The Integration of Carbon Capture, Utilization, and Storage (CCUS) in Waste-to-Energy Plants: A Review
Abstract
1. Introduction
2. Current Status of WtE Systems
- Anaerobic Digestion, which is applied specifically to organic waste that is biodegraded by microorganisms in an oxygen-free environment, producing biogas (with a 50–60% vol. methane content) that is exploited for electricity and/or heat production or as a fuel after upgrading processes.
- Gasification, in which waste is heated in a low-oxygen environment to produce a synthetic gas (syngas), which can be used to generate electricity or be converted into a fuel.
- Pyrolysis, which involves the thermal decomposition of waste in the absence of oxygen, generating bio-oil, syngas, and char.
- Incineration, through which waste is combusted in air at a high temperature, producing heat that can be used to generate electricity and/or provide district heating.
CO2 Emissions from Waste-to-Energy Plants and Applicability of CCUS Processes
3. Carbon Capture, Storage, and/or Utilization Strategies
3.1. Carbon Capture Strategies
3.1.1. Post-Combustion
3.1.2. Pre-Combustion
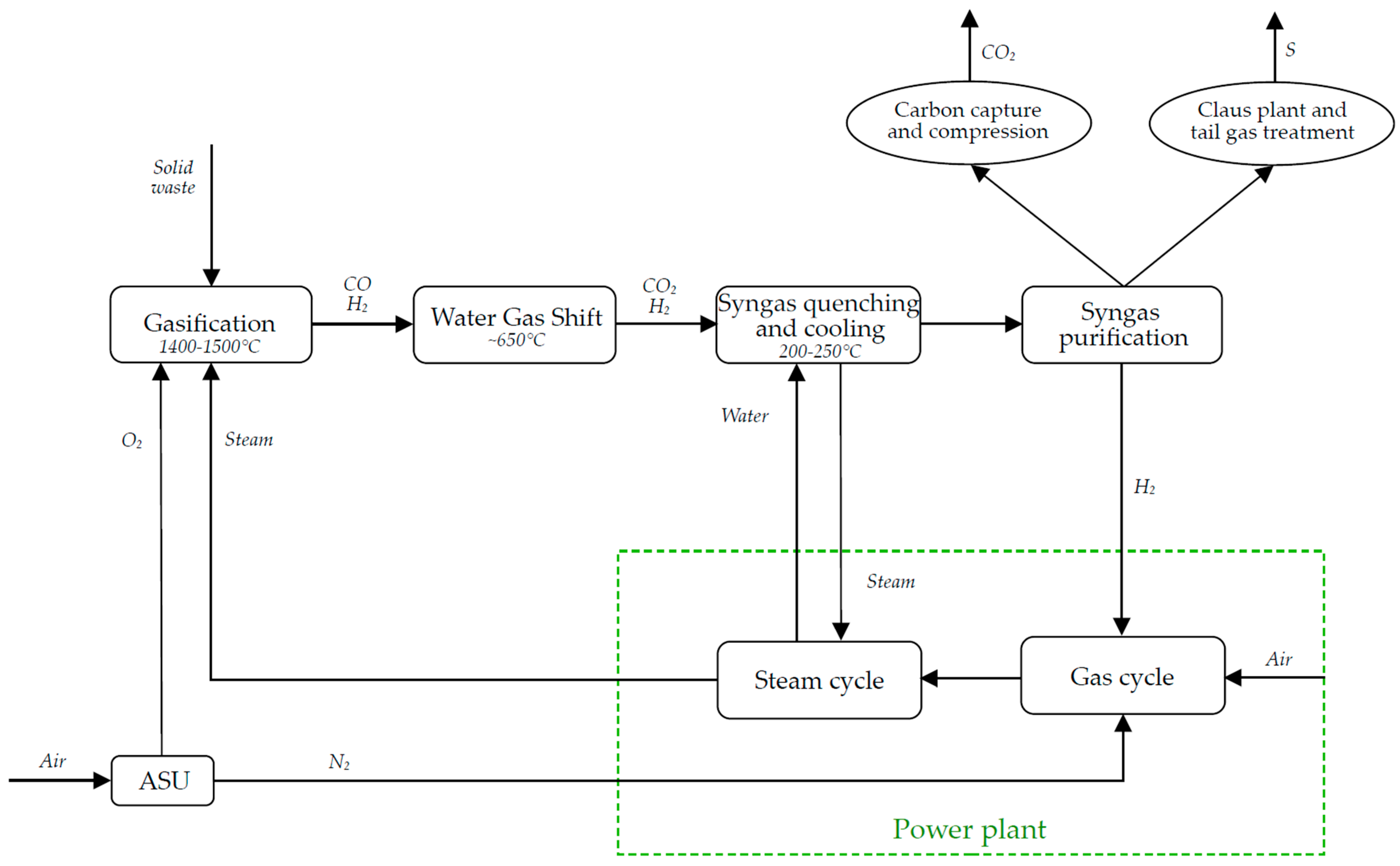
3.1.3. Oxyfuel Combustion
3.2. Storage and/or Utilization Strategies
3.2.1. CO2 Storage
3.2.2. CO2 Utilization
- SYNTHETIC HYDROCARBON FUELS AND CHEMICALS
- USE IN GREENHOUSES
- SODIUM BICARBONATE
- MINERAL CARBONATION AND CONCRETE CURING
- UREA
- ENHANCED HYDROCARBON RECOVERY
- POLYMERS
- MICRO-ALGAE
- OTHER APPLICATIONS
- Protein production, which is still at the demonstration phase. The investment costs for the electrolyzer are significant, but the potential revenue is considered low, because the protein demand can currently be fulfilled from other natural resources (animals and plants) [85].
- Lignin production, which is a mature technology that needs 0.22 tons of CO2 per ton of lignin produced [82].
- Food and beverages, even if their production is not considered a suitable application for waste-to-energy plants despite adequate CO2 purity standards, due to concerns regarding the possible contamination of the CO2. However, further developments may be foreseen [85].
- Semiconductor cleaning, e.g., in solar panels [85].
- Refrigerants, such as a coolant in industrial refrigerators and cooling facilities or dry ice [84].
- Fire-extinguishing gas used in portable cylinders [84].
| Utilization | TRL | Conversion Factor | Market Interest |
|---|---|---|---|
| SYNTHETIC HYDROCARBON FUELS AND CHEMICALS [50,82,84,87] | 9 (methanol) 3–5 (formic acid) | 1.4–1.7 tons of CO2/ton of methanol 1.0 ton of CO2/ton of formic acid 3.0 tons of CO2/ton of methane 2.0 tons of CO2/ton of DME | high |
| USE IN GREENHOUSES [82,89] | 9 | 100–600 kg of CO2/ha/hour | medium |
| SODIUM BICARBONATE [82,85,89] | 5–7 | 0.52 tons of CO2/ton of sodium bicarbonate 1.6 tons of sodium bicarbonate/ton of CO2 | high |
| MINERAL CARBONATION AND CONCRETE CURING [82] | 7–8 | 0.25 tons of CO2/ton of steel slag 0.03 tons of CO2/ton of blocks produced 0.12 tons of CO2/ton of precast concrete | high |
| UREA [82] | 9 | 0.74 tons of CO2/ton of urea | low |
| ENHANCED HYDROCARBON RECOVERY [91,95] | 9 | 0.8 Mtons of CO2/well/year 1.25 bbl of oil/ton of CO2 | low |
| POLYMERS [82,87] | 7 | 0.1–0.3 tons of CO2/ton of polyurethane 0.43 tons of CO2/ton of PPC | medium |
| MICRO-ALGAE [85,95] | 9 | 1.8–2.0 tons of CO2/ton of algae | uncertain |
4. Waste-to-Energy Plants Coupled with CCUS
4.1. Plants in Operation
4.2. Future Projects
| Place | WtE Company | Capture Technology | Waste Processed [tons/year] | CO2 Captured [tons/year] | Storage | Utilization | Status | |
|---|---|---|---|---|---|---|---|---|
| Denmark | Aalborg [103,104] | Reno-Nord | - | 223,500 | 180,000 | - | Methanol production | Completed in 2028 |
| Roskilde [105] | ARGO | - | 334,000 | - | Dedicated storage | - | Operating in 2030 | |
| Glostrup [106] | Vestforbrænding | 550,000 | 450,000 | Dedicated storage | - | Operating in 2025 | ||
| Copenhagen [107] | Amager Bakke | Amine | 600,000 | 500,000 | North Sea | - | Operating in 2025 | |
| Finland | Mustasaari [108,109] | Westenergy | - | 190,000 | 20,000 | - | Synthetic methane production | Scheduled to be built in 2023–2025 |
| Riihimäki [110] | Fortum | - | 270,000 | - | - | CO2-based high-quality raw materials | Pilot (capturing 15L CO2/min and converting it into 10L of methane) | |
| Italy | Corteolona [111] | A2A | HPC | 63,000 | - | - | - | Test installation |
| The Netherlands | Alkmaar [95,112] | HVC | Amine | 660,000 | 4000 | - | Greenhouse horticulture sector | The start of operations was in 2019 |
| 75,000 | - | Feasibility study ended in 2019 | ||||||
| Amsterdam [113] | AEB | Amine | 1,400,000 | 450,000 | - | Greenhouse horticulture sector | Feasibility study | |
| Hengelo [100] | Twence | Amine | 600,000 | 100,000 | - | Greenhouse horticulture sector | Large-scale plant planned for operation in 2025 | |
| Rozenburg [95,114] | AVR | - | 1,300,000 | 800,000 | - | Greenhouse horticulture sector; production of building materials; basic chemistry for plastics and biofuels | The capture facility is based on the previous experience of AVR in Duiven | |
| Norway | Bergen [115] | BIR | 220,000 | 100,000 | Northern Lights project | - | Feasibility study in 2021–2022, probably operating in 2030 | |
| Friederikstad [116] | Frevar KF/Kvitebjorn BIO-EL (2 facilities) | Amine | 120,000 | Northern Lights project | - | |||
| Heimdal [117] | Statkraft | Amine | 80,000 | 25 tons/hour | Northern Lights project | - | The feasibility study ended in autumn 2022 | |
| Klemetsrud [118,119] | Hafslund | Amine | 415,000 | 400,000 | Northern Lights project | - | Pilot plant [120], operating in 2026–2027 | |
| Kristiansand [121] | Returkraft | Membrane-based | 130,000 | 140,000 | Northern Lights project | - | Pilot in 2023, probably operating in 2030 | |
| Rådal [122,123] | BIR | - | 210,000 | - | Northern Lights project | - | Feasibility study conducted in 2021–22 | |
| - | Production of carbon nanofibers (CNFs) | Mobile test module (1.6 tons CNF/year) | ||||||
| Rakkestad [124] | Ostfold Energi | Amine | 10,000 | 10,000 | Storage in 2024 | Sell to the food and greenhouse industries at the beginning | Trial project completed in 2023 | |
| Tromso [125] | Kvitebjørn Varme | Amine | 110,000 | 100,000 | Considering various storage locations | - | Feasibility study, operating in 2030 | |
| Portugal | Porto [126] | LIPOR’s Energy Recovery Plant | - | 380,000 | 100,000 tons of captured biogenic CO2 | - | Sustainable aviation fuels (SAFs) | Feasibility study |
| Sweden | Malmö [127,128] | Sysav | Amine, HPC | 630,000 | 500,000 | Storage | - | Feasibility study, operating in 2030 |
| Helsingborg [129,130] | Öresundskraft | HPC | 160,000 | 210,000 | - | - | Mobile demonstration unit started operation in 2022 | |
| Switzerland | Niederurnen [112] | KVA Linth | Amine | 110,000 | 100,000 | Northern Lights project | - | Feasibility study |
| Dietikon [131] | Limeco | - | - | - | - | - | Feasibility study | |
| UK | Knottingley [132] | Enfinium | Amine | 1,500,000 | 1 ton/day (pilot) 1,200,000 from 2030 | North Sea | - | Pilot in 2024, operating in 2030 |
| Haverton Hill [133] | SUEZ | Amine | - | 240,000 | North Sea | - | Pre-Front end engineering design | |
| London [134] | Cory | Amine | 1,500,000 | 1300,000 | North Sea | - | Construction in 2026, operational by 2030 | |
| Runcorn [80] | Viridor | - | 1,100,000 | 900,000 | Hynet project | - | - | |
| Teeside [135] | Low Carbon and PMAC Energy | - | 500,000 | 400,000 | North Sea | - | Operating in 2030 | |
| Wilton [133] | SUEZ | - | 440,000 | - | North Sea | - | Pre-Front end engineering design | |
| - | Veolia [136] | Amine | - | - | - | Green fuels | Feasibility study | |
5. Techno-Economic and Environmental Aspects
5.1. Energy Requirements
| Source of Energy | Quantity | Notes | Electricity Penalty with CC * | |
|---|---|---|---|---|
| Power Only | CHP | |||
| Steam [35,38,39,40,41,42,43,84,137,138,139,141] | 2.45–4.71 GJ/ton CO2 | Majority of sources report around 3.6 GJ/ton of CO2 | ~25% | ~30% |
| Heat recovery [39,40,139] | 2.2–3.3 GJ/ton CO2 | With improvements, 2.5–5.4 GJ/ton CO2 | - | - |
| Electricity use for CO2 capture [39,40,43,91,139] | 15–44 kWh/ton waste 15–35 kWh/ton CO2 | - | 2–6% | 3–7% |
| Electricity use for CO2 conditioning: [40,91,139,141] | 99–160 kWh/ton CO2 | - | - | - |
| Compression [39,40,84,137,138,139] | 65–105 kWh/ton waste 86.1–105 kWh/ton CO2 | - | 8–14% | 10–17% |
| Drying [139] | 2 kWh/ton CO2 | - | <1% | <1% |
| Liquefaction [40,139] | 42 kWh/ton waste 64 kWh/ton CO2 | - | 5% | 7% |
5.2. Economic Considerations
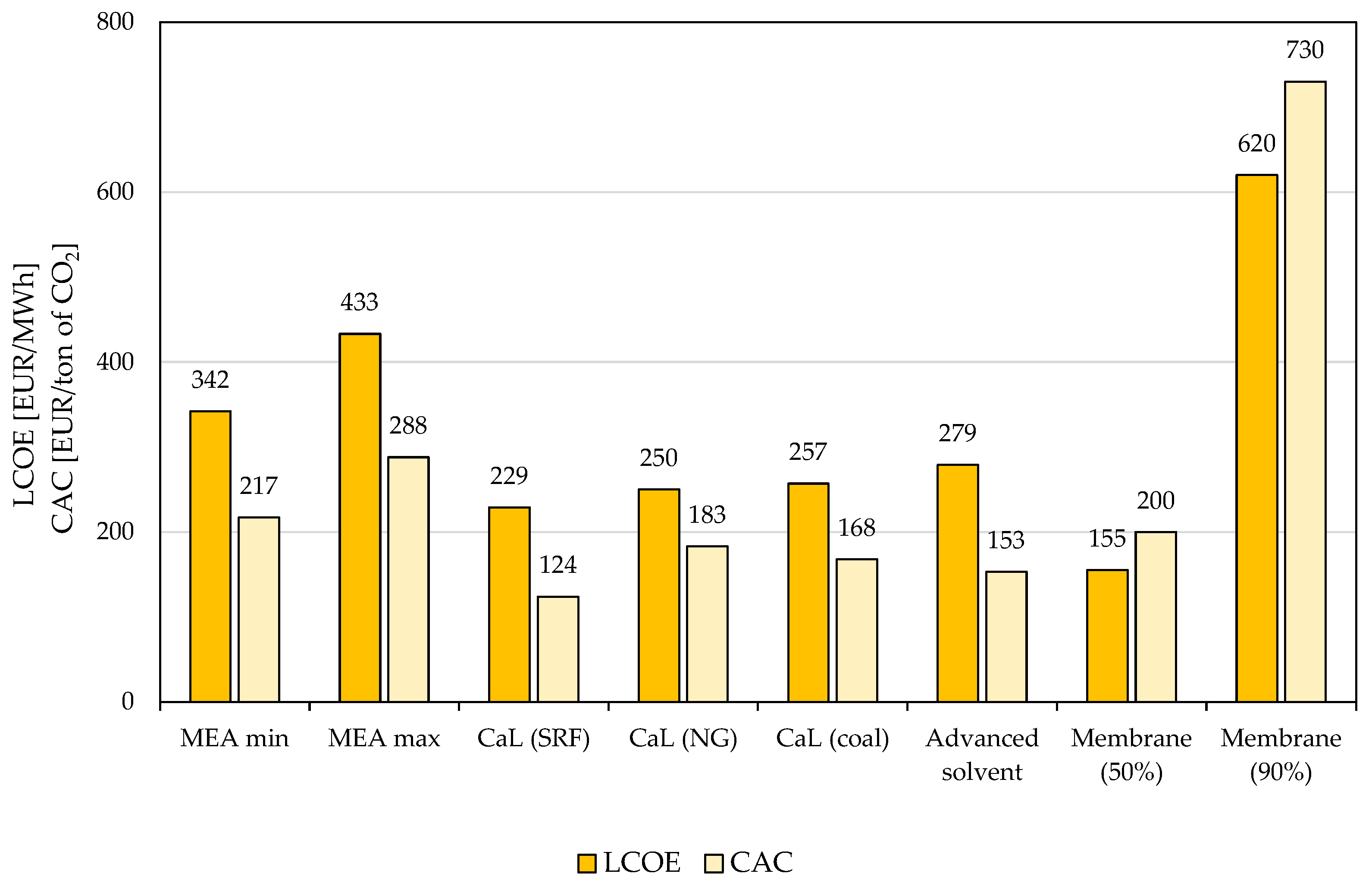
5.3. Life Cycle Assessment-Based Evaluations
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: Cambridge, UK, 2023; ISBN 978-1-00-932584-4. [Google Scholar]
- Paris Agreement to the United Nations Framework Convention on Climate Change, Dec. 12, 2015, T.I.A.S. No. 16-1104. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 25 March 2025).
- Radley-Gardner, O.; Beale, H.; Zimmermann, R. (Eds.) Regulation (EU) 2021/1119 of the European Parliament and of the Council of 30 June 2021 Establishing the Framework for Achieving Climate Neutrality and Amending Regulations (EC) No 401/2009 and (EU) 2018/1999 (‘European Climate Law’); Hart Publishing: Oxford, UK, 2016; ISBN 978-1-78225-864-3. [Google Scholar]
- World|Total Including LUCF|Greenhouse Gas (GHG) Emissions|Climate Watch. Available online: https://www.climatewatchdata.org/ghg-emissions?end_year=2021&start_year=1990 (accessed on 5 December 2024).
- Where Do Emissions Come from? 4 Charts Explain Greenhouse Gas Emissions by Sector—World Resources Institute. Available online: https://www.wri.org/insights/4-charts-explain-greenhouse-gas-emissions-countries-and-sectors (accessed on 18 March 2025).
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0. [Google Scholar]
- European Environment Agency, Briefing No 01/2025. Methane, Climate Change and Air Quality in Europe: Exploring the Connections. Available online: https://www.eea.europa.eu/en/analysis/publications/methane-climate-change-and-air-quality-in-europe-exploring-the-connections?activeTab=6397c084-2e5f-4545-a873-f99323d40846 (accessed on 24 March 2025). [CrossRef]
- CEWEP WtE Sustainability Roadmap Towards 2035. 2019. Available online: https://www.cewep.eu/wp-content/uploads/2019/09/WtE_Sustainability_Roadmap_Digital.pdf (accessed on 5 March 2025).
- Fahnestock, J.; Johansson, I.; Hasselqvist, M.; Mirata, M.; Nilsson, C.; Persson, A.; Petterson, A.; Sahlén, J. Waste Incineration for the Future. Scenario analysis and action plans. IEA Bioenergy 2019. Available online: https://www.ieabioenergy.com/wp-content/uploads/2019/04/Waste-Energy-for-the-Future-IEA-version.pdf (accessed on 11 December 2024).
- Climate Change: Deal on a More Ambitious Emissions Trading System (ETS)|News|European Parliament. Available online: https://www.europarl.europa.eu/news/en/press-room/20221212IPR64527/climate-change-deal-on-a-more-ambitious-emissions-trading-system-ets (accessed on 13 January 2025).
- FOEN, Federal Office for the Environment, Agreement with Managers of Waste Treatment Installations. Available online: https://www.bafu.admin.ch/bafu/en/home/themen/thema-klima/klimawandel-stoppen-und-folgen-meistern/schweizer-klimapolitik/branchenvereinbarungen/zielvereinbarung-uvek-abfallverwertungsanlagen-ch.html (accessed on 13 January 2025).
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 Capture Technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An Overview of Current Status of Carbon Dioxide Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, A. Comparative Assessment of CO2 Capture Technologies for Carbon-Intensive Industrial Processes. Prog. Energy Combust. Sci. 2012, 38, 87–112. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, M.; Jin, L.; Xu, M.; Li, J. Advancing Carbon Capture in Hard-to-Abate Industries: Technology, Cost, and Policy Insights. Clean Technol. Environ. Policy 2024, 26, 2077–2094. [Google Scholar] [CrossRef]
- Bertone, M.; Stabile, L.; Buonanno, G. An Overview of Waste-to-Energy Incineration Integrated with Carbon Capture Utilization or Storage Retrofit Application. Sustainability 2024, 16, 4117. [Google Scholar] [CrossRef]
- UTILITALIA White Paper on Municipal Waste Incineration. 2020. Available online: https://www.cewep.eu/wp-content/uploads/2021/03/WHITE-PAPER-DEFINITIVO-2-24-febbraio-2021.pdf (accessed on 12 November 2024).
- CEWEP Residual Waste Treatment Capacity Fact Sheet. 2020. Available online: https://www.cewep.eu/wp-content/uploads/2020/12/Residual-waste-treatment-capacity-factsheet-2020.pdf (accessed on 3 December 2024).
- Brettler Berenyi, E. Recycling and Waste-to-Energy: Are They Compatible? 2009 Update. June 2009. Available online: https://www.ecomaine.org/wp-content/uploads/2020/06/Berenyi-GAA-2009.pdf (accessed on 6 April 2025).
- Market Report Waste to Energy—The World Market for Waste Incineration Plants. Available online: https://www.ecoprog.com/publications/energy-management/waste-to-energy.htm (accessed on 5 January 2025).
- European Commission; Joint Research Centre. Best Available Techniques (BAT) Reference Document for Waste Incineration: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); EUR 29971 EN; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- ESWET, European Suppliers of Waste-to-Energy Technology. Annual Report 2019; European Suppliers of Waste-to-Energy Technology; Brussels, Belgium, 2019; Available online: https://eswet.eu/wp-content/uploads/2020/12/ESWET-AR-2019_Spreads_Small.pdf (accessed on 3 February 2025).
- CEWEP WtE Climate Roadmap. 2022. Available online: https://www.cewep.eu/wp-content/uploads/2022/06/CEWEP-WtE-Climate-Roadmap-2022.pdf.pdf (accessed on 12 December 2024).
- Blasenbauer, D.; Huber, F.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Bontempi, E.; Blondeau, J.; Chimenos, J.M.; Dahlbo, H.; et al. Legal Situation and Current Practice of Waste Incineration Bottom Ash Utilisation in Europe. Waste Manag. 2020, 102, 868–883. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of Municipal Solid Waste Incineration Fly Ash: State-of-the-Art Technologies and Future Perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the Management of MSW Incineration Ashes from Gas Cleaning: New Perspectives on Recovery of Secondary Raw Materials and Circular Economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- de Titto, E.; Savino, A. Environmental and Health Risks Related to Waste Incineration. Waste Manag. Res. 2019, 37, 976–986. [Google Scholar] [CrossRef]
- Zeman, F. Considering Carbon Capture and Storage for Energy Generation from Municipal Solid Waste. J. Environ. Eng. 2010, 136, 756–761. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). 2006 IPCC Guidelines for National Greenhouse Gas Inventories; The National Greenhouse Gas Inventories Programme, Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Hayama, Japan, 2006; Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/ (accessed on 5 November 2024).
- Mohn, J.; Szidat, S.; Zeyer, K.; Emmenegger, L. Fossil and Biogenic CO2 from Waste Incineration Based on a Yearlong Radiocarbon Study. Waste Manag. 2012, 32, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.W.; Fuglsang, K.; Pedersen, N.H.; Fellner, J.; Rechberger, H.; Astrup, T. Biogenic Carbon in Combustible Waste: Waste Composition, Variability and Measurement Uncertainty. Waste Manag. Res. J. Sustain. Circ. Econ. 2013, 31, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bioenergy with Carbon Capture and Storage—Analysis. Available online: https://www.iea.org/reports/bioenergy-with-carbon-capture-and-storage (accessed on 20 February 2025).
- Costa, G.; Baciocchi, R.; Polettini, A.; Pomi, R.; Hills, C.D.; Carey, P.J. Current Status and Perspectives of Accelerated Carbonation Processes on Municipal Waste Combustion Residues. Environ. Monit. Assess. 2007, 135, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Croymans, T.; Englebert, B.; Wightman, A.; Izquierdo, J. 11 Reasons Why Carbon Capture Should Be Prioritized in the Waste-to-Energy Sector. In Proceedings of the VENICE 2022—9th International Symposium on Energy from Biomass and Waste, Venice, Italy, 21–23 November 2022. [Google Scholar]
- Tota, V.; Viganò, F.; Gatti, M. Application of CCUS to the WtE Sector. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference, Abu Dhabi, United Arab Emirates, 15–18 March 2021. [Google Scholar]
- Akbar, F.M.; Hafiy, M.N.; Ibrahim, F.; Yudhistira, A.M. Effectiveness of Integrated Carbon Capture Technology in Waste-to-Energy Plants and Implementation Prospects. Sociae Polites 2021, 22, 30–47. [Google Scholar] [CrossRef]
- Magnanelli, E.; Mosby, J.; Becidan, M. Scenarios for Carbon Capture Integration in a Waste-to-Energy Plant. Energy 2021, 227, 120407. [Google Scholar] [CrossRef]
- Pour, N.; Webley, P.A.; Cook, P.J. Potential for Using Municipal Solid Waste as a Resource for Bioenergy with Carbon Capture and Storage (BECCS). Int. J. Greenh. Gas Control 2018, 68, 1–15. [Google Scholar] [CrossRef]
- Bisinella, V.; Hulgaard, T.; Riber, C.; Damgaard, A.; Christensen, T.H. Environmental Assessment of Carbon Capture and Storage (CCS) as a Post-Treatment Technology in Waste Incineration. Waste Manag. 2021, 128, 99–113. [Google Scholar] [CrossRef]
- Bisinella, V.; Nedenskov, J.; Riber, C.; Hulgaard, T.; Christensen, T.H. Environmental Assessment of Amending the Amager Bakke Incineration Plant in Copenhagen with Carbon Capture and Storage. Waste Manag. Res. J. Sustain. Circ. Econ. 2022, 40, 79–95. [Google Scholar] [CrossRef]
- Andersson, J. An Investigation of Carbon Capture Technologies for Sävenäs Waste-to-Energy Plant. Master’s Thesis, Luleå University of Technology Department of Civil, Environmental and Natural Resources Engineering, Luleå, Sweden, 2020. [Google Scholar]
- Su, D.; Herraiz, L.; Lucquiaud, M.; Thomson, C.; Chalmers, H. Thermal Integration of Waste to Energy Plants with Post-Combustion CO2 Capture. Fuel 2023, 332, 126004. [Google Scholar] [CrossRef]
- Tang, Y.; You, F. Multicriteria Environmental and Economic Analysis of Municipal Solid Waste Incineration Power Plant with Carbon Capture and Separation from the Life-Cycle Perspective. ACS Sustain. Chem. Eng. 2018, 6, 937–956. [Google Scholar] [CrossRef]
- Aouini, I.; Ledoux, A.; Estel, L.; Mary, S. Pilot Plant Studies for CO2 Capture from Waste Incinerator Flue Gas Using MEA Based Solvent. Oil Gas Sci. Technol. Rev. D’IFP Energ. Nouv. 2014, 69, 1091–1104. [Google Scholar] [CrossRef]
- Stolaroff, J. Carbonate Solutions for Carbon Capture: A Summary; Lawrence Livermore National Lab (LLNL): Livermore, CA, USA, 2013. [Google Scholar]
- Ayittey, F.K.; Obek, C.A.; Saptoro, A.; Perumal, K.; Wong, M.K. Process Modifications for a Hot Potassium Carbonate-based CO2 Capture System: A Comparative Study. Greenh. Gases Sci. Technol. 2020, 10, 130–146. [Google Scholar] [CrossRef]
- Haaf, M.; Hilz, J.; Unger, A.; Ströhle, J.; Epple, B. Methanol Production Via the Utilization of Electricity and CO2 Provided by a Waste Incineration Plant. In Proceedings of the 14th International Conference on Greenhouse Gas Control Technologies (GHGT-14), Melbourne, Australia, 21–25 October 2018. [Google Scholar]
- Haaf, M.; Anantharaman, R.; Roussanaly, S.; Ströhle, J.; Epple, B. CO2 Capture from Waste-to-Energy Plants: Techno-Economic Assessment of Novel Integration Concepts of Calcium Looping Technology. Resour. Conserv. Recycl. 2020, 162, 104973. [Google Scholar] [CrossRef]
- Durán, I.; Rubiera, F.; Pevida, C. Vacuum Swing CO2 Adsorption Cycles in Waste-to-Energy Plants. Chem. Eng. J. 2020, 382, 122841. [Google Scholar] [CrossRef]
- Llorach Naharro, P. Carbon Capture and Utilization from a Municipal Solid Waste-to-Energy Plant. Master’s Thesis, Chemical Engineering—Smart Chemical Factories, Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. [Google Scholar]
- Viganò, F.; Cretarola, L.; Spinelli, M. Molten Carbonate Fuel Cells (MCFC) for the Carbon Capture in Energy-from-Waste (EfW). In Proceedings of the VENICE 2022—9th International Symposium on Energy from Biomass and Waste, Venice, Italy, 21–23 November 2022. [Google Scholar]
- Cretarola, L.; Mazzolari, G.; Lena, E.D.; Spinelli, M.; Gatti, M.; Viganò, F. Carbon Capture for Energy-from-Waste Plants: Comparison of Three Appliable Technologies. In Proceedings of the CHANIA 2023 10th International Conference on Sustainable Solid Waste Management, Chania, Greece, 21–24 June 2023. [Google Scholar]
- Cormos, C.-C. Hydrogen and Power Co-Generation Based on Coal and Biomass/Solid Wastes Co-Gasification with Carbon Capture and Storage. Int. J. Hydrogen Energy 2012, 37, 5637–5648. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Z.; Li, H. SNG-Electricity Cogeneration through MSW Gasification Integrated with a Dual Chemical Looping Process. Chem. Eng. Process. Process Intensif. 2019, 145, 107665. [Google Scholar] [CrossRef]
- Ashkanani, H.E.; Wang, R.; Shi, W.; Siefert, N.S.; Thompson, R.L.; Smith, K.; Steckel, J.A.; Gamwo, I.K.; Hopkinson, D.; Resnik, K.; et al. Effect of Power Plant Capacity on the CAPEX, OPEX, and LCOC of the CO2 Capture Process in Pre-Combustion Applications. Int. J. Greenh. Gas Control 2021, 109, 103371. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-Energy Technology Integrated with Carbon Capture—Challenges and Opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Lucquiaud, M.; Herraiz, L.; Su, D.; Thomson, C.; Chalmers, H.; Becidan, M.; Ditaranto, M.; Roussanaly, S.; Anantharaman, R.; Moreno Mendaza, J.; et al. Negative Emissions in the Waste-to-Energy Sector: An Overview of the Newest-CCUS Programme. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference, Abu Dhabi, United Arab Emirates, 15–18 March 2021. [Google Scholar]
- Bergmo, P.E.S.; Emmel, B.U.; Anthonsen, K.L.; Aagaard, P.; Mortensen, G.M.; Sundal, A. Quality Ranking of the Best CO2 Storage Aquifers in the Nordic Countries. Energy Procedia 2017, 114, 4374–4381. [Google Scholar] [CrossRef]
- Building Nordic Excellence in CCS NORDICCS—The Nordic CCS Competence Centre, Top-Level Research Initiative. Available online: https://www.sintef.no/en/projects/2011/nordiccs-nordisk-ccs-kompetansesenter-/ (accessed on 3 October 2024).
- Ausfelder, F.; Baltac, S. IEA Special Report on Carbon Capture Utilisation and Storage, CCUS in Clean Energy Transitions. Energy Technol. Perspect. 2020, 174. Available online: https://www.iea.org/reports/ccus-in-clean-energy-transitions (accessed on 5 April 2025).
- Map of CCUS Projects in Europe—IOGP Europe. Available online: https://iogpeurope.org/resource/map-of-eu-ccus-projects/ (accessed on 5 November 2024).
- Antwerp@C Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2304 (accessed on 10 December 2024).
- Antwerp@C. Available online: https://ccushub.ogci.com/focus_hubs/antwerpc-kairosc/ (accessed on 12 January 2025).
- Antwerp@C. Investigates Potential for Halving CO2 Emissions in Port of Antwerp by 2030. Available online: https://newsroom.portofantwerpbruges.com/antwerpc-investigates-potential-for-halving-co2-emissions-in-port-of-antwerp-by-2030 (accessed on 15 February 2025).
- Global CCS. Institute Global Status of CCS 2024. Available online: https://www.globalccsinstitute.com/wp-content/uploads/2024/11/Global-Status-Report-6-November.pdf (accessed on 3 February 2025).
- C4—Carbon Capture Cluster Copenhagen Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2731 (accessed on 14 December 2024).
- C4 Carbon Capture Cluster Copenhagen. Available online: http://a-r-c.dk/c4/ (accessed on 12 December 2024).
- Copenhagen Energy Players Form CCS Alliance with Great Potential. Available online: https://stateofgreen.com/en/news/copenhagen-energy-groups-form-ccs-alliance/ (accessed on 13 December 2024).
- Project Greensand Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2730 (accessed on 20 December 2024).
- Greensand Project. Available online: https://greensandfuture.com/ (accessed on 26 February 2025).
- Aramis. Available online: https://ccushub.ogci.com/focus_hubs/aramis/ (accessed on 13 February 2025).
- Northern Lights Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2142 (accessed on 15 December 2024).
- Northern Lights/Longship. Available online: https://ccushub.ogci.com/focus_hubs/northern-lights/ (accessed on 13 January 2025).
- A Story About the Johansen Formation. Available online: https://ccsnorway.com/a-story-about-the-johansen-formation/ (accessed on 13 January 2025).
- Northern Lights Annual Report 2023. Available online: https://norlights.com/wp-content/uploads/2024/04/Northern-Lights-4061-SF8-Arsrapport-2023.pdf (accessed on 15 October 2024).
- V Net Zero Humber Cluster Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2736 (accessed on 12 February 2025).
- About-Viking CCS. Available online: https://www.vikingccs.co.uk/about (accessed on 6 December 2024).
- HyNet North West Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2165 (accessed on 10 January 2025).
- HyNet North West. Available online: https://hynet.co.uk/ (accessed on 10 January 2025).
- Government Shortlists Viridor’s Runcorn CCS Project. Available online: https://www.viridor.co.uk/news-and-insights/government-shortlists-viridor-s-runcorn-ccs-project-2/ (accessed on 11 January 2025).
- Northern Endurance Partnership. Available online: https://northernendurancepartnership.co.uk/ (accessed on 4 March 2025).
- Mikhelkis, L.; Govindarajan, V. Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden. Sustainability 2020, 12, 5922. [Google Scholar] [CrossRef]
- Galimova, T.; Ram, M.; Bogdanov, D.; Fasihi, M.; Khalili, S.; Gulagi, A.; Karjunen, H.; Mensah, T.N.O.; Breyer, C. Global Demand Analysis for Carbon Dioxide as Raw Material from Key Industrial Sources and Direct Air Capture to Produce Renewable Electricity-Based Fuels and Chemicals. J. Clean. Prod. 2022, 373, 133920. [Google Scholar] [CrossRef]
- Christensen, T.H.; Bisinella, V. Climate Change Impacts of Introducing Carbon Capture and Utilisation (CCU) in Waste Incineration. Waste Manag. 2021, 126, 754–770. [Google Scholar] [CrossRef] [PubMed]
- Shaliha, J.K.P. Internship Report: CO2 Utilisation and Power-to-X Possibility at Twence, University of Twente, Faculty of Engineering Technology, Master Programme Sustainable Energy Technology. 2017. Available online: https://essay.utwente.nl/85387/1/Report-Julia%20K.P.%20Shaliha%20-%20s1689819.pdf (accessed on 5 April 2025).
- Liu, H.; Ampah, J.D.; Zhao, Y.; Sun, X.; Xu, L.; Jiang, X.; Wang, S. A Perspective on the Overarching Role of Hydrogen, Ammonia, and Methanol Carbon-Neutral Fuels towards Net Zero Emission in the Next Three Decades. Energies 2022, 16, 280. [Google Scholar] [CrossRef]
- Nimmas, T.; Wongsakulphasatch, S.; Chanthanumataporn, M.; Vacharanukrauh, T.; Assabumrungrat, S. Thermochemical Transformation of CO2 into High-Value Products. Curr. Opin. Green Sustain. Chem. 2024, 47, 100911. [Google Scholar] [CrossRef]
- Naims, H. Economics of Carbon Dioxide Capture and Utilization—A Supply and Demand Perspective. Environ. Sci. Pollut. Res. 2016, 23, 22226–22241. [Google Scholar] [CrossRef]
- de Leeuw, M.; Koelemeijer, R. Decarbonisation Options for the Dutch Waste Incineration Industry; Netherlands Environmental Assessment Agency: Bilthoven, The Netherlands, 2022. [Google Scholar]
- Clerens, P.; Thuau, A. The Role of Waste-to-Energy (WtE) in the EU’s Long-Term Greenhouse Gas Emissions Reduction Strategy. Available online: https://books.vivis.de/wp-content/uploads/2023/01/2018_wm_025-036_clerens.pdf (accessed on 8 February 2025).
- Roussanaly, S.; Ouassou, J.A.; Anantharaman, R.; Haaf, M. Impact of Uncertainties on the Design and Cost of CCS from a Waste-to-Energy Plant. Front. Energy Res. 2020, 8, 17. [Google Scholar] [CrossRef]
- Singh, S.P. Effect of Temperature and Light on the Growth of Algae Species—A Review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar]
- News Release (10 August 2016): Toshiba Complete Installation of World’s First Commercial-Use CCU Syst|News|Toshiba. Available online: https://www.global.toshiba/ww/news/corporate/2016/08/pr1001.html (accessed on 10 February 2025).
- Giving CO2 an Economic Value: Carbon Capture Technology Helps Recycle Waste into Resources. Available online: https://asia.toshiba.com/highlights/giving-co2-an-economic-value-carbon-capture-technology-helps-recycle-waste-into-resources/ (accessed on 10 February 2025).
- IEAGHG Technical Report 2020 CCS on Waste-to-Energy. Available online: https://ieaghg.org/publications/ccs-on-waste-to-energy/ (accessed on 18 March 2025).
- Carbon Capture, Utilisation and Storage—Analysis. Available online: https://www.iea.org/reports/carbon-capture-utilisation-and-storage-2 (accessed on 12 February 2025).
- Huttenhuis, P.; Roeloffzen, A.; Versteeg, G. CO2 Capture and Re-Use at a Waste Incinerator. Energy Procedia 2016, 86, 47–55. [Google Scholar] [CrossRef]
- Le Havre: et si on Recyclait le CO2? Available online: https://www.veolia.fr/qui-sommes-nous/nos-engagements/nos-solutions-climat-france/havre-si-on-recyclait-co2 (accessed on 26 March 2025).
- CO2 Capture Plant. Available online: https://www.avr.nl/en/optimal-process/co2-capture-plant/ (accessed on 24 February 2025).
- Twence CO2 Capture Plant in Hengelo Sets an Example for The Netherlands. Available online: https://www.twence.com/news/twence-CO2-capture-plant-in-hengelo-sets-an-example-for-the-netherlands (accessed on 27 January 2025).
- Hu, G.; Nicholas, N.J.; Smith, K.H.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon Dioxide Absorption into Promoted Potassium Carbonate Solutions: A Review. Int. J. Greenh. Gas Control 2016, 53, 28–40. [Google Scholar] [CrossRef]
- European Commission. Stepping up Europe’s 2030 Climate Ambition Investing in a Climate-Neutral Future for the Benefit of Our People. J. Chem. Inf. Model. 2020, 53, 1689–1699. [Google Scholar]
- Reno-Nord har Skarpt Fokus på Plastaffaldet. Available online: https://renonord.dk/aktuelt/aktuelt/varmepumpe-skal-toemme-affaldsroeg-for-energi (accessed on 15 February 2025).
- Power-to-X Anlæg i Aalborg Skal Indfange CO2 Og Bruge Det Til Grønt Brændstof. Available online: https://renonord.dk/media/presse/pressemeddelelse_06.12.2021___ptx_til_aalborg.pdf (accessed on 12 February 2025).
- Møller, S.B. ARGO Will Capture CO2 by 2030 at the Latest. Available online: https://argo.dk/en/om-os/presse/nyheder/argo-vil-fange-co2-senest-i-2030/ (accessed on 5 December 2024).
- Technip Energies Selected for Vestforbrænding’s Carbon Capture Project at Waste-to-Energy Plant in Denmark|Technip Energies. Available online: https://www.ten.com/en/media/news/technip-energies-selected-vestforbraendings-carbon-capture-project-waste-energy-plant (accessed on 5 December 2024).
- Carbon Capture. Available online: http://a-r-c.dk/english/carbon-capture/ (accessed on 15 December 2024).
- Climate Positive Synthetic Methane Production Starts in the Vaasa Region in 2025. Available online: https://woimacorporation.com/climate-positive-synthetic-methane-production-starts-in-the-vaasa-region-in-2025/ (accessed on 11 January 2025).
- Hautamaa, S. EnergySampo CCU: Production of Synthetic Methane Starts at Westenergy in 2025. Available online: https://westenergy.fi/en/energysampo-ccu-production-of-synthetic-methane-starts-at-westenergy-in-2025/ (accessed on 16 December 2024).
- Fortum Launches a Ground-Breaking Pilot Project–Aims to Produce New Materials from the CO2 Emissions of Waste Incineration. Available online: https://www.fortum.com/media/2022/04/fortum-launches-ground-breaking-pilot-project-aims-produce-new-materials-co2-emissions-waste-incineration (accessed on 15 November 2024).
- Paul de Bruycker Presentation on CEWEP Congress 15th June 2023 Berlin: “WtE’s Role in the EU Green Deal”. Available online: https://www.cewep.eu/wp-content/uploads/2023/06/1.1-Paul-de-Bruycker.pdf (accessed on 23 November 2024).
- Born, J.-P. CATO meets the projects—Born Public. Presentation at CATO Workshop, The Netherlands, 4 December 2018. Available online: https://co2-cato.org/publish/pages/3375/_20181211_102020_23_2018-12-04_cato-meets-the-projects_born-public.pdf (accessed on 5 April 2025).
- AEB Amsterdam|Technology. Available online: https://www.aebamsterdam.com/technology/ (accessed on 22 January 2025).
- AVR to Capture CO2 in Holland. Available online: https://www.letsrecycle.com/news/avr-to-capture-co2-in-holland/ (accessed on 10 February 2025).
- Pedersen, A. BIR’s Carbon Capture Project and How Waste-to-Energy Can Contribute to Negative Emissions. Available online: https://www.uib.no/sites/w3.uib.no/files/attachments/20221101_energilab_v3.pdf (accessed on 25 February 2025).
- Frevar Capture Plant Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/2222 (accessed on 5 December 2024).
- Aker Carbon Capture Selected for Norwegian pre-FEED. Available online: https://bioenergyinternational.com/aker-carbon-capture-selected-for-norwegian-pre-feed/ (accessed on 5 December 2024).
- Hafslund Oslo Celsio—Klemetsrud CCS Project Details. Available online: https://www.geos.ed.ac.uk/sccs/project-info/1684 (accessed on 12 February 2025).
- Becidan, M.; Olsson, O. Deployment of Bio-CCS: Case Study on Waste-to-Energy; Fortum Oslo Varme (FOV): Oslo, Norway, 2021; Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/05/Becidan-2021-FINAL-IEA-Bio-BECCS-FOV-Case-study.pdf (accessed on 12 November 2024).
- Fagerlund, J.; Zevenhoven, R.; Thomassen, J.; Tednes, M.; Abdollahi, F.; Thomas, L.; Nielsen, C.J.; Mikoviny, T.; Wisthaler, A.; Zhu, L.; et al. Performance of an Amine-Based CO2 Capture Pilot Plant at the Fortum Oslo Varme Waste to Energy Plant in Oslo, Norway. Int. J. Greenh. Gas Control 2021, 106, 103242. [Google Scholar] [CrossRef]
- Air Products Part of Carbon Capture Pilot Project. Available online: https://gcenode.no/news/air-products-part-of-carbon-capture-pilot-project/ (accessed on 13 February 2025).
- BCS—The Test Module for Direct CO2 Capture from BIR Has Been Delivered|MarketScreener. Available online: https://www.marketscreener.com/quote/stock/BERGEN-CARBON-SOLUTIONS-A-121299686/news/BCS-The-test-module-for-direct-CO2-capture-from-BIR-has-been-delivered-40866335/ (accessed on 4 February 2025).
- Aker Carbon Capture to Explore Opportunities for CO2 Capture at BIR in Bergen. Available online: https://news.cision.com/aker-carbon-capture-as/r/aker-carbon-capture-to-explore-opportunities-for-co2-capture-at-bir-in-bergen,c3381268 (accessed on 2 February 2025).
- Trendafilova, P. Ostfold Energi to Test Its Carbon Capture Plant for Waste Incineration. Available online: https://carbonherald.com/ostfold-energi-to-test-its-carbon-capture-plant-for-waste-incineration/ (accessed on 16 January 2025).
- Saren Kvitebjørn Varme Has Received NOK 3.55 Million in Support from CLIMIT. Available online: https://sarenenergy.com/en/kvitebjorn-varme-has-received-nok-3-55-million-in-support-from-climit/ (accessed on 5 December 2024).
- Cutting-Edge Power-to-Liquid Project Transforms Municipal Waste-Derived CO2 into Sustainable Aviation Fuels (SAF). Available online: https://www.veolia.com/sites/g/files/dvc4206/files/document/2022/02/pr-power-to-liquid-municipal-waste-derived-CO2-sustainable-aviation-fuels-saf-portugal-17022022.pdf (accessed on 30 January 2025).
- Förstudie om CCS Visar På Två Lovande Tekniker för Avskiljning av Koldioxid|Sysav—Tar Hand om Och Återvinner Avfall. Available online: https://www.sysav.se/om-oss/pressrum/pressmeddelande/forstudie-om-ccs-visar-pa-tva-lovande-tekniker-for-avskiljning-av-koldioxid--3176860/ (accessed on 30 January 2025).
- Sysav: Front-Runner Towards a Carbon-Neutral Sweden—Ramboll Group. Available online: https://www.ramboll.com/projects/energy/sysav-waste-facility-pursues-net-zero (accessed on 28 January 2025).
- Växjö Energi First to Test New Carbon Capture Technology. Available online: https://www.veab.se/en/about/press/pressmeddelanden/2021/vaxjo-energi-first-to-test-new-carbon-capture-technology/ (accessed on 7 February 2025).
- First CapsolGoTM Unit Operational at O¨resundskraft’s Energy-from-Waste Plant|Live. Available online: https://live.euronext.com/en/node/11938820 (accessed on 6 December 2024).
- Aker Carbon Capture Awarded Feasibility Study by Waste to Energy Player in Switzerland. Available online: https://www.prnewswire.com/news-releases/aker-carbon-capture-awarded-feasibility-study-by-waste-to-energy-player-in-switzerland-302017587.html (accessed on 6 December 2024).
- Curds, P. UK-First Carbon Capture Pilot on Energy from Waste Facility Goes Live. Available online: https://enfinium.co.uk/uk-first-carbon-capture-pilot-on-energy-from-waste-facility-goes-live/ (accessed on 6 December 2024).
- Reduction of CO2 Emissions by 2030: SUEZ and BP Sign a Memorandum of Understanding to Explore—SUEZ Group. Available online: https://www.suez.com/en/news/press-releases/reduction-co2-emissions-by-2030-suez-and-bp-sign-memorandum-net-zero-teesside-uk-first-decarbonised-industrial-hub (accessed on 29 January 2025).
- Decarbonisation, C. Project Overview. Available online: https://corydecarbonisation.co.uk//the-project/project-overview/ (accessed on 5 December 2024).
- Redcar Energy Centre: BEIS’ Industrial Carbon Capture (ICC) Sequencing Process|RPS. Available online: https://www.rpsgroup.com/about-us/news/rps-helps-drive-redcar-energy-centre-to-the-next-stage-of-beis-industrial-carbon-capture-icc-sequencing-process/ (accessed on 6 December 2024).
- Veolia Feasibility Study Highlights Potential of UK’s First Carbon Capture Technology to Produce Sustainable Fuels Using Energy Recovery Facilities. Available online: https://www.veolia.co.uk/press-releases/veolia-feasibility-study-highlights-potential-uks-first-carbon-capture-technology (accessed on 6 December 2024).
- Chandel, M.K.; Kwok, G.; Jackson, R.B.; Pratson, L.F. The Potential of Waste-to-Energy in Reducing GHG Emissions. Carbon Manag. 2012, 3, 133–144. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, X.; Lai, Z.; Chen, Y. Energy Analysis and Environmental Impacts of a MSW Oxy-Fuel Incineration Power Plant in China. Energy Policy 2013, 60, 132–141. [Google Scholar] [CrossRef]
- Danish Energy Agency—Carbon Capture, Transport and Storage—Technology Descriptions and Projections for Long-Term Energy System Planning. 2024. Available online: https://ens.dk/en/analyses-and-statistics/technology-data-carbon-capture-transport-and-storage (accessed on 17 March 2025).
- Ros, J.A.; Monteiro, J.G.M.-S.; Goetheer, E.L.V. High Level Analysis of CO2 Capture in the Waste-to-Energy Sector. In Proceedings of the TCCS-11—Trondheim Conference on CO2 Capture, Transport and Storage, Trondheim, Norway, 21–23 June 2021. [Google Scholar]
- Boakes, E.; De Voogd, J.-K.; Wauters, G.; Van Caneghem, J. The Influence of Energy Output and Substitution on the Environmental Impact of Waste-to-Energy Operation: Quantification by Means of a Case Study. Clean Technol. Environ. Policy 2023, 25, 253–267. [Google Scholar] [CrossRef]
- Ding, G.; He, B.; Cao, Y.; Wang, C.; Su, L.; Duan, Z.; Song, J.; Tong, W.; Li, X. Process Simulation and Optimization of Municipal Solid Waste Fired Power Plant with Oxygen/Carbon Dioxide Combustion for near Zero Carbon Dioxide Emission. Energy Convers. Manag. 2018, 157, 157–168. [Google Scholar] [CrossRef]
- Lausselet, C.; Cherubini, F.; Oreggioni, G.D.; del Alamo Serrano, G.; Becidan, M.; Hu, X.; Rørstad, P.K.; Strømman, A.H. Norwegian Waste-to-Energy: Climate Change, Circular Economy and Carbon Capture and Storage. Resour. Conserv. Recycl. 2017, 126, 50–61. [Google Scholar] [CrossRef]
- Struthers, I.A.; Herraiz, L.; Muslemani, H.; Su, D.; Thomson, C.; Lucquiaud, M. Assessing the Negative Carbon Emissions Potential from the Waste-to-Energy Sector in Europe. In Proceedings of the 16th Greenhouse Gas Control Technologies Conference (GHGT-16), Lyon, France, 23–27 October 2022. [Google Scholar]
- Materazzi, M.; Chari, S.; Sebastiani, A.; Lettieri, P.; Paulillo, A. Waste-to-Energy and Waste-to-Hydrogen with CCS: Methodological Assessment of Pathways to Carbon-Negative Waste Treatment from an LCA Perspective. Waste Manag. 2024, 173, 184–199. [Google Scholar] [CrossRef]
- Sebastiani, A.; Paulillo, A.; Lettieri, P.; Materazzi, M. Retrofitting Waste-to-Energy with Carbon Capture and Storage in the UK: A Techno-Economic and Environmental Assessment. In Proceedings of the 31st European Biomass Conference and Exhibition, Bologna, Italy, 5–8 June 2023. [Google Scholar]


| Project Name | Company | Capacity [MtonsCO2/y] | CO2 Sources | Type of Storage | Storage Site | Type of Transport | Status | |
|---|---|---|---|---|---|---|---|---|
| Belgium | Antwerp@C [60,61,62,63,64] | Port of Antwerp, Air Liquide, BASF, Borealis, ExxonMobil, INEOS, Fluxys, and Total | 9 | Waste-to-energy, refining, (petro)chemical, iron and steel | - | North Sea (Norway, Netherlands, Denmark, UK) | Pipeline (Netherlands), ship (UK, Ireland, Norway) | In early development |
| Denmark | C4—Carbon Capture Cluster Copenhagen [61,65,66,67,68] | ARC, Argo, BIOFOS, Copenhagen Malmö Port (CMP), CTR, HOFOR, Vestforbrænding, VEKS, and Ørsted | 3 | Waste-to-energy, natural gas power plant | Deep saline formations, depleted oil and gas reservoirs | Danish North Sea | Pipeline (on shore), ship (to the site) | In planning, intended to be operational in year 2025 |
| Denmark | Greensand [61,65,69,70] | Wintershall Dea, INEOS Oil, Energy Cluster Denmark, Blue Water Shipping, SpotLight, Danish Technological Institute, Welltec, Semco Maritime, Maersk Drilling, GEUS, Geelmuyden Kiese, Ramboll, Aker Carbon Capture, Resen Waves, Magseis Fairfield, ESVAGT, DTU, Wind Power Lab, DHI, Dan-Unity CO2, University of Southampton, National Oceanography Centre, EUDP, Schlumberger New Energy | 1.5–8.0 | Waste-to-energy, cement | Depleted oil and gas reservoirs | Danish North Sea (Nini West field) | Pipeline, ship | In early development, intended to be operational in year 2025 |
| Netherlands | Aramis [61,65,71] | TotalEnergies, Shell, EBN, Gasunie | 5.0–20 | Waste-to-energy, (petro)chemicals, iron and steel, hydrogen, oil refining, cement | Deep saline formations, depleted oil and gas reservoirs | Dutch North Sea | Pipeline, ship | In early development, intended to be operational in year 2027 |
| Norway | Northern Lights (Langskip/ Longship) [61,65,72,73,74] | Northern Lights JV DA (Equinor, Shell, TotalEnergies) | 1.5–5.0 | Waste-to-energy, cement, hydrogen, biomass, steel, refineries | Deep saline formations | Johansen Formation | Pipeline (to the permanent storage site), ship (to a temporary storage site) | In advanced development, intended to be operational in year 2024 |
| Norway | Hafslund Oslo Celsio—Klemetsrud CCS Project (part of Longship project) [75] | Waste-to-Energy Agency of Oslo (EGE), Hafslund Eco, Infranode, and HitecVision | 0.4 | Waste-to-energy | Deep saline formations | Northern Lights site (Johansen Formation) | Pipeline, ship | In early development, intended to be operational in year 2026/2027 |
| UK | V Net Zero Humber Cluster (part of Humber Zero project) [76,77] | Harbour Energy (lead), VPI, Philips 66, EP UK Investments, Humber Zero, Prax | 3.6–11 | Waste-to-energy, refining, power plants | Depleted oil and gas reservoirs | Southern North Sea (Rotliegend gas fields and Bunter Formation aquifer) | Pipeline | In early development, intended to be operational in year 2026 |
| UK | HyNet North West [61,78,79,80] | Progressive Energy, Cadent, CF Fertilisers, Eni UK, Essar, Hanson, INOVYN (part of the INEOS Group), and the University of Chester | 0.8–10 | Waste-to-energy, hydrogen, refining, fertilizer, cement, other hard-to-abate industrial products | Depleted oil and gas reservoirs | Liverpool Bay | Pipeline | In early development, intended to be operational in year 2025 |
| UK | Northern Endurance Partnership [61,81] | NZT Power, BOC, CF Fertilisers, KELLAS, H2 Teeside, Suez, TV ERF, 8 Rivers | 27 | Waste-to-energy, gas-fired power station, fertilizer manufacturer, blue hydrogen production | Saline aquifer | Teeside, in the North Sea | Pipeline | Start up in 2026, operational in 2030 |
| Place | WtE Company | Capture Technology | Waste Processed [tons/Year] | CO2 Captured [tons/Year] | Storage | Utilization | Status | |
|---|---|---|---|---|---|---|---|---|
| France | Le Havre [98] | SARP Industries | Amine | 200,000 | 12,000 | - | Production of lubricant additives | Industrial unit in operation |
| Japan | Saga City [93,94] | Saga Municipality | Amine | 74,000 | 2500 | - | Algae cultivation | In operation since 2016 |
| The Netherlands | Duiven [99] | AVR | Amine | 400,000 | 60,000 | - | Greenhouse horticulture sector | In operation since 2019 |
| Hengelo [95,100] | Twence | Amine | 600,000 | 3000 | - | Production of NaHCO3 for acid gas removal | Small-scale plant in operation since 2014 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acampora, L.; Grilletta, S.; Costa, G. The Integration of Carbon Capture, Utilization, and Storage (CCUS) in Waste-to-Energy Plants: A Review. Energies 2025, 18, 1883. https://doi.org/10.3390/en18081883
Acampora L, Grilletta S, Costa G. The Integration of Carbon Capture, Utilization, and Storage (CCUS) in Waste-to-Energy Plants: A Review. Energies. 2025; 18(8):1883. https://doi.org/10.3390/en18081883
Chicago/Turabian StyleAcampora, Luigi, Serena Grilletta, and Giulia Costa. 2025. "The Integration of Carbon Capture, Utilization, and Storage (CCUS) in Waste-to-Energy Plants: A Review" Energies 18, no. 8: 1883. https://doi.org/10.3390/en18081883
APA StyleAcampora, L., Grilletta, S., & Costa, G. (2025). The Integration of Carbon Capture, Utilization, and Storage (CCUS) in Waste-to-Energy Plants: A Review. Energies, 18(8), 1883. https://doi.org/10.3390/en18081883






