Abstract
The effective recovery of valuable materials from spent LiFePO4 batteries is crucial for resource sustainability and environmental protection. This study investigates the recovery of phosphorus iron slag from waste LiFePO4 batteries, focusing on dissolution and impurity removal processes to produce battery-grade iron phosphate. Using high-temperature-activated dissolution, followed by precipitation/dissolution for impurity removal, we optimize conditions to ensure high recovery rates (up to 98.8% for FePO4 under optimized conditions) and product purity. Our findings demonstrate that the proposed method effectively transforms waste slag into valuable iron phosphate, significantly reducing raw material costs and contributing to sustainable battery recycling practices. The regenerated LiFePO4 cathode exhibits excellent electrochemical performance, achieving a discharge capacity of 160.7 mAh g−1 at 0.1 C, which meets market standard levels. This research provides a solid foundation for enhancing resource utilization and advancing circular economy principles in the battery industry.
1. Introduction
In the context of the global energy transition, lithium batteries have garnered widespread attention as a crucial energy storage technology [1,2,3,4]. With the rapid development of renewable energy, lithium batteries play a vital role in electric vehicles, renewable energy storage, and portable electronic devices. Among these, lithium-ion batteries using LiFePO4 cathode material have become particularly favored in the market due to their high safety, low cost, and long cycle life [5,6,7,8]. However, as early generations of LiFePO4 batteries gradually reach the end of their service life, the effective recovery of their resources, especially battery-grade phosphoric acid iron, has emerged as an urgent challenge that needs to be addressed [9,10]. Thus, exploring new recovery methods and technologies is essential for achieving sustainable development [11,12,13,14].
Currently, the recycling of used batteries primarily focuses on the extraction of high-value metals such as lithium, while elements like phosphorus and iron are often overlooked [15,16,17,18]. For instance, conventional sulfuric acid leaching methods achieve Li recovery rates of 85–93% but require high acid consumption (2–3 g H2SO4/g Fe) and fail to address FePO4 utilization [5,19,20,21]. This neglect not only impacts the overall resource utilization rate but also poses significant environmental pollution risks [22,23,24,25]. Recent studies highlight alternative approaches: organic acids like citric acid (lemon juice) achieve 94.8% Li recovery with minimal Fe loss (<4%) [26], while formic acid systems enable selective Li extraction (95% efficiency) with 50% lower acid consumption than mineral acids [27]. The waste generated from LiFePO4 batteries, particularly the iron phosphate slag, is typically regarded as a worthless byproduct [28,29]. However, this research advanced strategies such as high-temperature activation (800 °C) combined with precipitation/dissolution which have demonstrated FePO4 recovery rates exceeding 98.8%, surpassing conventional hydrometallurgical methods. To achieve the recycling of battery materials, effective strategies must be developed to treat these wastes and transform them into valuable products [30,31,32,33]. Notably, regenerated LiFePO4 from recycled FePO4 exhibits competitive electrochemical performance (160.7 mAh g−1 at 0.1 C), comparable to commercial counterparts (155–170 mAh g−1). This approach will not only enhance the comprehensive utilization of resources but also alleviate the pressure of waste disposal, thus promoting the development of a circular economy [26,34,35,36].
The core objective of this study is to explore the feasibility of using iron phosphate slag derived from the recycling of waste LiFePO4 batteries as a raw material for the production of battery-grade phosphoric acid iron [37]. Through systematic experimental design, we will focus on investigating the dissolution and impurity removal processes of iron phosphate slag to optimize preparation conditions and ensure the quality of the final product [38,39]. Specifically, we will compare the effectiveness of different dissolution methods and explore suitable impurity removal techniques to enhance the purity and stability of phosphoric acid iron. This process will provide a solid technical foundation for the realization of waste resource reutilization.
Through this research, we aim to offer new insights and methods for the resource utilization of waste batteries, thereby promoting the achievement of sustainable development goals. Additionally, the findings will provide theoretical support for related enterprises in improving their technologies within the battery recycling sector, facilitating the circular economy of battery materials. Ultimately, we hope to contribute to the sustainable development of the industry and promote the green recycling and reuse technologies for battery materials on a broader scale.
2. Materials and Method
2.1. Material Recovery
The chemical reagents used and their specifications are shown in Table 1.

Table 1.
Chemicals and specifications.
2.1.1. Dissolution of Iron Phosphate Residue
The iron phosphate slag in this study, derived from recycled lithium-ion batteries, primarily consists of iron phosphate and carbon, along with a significant amount of impurities. Two dissolution methods were employed: first, a direct dissolution method, where the waste iron phosphate was added to a solution of sulfuric acid at a specific concentration (6–8 M) and stirred for a designated period (4–6 h) to facilitate dissolution. Second, the iron phosphate slag was first subjected to high-temperature sintering in a muffle furnace, after which the waste iron phosphate was added to a solution of sulfuric acid at the same concentration (6–8 M) and stirred for a designated period (3–4 h) to achieve dissolution. All dissolution steps were performed under ambient atmospheric conditions, and the dissolution temperature was maintained at 60–70 °C.
2.1.2. Impurity Removal
A certain amount of a purification agent was added to the leachate to prevent aluminum from precipitating. Ammonium hydroxide was used to adjust the pH of the leachate to approximately 2. Under these conditions, the purification agent allows aluminum to remain in solution, while iron phosphate precipitates first. After solid–liquid separation, sulfuric acid is added to dissolve the precipitated iron phosphate, resulting in a qualified purification solution.
2.1.3. Regeneration of Iron Phosphate
The purified ferrous phosphate slag solution was placed in a flask, to which an equal volume of water was added. Under constant stirring at a temperature of 40 °C, ammonium hydroxide was introduced into the solution to adjust the pH to 2.2–2.5. The concentration of iron phosphate in the solution was monitored to ensure optimal conditions for precipitation. The solution was then filtered and washed until the conductivity was below 500 µS/cm, indicating the removal of impurities. The resulting filter cake was collected, and pure water was added, followed by phosphoric acid, ensuring thorough mixing. The slurry was then poured into a flask and aged by stirring for 3 h at 92 °C. After aging, the slurry was filtered and washed until the conductivity was below 200 µS/cm. The filter cake was subsequently dried at 105 °C for 12 h to obtain dihydrate iron phosphate. This dihydrate iron phosphate was placed in a ceramic crucible and sintered in a muffle furnace at a temperature of 560 °C for 4 h to yield the recovered anhydrous iron phosphate.
2.1.4. Synthesis of LiFePO4
Using the previously prepared iron phosphate precursor as the raw material, lithium carbonate and glucose were added in specific proportions (with a Li/Fe ratio of 1.05 and glucose constituting 10% of the total mass of lithium carbonate and iron phosphate). The solid content was controlled at 45%. The mixture was then milled in pure water until the D50 value of the particle size distribution reached 0.35–0.4 µm, determined using laser diffraction particle size analysis (Malvern Mastersizer 3000), resulting in a precursor mixture. This slurry was spray-dried to obtain a fully dried precursor. Subsequently, the dried precursor was placed in a quartz crucible and subjected to sintering in a tubular furnace under a nitrogen atmosphere at a temperature of 740 °C for 8 h, producing LiFePO4 cathode material.
2.2. Material Characterization
X-ray diffraction patterns were acquired using a Bruker Advance-D8 diffractometer, employing Cu Kα radiation as the source. The cell parameters were refined using GSAS software (version 2.0) following the Rietveld method. Solid samples (such as phosphorous iron slag) were digested using a mixed acid solution of HNO3 and HCl (aqua regia, 3:1 v/v) at 120 °C for 2 h. The solution was then filtered and diluted with deionized water, followed by elemental analysis via inductively coupled plasma optical emission spectrometry (ICP-OES, PerkinElmer Avio 500). Additionally, the morphologies of the materials were observed through scanning electron microscopy (SEM) images, utilizing a FEI Nova Nano SEM 230 instrument.
2.3. Electrochemical Test
To investigate the electrochemical properties of the fabricated cathode materials, coin-type (CR2016) cells were assembled in an argon-filled glove box. The electrode was prepared by combining the active material, acetylene black, and polyvinylidene fluoride (PVDF) in a weight ratio of 8:1:1 onto aluminum foils, using N-methyl-2-pyrrolidone (NMP) as the solvent. The prepared electrodes were then dried in a vacuum oven at 80 °C for 24 h. The mass loading of the active materials ranged from 1.5 to 2 mg/cm2. Charge and discharge measurements were conducted using the NEWARE battery test system.
3. Results and Discussion
3.1. Dissolution of Iron Phosphate Slag
3.1.1. Direct Dissolution Method
According to the literature, elevated temperatures (>70 °C) and extended dissolution durations (>6 h) promote the precipitation of FePO4, which may react to form abnormal phosphoric acid iron, adversely affecting its dissolution efficiency [27]. The optimal dissolution rate occurs at temperatures between 60 and 70 °C. At this temperature and under high acidity, dissolving for 4–6 h minimizes the precipitation of dissolved iron and phosphorus. Therefore, the chosen dissolution temperature was 60–70 °C, with a dissolution time set at 4–6 h.
Experiments were conducted under the same dissolution conditions using the direct dissolution method. After completion, the mixture was filtered, and the volumes of the filtrate and weights of the residue were recorded. The iron content in both the filtrate and residue was measured, with the recovery rate calculated using the following Formula (1):
Recovery Rate = (Filtrate iron concentration × Filtrate volume)/(Filtrate iron concentration × Filtrate volume + Residue iron content × Residue weight) × 100
Table 2 below presents the recovery rates from the direct dissolution method, calculated based on iron (notably, the recovery rate for phosphorus is higher than that for iron).

Table 2.
Recovery data for direct dissolution method.
This approach primarily validates the effects of 500 g, 600 g, 700 g, and 800 g sulfuric acid on the dissolution of iron phosphate slag. Higher quantities of sulfuric acid were not included in the experimental plan, as the acidity of the resulting leachate is closely related to the amount of sulfuric acid added. While increasing sulfuric acid might enhance recovery rates, excessive acidity would adversely affect subsequent reactions in phosphoric acid iron synthesis.
From the data presented in the table, it is evident that the overall recovery rate for iron phosphate slag is not high and exhibits instability, with a maximum of 83.3% and a minimum of only 39.82%. The low recovery rate may be related to the properties of the iron phosphate slag, where iron and phosphorus primarily exist as precipitated phosphoric acid iron and may have formed dihydrate phosphoric acid iron, leading to lower recovery rates. The instability in recovery rates could also stem from the raw materials, as different manufacturers may have varying processes for recovering iron phosphate slag, resulting in differing amounts of dihydrate phosphoric acid iron and ultimately unstable recovery rates.
An isolated analysis of the impact of sulfuric acid addition on the recovery filtrate did not reveal a consistent trend of increased recovery with greater sulfuric acid amounts. This suggests that the soluble portion of iron phosphate in the slag does not require substantial sulfuric acid, and the remaining insoluble components are difficult to dissolve even with increased acidity.
3.1.2. High-Temperature-Activated Dissolution Method
In this experimental approach, iron phosphate slag was first high-temperature-sintered in a muffle furnace before undergoing dissolution at a temperature of 60–70 °C for 3–4 h. Table 3 below presents the recovery rate data for the high-temperature-activated dissolution method.

Table 3.
Recovery data for high-temperature activation method.
The purpose of high-temperature activation is to ensure that all phosphoric acid iron in the iron phosphate slag exists in the form of anhydrous phosphoric acid iron. Unlike the dehydration process of phosphoric acid, the focus here is solely on the effect of activation temperature, with a constant activation time of 3 h.
During the high-temperature activation process, a visible transformation occurred in the appearance of the iron phosphate slag. The unactivated slag appeared as a black solid powder, while the slag activated at 500 °C and 560 °C exhibited a yellow-white surface, although agglomeration occurred during activation, with the interior remaining black. The slag activated at 800 °C turned entirely yellow-white, indicating a significant impact of activation temperature on the activation process.
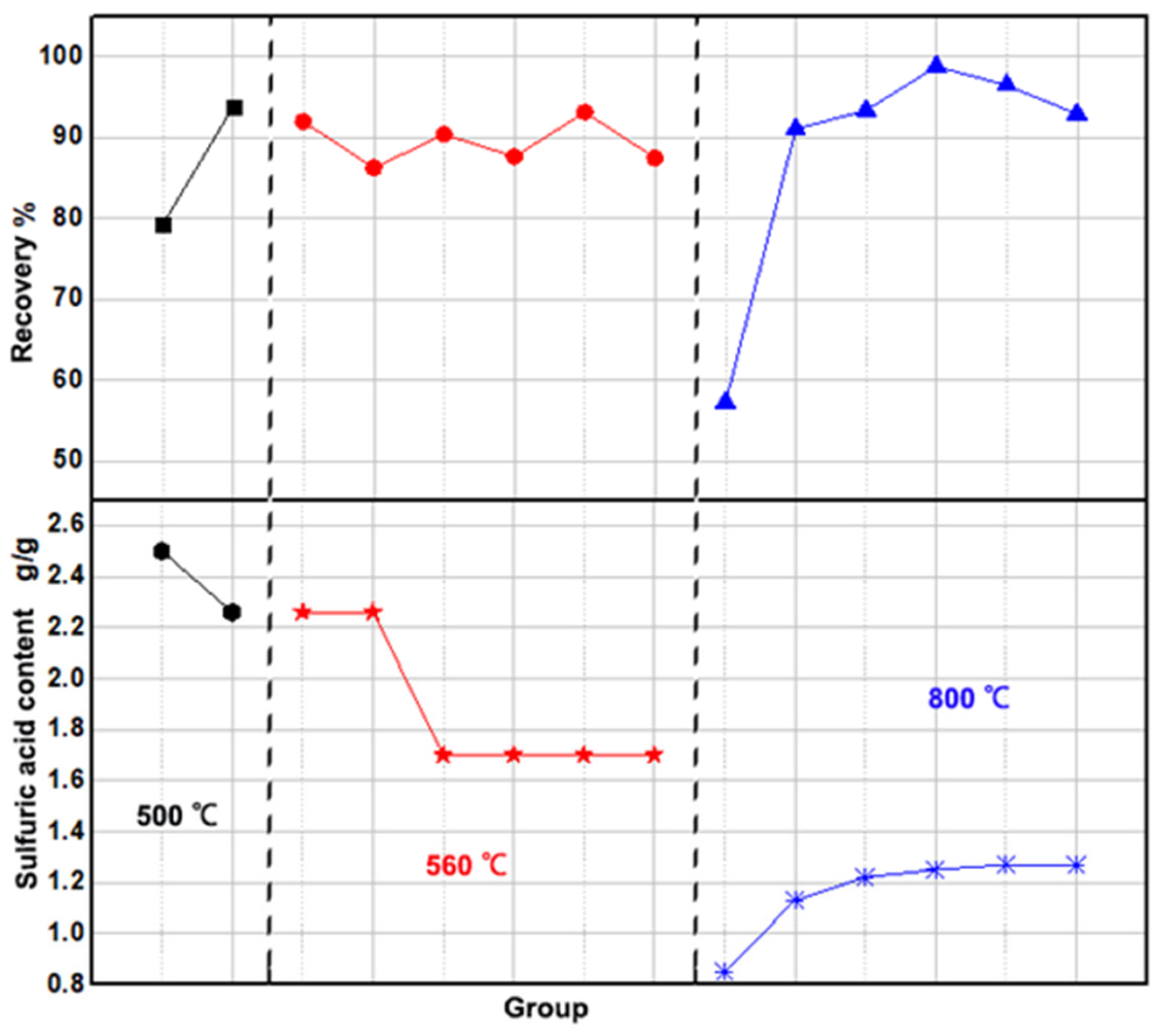
The following discussion examines the influence of activation temperature on the dissolution of iron phosphate slag. Overall, high-temperature activation is beneficial for improving the recovery rate of iron phosphate slag compared to the direct dissolution method, as shown in Figure 1. Under sufficient sulfuric acid conditions, the recovery rate can be maintained above 80%.
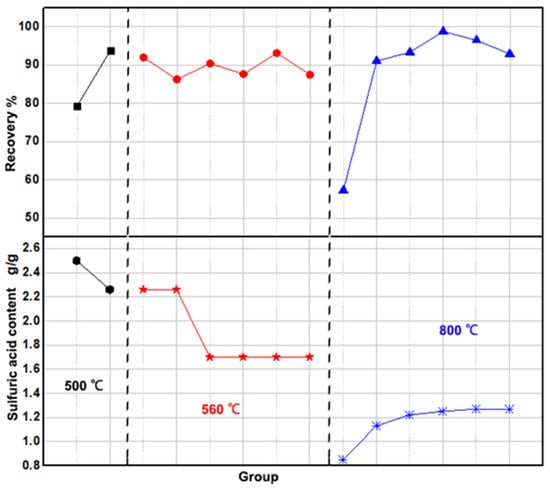
Figure 1.
Effect of activation temperature and amount of dissolved sulfuric acid on recovery.
At an activation temperature of 500 °C, the recovery rate exceeded 80%. However, in this case, experiments with higher sulfuric acid quantities did not yield higher recovery rates than those with lower quantities. For instance, the recovery rate with higher sulfuric acid was 79.28%, while that with lower acid reached 93.86%. This inconsistency may result from the incomplete activation of the slag at 500 °C, leading to uneven distribution within the material and, consequently, unstable recovery rates.
Raising the temperature to 560 °C stabilized recovery above 85%, peaking at 93.14% with reduced sulfuric acid. At 800 °C, recovery exceeded 90%, reaching 98.8%, while acid use dropped by about 50%. However, excessively high temperatures may volatilize phosphorus and convert iron to ferric oxide, making 800 °C the optimal choice. The amount of sulfuric acid also plays a critical role in recovery. The study found that a peak recovery of 98.8% occurred at 1.25 g sulfuric acid, with the optimal range for effective recovery being 1.22 to 1.27 g.
In summary, the high-temperature-activated dissolution method achieves over 90% recovery, presenting a more efficient alternative to direct dissolution with roughly 50% less sulfuric acid. The ideal conditions for optimal recovery are an activation temperature of 800 °C, 1.27 g sulfuric acid per gram of iron, a dissolution temperature of 60–70 °C, and a dissolution time of 3–4 h.
3.2. Impurity Removal from Solution
The filtrate of Group 12 obtained from the dissolution of phosphorus iron slag showed impurity contents as presented in Table 4. The main impurities were aluminum (Al) and titanium (Ti), with Al exceeding 300 ppm and Ti exceeding 150 ppm, while other impurities were relatively low. The Al originates from the battery current collectors, and Ti comes from the doping of lithium iron. Both Al and Ti exist in the form of metal salts. If Al and Ti are not removed and phosphorus iron is synthesized directly, these impurities will contaminate the product, resulting in excessive levels of Al and Ti. Currently, the manufacturing of LiFePO4 typically incorporates Ti during production; therefore, it may be feasible to retain the Ti element while removing Al.

Table 4.
Impurity content of phosphorus iron slag after dissolution in Group 12.
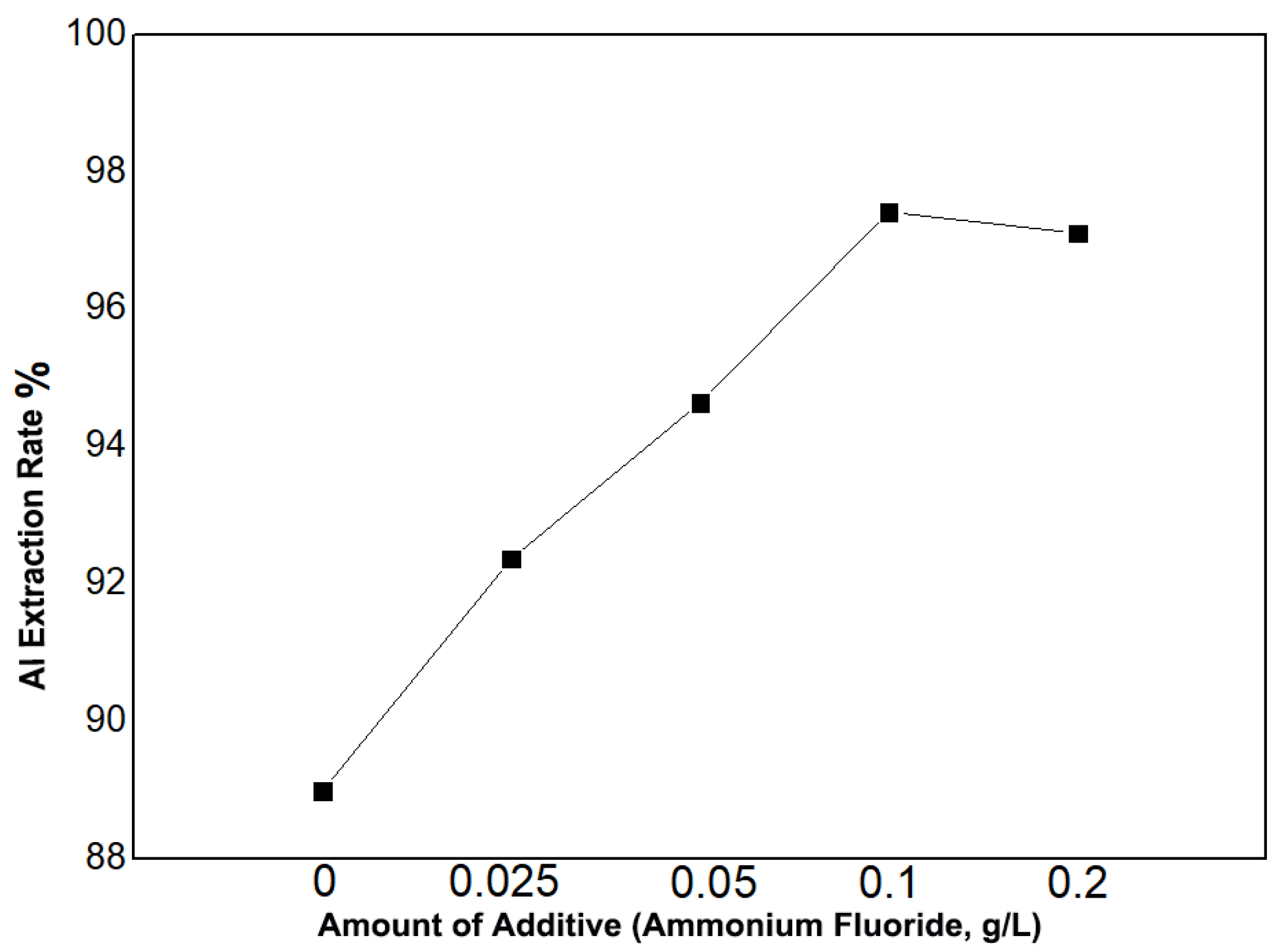
For the impurity removal process, we employed a precipitation/dissolution method. First, the removing agent was dissolved in the leachate, and then ammonia was diluted tenfold. The diluted ammonia was added to the leachate, adjusting the pH to 2, followed by stirring for 30 min. The mixture was then filtered, and the filter cake was mixed with pure water and sulfuric acid to ensure the complete dissolution of the precipitate. As shown in Figure 2, the addition of ammonium fluoride to the leachate effectively removed aluminum, and within a certain range, the aluminum removal efficiency increased with the amount of ammonium fluoride added. Without ammonium fluoride, the aluminum removal efficiency was 88.99%. With the addition of 0.025 g/L of ammonium fluoride, the aluminum removal rate increased to 92.37%. When the ammonium fluoride concentration was increased to 0.1 g/L, the aluminum removal rate reached 97.42%. However, further increasing the ammonium fluoride amount to 0.2 g/L resulted in a slight decrease in aluminum removal efficiency to 97.11%. Therefore, the optimal amount of the removing agent was determined to be 0.1 g/L.
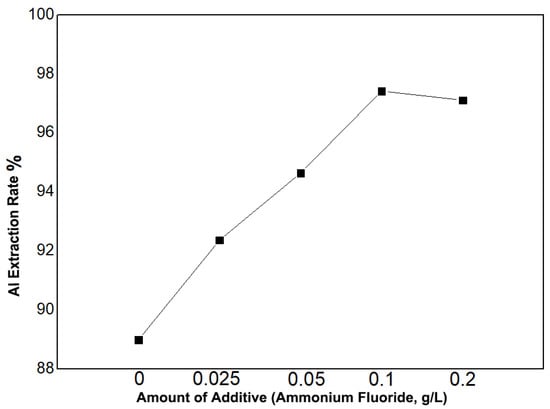
Figure 2.
Effect of ammonium fluoride addition on aluminum removal efficiency.
3.3. Regeneration for the Preparation of Battery-Grade Iron Phosphate
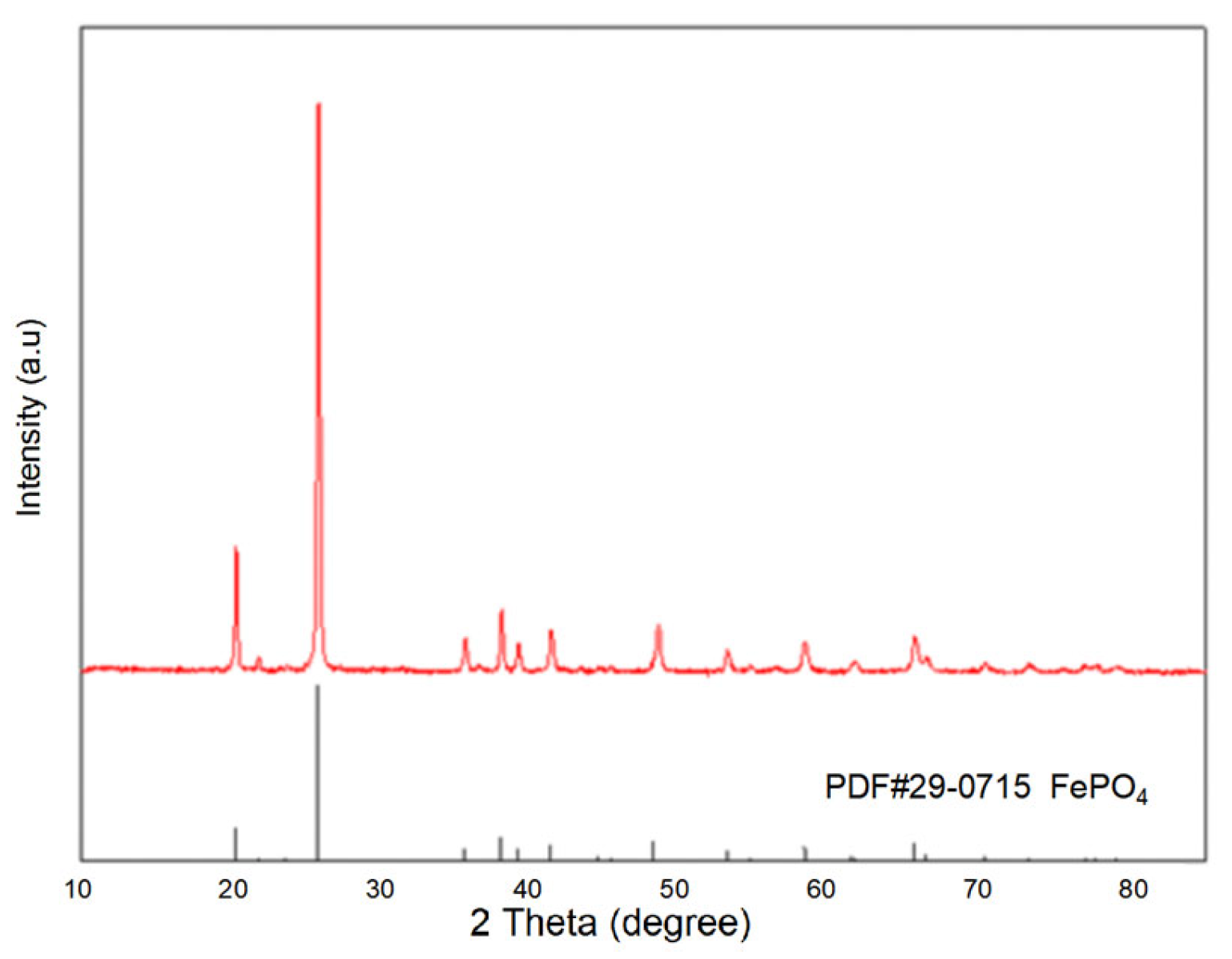
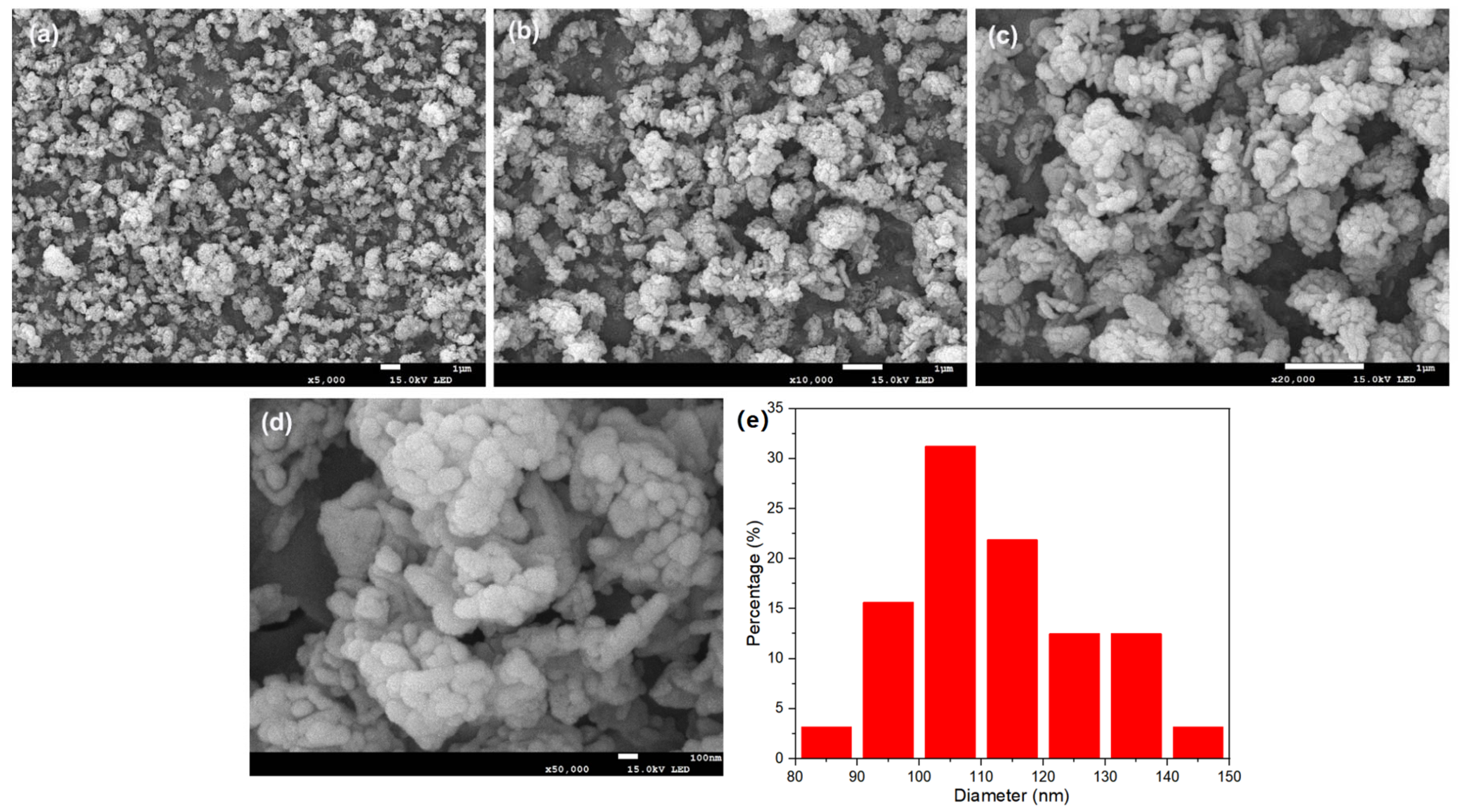
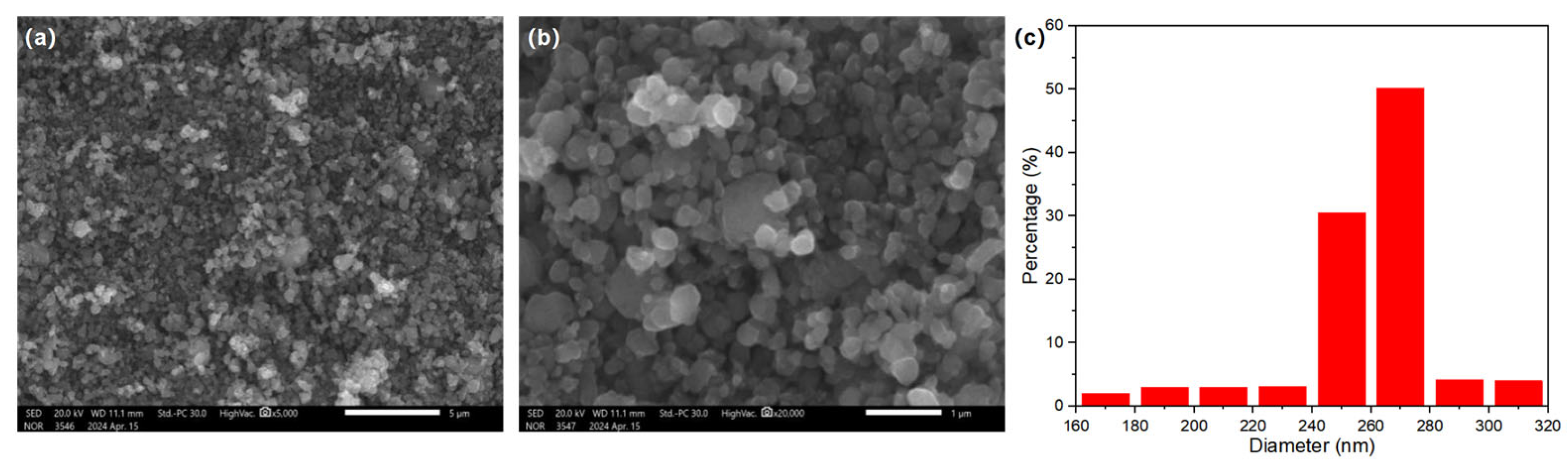
Using the purified filtrate, recovered iron phosphate was prepared. The synthesized iron phosphate is shown in Figure 3. The XRD patterns indicate sharp diffraction peaks and distinct characteristic peaks, indicating the high crystallinity of the sample. Comparison with standard cards revealed that the sample pattern was consistent with the standard card for iron phosphate (29-0715), with no additional peaks, suggesting the high purity of the sample. In the SEM results of the synthesized FePO4 (Figure 4), the particles were observed to be uniformly dispersed, with a diameter of approximately 100 nm. This uniform dispersion is crucial for the subsequent synthesis of LiFePO4. The smaller particle size not only increases the reaction surface area but also enhances the contact between reactants, facilitating a more complete reaction. The fine FePO4 particles are likely to interact more effectively with the lithium source, potentially leading to a more efficient synthesis process for LiFePO4 and improved electrochemical performance. These findings underscore the importance of optimizing particle size and distribution for achieving high-quality LiFePO4 synthesis.
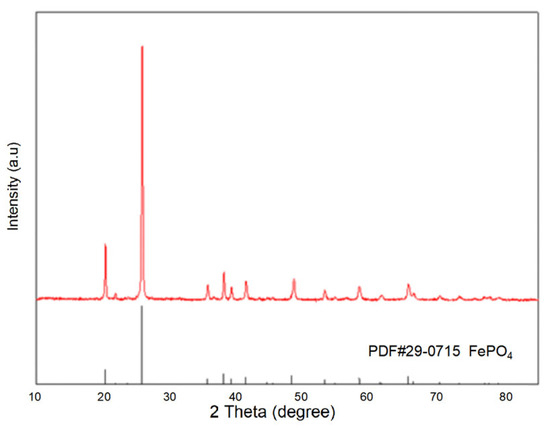
Figure 3.
The XRD pattern of FePO4 prepared under optimized conditions for Group 12.
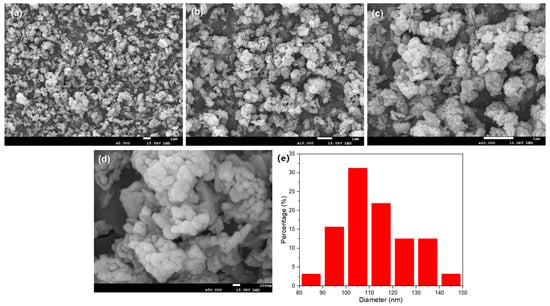
Figure 4.
(a–d) SEM images of the as-prepared FePO4 at different magnifications; (e) particle size distribution histogram of FePO4.
3.4. Regeneration of LiFePO4
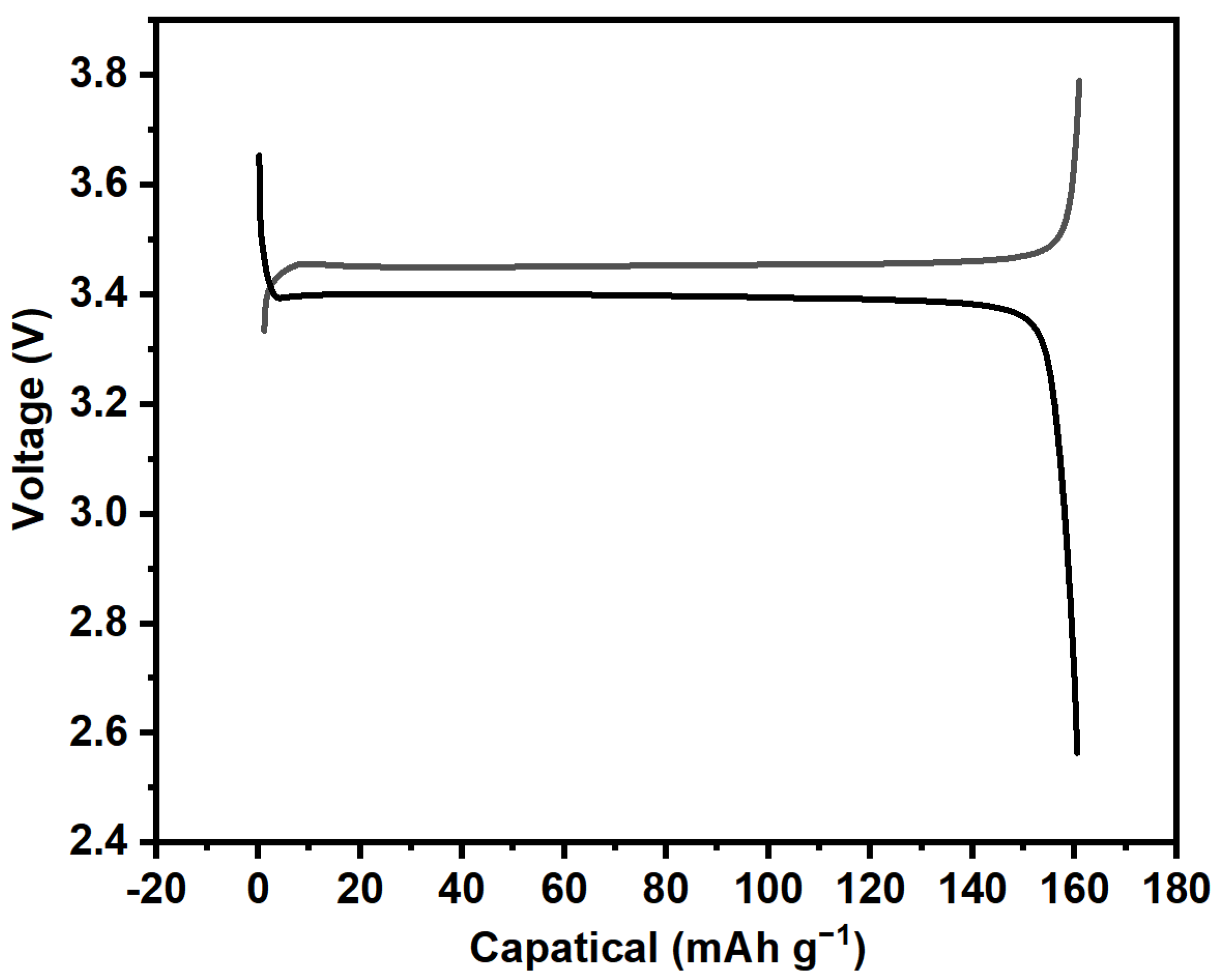
Using the previously prepared iron phosphate precursor as the raw material, LiFePO4 cathode material was regenerated. The images of the obtained LiFePO4 are shown in Figure 5. It can be observed that the prepared LiFePO4 cathode material exhibited a relatively uniform distribution with overall good dispersion, showing only slight agglomeration. Additionally, as shown in the charge and discharge curves in Figure 6, the regenerated LiFePO4 exhibited a discharge capacity of 160.7 mAh g−1, with a coulombic efficiency of 99%. This performance is nearly comparable to that of commercially available LiFePO4. Furthermore, measurements of the tap density revealed a value of 2.45 g cm−3. These results indicate that LiFePO4 synthesized from recycled iron phosphate has promising application potential.
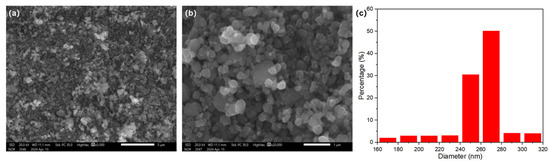
Figure 5.
(a,b) SEM images of LiFeO4 at different magnifications; (c) particle size distribution histogram of LiFePO4.
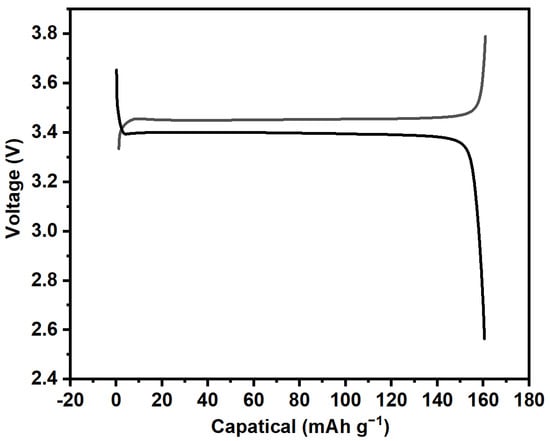
Figure 6.
Charge and discharge curves of the as-prepared LiFePO4 at a current density of 0.1 C.
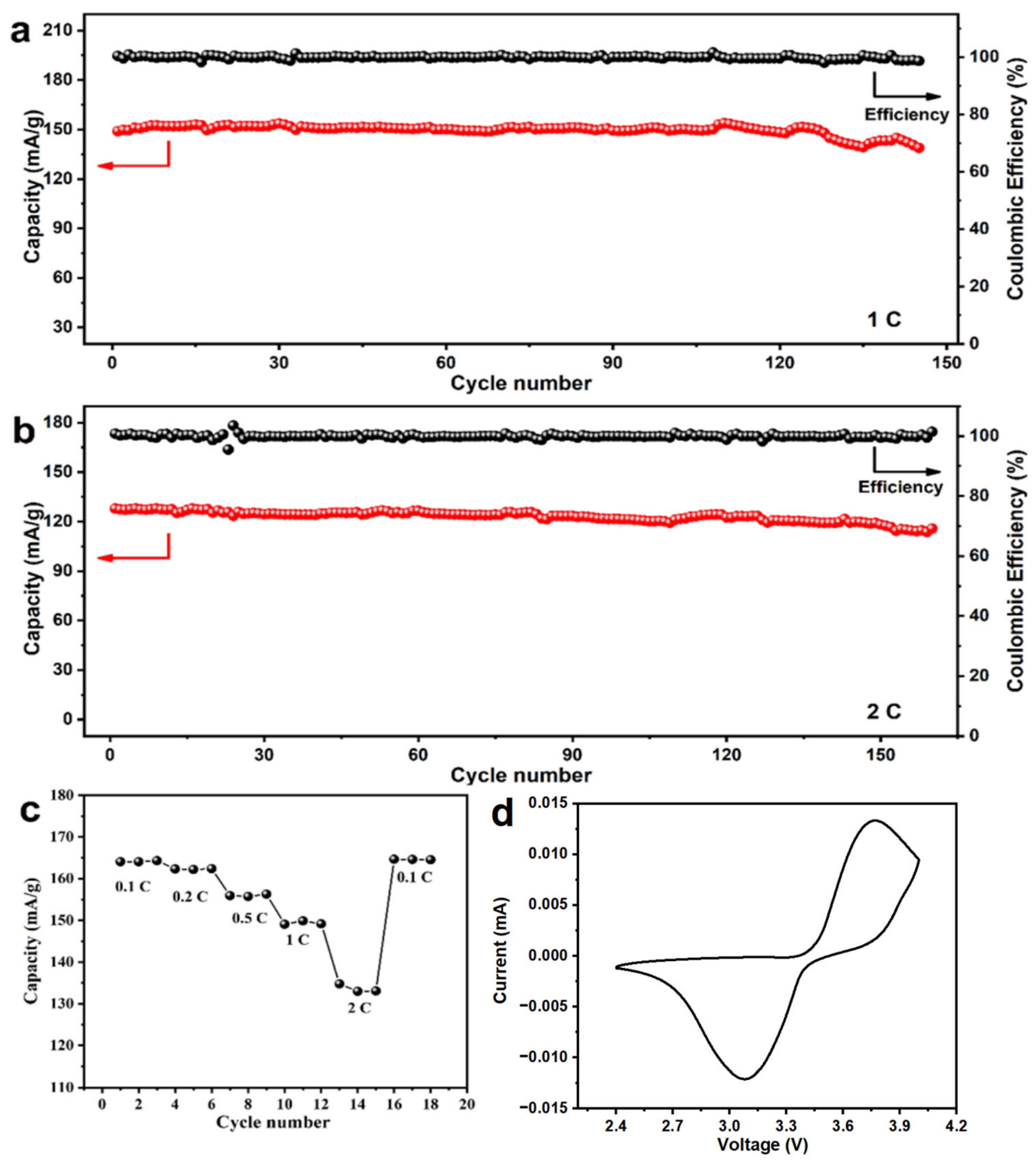
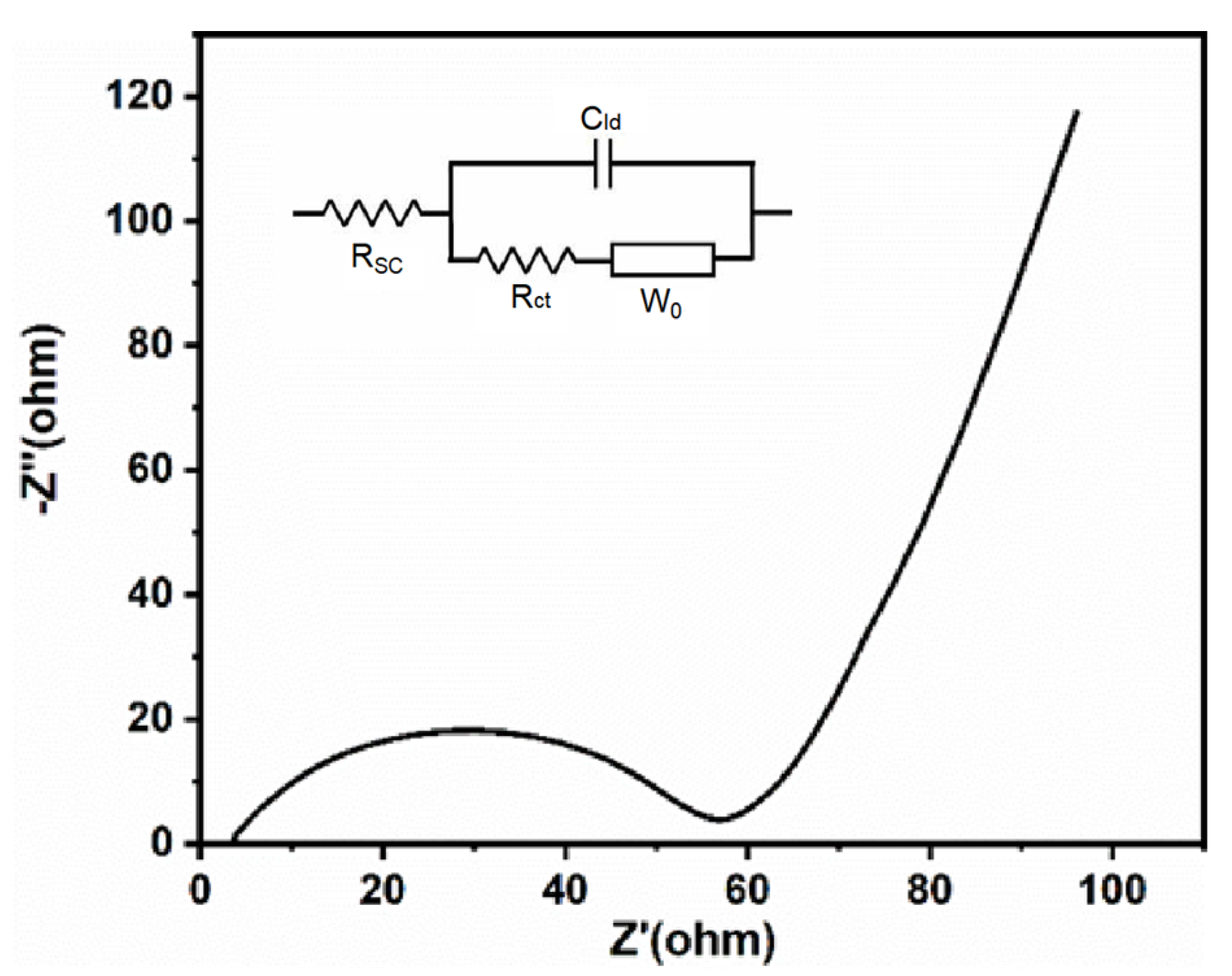
As shown in Figure 7a, the prepared LiFePO4 exhibited a reversible discharge capacity of 150 mAh g−1 at a current density of 1 C. After 145 cycles, it retained 92.75% of its initial capacity. Additionally, at 2 C, the initial specific capacity was 127.85 mAh g−1, and after 160 cycles, the capacity faded by only 9.56%, as shown in Figure 7b. Furthermore, its reversible specific capacities at 0.1 C, 0.2 C, 0.5 C, 1 C, and 2 C were 164.07, 162.33, 155.92, 149.88, and 134.77 mAh g−1, respectively (Figure 7c). According to the CV results (Figure 7d), the discharge plateau was at 3.4 V, and the charge plateau was just below 3.5 V, maintaining a small overpotential, which was consistent with the charge/discharge curves. The EIS results (Figure 8) showed that the internal resistance of the battery was only about 58 ohms, indicating excellent kinetics due to its relatively low internal resistance. Additionally, the Solution Resistance (RSC) was extracted from the high-frequency intercept (>104 Hz) on the real axis, with a fitted value of RSC = 5.2 Ω. This indicates low ohmic resistance due to the high ionic conductivity of the electrolyte and good electrode-electrolyte contact. The Charge Transfer Resistance (RCT) was determined from the diameter of the semicircle in the mid-frequency range (102–100 Hz), yielding a fitted value of RCT = 52.8 Ω. This reflects efficient charge transfer kinetics at the electrode/electrolyte interface, which is critical for fast Li+ intercalation/deintercalation. Furthermore, the Warburg Impedance (W0), represented by the linear slope in the low-frequency region (<10−1 Hz), is associated with solid-state Li+ diffusion; a near-45° slope confirms diffusion-dominated behavior.
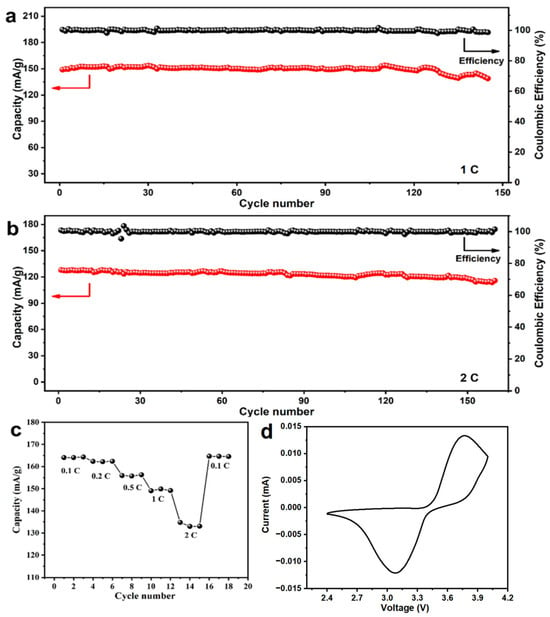
Figure 7.
Electrochemical performance of prepared LiFePO4 at (a) 1 C; (b) 2 C; (c) different rates and (d) CV curve.
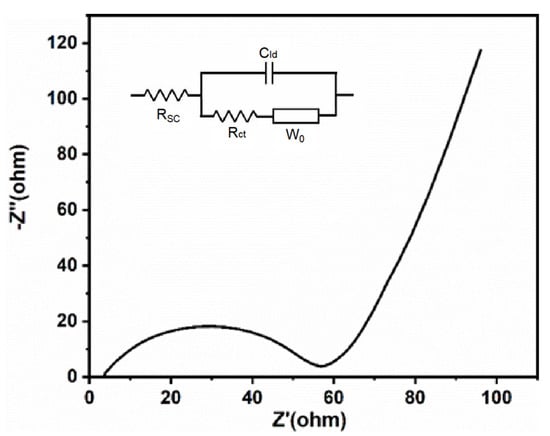
Figure 8.
EIS curve and equivalent circuit model of recovered LiFePO4.
4. Conclusions
This study used waste phosphorus iron slag from the recycling of old LiFePO4 batteries as raw material to investigate the recovery and dissolution conditions of phosphorus iron slag, as well as impurity removal methods. It also explored the process conditions for synthesizing battery-grade iron phosphate from the leachate of phosphorus iron slag. Moreover, the LiFePO4 cathode material produced from the reaction of iron phosphate waste residue with lithium carbonate reached the performance level of mainstream LiFePO4 cathode materials in the market, demonstrating excellent electrochemical properties. In summary, utilizing phosphorus iron slag as the raw material, employing high-temperature-activated dissolution followed by precipitation/dissolution for impurity removal, and synthesizing battery-grade iron phosphate from the treated leachate is a viable route. This method facilitates the resource utilization of waste phosphorus iron slag from old LiFePO4 batteries, reduces raw material costs, and holds significant practical implications.
Author Contributions
Conceptualization, P.L.; Methodology, P.L.; Software, P.L.; Validation, P.L.; Formal analysis, P.L.; Data curation, P.L.; Writing—original draft, P.L.; Writing—review and editing, Y.W., T.C. and K.Z.; Visualization, P.L., K.L. and L.Z.; Supervision, W.L. and K.L.; Project administration, L.Z., T.C. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 22272204), the Hunan Provincial Natural Science Foundation Project (No.2023JJ40276), and the Beijing Nova Program (No. 20220484153).
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, J.; Neiber, R.R.; Park, J.; Ali Soomro, R.; Greene, G.W.; Ali Mazari, S.; Young Seo, H.; Hong Lee, J.; Shon, M.; Wook Chang, D.; et al. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery. Chem. Eng. J. 2022, 431, 133993–134008. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, C.; Zhang, X.; Wang, H.; Song, J.; Zhang, K.; Yang, T.; Zuo, Y.; Yang, Y.; Gao, C.; et al. An Ultrasmall Ordered High-Entropy Intermetallic with Multiple Active Sites for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2024, 146, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liang, S.; Yu, Y.; Cao, L.; Yang, C.; Liu, X.; Guo, K.; Müller-Buschbaum, P.; Cheng, Y.J.; Wang, C. A chronicle of titanium niobium oxide materials for high-performance lithium-ion batteries: From laboratory to industry. Carbon Neutralization 2024, 3, 1036–1091. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, X.; Fang, R.; Lu, C.; Wang, K.; Gan, Y.; He, X.; Zhang, J.; Huang, H.; Zhang, W.; et al. Supercritical carbon dioxide technology in synthesis, modification, and recycling of battery materials. Carbon Neutralization 2023, 2, 169–185. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, L.; Wang, W.; Liu, Y.; Ma, Q.; Mu, D.; Li, R.; Dai, C. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv. 2016, 6, 43613–43625. [Google Scholar] [CrossRef]
- Yin, R.-X.; Zhu, W.-G.; Zhao, Z.-W.; Xu, W.-H.; Liu, X.-H.; He, L.-H. Lithium recovery from brine by PEG-modified porous LiFePO4/FePO4 electrode system. Sep. Purif. Technol. 2024, 338, 126375–126383. [Google Scholar] [CrossRef]
- Du, H.; Kang, Y.; Li, C.; Zhao, Y.; Tian, Y.; Lu, J.; Chen, Z.; Gao, N.; Li, Z.; Wozny, J.; et al. Recovery of lithium salt from spent lithium-ion battery by less polar solvent wash and water extraction. Carbon Neutralization 2023, 2, 416–424. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, H.; Fang, Y.; Li, W.; Lan, S.; Wei, S.; Yin, Z.; Tang, Y.; Ren, Y.; Liu, Q. Typical cathode materials for lithium-ion and sodium-ion batteries: From structural design to performance optimization. Carbon Neutralization 2023, 2, 339–377. [Google Scholar] [CrossRef]
- Hu, G.; Huang, K.; Du, K.; Peng, Z.; Cao, Y. Efficient recovery and regeneration of FePO4 from lithium extraction slag: Towards sustainable LiFePO4 battery recycling. J. Clean. Prod. 2024, 434, 140091–140101. [Google Scholar] [CrossRef]
- Bai, L.; Liu, G.; Fu, Y.; Sun, W.; Zeng, X.; Shao, R.; Ou, H.; Liang, Y.; Yuan, F. Approach towards the Purification Process of FePO4 Recovered from Waste Lithium-Ion Batteries. Processes 2024, 12, 1861. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in Nanostructured Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Chen, T.; Cai, J.; Wang, H.; Gao, C.; Yuan, C.; Zhang, K.; Yu, Y.; Xiao, W.; Luo, T.; Xia, D. Symbiotic reactions over a high-entropy alloy catalyst enable ultrahigh-voltage Li–CO2 batteries. Energy Environ. Sci. 2025, 18, 853–861. [Google Scholar] [CrossRef]
- Gören, A.; Costa, C.M.; Silva, M.M.; Lanceros-Mendez, S. Influence of fluoropolymer binders on the electrochemical performance of C-LiFePO4 based cathodes. Solid State Ion. 2016, 295, 57–64. [Google Scholar] [CrossRef]
- Yang, S.; Song, Y.; Ngala, K.; Zavalij, P.Y.; Stanley Whittingham, M. Performance of LiFePO4 as lithium battery cathode and comparison with manganese and vanadium oxides. J. Power Sources 2003, 119–121, 239–246. [Google Scholar] [CrossRef]
- Kakarla, A.K.; Narsimulu, D.; Bandi, H.; Shanthappa, R.; Yu, J.S. Facile synthesis of N-doped reduced graphene oxide matrix-covered porous Fe2VO4 hybrid composite nanostructures as anode material for lithium-ion batteries. J. Alloys Compd. 2023, 960, 170784. [Google Scholar] [CrossRef]
- Kakarla, A.K.; Narsimulu, D.; Patnam, H.R.; Shanthappa, R.; Yu, J.S. Structural and electrochemical properties of mesoporous FeVO4 as a negative electrode for lithium-ion battery. Int. J. Energy Res. 2022, 46, 13590–13601. [Google Scholar] [CrossRef]
- Zhang, Y.; Ru, J.; Hua, Y.; Cheng, M.; Lu, L.; Wang, D. Priority Recovery of Lithium From Spent Lithium Iron Phosphate Batteries via H2O-Based Deep Eutectic Solvents. Carbon Neutralization 2025, 4, e186. [Google Scholar] [CrossRef]
- Tang, R.; Dong, J.; Wang, C.; Yin, A.; Lu, Y.; Li, N.; Shen, W.; Zhang, J.; Yan, K.; Zhao, G.; et al. A Comprehensive Review of the Research Progress on the Low-Temperature Performance of LiFePO4 Batteries. Carbon Neutralization 2025, 4, e70001. [Google Scholar] [CrossRef]
- Zhou, Y.; Pang, M.; Zhang, M.; Yuan, Y.; Yang, Y.; Qin, F.; Liu, W.; Chen, T.; Liu, K. Controlling crystallographic orientation through composite phase regulation to unlock oxide cathode performance. Chem. Eng. J. 2024, 501, 157527. [Google Scholar] [CrossRef]
- Nigl, T.; Schwarz, T.E.; Walch, C.; Baldauf, M.; Rutrecht, B.; Pomberger, R. Characterisation and material flow analysis of end-of-life portable batteries and lithium-based batteries in different waste streams in Austria. Waste Manag. Res. 2020, 38, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Fung, K.Y.; Ng, K.M.; Wibowo, C. Process Development for the Recycle of Spent Lithium Ion Batteries by Chemical Precipitation. Ind. Eng. Chem. Res. 2014, 53, 18245–18259. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Chen, T.; Ouyang, B.; Fan, X.; Zhou, W.; Liu, W.; Liu, K. Oxide cathodes for sodium-ion batteries: Designs, challenges, and perspectives. Carbon Energ. 2022, 4, 170–199. [Google Scholar] [CrossRef]
- Kumar, J.; Shen, X.; Li, B.; Liu, H.; Zhao, J. Selective recovery of Li and FePO4 from spent LiFePO4 cathode scraps by organic acids and the properties of the regenerated LiFePO4. Waste Manag. Res. 2020, 113, 32–40. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, N.; Noor, T.; Zaman, N. Selective lithium recovery from spent LFP Li-ion batteries using organic acids. Ionics 2024, 31, 273–286. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Gao, R.; Xu, Z. Pyrolysis and utilization of nonmetal materials in waste printed circuit boards: Debromination pyrolysis, temperature-controlled condensation, and synthesis of oil-based resin. J. Hazard. Mater. 2019, 364, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhou, H.; Huang, Z.; Tao, S.; Zhai, B.; Liu, L.; Hu, L. Regeneration cathode material mixture from spent lithium iron phosphate batteries. J Mater. Sci.-Mater. Electron. 2018, 29, 9283–9290. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217–102237. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2016, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Yao, Y.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. A green and effective room-temperature recycling process of LiFePO4 cathode materials for lithium-ion batteries. Waste Manag. Res. 2019, 85, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. Res. 2019, 89, 83–93. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Y.; Liu, Y.; Zhang, M.; Liu, X.; Yuan, Y.; Zhou, Y.; Liu, W.; Chen, T.; Liu, K. Optimizing interfacial modification for enhanced performance of Na3V2(PO4)3 cathode in sodium-ion batteries. Chem. Eng. J. 2024, 495, 153396. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries. J. Hazard. Mater. 2014, 271, 50–56. [Google Scholar] [CrossRef]
- Chen, D.; Rao, S.; Wang, D.; Cao, H.; Xie, W.; Liu, Z. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid- l-ascorbic acid system. Chem. Eng. J. 2020, 388, 124321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).