Resource Recovery and Synthesis of Battery-Grade FePO4 from Waste LiFePO4 Battery Slag
Abstract
1. Introduction
2. Materials and Method
2.1. Material Recovery
2.1.1. Dissolution of Iron Phosphate Residue
2.1.2. Impurity Removal
2.1.3. Regeneration of Iron Phosphate
2.1.4. Synthesis of LiFePO4
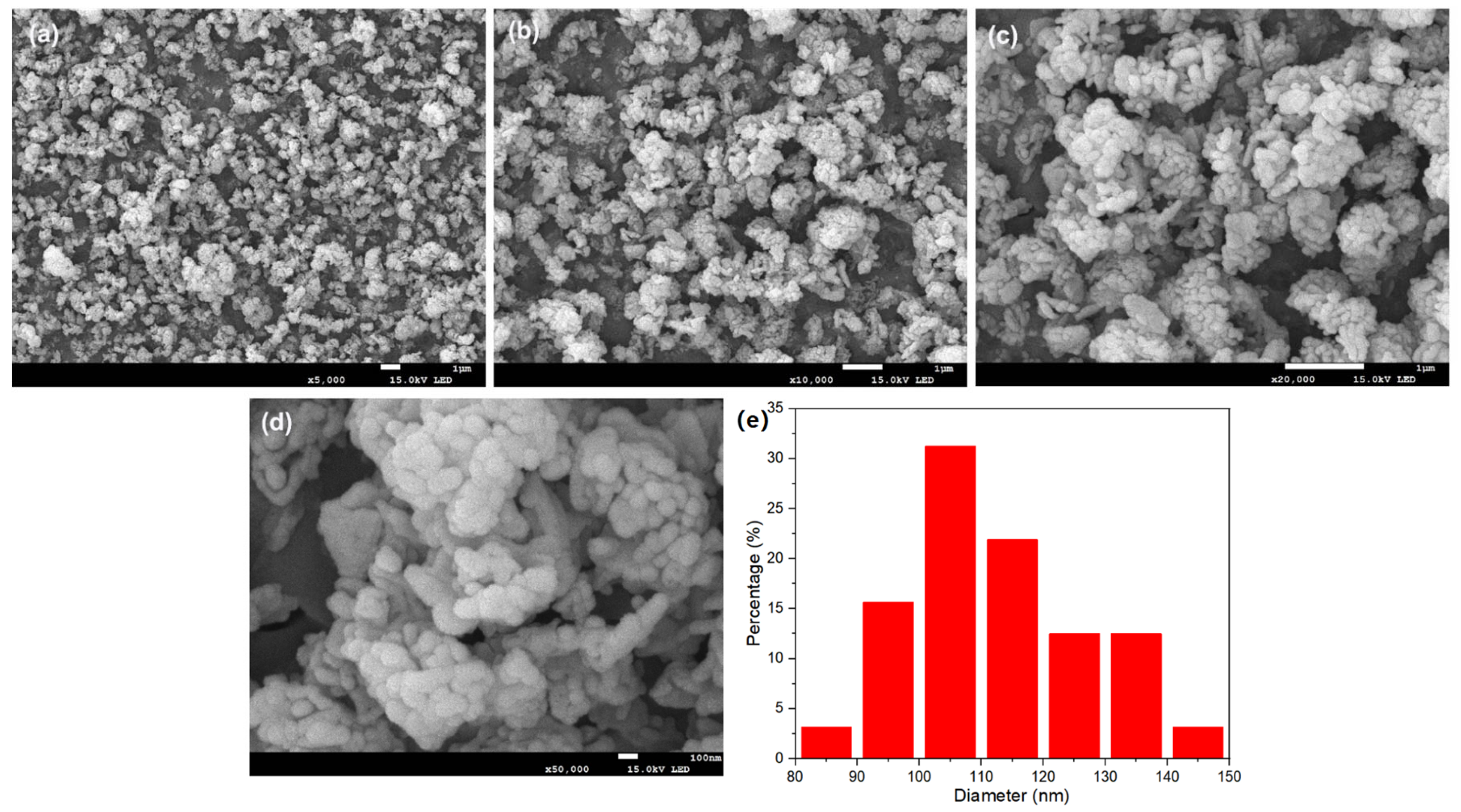
2.2. Material Characterization
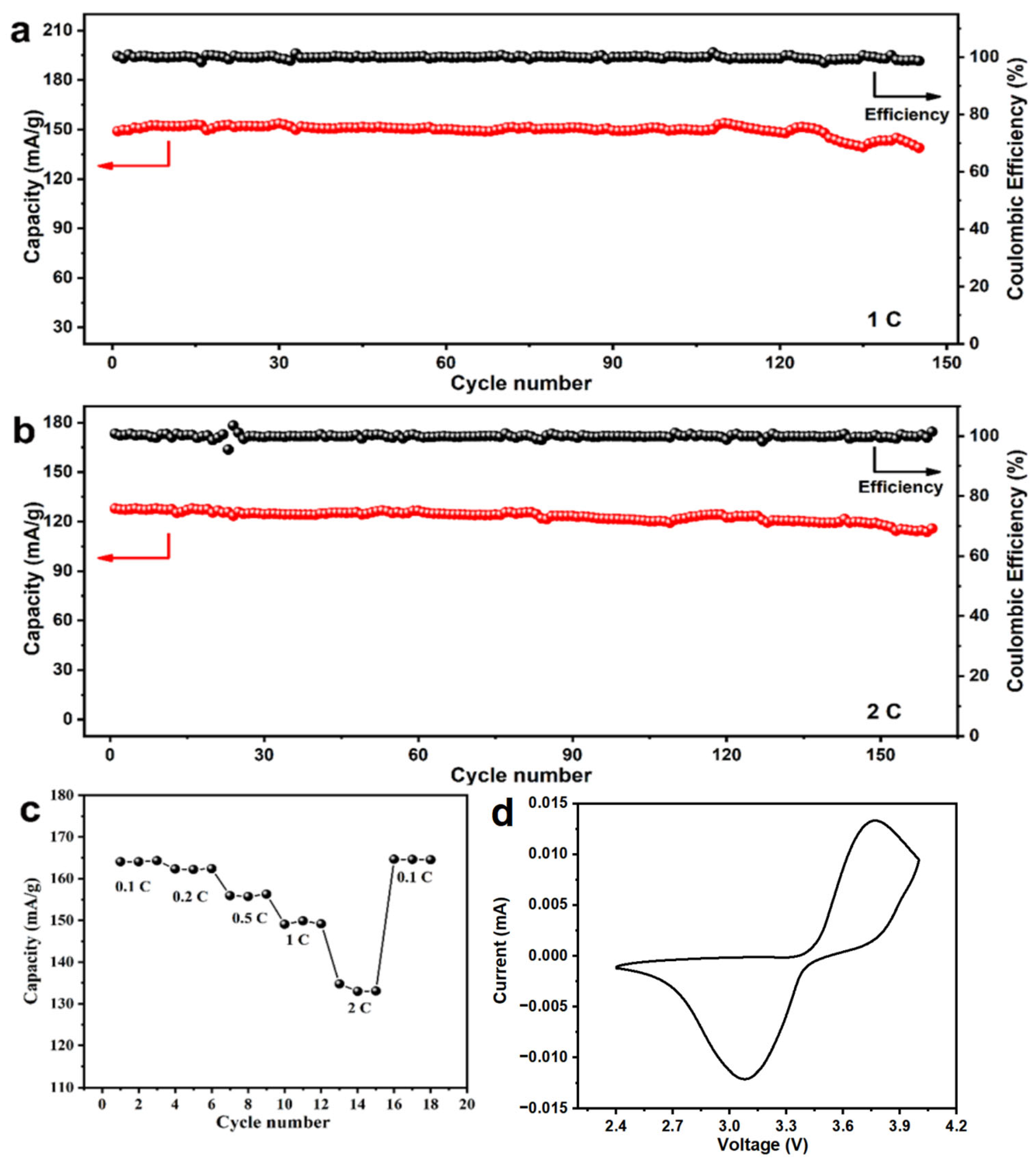
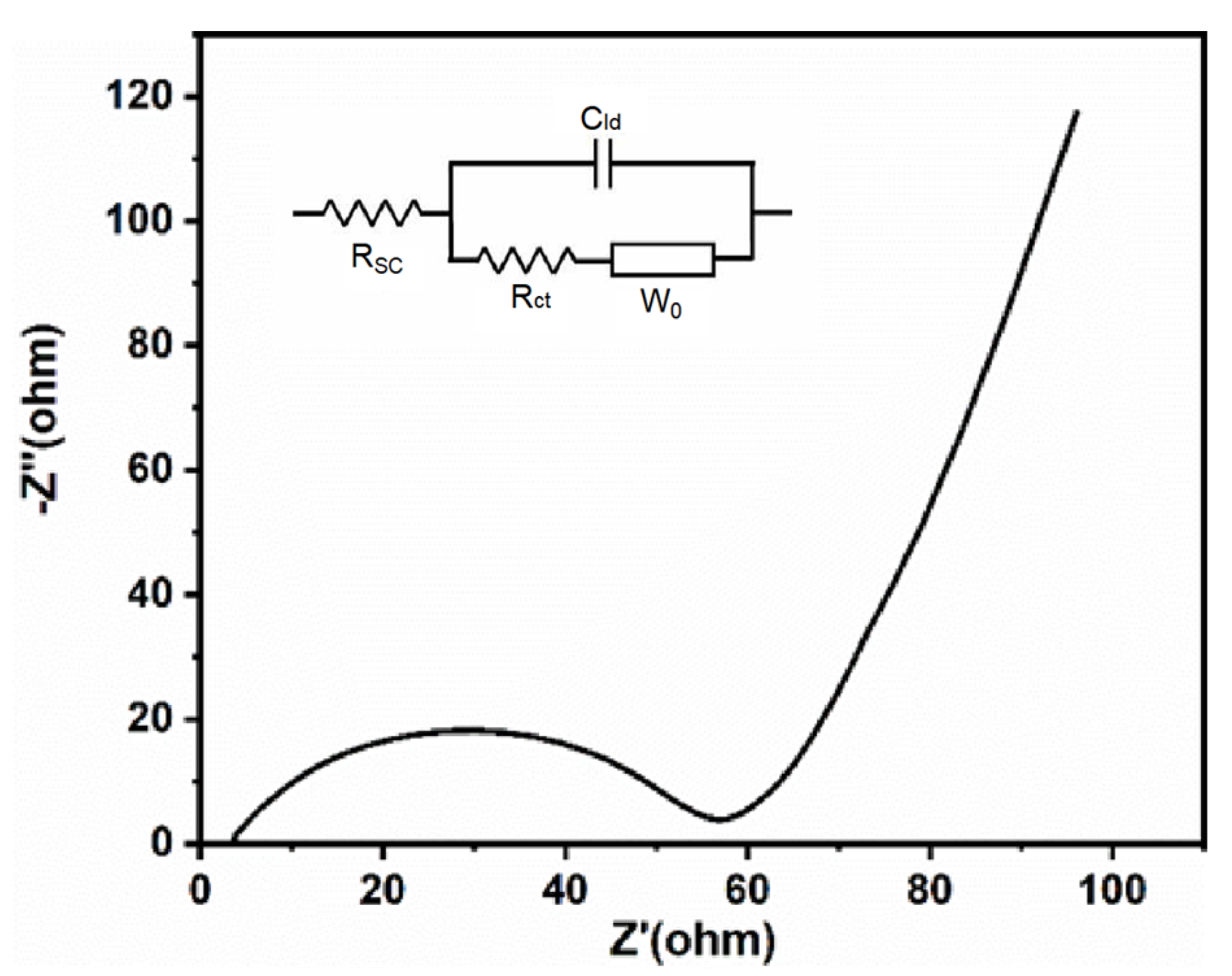
2.3. Electrochemical Test
3. Results and Discussion
3.1. Dissolution of Iron Phosphate Slag
3.1.1. Direct Dissolution Method
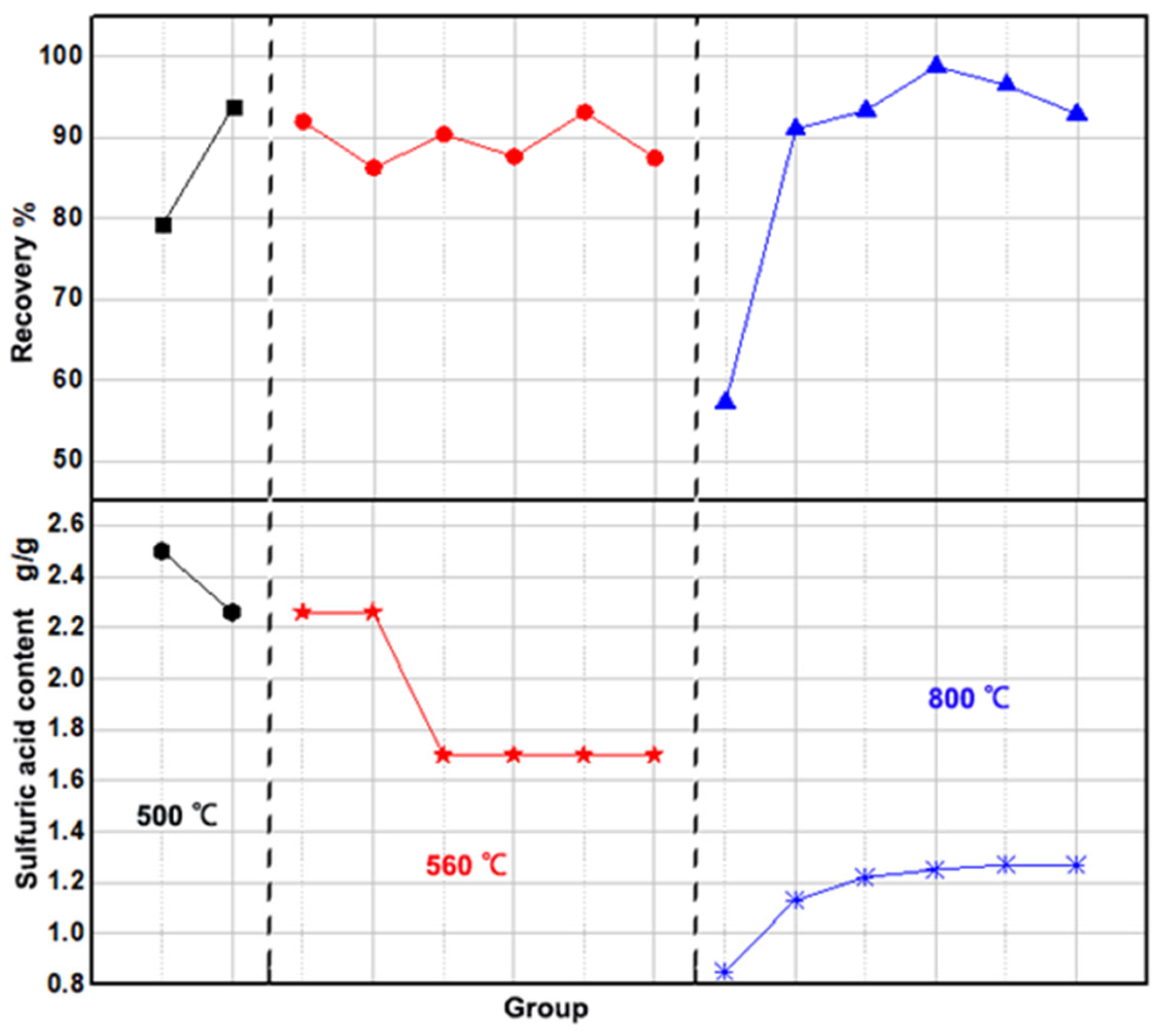
3.1.2. High-Temperature-Activated Dissolution Method
3.2. Impurity Removal from Solution
3.3. Regeneration for the Preparation of Battery-Grade Iron Phosphate
3.4. Regeneration of LiFePO4
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, J.; Neiber, R.R.; Park, J.; Ali Soomro, R.; Greene, G.W.; Ali Mazari, S.; Young Seo, H.; Hong Lee, J.; Shon, M.; Wook Chang, D.; et al. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery. Chem. Eng. J. 2022, 431, 133993–134008. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, C.; Zhang, X.; Wang, H.; Song, J.; Zhang, K.; Yang, T.; Zuo, Y.; Yang, Y.; Gao, C.; et al. An Ultrasmall Ordered High-Entropy Intermetallic with Multiple Active Sites for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2024, 146, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liang, S.; Yu, Y.; Cao, L.; Yang, C.; Liu, X.; Guo, K.; Müller-Buschbaum, P.; Cheng, Y.J.; Wang, C. A chronicle of titanium niobium oxide materials for high-performance lithium-ion batteries: From laboratory to industry. Carbon Neutralization 2024, 3, 1036–1091. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, X.; Fang, R.; Lu, C.; Wang, K.; Gan, Y.; He, X.; Zhang, J.; Huang, H.; Zhang, W.; et al. Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials. Carbon Neutralization 2023, 2, 169–185. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, L.; Wang, W.; Liu, Y.; Ma, Q.; Mu, D.; Li, R.; Dai, C. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv. 2016, 6, 43613–43625. [Google Scholar] [CrossRef]
- Yin, R.-X.; Zhu, W.-G.; Zhao, Z.-W.; Xu, W.-H.; Liu, X.-H.; He, L.-H. Lithium recovery from brine by PEG-modified porous LiFePO4/FePO4 electrode system. Sep. Purif. Technol. 2024, 338, 126375–126383. [Google Scholar] [CrossRef]
- Du, H.; Kang, Y.; Li, C.; Zhao, Y.; Tian, Y.; Lu, J.; Chen, Z.; Gao, N.; Li, Z.; Wozny, J.; et al. Recovery of lithium salt from spent lithium-ion battery by less polar solvent wash and water extraction. Carbon Neutralization 2023, 2, 416–424. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, H.; Fang, Y.; Li, W.; Lan, S.; Wei, S.; Yin, Z.; Tang, Y.; Ren, Y.; Liu, Q. Typical cathode materials for lithium-ion and sodium-ion batteries: From structural design to performance optimization. Carbon Neutralization 2023, 2, 339–377. [Google Scholar] [CrossRef]
- Hu, G.; Huang, K.; Du, K.; Peng, Z.; Cao, Y. Efficient recovery and regeneration of FePO4 from lithium extraction slag: Towards sustainable LiFePO4 battery recycling. J. Clean. Prod. 2024, 434, 140091–140101. [Google Scholar] [CrossRef]
- Bai, L.; Liu, G.; Fu, Y.; Sun, W.; Zeng, X.; Shao, R.; Ou, H.; Liang, Y.; Yuan, F. Approach towards the Purification Process of FePO4 Recovered from Waste Lithium-Ion Batteries. Processes 2024, 12, 1861. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in Nanostructured Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Chen, T.; Cai, J.; Wang, H.; Gao, C.; Yuan, C.; Zhang, K.; Yu, Y.; Xiao, W.; Luo, T.; Xia, D. Symbiotic reactions over a high-entropy alloy catalyst enable ultrahigh-voltage Li–CO2 batteries. Energy Environ. Sci. 2025, 18, 853–861. [Google Scholar] [CrossRef]
- Gören, A.; Costa, C.M.; Silva, M.M.; Lanceros-Mendez, S. Influence of fluoropolymer binders on the electrochemical performance of C-LiFePO4 based cathodes. Solid State Ion. 2016, 295, 57–64. [Google Scholar] [CrossRef]
- Yang, S.; Song, Y.; Ngala, K.; Zavalij, P.Y.; Stanley Whittingham, M. Performance of LiFePO4 as lithium battery cathode and comparison with manganese and vanadium oxides. J. Power Sources 2003, 119–121, 239–246. [Google Scholar] [CrossRef]
- Kakarla, A.K.; Narsimulu, D.; Bandi, H.; Shanthappa, R.; Yu, J.S. Facile synthesis of N-doped reduced graphene oxide matrix-covered porous Fe2VO4 hybrid composite nanostructures as anode material for lithium-ion batteries. J. Alloys Compd. 2023, 960, 170784. [Google Scholar] [CrossRef]
- Kakarla, A.K.; Narsimulu, D.; Patnam, H.R.; Shanthappa, R.; Yu, J.S. Structural and electrochemical properties of mesoporous FeVO4 as a negative electrode for lithium-ion battery. Int. J. Energy Res. 2022, 46, 13590–13601. [Google Scholar] [CrossRef]
- Zhang, Y.; Ru, J.; Hua, Y.; Cheng, M.; Lu, L.; Wang, D. Priority Recovery of Lithium From Spent Lithium Iron Phosphate Batteries via H2O-Based Deep Eutectic Solvents. Carbon Neutralization 2025, 4, e186. [Google Scholar] [CrossRef]
- Tang, R.; Dong, J.; Wang, C.; Yin, A.; Lu, Y.; Li, N.; Shen, W.; Zhang, J.; Yan, K.; Zhao, G.; et al. A Comprehensive Review of the Research Progress on the Low-Temperature Performance of LiFePO4 Batteries. Carbon Neutralization 2025, 4, e70001. [Google Scholar] [CrossRef]
- Zhou, Y.; Pang, M.; Zhang, M.; Yuan, Y.; Yang, Y.; Qin, F.; Liu, W.; Chen, T.; Liu, K. Controlling crystallographic orientation through composite phase regulation to unlock oxide cathode performance. Chem. Eng. J. 2024, 501, 157527. [Google Scholar] [CrossRef]
- Nigl, T.; Schwarz, T.E.; Walch, C.; Baldauf, M.; Rutrecht, B.; Pomberger, R. Characterisation and material flow analysis of end-of-life portable batteries and lithium-based batteries in different waste streams in Austria. Waste Manag. Res. 2020, 38, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Fung, K.Y.; Ng, K.M.; Wibowo, C. Process Development for the Recycle of Spent Lithium Ion Batteries by Chemical Precipitation. Ind. Eng. Chem. Res. 2014, 53, 18245–18259. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Chen, T.; Ouyang, B.; Fan, X.; Zhou, W.; Liu, W.; Liu, K. Oxide cathodes for sodium-ion batteries: Designs, challenges, and perspectives. Carbon Energ. 2022, 4, 170–199. [Google Scholar] [CrossRef]
- Kumar, J.; Shen, X.; Li, B.; Liu, H.; Zhao, J. Selective recovery of Li and FePO4 from spent LiFePO4 cathode scraps by organic acids and the properties of the regenerated LiFePO4. Waste Manag. Res. 2020, 113, 32–40. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, N.; Noor, T.; Zaman, N. Selective lithium recovery from spent LFP Li-ion batteries using organic acids. Ionics 2024, 31, 273–286. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Gao, R.; Xu, Z. Pyrolysis and utilization of nonmetal materials in waste printed circuit boards: Debromination pyrolysis, temperature-controlled condensation, and synthesis of oil-based resin. J. Hazard. Mater. 2019, 364, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhou, H.; Huang, Z.; Tao, S.; Zhai, B.; Liu, L.; Hu, L. Regeneration cathode material mixture from spent lithium iron phosphate batteries. J Mater. Sci.-Mater. Electron. 2018, 29, 9283–9290. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217–102237. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2016, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Yao, Y.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. A green and effective room-temperature recycling process of LiFePO4 cathode materials for lithium-ion batteries. Waste Manag. Res. 2019, 85, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. Res. 2019, 89, 83–93. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Y.; Liu, Y.; Zhang, M.; Liu, X.; Yuan, Y.; Zhou, Y.; Liu, W.; Chen, T.; Liu, K. Optimizing interfacial modification for enhanced performance of Na3V2(PO4)3 cathode in sodium-ion batteries. Chem. Eng. J. 2024, 495, 153396. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries. J. Hazard. Mater. 2014, 271, 50–56. [Google Scholar] [CrossRef]
- Chen, D.; Rao, S.; Wang, D.; Cao, H.; Xie, W.; Liu, Z. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid- l-ascorbic acid system. Chem. Eng. J. 2020, 388, 124321. [Google Scholar] [CrossRef]




| Chemical Name | Chemical Formula | Specifications |
|---|---|---|
| Ammonium Hydroxide | NH4OH | 28–30% solution |
| Phosphoric Acid | H3PO4 | 85% (concentrated) |
| Lithium Carbonate | Li2CO3 | ≥99% purity |
| Glucose | C6H12O6 | ≥99% purity |
| Acetylene Black | C8H8 | Conductive carbon black |
| Polyvinylidene Fluoride | PVDF | ≥98% purity |
| Nitrogen | N2 | ≥99.99% purity (for inert atmosphere) |
| Deionized Water | H2O | Conductivity < 0.1 µS/cm |
| Aqua Regia | N/A | HNO3:HCl (3:1 v/v) for analysis |
| Test Group | Amount of Iron Phosphate Slag (g) | Amount of Acid Added (g) | Sulfuric per g of Iron | Recovery Rate |
|---|---|---|---|---|
| Group 1 | 1000 | 500 g sulfuric acid | 2.43 | 83.3% |
| Group 2 | 1000 | 600 g sulfuric acid | 2.92 | 40.19% |
| Group 3 | 1000 | 700 g sulfuric acid | 3.4 | 39.82% |
| Group 4 | 1000 | 800 g sulfuric acid | 3.89 | 78.48% |
| Activation Temperature | Group | Amount of Iron Phosphate Slag (g) | Amount of Acid Added (g) | Sulfuric Acid per g of Iron | Recovery Rate |
|---|---|---|---|---|---|
| 500 °C | Group T1 | 1000 | 875 | 2.5 | 79.28% |
| Group T2 | 1000 | 800 | 2.26 | 93.86% | |
| 560 °C | Group T3 | 1000 | 800 | 2.26 | 91.89% |
| Group T4 | 1000 | 800 | 2.26 | 86.26% | |
| Group T5 | 1000 | 600 | 1.7 | 90.43% | |
| Group T6 | 1000 | 600 | 1.7 | 87.64% | |
| Group T7 | 1000 | 600 | 1.7 | 93.14% | |
| Group T8 | 1000 | 600 | 1.7 | 87.50% | |
| 800 °C | Group T9 | 1000 | 300 | 0.85 | 57.23% |
| Group T10 | 1000 | 400 | 1.13 | 91.04% | |
| Group T11 | 1000 | 430 | 1.22 | 93.35% | |
| Group T12 | 1000 | 440 | 1.25 | 98.80% | |
| Group T13 | 1000 | 450 | 1.27 | 96.48% | |
| Group T13 | 1000 | 450 | 1.27 | 92.92% |
| Group | Element Content (ppm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ca | Cd | Co | Cr | Cu | Mg | Mn | Na | Ni | K | Pb | Ti | Zn | |
| Group 12 | 323.1 | 5.331 | 0.09 | 2.882 | 3.861 | 0.968 | 5.111 | 12.29 | 106.1 | 11.78 | 5.011 | 3.33 | 149.6 | 1.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, Y.; Zhu, L.; Zhang, K.; Liu, W.; Chen, T.; Liu, K. Resource Recovery and Synthesis of Battery-Grade FePO4 from Waste LiFePO4 Battery Slag. Energies 2025, 18, 1829. https://doi.org/10.3390/en18071829
Li P, Wang Y, Zhu L, Zhang K, Liu W, Chen T, Liu K. Resource Recovery and Synthesis of Battery-Grade FePO4 from Waste LiFePO4 Battery Slag. Energies. 2025; 18(7):1829. https://doi.org/10.3390/en18071829
Chicago/Turabian StyleLi, Puliang, Yang Wang, Liying Zhu, Kun Zhang, Weifang Liu, Tao Chen, and Kaiyu Liu. 2025. "Resource Recovery and Synthesis of Battery-Grade FePO4 from Waste LiFePO4 Battery Slag" Energies 18, no. 7: 1829. https://doi.org/10.3390/en18071829
APA StyleLi, P., Wang, Y., Zhu, L., Zhang, K., Liu, W., Chen, T., & Liu, K. (2025). Resource Recovery and Synthesis of Battery-Grade FePO4 from Waste LiFePO4 Battery Slag. Energies, 18(7), 1829. https://doi.org/10.3390/en18071829




