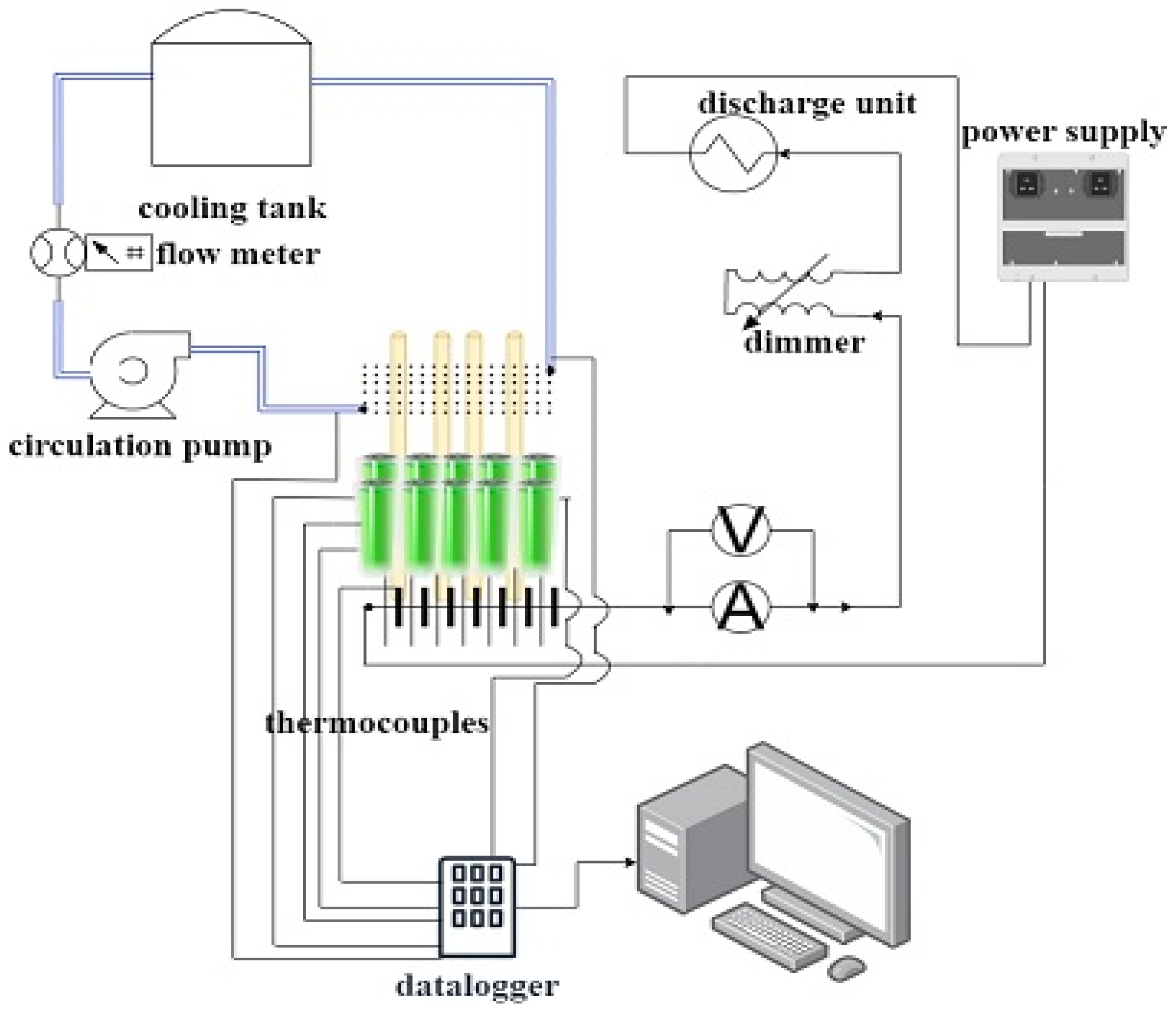
Findings
In this study, we aimed to keep the battery packs consisting of 24 cells at different current intensities (1C, 2C, and 3C) in the optimum operating range of 15–40 °C with straight heat pipe, pulsating heat pipe, immersion method, and heat pipe immersion method hybrid cooling systems. The experimental studies were compared with each other by percentage efficiency calculation.
In
Table 1 and
Figure 2, the experiments carried out in the straight heat pipe system are compared.
CuO nanofluid-doped heat pipes were 13.25% more efficient than pure water in reducing the average cell temperature and 7.22% more efficient than Al2O3-doped heat pipes.
In
Table 2 and
Figure 3, the experiments performed in the straight heat pipe system are compared.
CuO nanofluid-doped heat pipes were 28.41% more efficient than pure water in reducing the average cell temperature and 13.1% more efficient than Al2O3 nanoparticle-doped heat pipes.
In
Table 3 and
Figure 4, the experiments performed in the straight heat pipe system are compared.
CuO nanofluid-doped heat pipes provided 26.33% efficiency in reducing the average cell temperature compared with pure water, while their efficiency was 9.81% compared with Al2O3-doped heat pipes.
The most successful results in reducing the average cell temperature in plain heat pipe experiments occurred under 2C current intensity and in experiments with CuO-doped nanofluids. The reason for the low efficiency at 1C current intensity is that the heat pipe cannot reach the optimum operating temperature.
The reason why the time scales in the graphs were t = 3000 s at 1C current intensity, t = 1500 s at 2C current intensity, and t = 1000 s at 3C current intensity was that they are the preferred times in order to not consume the total energy of the batteries depending on the current speed and not to reach the cut-off voltage. When lithium-ion batteries are completely discharged, the life of the batteries decreases and may pose a risk of explosion in terms of safety. Therefore, it is important to use lithium-ion batteries within safe limits without completely discharging them. Under normal conditions, the 1C current rate refers to the discharge rate of the battery in exactly 1 h and is 3600 s. Likewise, half an hour for 2C is 1800 s, and for 3C, it is one-third of 1 h, i.e., 1200 s. In order to eliminate the risk of non-rechargeability and explosion if the lithium-ion batteries are completely discharged by exceeding the cut-off voltage, these times were selected as 50 min (3000 s) for 1C, 25 min (1500 s) for 2C, and 16.7 min (1000 s) for 3C.
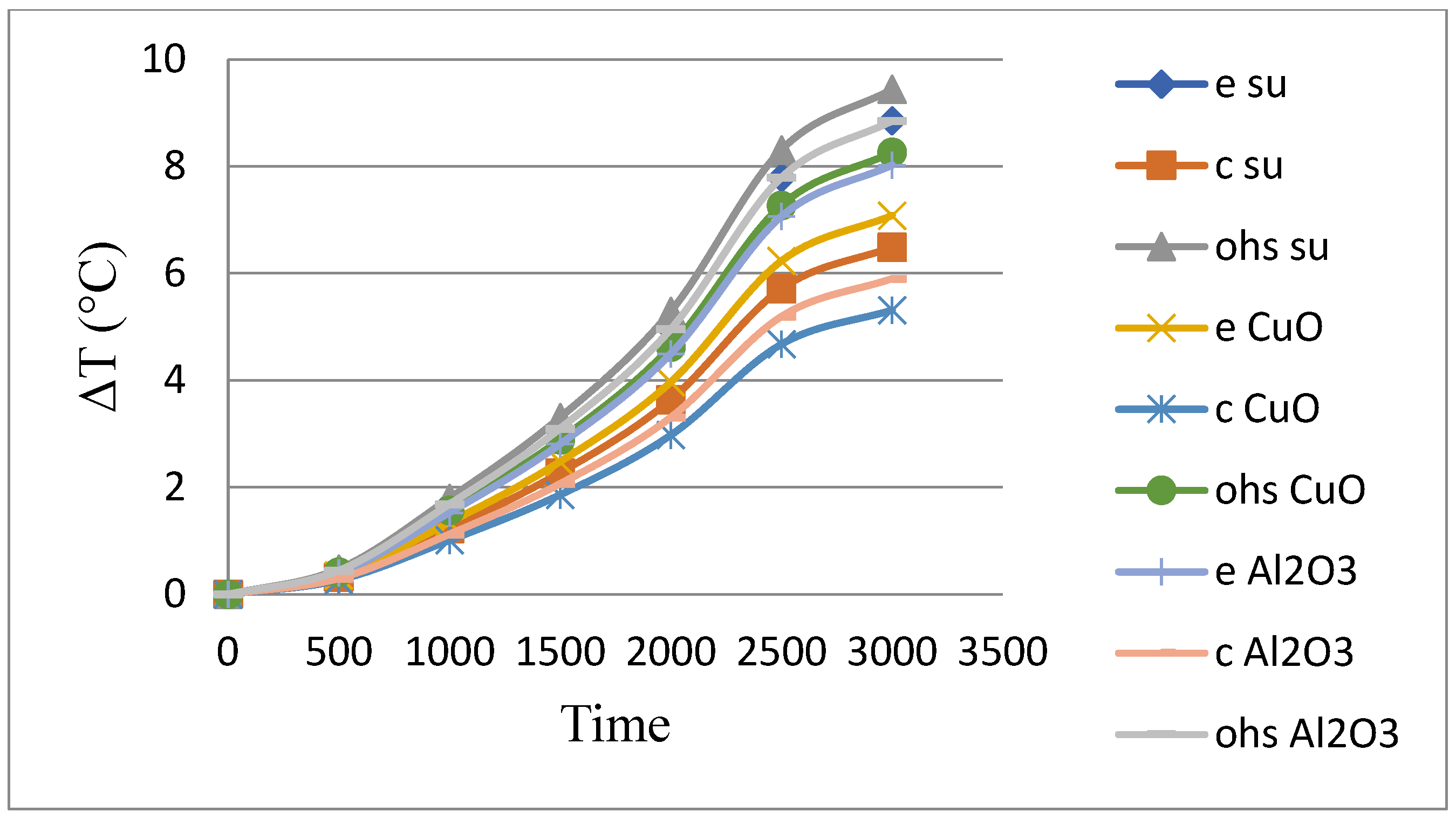
As shown in
Table 4 and
Figure 5, the experiments performed in the straight heat pipe system using methanol-based fluid are compared.
CuO nanofluid doped heat pipes were 12.87% more efficient than methanol in reducing the average cell temperature and 6.38% more efficient than Al2O3 doped heat pipes.
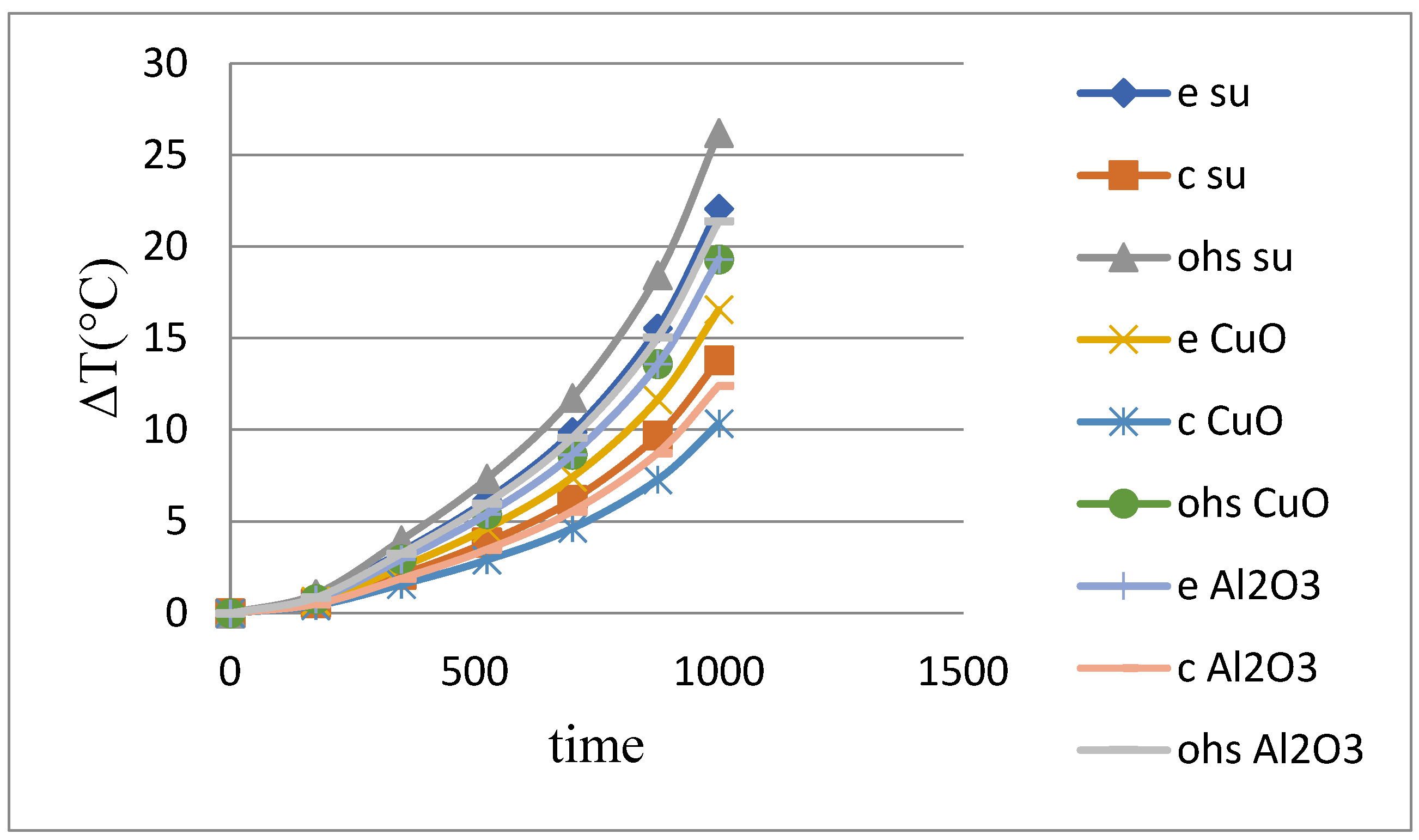
As shown in
Table 5 and
Figure 6, the experiments performed in the straight heat pipe system using methanol-based fluid are compared.
CuO nanofluid-doped heat pipes were 28.28% more efficient than methanol in reducing the average cell temperature, while Al2O3-doped heat pipes were 12.88% more efficient.
As shown in
Table 6 and
Figure 7, the experiments performed in the straight heat pipe system using methanol-based fluid are compared.
CuO nanofluid-doped heat pipes were 26.14% more efficient than methanol in reducing the average cell temperature, while Al2O3-doped heat pipes were 11.02% more efficient.
As shown in
Table 7 and
Figure 8, the experiments performed in the straight heat pipe system using water-based fluid are compared.
CuO nanofluid-doped heat pipes were 19.54% more efficient than water in reducing the average cell temperature, while Al2O3-doped heat pipes were 10.26% more efficient than water.
As shown in
Table 8 and
Figure 9, the experiments performed in the pulsating heat pipe system using water-based fluid are compared.
CuO nanofluid-doped heat pipes were 29.26% more efficient than water in reducing the average cell temperature, while Al2O3-doped heat pipes were 13.91% more efficient than water.
As shown in
Table 9 and
Figure 10, the experiments performed in the pulsating heat pipe system using water-based fluid are compared.
CuO nanofluid-doped heat pipes were 25.53% more efficient than water in reducing the average cell temperature, while Al2O3-doped heat pipes were 9.68% more efficient.
As can be seen in
Table 10 and
Figure 11, pulsating heat using methanol-based fluid experiments performed in the tubular system are compared.
CuO nanofluid-doped heat pipes were 13.3% more efficient than water in reducing the average cell temperature and 7.14% more efficient than Al2O3-doped heat pipes.
As shown in
Table 11 and
Figure 12, the experiments performed in the pulsating heat pipe system using methanol-based fluid are compared.
CuO nanofluid-doped heat pipes provide 28.74% efficiency compared with water in reducing the average cell temperature, while it is 13.14% efficient compared with Al2O3 doped heat pipes.
As shown in
Table 12 and
Figure 13, the experiments performed in the pulsating heat pipe system using methanol-based fluid are compared.
CuO nanofluid-doped heat pipes provide 26.14% efficiency compared with water in reducing the average cell temperature, while it is 23.03% efficient compared with Al2O3-doped heat pipes.
In the experiments using the immersion method, the battery cells were placed in the Thermasolv im2 liquid pool and the results were tested. Although the immersion method alone provides successful cooling, heat pipe-reinforced systems provide important contributions at high current capacities.
As can be seen in
Figure 14, in the study carried out only with the immersion method, temperature differences of 3.6 °C, 6.1 °C, and 8.7 °C at 2C current intensity and 8.7 °C at 3C current intensity were determined at the pool temperature under 1C current intensity. Here, the pool temperature is equal to the evaporator temperature as well as the average cell temperature, since the coils are completely inside the pool.
When the immersion method and heat pipe systems were combined and a hybrid thermal management design was created, as shown in
Figure 15, it can be seen that the evaporator temperature difference (e) in the studies carried out with the pulsating heat pipe with water-based fluid (im2 php water) at 1C current intensity was 2.5 °C and the evaporator temperature difference (e) in the studies carried out with the pulsating heat pipe with water/CuO nanosolution base fluid (im2 php water/CuO) was 1, an evaporator temperature difference (e) of 2 °C was determined in the studies carried out with a pulsating heat pipe (im2 php water/Al
2O
3) with 9 °C water/Al
2O
3 nanosolution-based fluid.
The evaporator temperature difference (e) was 2.6 °C in studies with methanol-based fluidized pulsating heat pipe (im2 php methanol); the evaporator temperature difference (e) was 2.6 °C in studies with methanol/CuO nanosolution-based fluidized pulsating heat pipe (im2 php methanol/CuO), and the evaporator temperature difference (e) was determined as 2.3 °C in the studies carried out with the pulsating heat pipe (im2 php methanol/Al2O3) at 3 °C with methanol/Al2O3 nanosolution-based fluid. The most successful system was the im2 php water/CuO hybrid design with 47.22% efficiency compared with the immersion method.
Figure 16 shows that the evaporator temperature difference (e) in the studies carried out with the water-based fluid pulsating heat pipe (im2 php water) at 2C current intensity was 3.8 °C and the evaporator temperature difference (e) in the studies carried out with the water/CuO nanosolution-based fluid pulsating heat pipe (im2 php water/CuO) was 3.8 °C and 2 °C, and for the water/Al
2O
3 nanosolution-based fluid pulsating heat pipe (im2 php water/Al
2O
3), an evaporator temperature difference (e) of 3.4 °C was determined.
The evaporator temperature difference (e) was 4 °C in the studies carried out with the methanol-based fluidized pulsating heat pipe (im2 php methanol), the evaporator temperature difference (e) was 3.4 °C in the studies carried out with the methanol/CuO nanosolution-based fluidized pulsating heat pipe (im2 php methanol/CuO), and the evaporator temperature difference (e) was 3.7 °C in the studies carried out with the methanol/Al2O3 nanosolution-based fluidized pulsating heat pipe (im2 php methanol/Al2O3).
Here, the most successful system was im2 php water/CuO hybrid design with 47.54% efficiency compared with the immersion method.
Figure 17 shows that the evaporator temperature difference (e) in the studies carried out with the pulsating heat pipe with water-based fluid (im2 php water) at 3C current intensity was 6 °C, the evaporator temperature difference (e) in the studies carried out with the pulsating heat pipe with water/CuO nanosolution-based fluid (im2 php water/CuO) was 4 °C, and the evaporator temperature difference (e) for the water/Al
2O
3 nanosolution base fluid pulsating heat pipe (im2 php water/Al
2O
3) was 5 °C.
The evaporator temperature difference (e) was 6.4 °C in studies with a methanol-based fluidized pulsating heat pipe (im2 php methanol), the evaporator temperature difference (e) was 4.4 °C in studies with a methanol/CuO nanosolution-based fluidized pulsating heat pipe (im2 php methanol/CuO), and the evaporator temperature difference (e) was found to be 5.3 °C in the studies carried out with the pulsating heat pipe (im2 php methanol/Al2O3) with 6 °C methanol/Al2O3 nanosolution-based fluid. Here, the most successful system was the im2 php water/CuO hybrid design with 49.43% efficiency compared with the immersion method.
In the study, uncertainty analysis was performed and the results were examined.
S: Measured dimension
X1, X2,…, Xn: Variables affecting measurement
W1, W2,…, Wn: The error rate of the independent variable is;
The total uncertainty in our experiment was calculated using the uncertainty analysis formulas and/or equations mentioned above according to the measurement accuracy rates shown in
Table 13. The total error rate was calculated as Ws = ±7.418 and was considered acceptable.