Abstract
This work demonstrates the feasibility of using biochars derived from a variety of waste feedstocks, such as food organics and garden organics (FOGOs), garden organics (GOs), and biosolids (BSs), provided by Barwon Water (BW) and South East Water (SEW), as active electrode material for supercapacitor application. Four different biochars were produced by the co-pyrolysis of pre-treated mixed waste feedstocks, which were fabricated into a two-electrode symmetric supercapacitor set-up to evaluate their energy storage potential. Two different approaches, (i) carbon nanoparticle coating/modification and (ii) thermochemical activation, were employed to improve the electrochemical properties of the biochars. Potassium hydroxide-activated biochar derived from BW’s triple waste feedstock mixture (comprising 70% GOs, 20% FOGOs, and 10% BSs) demonstrated the highest specific capacitance (30.33 F/g at 0.1 A/g), energy density (4.21 Wh/kg), and power density (2.15 kW/kg) among the tested samples. Such waste-derived biochar offers several benefits for energy storage, including cost-efficiency and sustainable alternatives to traditional electrode materials. The biochar’s electrochemical performance can be further improved by improving the feedstock quality by different pre-treatments.
1. Introduction
In the current era of industrialisation and urbanisation, sustainability goals mandate the efficient harnessing of renewable energy. However, it is undeniable that the intermittent nature of renewable energy leads to fluctuations in energy production. In addition, the intermittent nature of renewable energy sources further necessitates the development of efficient energy storage devices. Continuous efforts are being made to identify exquisite materials for energy storage [1,2,3]. Electrochemical devices, such as batteries and supercapacitors, have been the preferred choice for storing energy. Batteries are conventionally used as energy storage devices; however, potential limitations, such as limited stability, low power density, and longer charge–discharge cycles, limit the applications [4]. On the contrary, a supercapacitor is an electrochemical device that can store and release potential energy electrostatically and has excellent properties, such as a fast charge–discharge rate, high coulombic efficiency, high power density, and long-cycle stability [5]. Therefore, using supercapacitors for energy storage has substantially transformed the renewable energy sector [6]. Supercapacitors have seen potential applications in the automobile industry, electrical grids, scenarios that require uninterruptable power supply, and power tools. The dominated energy storage mechanism in a supercapacitor is reported as electrostatic electric double-layer capacitance (EDLC) and electrochemical pseudocapacitance [7]. While electric double-layer capacitors have excellent electrochemical stability and a high specific surface area, their average energy density is low [8]. Similarly, pseudocapacitors exhibit large capacitance but low power density and poor cycling stability [9]. Such limitations can be addressed using a hybrid ion micro-supercapacitor, which uses a capacitor-type cathode and a battery-type anode, thereby achieving a balance between energy and power density [10,11,12].
Carbon materials, such as graphite, carbon nanotube, fullerene, etc., have been used as electrode materials for their significant electrical conductivity and electrochemical stability. However, they are expensive and time-consuming for bulk production. In recent years, biochar-based carbon materials have gained increasing research interest as sustainable energy sources for electrochemical energy storage devices [13]. Biochar can be produced by thermochemical techniques, such as pyrolysis, gasification, torrefaction, hydrothermal carbonisation, and flash carbonisation [14]. Of all these techniques, pyrolysis is the most commonly used to produce solid carbon-rich biochar residue, which involves chemical decomposition induced in organic materials (biomass including lignocellulosic, livestock, and aquatic wastes) by heat in the absence of oxygen [15]. Moreover, the quality of obtained biochar can be improved by pre-treatment of the feedstocks using different methods, such as drying, vacuum freeze-drying, pelleting, and ultrasonic vibration-assisted pelleting, which significantly affect the surface and internal structure of the biochar produced [16]. In addition, several activation methods, including chemical (acids, bases, and salts) activation, physical (steam, air, and carbon dioxide), thermal, and electrochemical modifications, can also be employed to increase the surface area and porosity of biochars for improved energy storage potential [17].
Several research studies have demonstrated the promising potential of N self-doped biochar in the field of supercapacitors [18,19,20]. The inherent presence of nitrogen and increase in the specific surface area upon physical (e.g., microwave [21]) and chemical (e.g., potassium hydroxide [22]) activation could significantly increase the double-layer capacitance of biochar, making it an attractive electrode material for supercapacitor applications [23]. Introducing functional groups onto the surface of biochar and the thermochemical activation of biochar could significantly enhance the energy storage mechanism by enhancing the energy and power density of biochar. This way, the advantages of both EDLC and pseudocapacitance mechanisms can be imparted to the biochar [7,24]. For example, Wang et al. [21] followed a two-step approach to fabricate Ni-loaded biochar-based electrodes: (i) carbonisation of sewage sludge and manure waste at 600 °C for 4 h and (ii) nickel (Ni) loading on biochar by batch sorption. The Ni-loaded biochar electrodes were then used in supercapacitors, which exhibited specific capacitance in the range of 33–39 F/g [21]. Lately, biochar derived from various biosolid (BS) wastes, including digested sludge [22], sewage sludge [25], microalgal sludge [23], and floc sludge [26], has gained increasing attention as electrode material for supercapacitor applications. In addition, biochar derived from other waste sources, such as food organics (FOs) and garden organics (GOs), have also shown promising potential for supercapacitor applications [27,28]. However, biochar derived from mixed waste feedstock has not yet been investigated for supercapacitor applications and possesses challenges associated with pre-treatment and diversity in the carbon structure. Such a mixed waste approach could offer several benefits for commercialising supercapacitors, including sustainable alternatives to traditional electrode materials and lower costs. Moreover, the mixed waste valorisation approach provides a technical solution for smarter waste management and has emerged as a sustainable solution to transform environmental waste into value-added products for a circular economy. Moreover, the supercapacitor market is estimated to be at USD 1504.29 billion by 2031 [29]. Therefore, developing mixed waste-derived biochar-based electrode material for energy storage systems is a promising upcycling approach that is economical and environmentally friendly.
In this work, we demonstrate the feasibility of using mixed waste obtained from Barwon Water (BW) and South East Water (SEW) as feedstock material for biochar production and applying the obtained biochar as active electrode material for supercapacitor applications. Such a mixed waste upcycling approach will provide a sustainable platform for industrial waste management, support the development of new materials and technologies for cost-effective energy solutions, and promote a circular economy. Therefore, the first part of this work was focused on producing (via pyrolysis) biochar from mixed waste feedstock obtained from BW and SEW and their physicochemical characterisation; the second part was focused on the fabrication of a supercapacitor using the obtained biochar and their electrochemical performance evaluation; and the third part was focused on the post-treatment (chemical activation and carbon coating) of biochars to improve their physical and electrochemical properties, such as specific capacitance and energy density.
2. Materials and Methods
2.1. Materials
Carbon-rich waste feedstock materials (Table 1) were obtained from Barwon Water (BW) (Geelong, VIC, Australia) and South East Water (SEW) (Seaford, VIC, Australia). Activated carbon powder (<44 µm) and potassium hydroxide (KOH) were procured from ChemSupply (Adelaide, SA, Australia). Poly-vinylidene fluoride (PVDF: average molecular weight ~180,000) and 1-methyl-2-pyrrolidinone (NMP) were purchased from Sigma-Aldrich (Sydney, NSW, Australia). Conductive copper tape with a thickness of 35 µm was procured from RS Components (Sydney, NSW, Australia). Celgard® 3501 microporous polypropylene (PP) membrane was purchased from Celgard (Charlotte, NC, USA).

Table 1.
Waste feedstocks used for biochar synthesis.
2.2. Biochar Production
The biochar was produced from mixed waste feedstock through a controlled pyrolysis process. The mixed waste underwent a sequential processing protocol comprising pre-treatment, pyrolysis, and post-treatment to make biochar and activated biochar. The pre-treatment steps included (i) drying in a hot-air oven for 6 h, (ii) mechanical milling, and (iii) particle size classification via sieving. Pyrolysis was performed in both a lab-scale reactor and the PYROCO Mark-1 pilot-scale system, a patented fluidised bed heat exchanger technology developed by RMIT [30]. The pyrolysis temperature was maintained at 600 °C for both lab- and pilot-scale experiments, with solid residence times of 3 h and 1 h, respectively. Figure 1 illustrates the lab-scale procedure for the selected co-pyrolysis experiments. Table 2 summarises all the biochar materials synthesised and tested for supercapacitor applications. Post-treatment methods for biochar activation included chemical vapour deposition (CVD) via biogas decomposition and chemical activation using potassium hydroxide (KOH). The CVD process enhanced the carbon content of the biochar through the deposition of carbon nanospheres (CNSs), which exhibit high carbon purity, nanostructured morphology, and crystallinity properties advantageous for energy storage applications. During CVD, biogas was catalytically decomposed over a bed of biosolids-derived biochar particles at 900 °C, with a wet hourly space velocity (WHSV) of 0.3 s−1. A biochar slurry was first prepared for KOH activation by dispersing the biochar powder in an aqueous KOH solution (comprising 50 vol. % ethanol). The slurry with a biochar/KOH weight ratio of 1:3 was oven-dried at 100 °C overnight. The resulting dry solid mixture was then activated at 800 °C for 2 h under nitrogen in a tubular furnace. The activation temperature and biochar-to-KOH ratio used in this work is based on the literature reported for obtaining activated biochar with a high specific surface area and capacitance [22]. The obtained product was then ground to powder (using mortar and pestle), dialysed for several days in distilled water (to remove unreacted ions and any by-products), and oven-dried under vacuum at 150 °C overnight (to remove any bound water). For electrode fabrication, the synthesised biochar samples were ring-milled (for 10 s) and mechanically sieved to obtain <100 µm and 100–300 µm size fractions of biochar powder.
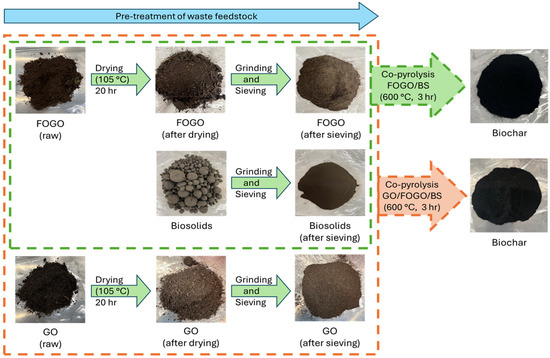
Figure 1.
Schematic of co-pyrolysis of waste feedstocks: garden organics (GOs), food organics and garden organics (FOGOs), and biosolids (BSs).

Table 2.
Summary of biochar materials synthesised and tested for supercapacitor applications.
2.3. Characterisation of Biochar
The surface morphology and pore structure of synthesised biochar samples were investigated using an FEI Verios 460L scanning electron microscope (SEM) (Melbourne, VIC, Australia). Before analysis, all samples were coated with gold (thickness of 20 nm) using a Leica ACE600 vacuum sputter coater(Sydney, NSW, Australia).
The thermal degradation profile of feedstock materials was analysed (under an inert atmosphere with a nitrogen flow rate of 20 mL/min) using a TA Instrument Discovery SDT 650 thermogravimetric analyser (TGA) (Rydalmere, NSW, Australia). The analysis was performed in triplicate using ceramic alumina pans with a volume of 90 µL. Five different pyrolysis temperatures and three heating rates were used for proximate analysis.
The carbon (C), hydrogen (H), nitrogen (N), and sulphur (S) content of synthesised biochar samples were measured using a PerkinElmer 2400 series II CHNS/O analyser (Shelton, CT, USA). All samples were analysed in triplicate.
The functional groups in synthesised biochar samples were investigated using a PerkinElmer Spectrum 100 Fourier-transform infrared (FTIR) (Rydalmere, NSW, Australia) spectrometer equipped with an attenuated total reflectance (ATR) accessory.
The crystallographic structure information of the synthesised biochar samples was investigated using a Bruker D4 ENDEAVOUR powder XRD (Sydney, NSW, Australia), operated with Cu Kα radiation. The diffraction patterns were collected for a 2θ (the angle between the transmitted beam and reflected beam) value between 10 and 60°, measured at a scan rate of 1.2°/min with a step size of 0.02°.
The nature of the disorder or defects in the carbon structure of synthesised biochar samples was investigated using a Perkin-Elmer Raman Station 400F spectrometer (Melbourne, VIC, Australia) operated with 785 nm laser excitation. The Raman spectrum was collected in the 1050–1750 cm−1 range with 10 accumulations and an acquisition time of 10 s. The spectrum was collected from 10 different spots of the sample and averaged.
The surface area, mesopore size, and volume of the synthesised biochar samples were determined using a Micromeritics TriStar II 3020 gas adsorption Brunauer–Emmett–Teller (BET) (Norcross, GA, USA) analyser at 77 K temperature. Before analysis, the sample was degassed under vacuum at 180 °C for 24 h using a VacPrep™ 061 degasser (Norcross, GA, USA).
2.4. Supercapacitor Fabrication and Testing
Biochar electrodes, comprising synthesised biochar samples (as active material) and PVDF (as polymer binder) at different weight ratios, were fabricated to evaluate their suitability as active electrode material for supercapacitor applications. PVDF solution (prepared using NMP as solvent) was added to the biochar and mechanically stirred overnight at room temperature to obtain biochar ink. The prepared ink was then solvent-casted on copper tape and oven-dried at 70 °C overnight. The obtained electrodes on copper tape were further vacuum-dried at 120 °C for 2 h to ensure the complete removal of NMP. The electrode mass loading and assembled pressure of the fabricated supercapacitors were in the range of 9–11 mg/cm2 and 2–3 kPa, respectively. A custom-made Teflon cell set-up was used to assemble the biochar supercapacitor with a two-electrode symmetric cell configuration using copper tape (as a collector), fabricated biochar electrode, porous PP membrane (as a separator), and aqueous 0.5 M KOH (as an electrolyte).
Cyclic voltammetry (CV)—at a scan rate of 5.0 mV/s—cyclic charge–discharge (CCD)—at a current density of 0.1 A/g—and electrochemical impedance spectroscopy (EIS) techniques were used to evaluate the electrochemical performance of the fabricated biochar supercapacitor. The specific capacitance (CS) in F/g of the fabricated biochar electrode was calculated from the CV data using the following equation [23]:
where ‘A’ is the area inside the CV curve (AV), ‘k’ is the scan rate (V/s), ‘m’ is the total mass (g) of active electrode material, and ‘ΔV’ is the potential window (V). The specific capacitance (CS) in F/g and energy density (E) in Wh/kg of fabricated biochar electrode were calculated from the CCD data using the following equations [23]:
where ‘I’ is the constant discharge current (A), ‘Δt’ is the discharge time (s), ‘m’ is the total mass (g) of active electrode material, and ‘ΔV’ is the potential window (V). Effective series resistance (RES) in Ω and power density (P) in kW/kg were calculated from the CCD data using the following equations [31]:
where ‘Vd’ is the voltage drop (V), ‘I’ is the discharge current (A), ‘ΔV’ is the potential window (V), and ’m’ is the total mass (g) of active electrode material.
3. Results and Discussion
3.1. Proximate and Ultimate Analysis of Feedstock and Biochar Samples
Table 3 presents the proximate and elemental analysis results for biochar samples obtained from different feedstocks and their combinations. The analysis provides information about crucial parameters such as moisture content, volatile matter, fixed carbon, and ash content. Table 3 provides insights into how the properties of biochar changed with respect to feedstock. The variations in the content of biochar reflect the specific characteristics and properties of the feedstocks and their influence on the resulting biochar composition. Based on the information obtained from Table 3, it was noticed that the carbon percentage in the biochar samples increases when using a combination of feedstocks. Specifically, the highest carbon percentage was observed in the biochar sample derived from the combination of BSs (feedstock 1), GOs (feedstock 2), and FOGOs (feedstock 3). The order of the carbon percentage, from highest to lowest, is LBC-BW(GOs/FOGOs/BSs) > LBC-BW(FOGOs/BSs). These findings are significant as a higher carbon content is desirable for investigating the electrochemical properties of biochar samples as potential alternative carbon electrodes in supercapacitor applications. The increased carbon percentage indicates a higher carbon content and potentially improved electrical conductivity, which are favourable properties for supercapacitor electrode materials. Further analysis and characterisation of these biochar samples can shed light on their specific electrochemical properties and determine their suitability as carbon electrodes in supercapacitors.

Table 3.
Proximate and ultimate analysis results of feedstock and biochar samples.
3.2. FTIR of Biochar Samples
The FTIR spectra were analysed in this study to investigate the functional groups present in the synthesised biochar samples (Figure 2). The FTIR analysis revealed several characteristic peaks corresponding to specific functional groups. In all biochar samples, a broad peak between 3500 and 3250 cm−1 indicated the stretching vibrations of hydroxyl groups (–OH). The broad peak between 1507 and 1610 cm−1 represents the stretching of C=C. Peaks at 2914 and 2843 cm−1 were attributed to methylene groups’ aliphatic C–H and CH3 stretching. Peaks at 1590 and 1008 cm−1 represent aromatic C=C and C–O stretching vibrations, respectively, while a peak at 1314 cm−1 was attributed to CH3 deformation. Additionally, a peak around 880–900 cm−1 was associated with C–H bending in aromatic compounds, indicating the aromatic nature of the biochar. Another peak around 680 cm−1 corresponded to C–C out-of-plane bending, confirming the presence of polycyclic aromatic hydrocarbons (PAHs) in the biochar. These peaks confirmed the presence of hydrogen and oxygen-containing functionalities in the biochar samples.
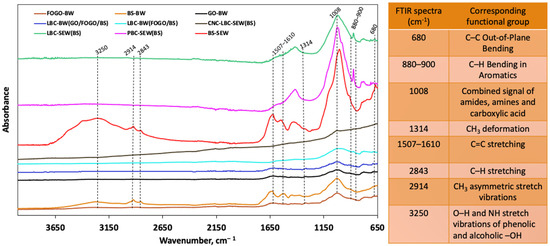
Figure 2.
Fourier-transform infrared (FTIR) spectra and corresponding chemical functional groups in synthesised biochars.
3.3. SEM and XRD Analysis of Biochar Samples
SEM analysis was conducted to examine the morphology of the synthesised biochar samples. Based on the SEM images shown in Figure 3, it is evident that the biochar produced from the combined feedstock LBC-BW(GOs/FOGOs/BSs) and LBC-SEW(BSs) exhibits a higher presence of elliptical pores on its surface compared to the biochar from the LBC-BW(FOGOs/BSs) feedstock. This morphological characteristic of the biochar with increased elliptical pores is expected to be advantageous for further electrochemical analysis. The abundance of elliptical pores provides a larger surface area and enhanced accessibility for electrolyte penetration and ion diffusion, which are crucial for electrochemical reactions in energy applications. This morphological feature can improve electrode–electrolyte interactions, enhance charge transfer kinetics, and increase electrochemical performance. Therefore, the observed elliptical pores in the biochar derived from the combined feedstock highlight its potential as an alternative carbon electrode material for supercapacitor applications and support the potential suitability of this biochar for further electrochemical analysis. The varied microstructures of biochar samples result from differences in their waste feedstock composition, mineral content, and processing conditions, which directly impact their energy storage performance by altering surface area, electrical conductivity, and ion accessibility.
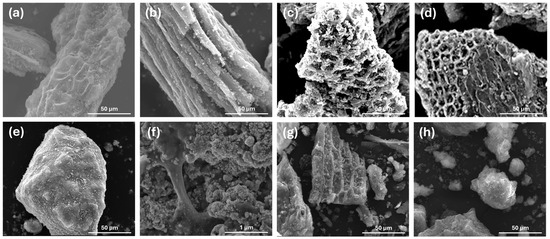
Figure 3.
Scanning electron microscope (SEM) images of (a) LBC-BW(FOGOs/BSs), (b) LBC-BW(GOs/FOGOs/BSs), (c) PBC-SEW(BSs), (d) LBC-SEW(BSs), (e) CNC-LBC-SEW(BSs) at 2000× magnification, and (f) CNC-LBC-SEW(BSs) at 100,000× magnification. SEM images of (g) KOH-LBC-BW(GOs/FOGOs/BSs) and (h) KOH-CNC-LBC-SEW(BSs) at 2000× magnification.
Figure 4 compares the XRD pattern of synthesised biochars and commercial activated carbon powder. The two broad peaks were observed around 2θ values of 24.5° and 42.9° corresponding to the diffraction of (002) and (100) carbon planes, which indicate the amorphous or disordered nature of the carbon in the samples [32]. All the other sharp peaks can be attributed to inorganic components in the biochars. The sharp peaks observed at 2θ values of 21.0°, 26.7°, 29.5°, 36.1°, and 43.3° indicate the presence of quartz, and 2θ values of 25.6°, 27.5°, 28.4°, 31.3°, 34.7°, 36.6°, 39.6°, 40.4°, 42.5°, 47.6°, 48.5°, and 50.2° indicate the presence of calcite in the biochars [33]. Quartz and calcite have been previously reported to be common inorganic contaminants in garden and food waste-derived biochars [34,35]. Quartz mainly originates from soil particles in garden organics, whereas calcite from calcium-rich materials like eggshells and biosolids remains stable during pyrolysis. Variations in peak intensity between different biochars may indicate differences in mineral retention across production scales. Furthermore, the quartz peak could also overlap with the graphite (002) diffraction peak commonly observed around a 2θ value of 26.6° [32], which is further investigated using Raman spectroscopy. The inorganic components in biochars can be removed by using acid and alkaline solutions; however, they were used in this study to investigate the potential of as-prepared (non-treated) biochars for electrode application, which could significantly reduce costs and the number of production processes.
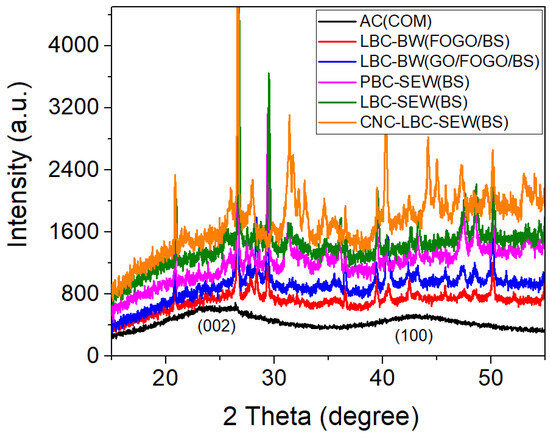
Figure 4.
X-ray diffraction (XRD) pattern of synthesised biochars and commercial activated carbon powder.
3.4. Raman and BET Analysis of Biochar Samples
Figure 5 compares the Raman spectrum of synthesised biochars and commercially available activated carbon powder. The two prominent Raman bands (D and G) commonly observed around 1350 and 1580 cm−1 for carbon materials correspond to the amorphous and graphitic carbon structures, respectively, where the intensity ratio of D and G bands (ID/IG) reflects the degree of disorder [36]. D’ bands around 1150, 1210, 1515, and 1695 cm−1 have also been reported for biochar samples [37,38]. The ID/IG ratio obtained from the Raman spectrum of the biochars is provided in Table 4. In general, the degree of disorder of all the biochar samples was observed to be less than commercial activated carbon. Moreover, KOH activation further increased the defects expected from pore formation. Further, the reaction of KOH with carbon results in the formation of potassium carbonate and the evolution of gases like carbon dioxide, contributing to pore formation [15]. Among the synthesised biochars, the LBC-BW(FOGOs/BSs) showed the relatively lowest structural defects, whereas the LBC-SEW(BSs) showed the highest. However, the ID/IG ratio of LBC-SEW(BSs) decreased with the carbon nanomaterial coating due to the increased volume fraction of ordered carbon. The synthesised biochar samples exhibited a BET surface area between 4.7 and 116.2 m2/g (Table 4), which is in the range reported in the literature for food waste, poultry litter, and paper sludge-derived biochar but lower than sawdust and rice husk-derived biochar [39]. However, the KOH activation of LBC-BW(GOs/FOGOs/BSs) and CNC-LBC-SEW(BSs) biochar samples showed ~7- and ~20-fold increases in the surface area, which can be attributed to their increase in mesopore size and volume. These structural defects could affect the electrical conductivity of the obtained biochars and consequently supercapacitor performance.
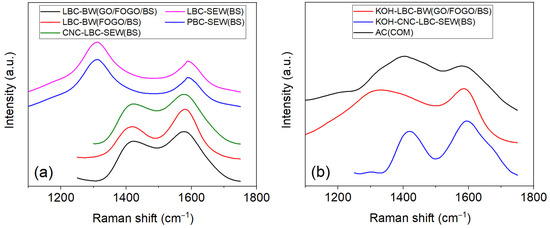
Figure 5.
Raman spectra of (a) as-synthesised biochars and (b) KOH-treated biochars compared to commercial activated carbon powder.

Table 4.
Raman and BET analysis results of biochars and commercial active carbon.
3.5. Optimisation of Biochar Ink and Electrode Composition
The optimisation of supercapacitor performance and biochar ink and its composition for solution-casting was first studied using the LBC-BW(GOs/FOGOs/BSs) sample. Various formulations comprising 80–90 wt% biochar (at two size fractions, <100 µm and 100–300 µm), 5–15 wt% PVDF polymer, 5–10 wt% commercial active carbon, and NMP solvent with a solids-to-NMP ratio of 0.25–0.65 (w/v) were prepared. The solids-to-NMP ratio of 0.45 (w/v) was observed to be optimal, whereas 0.25 (w/v) was observed to be too thin (overspreading), and 0.65 (w/v) was too thick to spread evenly. The supercapacitor performance of the electrodes prepared using these inks was analysed using CV (Figure 6). The specific capacitance calculated from the CV data is given in Table 5. The formulation comprising 90 wt% biochar (<100 µm) and 10 wt% PVDF was established to be the optimal composition for solution-casting and supercapacitor performance (highest specific capacitance) and therefore used for the fabrication of all other biochar supercapacitors and commercial activated carbon (for comparison). The obtained results can be related to (i) the surface area of biochar, where smaller particles have a higher surface area, which allows for more electrolyte ions to diffuse and charge to be stored in the electrode, (ii) concentration of PVDF, where insufficient binder can result in weak binding between the current collector and active material, and (iii) carbon structure, where a lower Raman ID/IG ratio indicates a more disordered local carbon structure, which is associated with higher capacitance. A 10 wt% PVDF concentration has also been reported in the literature as the optimal binder content for symmetric supercapacitors [40].
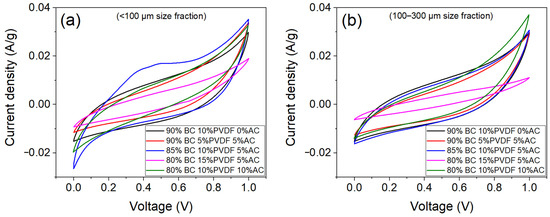
Figure 6.
Cyclic voltammetry curves (at 5 mV/s scan rate) of biochar supercapacitors fabricated using (a) <100 µm and (b) 100–300 µm size fraction LBC-BW(GOs/FOGOs/BSs).

Table 5.
Specific capacitance (CS) values (calculated from cyclic voltammetry data) of the LBC-BW(GOs/FOGOs/BSs) supercapacitors fabricated using various compositions for optimisation.
3.6. Supercapacitor Performance of As-Prepared Biochars
Figure 7a shows the CV curves (at 5 mV/s scan rate) of supercapacitors fabricated using as-prepared biochars (<100 µm) and commercial activated carbon at an optimised biochar/PVDF weight ratio of 9:1. The lack of the quasi-rectangular shape of the curves indicates poor capacitive behaviour and high ohmic resistance of biochars and commercial activated carbon electrodes. Figure 7b shows the CCD curves (at 0.1 A/g current density) of supercapacitors fabricated using as-prepared biochars and commercial activated carbon. The non-symmetric nature of the curves along with a high discharge voltage drop (>40.0%) indicates the poor charge carrier mobility of electrodes, which can be attributed to the amorphous nature of the carbon measured by XRD, and the higher ID/IG ratio (>0.86) measured by Raman spectroscopy. The biochar samples exhibited discharge time in the following order: PBC-SEW(BSs) (3.22 s) > LBC-BW(GOs/FOGOs/BSs) (2.45 s) > LBC-BW(FOGOs/BSs) (1.24 s) > LBC-SEW(BSs) (0.60 s), as shown in Figure 7b. Although specific capacitance has been reported using both CV and CCD data in the literature, CCD data were considered to be more appropriate for calculating energy and power density as they have additional valuable parameters such as discharge time and potential drop. A supercapacitor’s charging and discharging time is affected by several parameters, including the quality of the electrode materials, electrolytes, and the device’s architecture. Particularly, the physical properties of electrode material, such as surface area, pore size/structure/volume, and electrical conductivity, primarily impact the specific capacitance and the resulting performance; where a high surface area provides more active sites for charge storage, optimal pore parameters can provide a high electrolyte accessible surface area, and high electrical conductivity allows electrons to move efficiently [2]. The specific capacitance, energy density, series resistance, and power density calculated (using Equations (2)–(5)) from the CCD data are given in Table 6. The obtained specific capacitance values were observed to be influenced mainly by the biochar surface area. The PBC-SEW(BSs) sample exhibited the highest specific capacitance (1.29 F/g) and energy density (0.18 Wh/kg) among the synthesised biochar samples, followed by LBC-BW(GOs/FOGOs/BSs) and LBC-BW(FOGOs/BSs), which can be attributed to the fixed carbon content and surface area. The electrochemical performance of PBC-SEW(BSs) biochar supercapacitors is in the range of binder-free monolithic biochar supercapacitors (1.0 F/g) [41] but significantly lower than sludge-derived biochar supercapacitors (75–123 F/g) previously reported in the literature [22,23,25,26]. This can be attributed to the biochar samples’ relatively high inorganic and ash content, which is also reflected in the calculated high effective series resistance of the biochar supercapacitors (Table 6). The commercial activated carbon exhibited specific capacitance and energy density between the LBC-BW(GOs/FOGOs/BSs) and PBC-SEW(BSs) samples. The electrochemical performance of the as-prepared biochar samples falls short when compared to the performance of other supercapacitor electrode materials reported in the literature, such as MXene (120–209 F/g) [42], graphene (211 F/g) [43], and hierarchical architectures of oxide materials (650–1635 F/g) [44,45]. However, thermochemical activation can improve the electrochemical performance of the as-prepared biochars, such as KOH treatment at elevated temperatures and carbon nanoparticle coating.
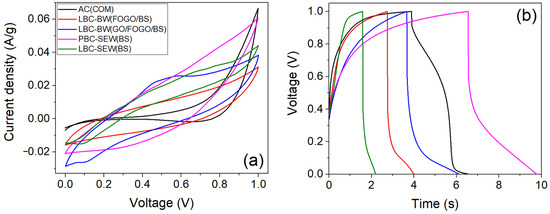
Figure 7.
(a) Cyclic voltammetry (at 5 mV/s scan rate) and (b) cyclic charge–discharge curves (at 0.1 A/g current density) of supercapacitors fabricated using as-prepared biochars and commercial activated carbon powder.

Table 6.
Specific capacitance (CS), energy density (E), effective series resistance (RES), and power density (P) values of biochar supercapacitors calculated from cyclic charge–discharge data.
3.7. Effect of Different Treatment Methods on Biochar Supercapacitor Performance
The key properties required for supercapacitor electrodes are large mesopore volume, high active surface area, and charge carrier mobility [5]. Therefore, to improve these properties in biochar samples, two approaches, thermochemical activation and carbon coating, were applied to selected samples. The LBC-BW(GOs/FOGOs/BSs) sample exhibited the highest power density (44.64 W/kg), and the LBC-SEW sample showed the highest series resistance (134.92 Ω) among the as-prepared biochar electrodes. Therefore, the LBC-BW(GOs/FOGOs/BSs) sample was treated (activated) with KOH to improve its mesopore volume and surface area further. Conversely, the LBC-SEW sample was carbon-coated to enhance its charge carrier mobility further, thereby improving the supercapacitor performance. Figure 8a compares the CV curves (at 5 mV/s scan rate) of CNC-LBC-SEW(BSs), KOH-CNC-LBC-SEW(BSs), LBC-BW(GOs/FOGOs/BSs), and KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitors. The KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor showed a relatively quasi-rectangular shape, which indicates a good and improved capacitive behaviour compared to its as-prepared counterpart supercapacitor. On the other hand, no significant difference in the curve pattern was observed between the CNC-LBC-SEW(BSs) and KOH-CNC-LBC-SEW(BSs) supercapacitors. Furthermore, Figure 8b shows the CCD curves (at current density of 0.1 A/g) of CNC-LBC-SEW(BSs), KOH-CNC-LBC-SEW(BSs), LBC-BW(GOs/FOGOs/BSs), and KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitors. The CCD curve of KOH-LBC-BW(GOs/FOGOs/BSs) showed a near-symmetrical triangular shape, indicating excellent charging/discharging reversibility. Moreover, no apparent voltage or IR drop, 2.5% for KOH-LBC-BW(GOs/FOGOs/BSs), 7.3% for CNC-LBC-SEW(BSs), and 6.7% for KOH-CNC-LBC-SEW(BSs), was observed for the discharge curve of treated biochar supercapacitors.
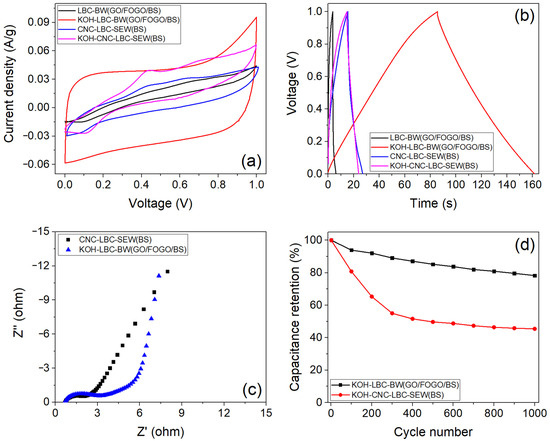
Figure 8.
(a) Cyclic voltammetry curves (at 5 mV/s scan rate), (b) cyclic charge–discharge curves (at 0.1 A/g current density), and (c) Nyquist plots of treated biochar supercapacitors. (d) Capacitance retention of KOH-activated biochar supercapacitors during 1000 cycles (at a current density of 0.1 A/g).
The KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor exhibited the highest discharge time of 75.8 s at 0.1 A/g current density, a 30-fold increase compared to the respective as-prepared or non-activated counterpart. The discharge time of the KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor decreased to 4.2 s at a higher current density (1.0 A/g), which indicates good rate capability of the biochar electrode, promoting faster charge/discharge. Moreover, the KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor demonstrated the highest specific capacitance (30.33 F/g at 0.1 A/g) and energy density (4.21 Wh/kg) among the tested samples, which is a 30-fold increase compared to the respective non-activated biochar supercapacitor. However, the specific capacitance of KOH-LBC-BW(GOs/FOGOs/BSs) is relatively low compared to values reported for single-source feedstock-derived biochars, which might be related to the inherent property of the biochar obtained from mixed waste feedstock [27,28]. The enhanced performance of KOH-activated biochar can be attributed to an increase in the surface area of the KOH-activated biochar (due to the formation of mesoporous structures by gaseous reaction products like carbon dioxide), an increase in O-containing functional groups (convert them to unstable forms), and the promotion of aromatisation, leading to an increase in the electron exchange capacity [46]. The KOH-LBC-BW(GOs/FOGOs/BSs) sample was measured to have a BET surface area of 845.8 m2/g and pore volume of 0.174 cm2/g, which is around a 728% and 446% increase, respectively, compared to its non-activated counterpart, justifying the enhanced performance of the material. In addition, the lower effective series resistance (5.18 Ω) calculated for the KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor is also supported by the EIS Nyquist plot (Figure 8c), which shows the frequency-dependent behaviour of the fabricated biochar electrode, where a linear vertical line under low frequencies represents equilibrium differential capacitance, and a low slope with increasing frequency represents the fast diffusion of ions at the electrochemical interfaces of the interconnected conductive porous structure of the electrode, which supports the enhanced performance [47]. The KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor exhibited the highest power density of 2.15 kW/kg, along with a capacitance retention of 78.3% for 1000 cycles (Figure 8d), which is in the similar range reported for the microbial sludge-derived biochar supercapacitor [23].
On the other hand, the CNC-LBC-SEW(BSs) supercapacitor exhibited a discharge time of 11.5 s, which is a 19-fold increase compared to the respective as-prepared or uncoated counterpart. The enhanced performance of carbon nanomaterial-coated biochar can be attributed to increased electrical conductivity, which is supported by decreased structural defect (i.e., decrease in Raman ID/IG ratio) and effective series resistance [48]. However, no significant increase in discharge time was observed for the KOH-LCNMBC-SEW supercapacitor. While KOH treatment significantly improved the surface area and mesopore volume of LCNMBC-SEW (Table 4), the supercapacitor performance of the samples did not show any improvement; this may be due to the fact that the carbon nanospheres in the samples could potentially affect the hierarchical pore (micro to macro) size and structure in the fabricated electrodes, thereby limiting the electrolyte and subsequently specific capacitance [49]. The KOH-CNC-LBC-SEW(BSs) supercapacitor exhibited a power density of 0.649 kW/kg and a capacitance retention of 46.1% for 1000 cycles (Figure 8d), which is significantly less when compared to the KOH-LBC-BW(GOs/FOGOs/BSs) supercapacitor.
This study provides a platform for upcycling mixed waste feedstock from industry and household waste into carbonaceous biochar material, which can potentially address the environmental waste management issues and advance the commercialisation of energy storage devices, such as supercapacitors at an affordable cost and provide circular economy opportunities. As biochar quality largely depends on the quality of raw material used for pyrolysis, it is necessary to improve the quality of waste feedstock, particularly mixed waste feedstock, by applying suitable pre-treatment methods. Here, we have used common pre-treatment methods such as drying, milling, and sieving. However, additional processes for removing inorganic content and/or enriching carbon content in feedstocks could improve biochar quality for energy storage applications. As demonstrated in this study, carbon nanoparticle coating significantly enhanced the specific capacitance of the biochar; however, issues related to the hierarchical pore size and structure (micro to macro scale) of fabricated electrodes still need to be addressed for the further improvement in CVD-treated biochar for supercapacitor applications. Addressing these issues could further the reality of a sustainable future with biochar-based energy storage devices produced via the valorisation of mixed waste feedstocks. To ensure the large-scale consistency of mixed waste feedstock-derived biochar, we can implement a three-tier quality control system combining AI-assisted real-time waste stream monitoring, automated sorting technologies (to ensure material composition), and standardised feedstock blending to achieve batch feedstock to obtain a performance variation of less than 8% [50].
4. Conclusions
In summary, biochars were synthesised from various waste feedstock provided by BW and SEW. Various analysis techniques were employed to determine the physical and chemical properties of the feedstock and biochars. Proximate and ultimate analysis revealed an increased carbon percentage in biochar samples produced from combined feedstocks. SEM showed the presence of porous structures in as-prepared biochars. The biochars comprise amorphous carbon with structural defects. Furthermore, four different biochars (two from BW waste and two from SEW waste) were investigated for their suitability as active electrode material for supercapacitor applications. Two different methods, such as carbon nanomaterial coating and KOH activation, were applied to the biochars that exhibited the lowest and highest specific capacitance to improve their electrochemical performance. Both methods significantly increased the specific capacitance, energy density, and power density of the fabricated biochar supercapacitors. The carbon nanomaterial coating decreased the mesopore size and volume and increased the surface area. However, KOH activation showed relatively higher performance, which was attributed to increased mesopore size, volume, and surface area. The waste feedstock-derived biochars prepared in this work have the potential to be active electrode materials for supercapacitor applications.
Author Contributions
S.P. (Sudhakar Pabba): Methodology, Investigation, Data Curation, Writing—Original Draft. R.B.: Methodology, Investigation, Data Curation, Writing—Original Draft. A.K.V.: Methodology, Investigation, Data Curation, Writing—Review and Editing. G.V.: Methodology, Investigation. M.K.J.: Data Curation, Writing—Review and Editing. I.G.H.: Investigation, Data Curation. N.R.C.: Resources, Supervision, Visualisation, Writing—Review and Editing. A.S. (Aravind Surapaneni): Methodology, Investigation. M.T.: Data Curation, Writing—Review and Editing. A.S. (Abhishek Sharma): Methodology, Investigation. S.P. (Savankumar Patel): Methodology, Investigation, Writing—Review and Editing. K.S.: Funding Acquisition, Resources, Supervision, Visualisation, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the ARC Training Centre for the Transformation of Australia’s Biosolids Resource and ARC Research Hub for TREMS at RMIT University. The authors acknowledge the facilities and technical assistance of the RMIT Microscopy and Microanalysis Facility (RMMF).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
Author Michael Thomas was employed by the company Barwon Water. Author Aravind Surapaneni was employed by the company South East Water. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rawat, S.; Boobalan, T.; Krishna, B.B.; Sathish, M.; Hotha, S.; Bhaskar, T. Biochar for Supercapacitor Application: A Comparative Study. Chem. Asian J. 2022, 17, e202200982. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Baruch-Mordo, S.; Kiesecker, J.M.; Kennedy, C.M.; Oakleaf, J.R.; Opperman, J.J. From Paris to practice: Sustainable implementation of renewable energy goals. Environ. Res. Lett. 2019, 14, 024013. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable biochar for advanced electrochemical/energy storage applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, M.A.; Humayun, M.; Usman, M.; Shah, S.S.; Bibi, S.; Hasnain, B.S.; Ahmad, S.M.; Khan, A.; Shah, N.; et al. A Review of Supercapacitors: Materials Design, Modification, and Applications. Energies 2021, 14, 7779. [Google Scholar] [CrossRef]
- Elmorshedy, M.F.; Elkadeem, M.; Kotb, K.M.; Taha, I.B.; Mazzeo, D. Optimal design and energy management of an isolated fully renewable energy system integrating batteries and supercapacitors. Energy Convers. Manag. 2021, 245, 114584. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Gupta, S.; Lee, C.-Y.; Tai, N.-H. Effects of physical and chemical activations on the performance of biochar applied in supercapacitors. Appl. Surf. Sci. 2023, 610, 155560. [Google Scholar] [CrossRef]
- Hsieh, C.-E.; Chang, C.; Gupta, S.; Hsiao, C.-H.; Lee, C.-Y.; Tai, N.-H. Binder-free CoMn2O4/carbon nanotubes composite electrodes for high-performance asymmetric supercapacitor. J. Alloys Compd. 2022, 897, 163231. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Gupta, S.; Hsiao, C.-H.; Lee, C.-Y.; Tai, N.-H. Multilayered nickel oxide/carbon nanotube composite paper electrodes for asymmetric supercapacitors. Electrochim. Acta 2020, 354, 136744. [Google Scholar]
- Chen, Y.; Li, S.; Chen, J.; Gao, L.; Guo, P.; Wei, C.; Fu, J.; Xu, Q. Sulfur-bridged bonds enabled structure modulation and space confinement of MnS for superior sodium-ion capacitors. J. Colloid Interface Sci. 2024, 664, 360–370. [Google Scholar] [CrossRef]
- Ma, J.; Qin, J.; Zheng, S.; Fu, Y.; Chi, L.; Li, Y.; Dong, C.; Li, B.; Xing, F.; Shi, H.; et al. Hierarchically Structured Nb2O5 Microflowers with Enhanced Capacity and Fast-Charging Capability for Flexible Planar Sodium Ion Micro-Supercapacitors. Nano-Micro Lett. 2024, 16, 67. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, V.S.S.; Antunes, E.; Baig, Z.; Rehman, S.; Murugavelh, S.; Leu, S.Y.; Sarmah, A.K. Pyrolysis of anaerobic digested residues in the presence of catalyst-sorbent bifunctional material: Pyrolysis characteristics, kinetics and evolved gas analysis. Bioresour. Technol. 2022, 351, 127022. [Google Scholar] [CrossRef]
- Meng, F.; Wang, D.; Zhang, M. Effects of different pretreatment methods on biochar properties from pyrolysis of corn stover. J. Energy Inst. 2021, 98, 294–302. [Google Scholar] [CrossRef]
- Prabakar, P.; Mustafa Mert, K.; Muruganandam, L.; Sivagami, K. A comprehensive review on biochar for electrochemical energy storage applications: An emerging sustainable technology. Front. Energy Res. 2024, 12, 1448520. [Google Scholar]
- Gao, Y.; Sun, R.; Li, A.; Ji, G. In-situ self-activation strategy toward highly porous biochar for supercapacitors: Direct carbonization of marine algae. J. Electroanal. Chem. 2021, 882, 114986. [Google Scholar]
- Makinde, W.O.; Hassan, M.A.; Pan, Y.; Guan, G.; López-Salas, N.; Khalil, A.S. Sulfur and nitrogen co-doping of peanut shell-derived biochar for sustainable supercapacitor applications. J. Alloys Compd. 2024, 991, 174452. [Google Scholar]
- Xiaorui, L.; Haiping, Y. A state-of-the-art review of N self-doped biochar development in supercapacitor applications. Front. Energy Res. 2023, 11, 1135093. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Pei, L.; Ying, D.; Xu, X.; Zhao, L.; Jia, J.; Cao, X. Converting Ni-loaded biochars into supercapacitors: Implication on the reuse of exhausted carbonaceous sorbents. Sci. Rep. 2017, 7, 41523. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Fan, H.-X.; Dai, X.-H.; Yuan, S.-J. Digested sludge-derived three-dimensional hierarchical porous carbon for high-performance supercapacitor electrode. R. Soc. Open Sci. 2018, 5, 172456. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, D.Y.; Lee, J.H.; Lee, S.K.; Chun, Y.; Yoo, H.Y.; Lee, H.U.; Kwak, H.S.; Park, C.; Lee, J.H.; et al. High potential of microalgal sludge biochar for a flexible all-solid-state microsupercapacitor. J. Energy Storage 2021, 44, 103458. [Google Scholar] [CrossRef]
- Piwek, J.; Slesinski, A.; Fic, K.; Aina, S.; Vizintin, A.; Tratnik, B.; Tchernychova, E.; Lobera, M.P.; Bernechea, M.; Dominko, R. High frequency response of adenine-derived carbon in aqueous electrochemical capacitor. Electrochim. Acta 2022, 424, 140649. [Google Scholar]
- Li, X.; Hao, T.; Tang, Y.; Chen, G. A “Seawater-in-Sludge” approach for capacitive biochar production via the alkaline and alkaline earth metals activation. Front. Environ. Sci. Eng. 2020, 15, 3. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, R.; Wang, W.; Zhao, H. Recovery and reuse of floc sludge for high-performance capacitors. Front. Environ. Sci. Eng. 2021, 16, 78. [Google Scholar] [CrossRef]
- Ismail, I.S.; Othman, M.F.H.; Rashidi, N.A.; Yusup, S. Recent progress on production technologies of food waste–based biochar and its fabrication method as electrode materials in energy storage application. Biomass Convers. Biorefinery 2023, 13, 14341–14357. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Pandit, B.; Dang, V.D.; Doong, R.-A. Agricultural waste to real worth biochar as a sustainable material for supercapacitor. Sci. Total Environ. 2023, 869, 161441. [Google Scholar] [CrossRef]
- Kwarciany, R.; Fiedur, M.; Saletnik, B. Opportunities and Threats for Supercapacitor Technology Based on Biochar—A Review. Energies 2024, 17, 4617. [Google Scholar] [CrossRef]
- Shah, K. A Pyrolysis Reaction System and Method of Pyrolysing an Organic Feed. Australia. 2019. Available online: https://patentscope.wipo.int/search/en/WO2019227162 (accessed on 15 January 2025).
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef]
- Husain, Z.; Shakeelur Raheman, A.R.; Ansari, K.B.; Pandit, A.B.; Khan, M.S.; Qyyum, M.A.; Lam, S.S. Nano-sized mesoporous biochar derived from biomass pyrolysis as electrochemical energy storage supercapacitor. Mater. Sci. Energy Technol. 2022, 5, 99–109. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Liu, S.-C.; Chen, H.-R.; Chang, Y.-M.; Tsai, Y.-L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Mujtaba, G.; Hayat, R.; Hussain, Q.; Ahmed, M. Physio-Chemical Characterization of Biochar, Compost and Co-Composted Biochar Derived from Green Waste. Sustainability 2021, 13, 4628. [Google Scholar] [CrossRef]
- Boakye, P.; Nuagah, M.B.; Oduro-Kwarteng, S.; Appiah-Effah, E.; Kanjua, J.; Antwi, A.B.; Darkwah, L.; Sarkodie, K.; Sokama-Neuyam, Y.A. Pyrolysis of municipal food waste: A sustainable potential approach for solid food waste management and organic crop fertilizer production. Sustain. Environ. 2023, 9, 2260057. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J. Effects of pressure on morphology and structure of bio-char from pressurized entrained-flow pyrolysis of microalgae. Data Brief 2018, 18, 422–431. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, J.; Zhang, W.; Tian, H.; Hu, Z.; Ruan, M.; Song, Z.; Liu, L. Effect of Char Structure Evolution During Pyrolysis on Combustion Characteristics and Kinetics of Waste Biomass. J. Energy Resour. Technol. 2018, 140, 072203. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Daraghmeh, A.; Hussain, S.; Servera, L.; Xuriguera, E.; Cornet, A.; Cirera, A. Impact of binder concentration and pressure on performance of symmetric CNFs based supercapacitors. Electrochim. Acta 2017, 245, 531–538. [Google Scholar] [CrossRef]
- Jiang, J. High Temperature Monolithic Biochar Supercapacitor Using Ionic Liquid Electrolyte. J. Electrochem. Soc. 2017, 164, H5043. [Google Scholar] [CrossRef]
- Hussain, I.; Arifeen, W.U.; Khan, S.A.; Aftab, S.; Javed, M.S.; Hussain, S.; Ahmad, M.; Chen, X.; Zhao, J.; Rosaiah, P.; et al. M4X3 MXenes: Application in Energy Storage Devices. Nano-Micro Lett. 2024, 16, 215. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mata, J.; Dutta, N.K.; Choudhury, N.R. 3D printed graphene aerogels using conductive nanofibrillar network formulation. Nano Trends 2023, 2, 100011. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Ethanol-directed morphological evolution of hierarchical CeOx architectures as advanced electrochemical capacitors. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar] [CrossRef]
- Chen, N.; Younis, A.; Huang, S.; Chu, D.; Li, S. Advanced three-dimensional hierarchical Pr6O11@Ni-Co oxides-based core-shell electrodes for supercapacitance application. J. Alloys Compd. 2019, 783, 772–778. [Google Scholar] [CrossRef]
- Lü, F.; Lu, X.; Li, S.; Zhang, H.; Shao, L.; He, P. Dozens-fold improvement of biochar redox properties by KOH activation. Chem. Eng. J. 2022, 429, 132203. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Q.; Liang, Q. Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials 2022, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Jin, B.; Tang, H.; Ma, L.; Zhang, R.; Ran, J.; Zhang, H. Optimizing porous structure of carbon electrodes for temperature-independent capacitance at sub-zero temperatures. Chem. Eng. J. 2022, 441, 136053. [Google Scholar] [CrossRef]
- Olawade, D.B.; Fapohunda, O.; Wada, O.Z.; Usman, S.O.; Ige, A.O.; Ajisafe, O.; Oladapo, B.I. Smart waste management: A paradigm shift enabled by artificial intelligence. Waste Manag. Bull. 2024, 2, 244–263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).