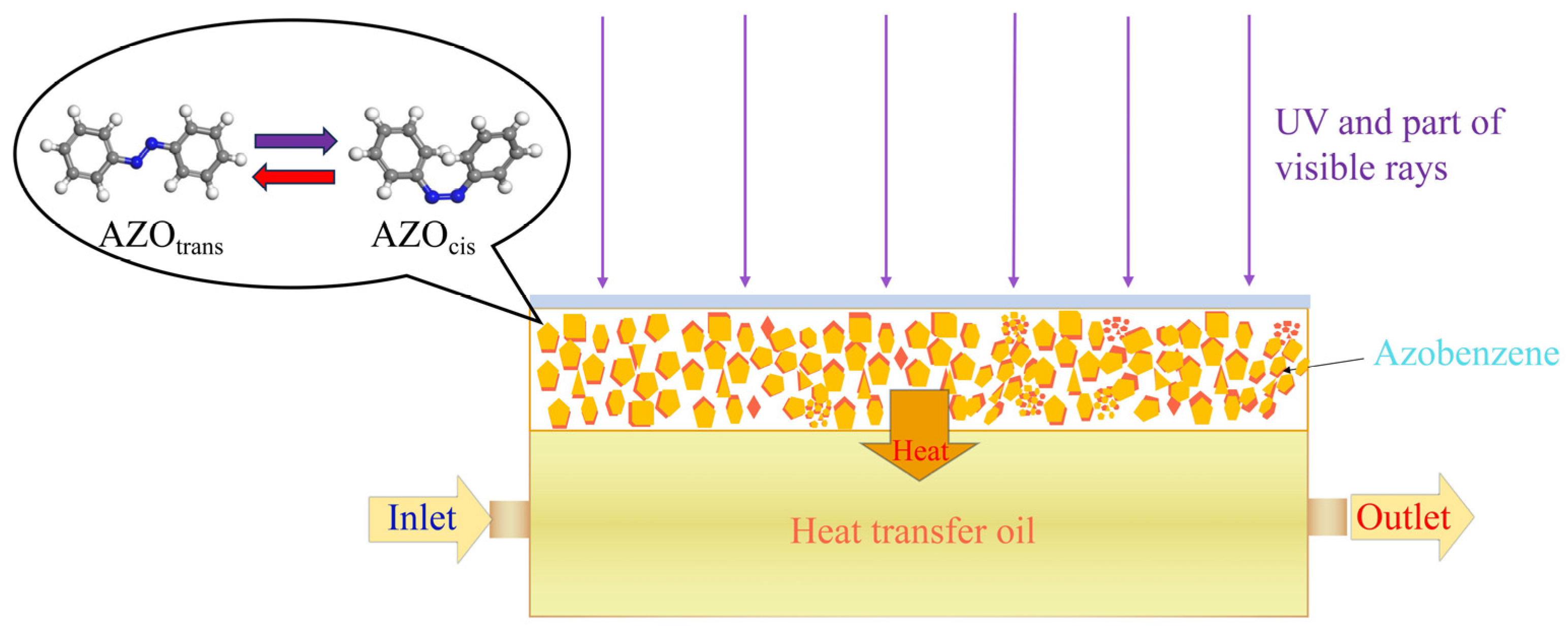
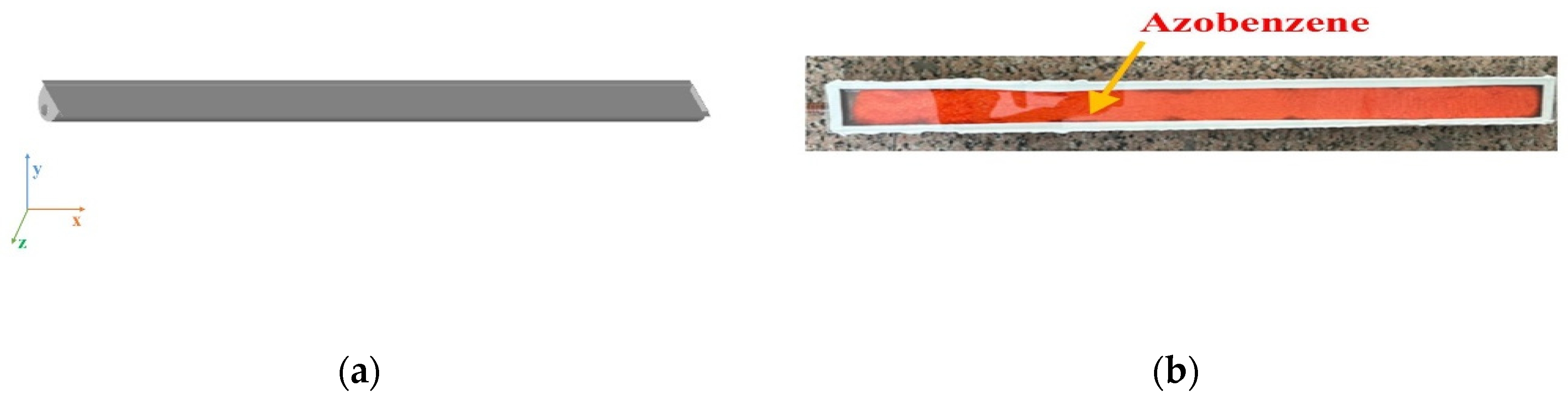
2.3. Design of the MOST Device
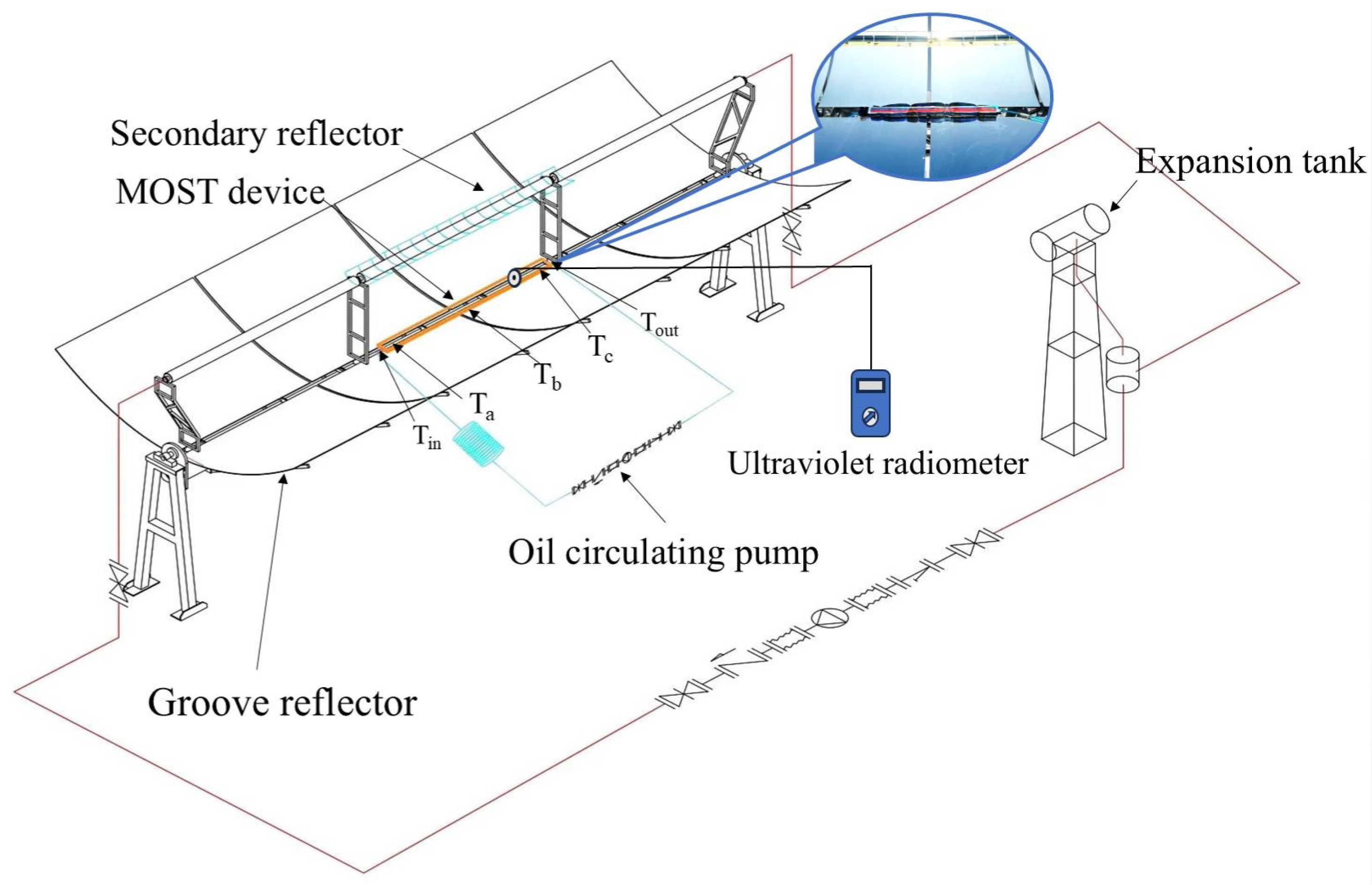
2.4. Laboratory Installation and Operation Monitoring System
2.5. Thermal Conductivity Model of the MOST Device and Mathematical Model
- The AZO (azobenzene) molecules are assumed to behave independently without intermolecular interactions, and their mutual influences are neglected in the reaction system.
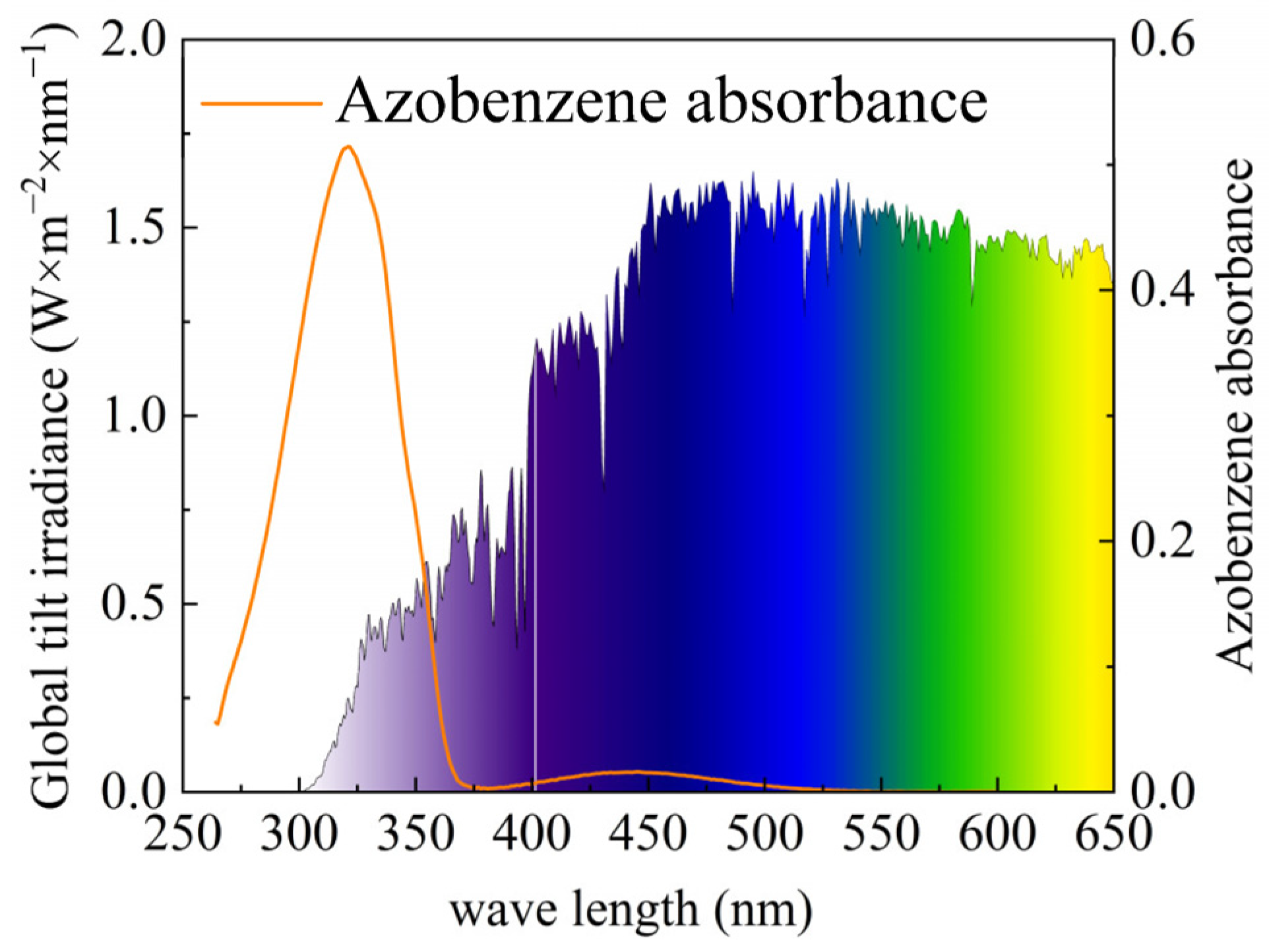
- AZO molecules exhibit threshold-dependent spectral absorption characteristics: all photons with wavelengths below 490 nm are absorbed, with their energy being converted into stored chemical potential and subsequently released as thermal energy.
- In the MOST device, all energy losses exclusively manifest as thermal dissipation, with no other forms of energy loss occurring.
3. Experimental Analysis of the MOST Device
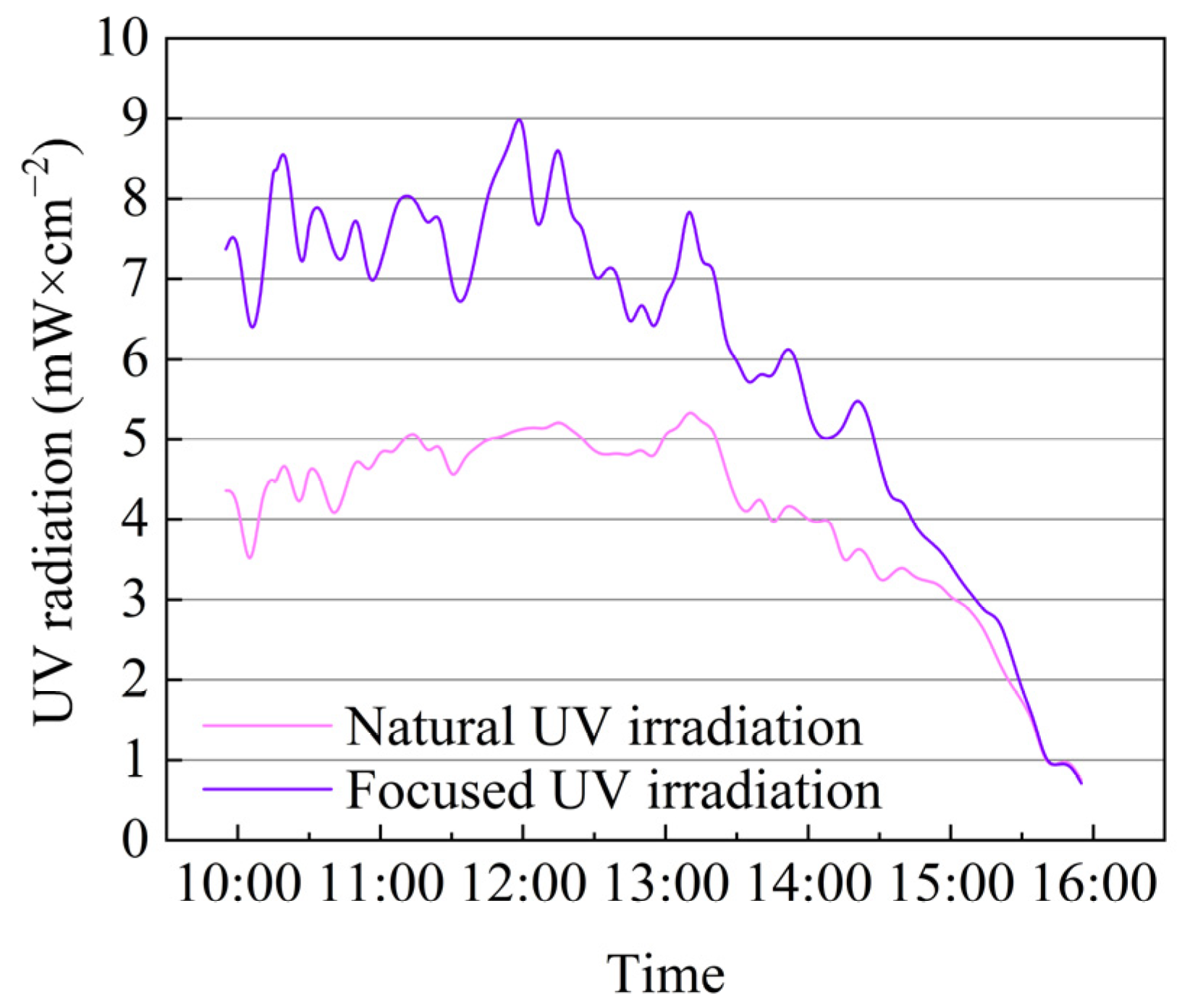
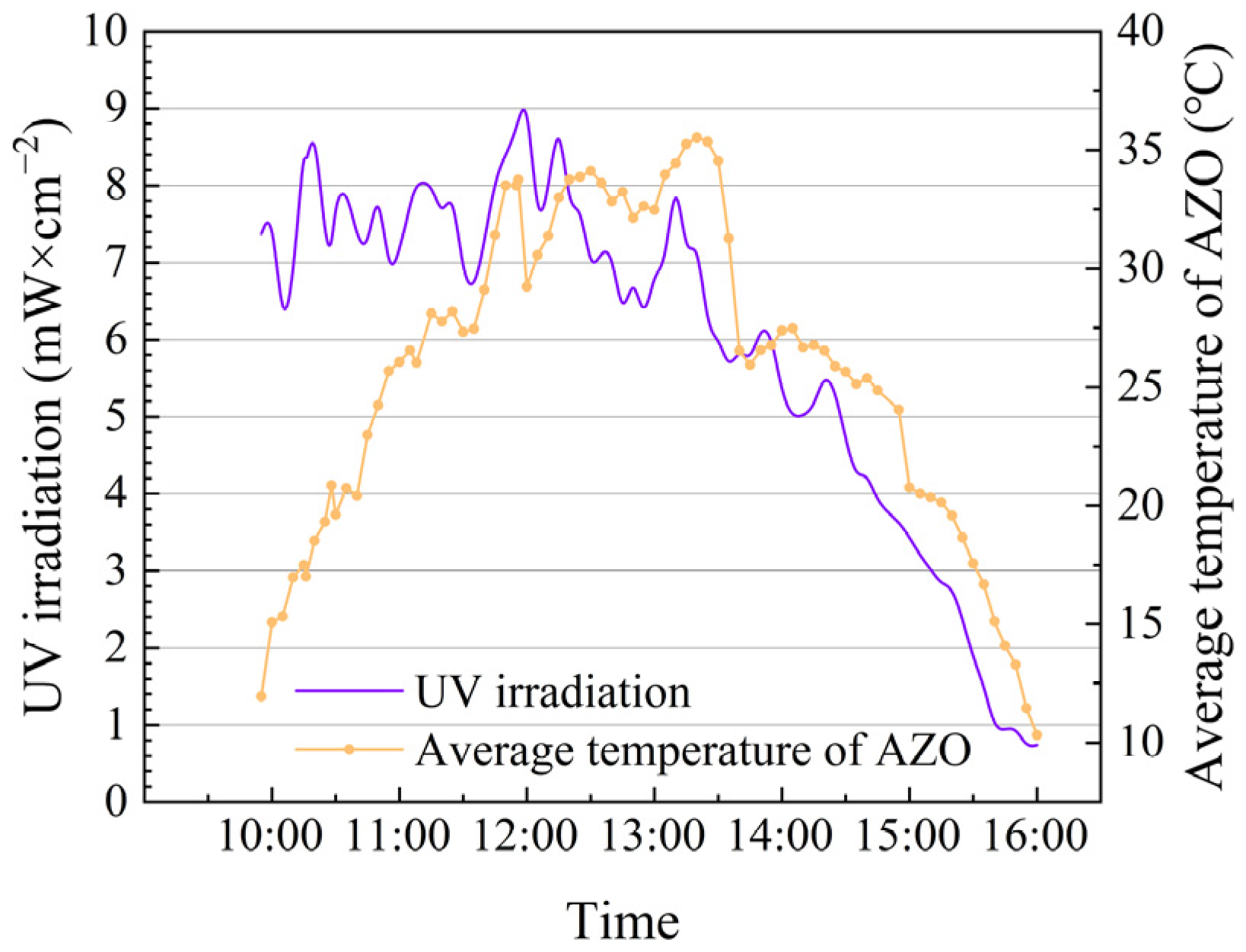
3.1. Convergence Effect of Ultraviolet Light
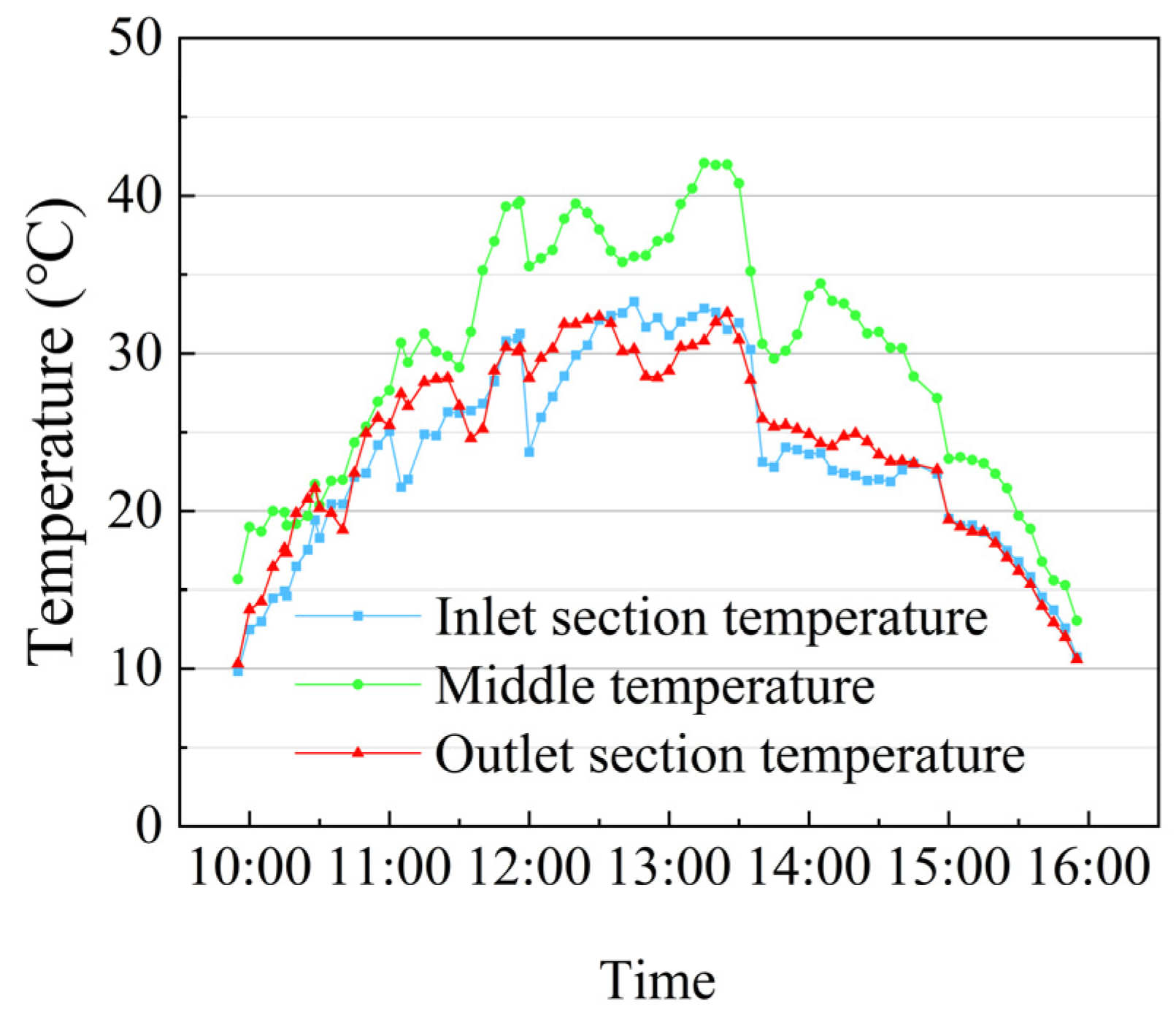
3.2. The Temperature of Heat Transfer Oil and AZO
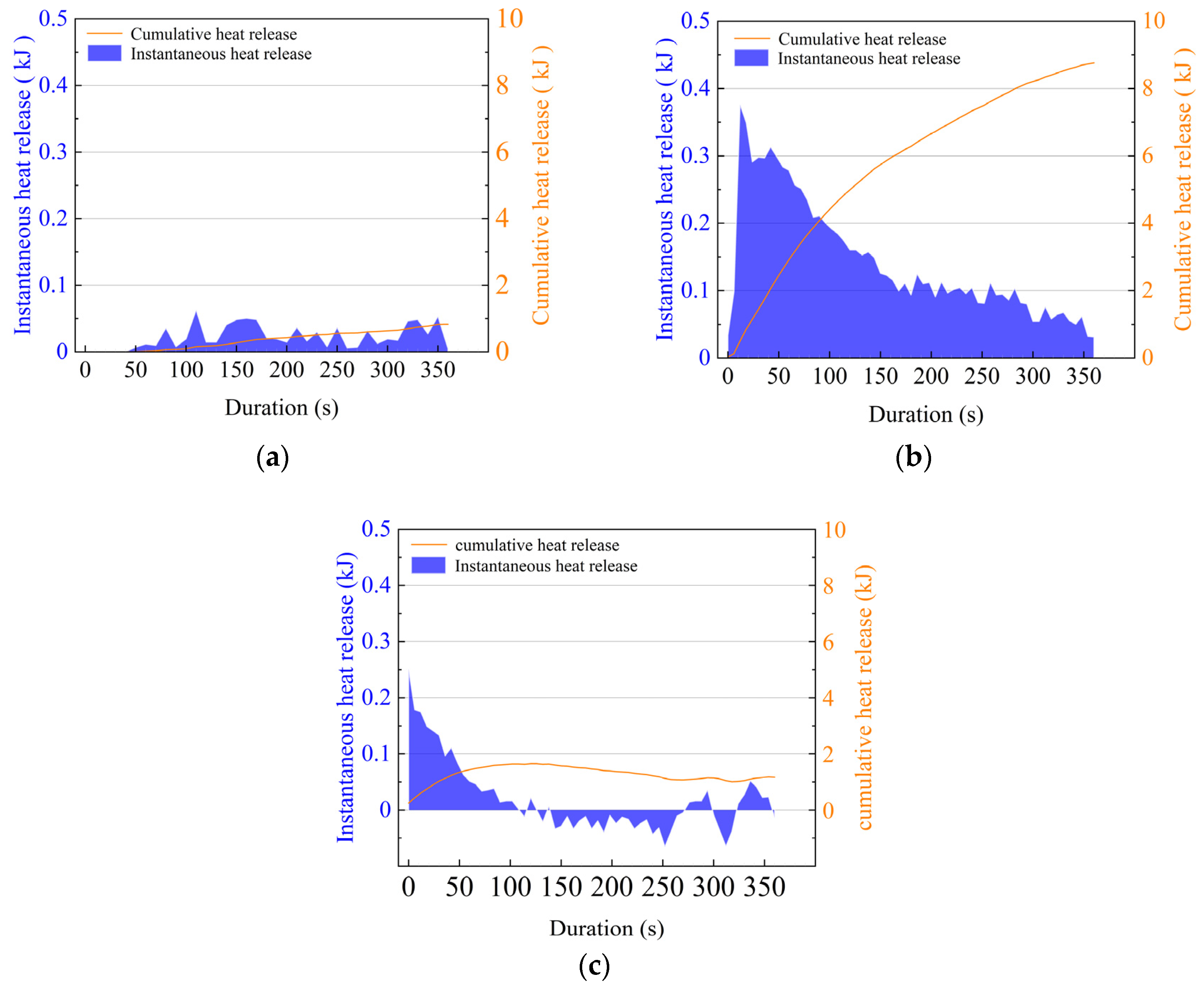
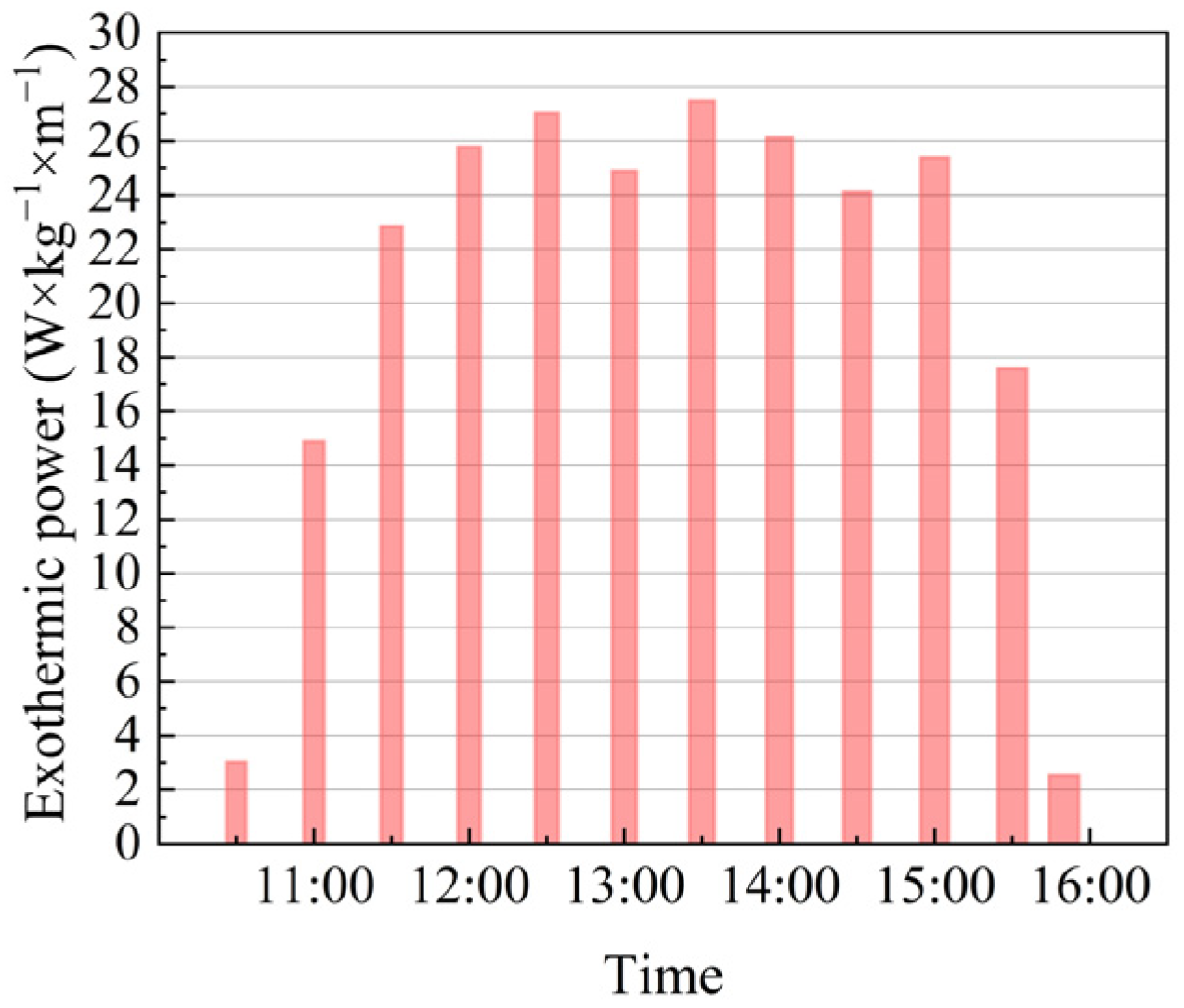
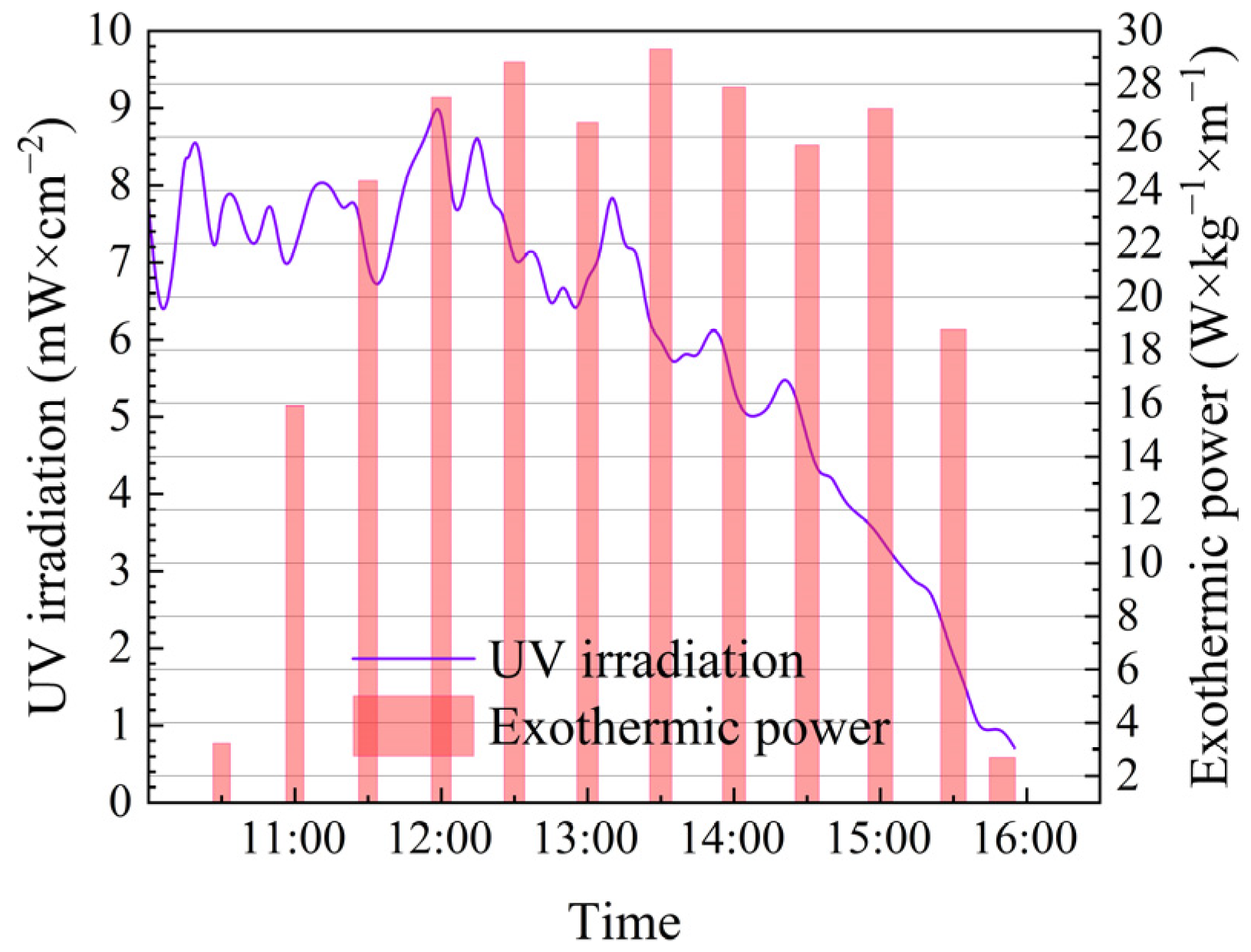
3.3. Exothermic Quantity and Exothermic Power
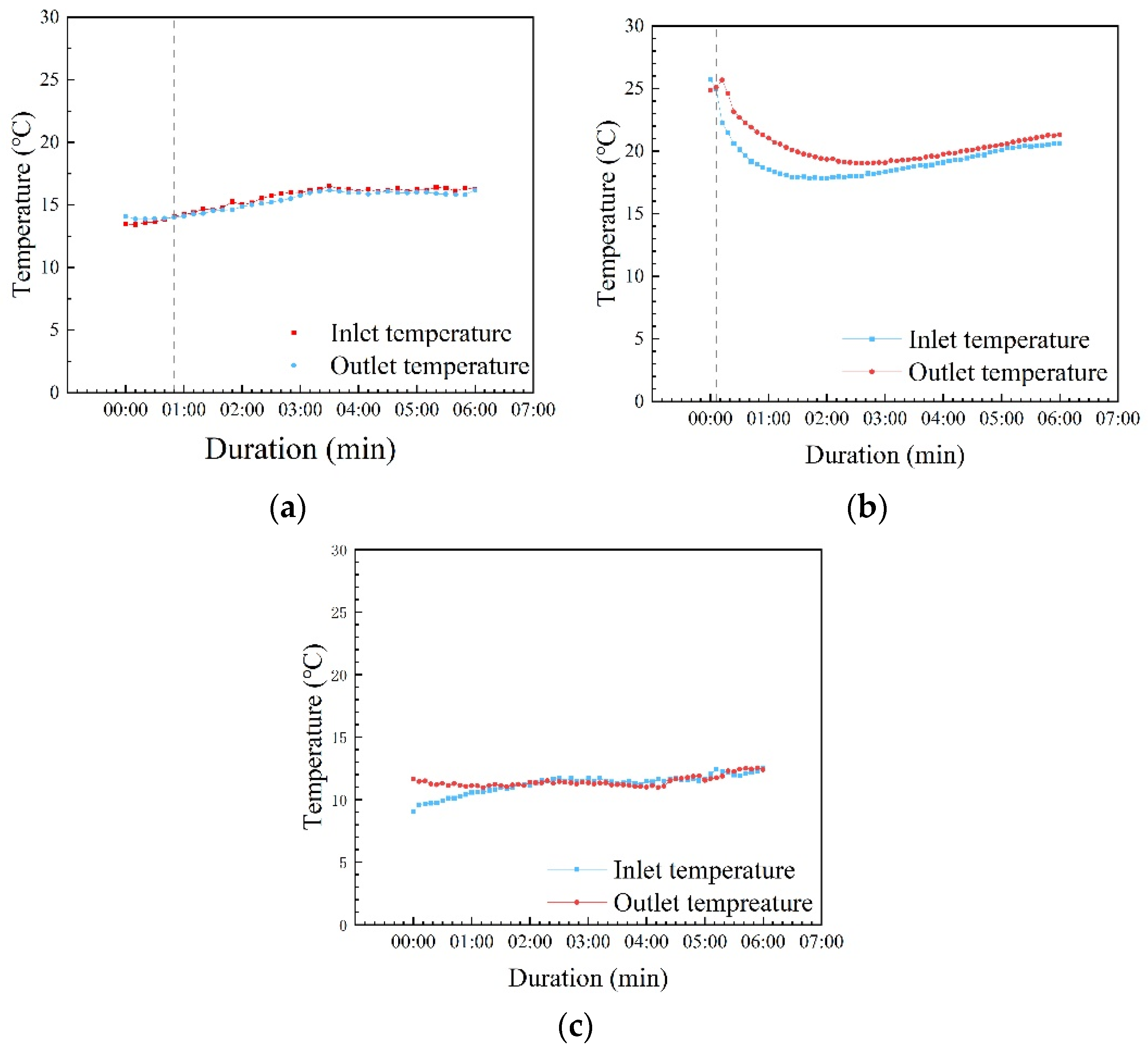
3.4. UV Irradiation and MOST Device
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Taylor, R.; Hao, Y.; Besagni, G.; Markides, C.N.; Li, G. Solar energy utilisation: Current status and roll-out potential. Appl. Therm. Eng. 2022, 209, 118285. [Google Scholar] [CrossRef]
- Wu, H.; Xie, G.; Jie, Z.; Hui, X.; Yang, D.; Du, C. Research progress about chemical energy storage of solar energy. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 052070. [Google Scholar] [CrossRef]
- Hinojosa, J.F.; Moreno, S.F.; Maytorena, V.M. Low-temperature applications of phase change materials for energy storage: A descriptive review. Energies 2023, 16, 3078. [Google Scholar] [CrossRef]
- Pachori, H.; Choudhary, T.; Sheorey, T. Significance of thermal energy storage material in solar air heaters. Mater. Today Proc. 2022, 56, 126–134. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Ravichandran, M.; Mohanavel, V.; Karthick, A.; Aswin, L.S.R.L.; Priyanka, S.S.H.; Kumar, S.K.; Kumar, S.P. Review on phase change materials for solar energy storage applications. Environ. Sci. Pollut. Res. 2021, 29, 9491–9532. [Google Scholar] [CrossRef]
- Moth-Poulsen, K.; Ćoso, D.; Börjesson, K.; Vinokurov, N.; Meier, S.K.; Majumdar, A.; Vollhardtc, K.P.C.; Segalman, R.A. Molecular solar thermal (MOST) energy storage and release system. Energy Environ. Sci. 2012, 5, 8534. [Google Scholar] [CrossRef]
- Quant, M.; Lennartson, A.; Dreos, A.; Kuisma, M.; Erhart, P.; Börjesson, K.; Moth-Poulsen, K. Low Molecular Weight Norborna-diene Derivatives for Molecular Solar-Thermal Energy Storage. Chem. Eur. J. 2016, 22, 13265–13274. [Google Scholar] [CrossRef]
- Dreos, A.; Börjesson, K.; Wang, Z.; Roffey, A.; Norwood, Z.; Kushnir, D.; Moth-Poulsen, K. Exploring the potential of a hybrid device combining solar water heating and molecular solar thermal energy storage. Energy Environ. Sci. 2017, 10, 728–734. [Google Scholar] [CrossRef]
- Wang, Z.; Hölzel, H.; Fernandez, L.; Aslam, A.S.; Baronas, P.; Orrego-Hernández, J.; Ghasemi, S.; Campoy-Quiles, M.; Moth-Poulsen, K. Hybrid solar energy device for simultaneous electric power generation and molecular solar thermal energy storage. Joule 2024, 8, 2607–2622. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Hu, Z.; Orrego-Hernández, J.; Mu, E.; Zhang, Z.Y.; Jevric, M.; Liu, Y.; Fu, X.; Wang, F.; et al. Chip-scale solar thermal electrical power generation. Cell Rep. Phys. Sci. 2022, 3, 100789. [Google Scholar] [CrossRef]
- Bauer, U.; Fromm, L.; Weiß, C.; Bachmann, P.; Späth, F.; Düll, F.; Steinhauer, J.; Hieringer, W.; Görling, A.; Hirsch, A.; et al. Controlled Catalytic Energy Release of the Norbornadiene/Quadricyclane Molecular Solar Thermal Energy Storage System on Ni(111). J. Phys. Chem. C 2018, 123, 7654–7664. [Google Scholar] [CrossRef]
- Uda, M.; Momotake, A.; Arai, T. Synthesis and isomerization of azobenzene dendrimers. Photochem. Photobiol. Sci. 2003, 2, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Feng, J.; Li, W.Y.; Wang, G.; Feng, W.; Yu, H. Azobenzene-containing polymer for solar thermal energy storage and release: Advances, challenges, and opportunities. Prog. Polym. Sci. 2024, 149, 101782. [Google Scholar] [CrossRef]
- Dong, L.; Feng, Y.; Wang, L.; Feng, W. Azobenzene-based solar thermal fuels: Design, properties, and applications. Chem. Soc. Rev. 2018, 47, 7339–7368. [Google Scholar] [CrossRef]
- Adamson, A.W.; Vogler, A.; Kunkely, H.; Wachter, R. Photocalorimetry. Enthalpies of photolysis of trans-azobenzene, ferriox-alate and cobaltioxalate ions, chromium hexacarbonyl, and dirhenium decarbonyl. J. Am. Chem. Soc. 1978, 100, 1298–1300. [Google Scholar] [CrossRef]
- Giménez-Gómez, A.; Magson, L.; Merino-Robledillo, C.; Hernáez-Troya, S.; Sanosa, N.; Sampedro, D.; Funes-Ardoiz, I. State-of-the-art and challenges towards a Molecular Solar Thermal (MOST) energy storage device. React. Chem. Eng. 2024, 9, 1629–1640. [Google Scholar] [CrossRef]
- Wang, Z.; Losantos, R.; Sampedro, D.; Morikawa, M.A.; Börjesson, K.; Kimizuka, N.; Moth-Poulsen, K. Demonstration of an azobenzene derivative based solar thermal energy storage system. J. Mater. Chem. A 2019, 7, 15042–15047. [Google Scholar] [CrossRef]
- Wang, Z.; Moïse, H.; Cacciarini, M.; Nielsen, M.B.; Morikawa, M.A.; Kimizuka, N.; Moth-Poulsen, K. Liquid-based multijunction molecular solar thermal energy collection device. Adv. Sci. 2021, 8, 2103060. [Google Scholar] [CrossRef]
- Sun, W.; Shangguan, Z.; Zhang, X.; Dang, T.; Zhang, Z.Y.; Li, T. Solar Efficiency of Azo-Photoswitches for Energy Conversion: A Comprehensive Assessment. ChemSusChem 2023, 16, e202300582. [Google Scholar] [CrossRef]
- Öztürk, M.; Bezir, N.Ç.; Özek, N. Optical, Energetic and Exergetic Analyses of Parabolic Trough Collectors. Chin. Phys. Lett. 2007, 24, 1787. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Q. A novel spectrum allocation method in the photovoltaic-thermochemical hybrid solar system. J. Power Sources 2021, 513, 230541. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J. A novel solution method for reflector shape of solar Compound Parabolic Concentrator and verification. Renew. Energy 2022, 192, 385–395. [Google Scholar] [CrossRef]
- Canavarro, D.; Chaves, J.; Collares-pereira, M. A novel Compound Elliptical-type Concentrator for parabolic primaries with tubular receiver. Sol. Energy 2016, 134, 383–391. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, G.; Luo, Z.; Ni, M.; Yang, T.; Xu, W. Comparison of different types of secondary mirrors for solar application. Optik 2014, 125, 1106–1112. [Google Scholar] [CrossRef]
- Lee, C.C. Optical interference coatings for optics and photonics. Appl. Opt. 2012, 52, 73–81. [Google Scholar] [CrossRef]
- Lednev, I.K.; Ye, T.Q.; Matousek, P.; Towrie, M.; Foggi, P.; Neuwahl, F.V.R.; Umapathy, S.; Hester, R.E.; Moore, J.N. Femtosecond time-resolved UV-visible absorption spectroscopy of trans-azobenzene: Dependence on excitation wavelength. Chem. Phys. Lett. 1998, 290, 68–74. [Google Scholar] [CrossRef]
- Paulescu, E.; Paulescu, M. A new clear sky solar irradiance model. Renew. Energy 2021, 179, 2094–2103. [Google Scholar] [CrossRef]
- Ramasamy, A.K.; Ganesh, M.; Rajamani, K.; Loganathan, A.K.; Rangaswamy, R. Investigation of concentrated solar collector with discretized flat mirrors in parabolic arrangement. Energy Sustain. Dev. 2021, 64, 25–34. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Moghimi, M.A. Reducing the optical end losses of a linear Fresnel reflector using novel techniques. Sol. Energy 2019, 186, 247–256. [Google Scholar] [CrossRef]
- Leroux, Y.R.; Hapiot, P. Photo-modulation of the permeation in azobenzene derivatives monolayer films electrografted on carbon substrates. Electrochem. Commun. 2013, 33, 107–110. [Google Scholar] [CrossRef]
- Henzl, J.; Mehlhorn, M.; Gawronski, H.; Rieder, K.H.; Morgenstern, K. Reversible cis–trans Isomerization of a Single Azobenzene Molecule. Angew. Chem. Int. Ed. 2006, 45, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, Z.; Liu, Y.; Zhang, X.; Cui, Z. Thermodynamic analysis of a combined cooling, heating, and power system integrated with full-spectrum hybrid solar energy device. Energy Convers. Manag. 2021, 228, 113596. [Google Scholar] [CrossRef]
- Fang, J.; Liu, Q.; Guo, S.; Lei, J.; Jin, H. Spanning solar spectrum: A combined photochemical and thermochemical process for solar energy storage. Appl. Energy 2019, 247, 116–126. [Google Scholar] [CrossRef]
- Börjesson, K.; Lennartson, A.; Moth-Poulsen, K. Efficiency Limit of Molecular Solar Thermal Energy Collecting Devices. ACS Sustain. Chem. Eng. 2013, 1, 585–590. [Google Scholar] [CrossRef]
- Xu, L.; Sun, F.; Ma, L.; Li, X.; Lei, D.; Yuan, G.; Zhu, H.; Zhang, Q.; Xu, E.; Wang, Z. Analysis of optical and thermal factors’ effects on the transient performance of parabolic trough solar collectors. Sol. Energy 2019, 179, 195–209. [Google Scholar] [CrossRef]
- Wang, Z.; Roffey, A.; Losantos, R.; Lennartson, A.; Jevric, M.; Petersen, A.U.; Quant, M.; Dreos, A.; Wen, X.; Sampedro, D.; et al. Macroscopic heat release in a molecular solar thermal energy storage system. Energy Environ. Sci. 2019, 12, 187–193. [Google Scholar] [CrossRef]


| Project | Data |
|---|---|
| Reflector area | 15.3 m2 |
| Total length of collector tube | 6000 mm |
| Collector opening width | 2550 mm |
| focal length | 850 mm |
| Mirror emissivity | 0.9 |
| Outer diameter of collector tube | 90 mm |
| Inner diameter of collector tube | 45 mm |
| Thermal conductivity of collector tube | 54 W·m−1·K−1 |
| Collector Absorption Rate | 0.9 |
| Specific heat capacity of heat conducting oil (26 °C) | 0.76 kJ·kg−1·K−1 |
| Project | Norm (mm) |
|---|---|
| reflector length | 200 |
| reflector Thickness | 2 |
| reflector Arc Length | 177.89 |
| reflector diameter depth | 30 |
| Mirror cutoff wavelength | 390 |
| Focal length of transmitted light | 50 |
| Focal length of reflected light | 800 |
| Project | Norm |
|---|---|
| structural material | copper (pure copper, as opposed alloy) |
| thermal conductivity (W·m−1·K−1) | 401 |
| length (mm) | 1000 |
| high (mm) | 35 |
| maximum width (mm) | 70 |
| Fuel zone size (mm) | 1000 × 70 × 5 |
| Diameter of heat conduction oil orifice (mm) | 10 |
| Azobenzene charge (kg) | 0.83 |
| Chemical Name | Abbreviation | Formula | CAS | Purity | Molar Mass |
|---|---|---|---|---|---|
| Azobenzene | [AZO] | C12H10N2 | 103-33-3 | ≥97% | 182.22 |
| Order of Experiments | Cumulative Heat Release (kJ) | Peak Transient Exotherm (kJ·kg−1·m−1) | Exothermic Power of the MOST Device (W·kg−1·m−1) |
|---|---|---|---|
| 1 | 0.87 | 0.07 | 3.22 |
| 2 | 4.75 | 0.25 | 15.89 |
| 3 | 7.28 | 0.41 | 24.38 |
| 4 | 9.04 | 0.54 | 27.50 |
| 5 | 8.61 | 0.51 | 28.82 |
| 6 | 7.93 | 0.45 | 26.55 |
| 7 | 8.76 | 0.45 | 29.31 |
| 8 | 8.33 | 0.58 | 27.88 |
| 9 | 7.68 | 0.33 | 25.71 |
| 10 | 8.32 | 0.51 | 27.08 |
| 11 | 5.61 | 0.32 | 18.77 |
| 12 | 1.17 | 0.25 | 2.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Guo, C.; Zhang, Y.; Wang, H.; Liu, W.; Jin, J.; Guo, S.; Cuce, E. Exploring Options for the Application of Azobenzene for Molecular Solar Thermal Energy Storage: Integration with Parabolic Trough Solar Systems. Energies 2025, 18, 2298. https://doi.org/10.3390/en18092298
Zhang L, Guo C, Zhang Y, Wang H, Liu W, Jin J, Guo S, Cuce E. Exploring Options for the Application of Azobenzene for Molecular Solar Thermal Energy Storage: Integration with Parabolic Trough Solar Systems. Energies. 2025; 18(9):2298. https://doi.org/10.3390/en18092298
Chicago/Turabian StyleZhang, Li, Changcheng Guo, Yazhu Zhang, Haofeng Wang, Wenjing Liu, Jing Jin, Shaopeng Guo, and Erdem Cuce. 2025. "Exploring Options for the Application of Azobenzene for Molecular Solar Thermal Energy Storage: Integration with Parabolic Trough Solar Systems" Energies 18, no. 9: 2298. https://doi.org/10.3390/en18092298
APA StyleZhang, L., Guo, C., Zhang, Y., Wang, H., Liu, W., Jin, J., Guo, S., & Cuce, E. (2025). Exploring Options for the Application of Azobenzene for Molecular Solar Thermal Energy Storage: Integration with Parabolic Trough Solar Systems. Energies, 18(9), 2298. https://doi.org/10.3390/en18092298






