Surfactant–Polymer Formulation for Chemical Flooding in Oil Reservoirs
Abstract
1. Introduction
- -
- When there is an unfavourable mobility ratio between oil and water;
- -
- When the mobility ratio is favourable but the oil viscosity is low in reservoirs with a certain degree of heterogeneity.
- (1)
- Reduction ininterfacial tension (IFT) at the oil–water interface;
- (2)
- Alteration of rock wettability.
2. Materials and Methods
2.1. Experimental Materials and Equipment
2.1.1. Polymer and Surfactant Samples
- 1.
- Anionic surfactants based on sodium alkylbenzene sulfonate, diethanolamide of lauric acid, sodium laureth sulphate, dodecylbenzene sulfonate, and alpha-olefin sulfonate;
- 2.
- Amphoteric surfactants based on betaine and oleyl betaine.
2.1.2. Brine Model
2.1.3. Core Samples and Oil Samples
2.1.4. Experimental Equipment
2.2. Experimental Methods
2.2.1. Requirements for Polymers and Experimental Methodology
- Compatibility with the reservoir water (absence of lumps or precipitates during solution preparation). The methodology is based on the periodic visual assessment of polymer powder dissolution during solution preparation. As the polymer dissolves, the solution becomes homogeneous, and no polymer particles should remain. The acceptable dissolution time should not exceed 240 min.
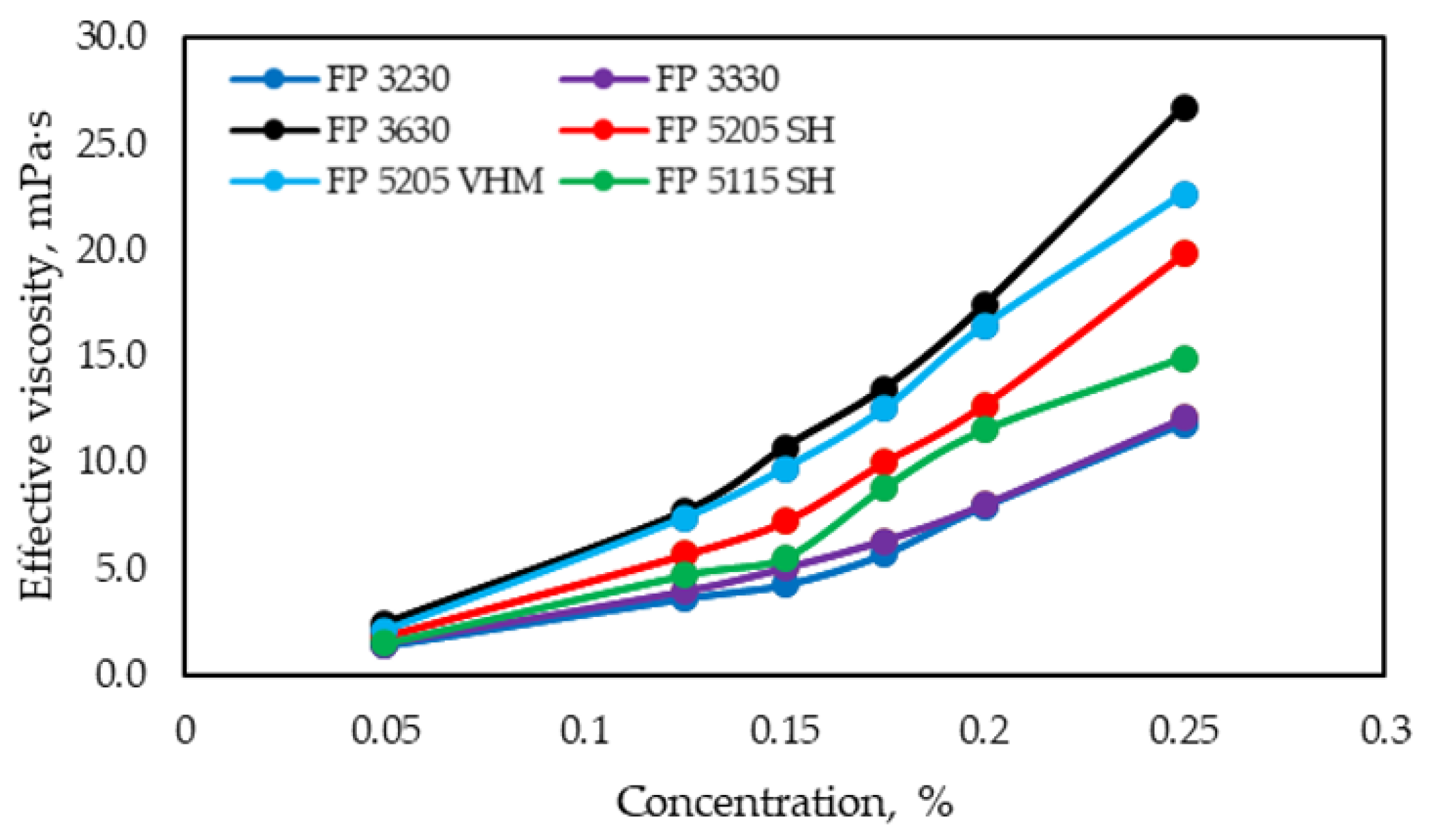
- Rheological properties, specifically achieving maximum solution viscosity at minimum concentration. The methodology involves measuring the viscosity of polymer solutions at various spindle rotation speeds (shear rates) using a rotational viscometer.
- Resistance to mechanical degradation. The mechanical stability coefficient is calculated as the ratio of the viscosity of the degraded solution to the original solution. The mechanical stability coefficient should be above 0.85. The methodology involves measuring the viscosity of the polymer solution before and after vigorous stirring using a paddle mixer at high rotation speeds (2000 rpm and above).
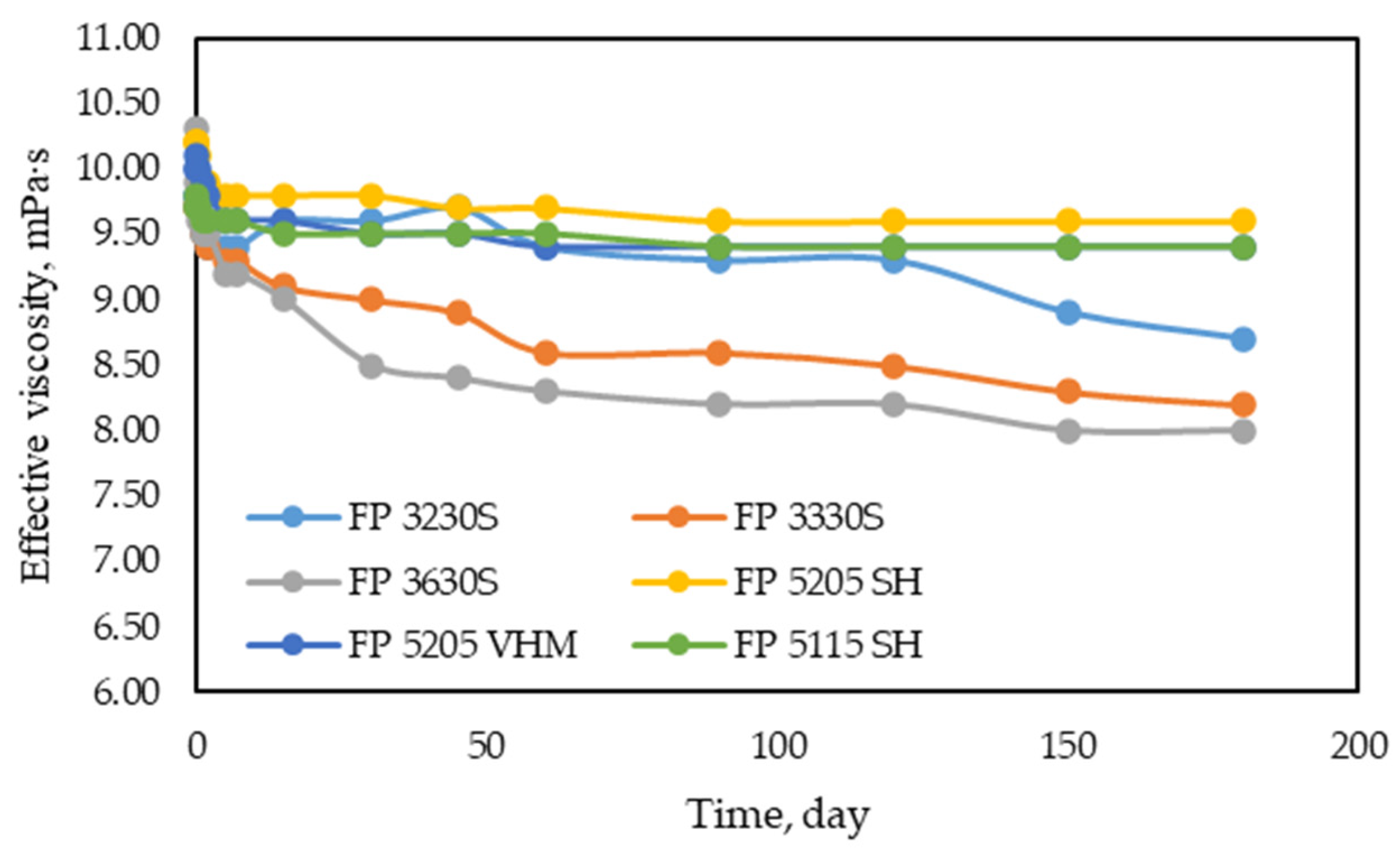
- Thermal stability of polymers. This is determined as the ratio of the viscosity of the solution after heating and prolonged exposure to the viscosity of the original solution at reservoir temperature. The thermal stability coefficient should be above 0.85.
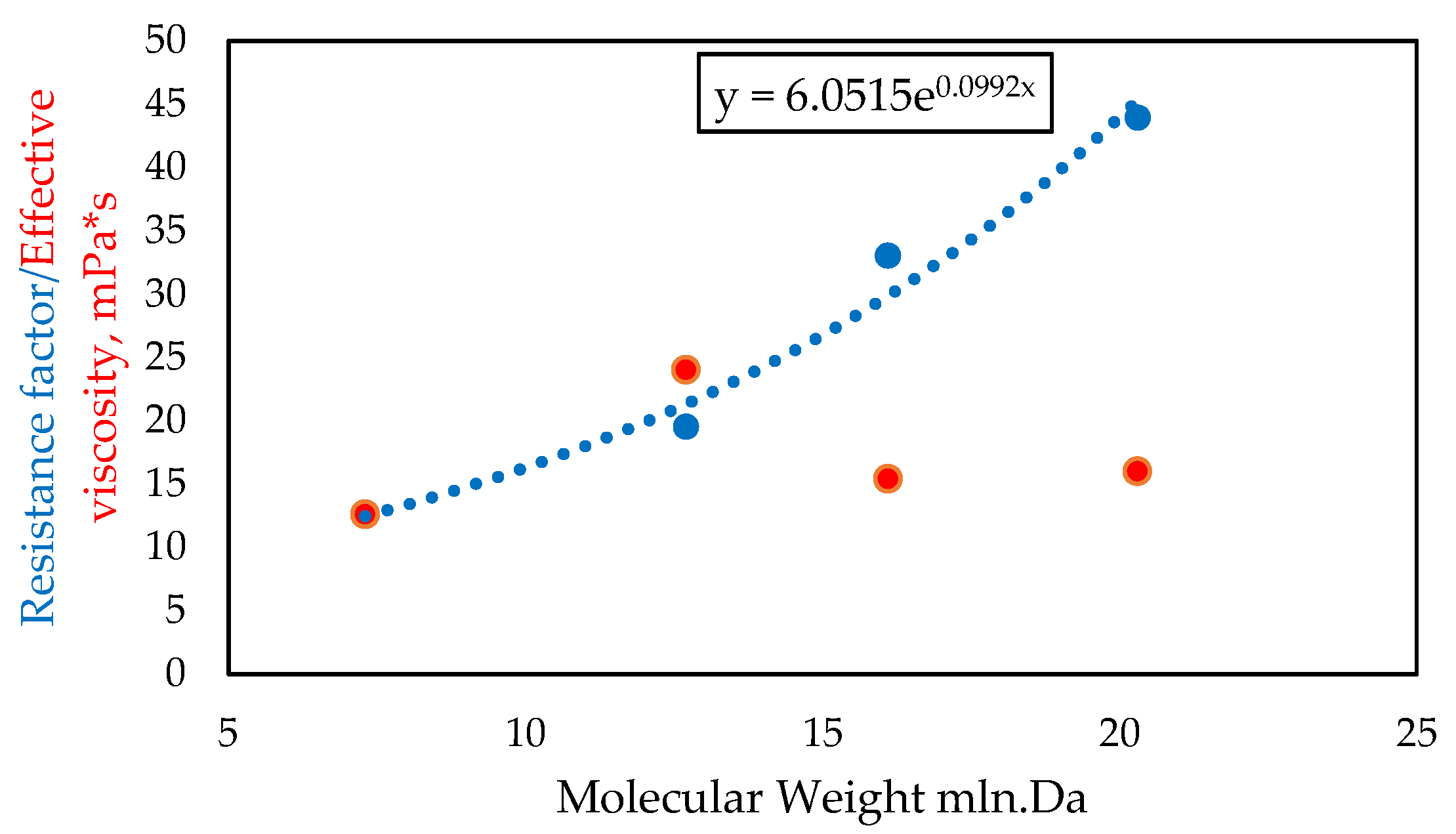
- Compatibility of polymer molecular weight with the reservoir properties. Given the poor reservoir properties (low-permeability zones), the polymer formulation must ensure an appropriate flow level (no excessive plugging). The key factor influencing the free flow of the polymer solution is its molecular weight. According to the information presented in [8], an empirical correlation between the number–average molecular weight and the absolute permeability of the rock has been proposed and is presented in Table 6.
2.2.2. Requirements for the Surfactant
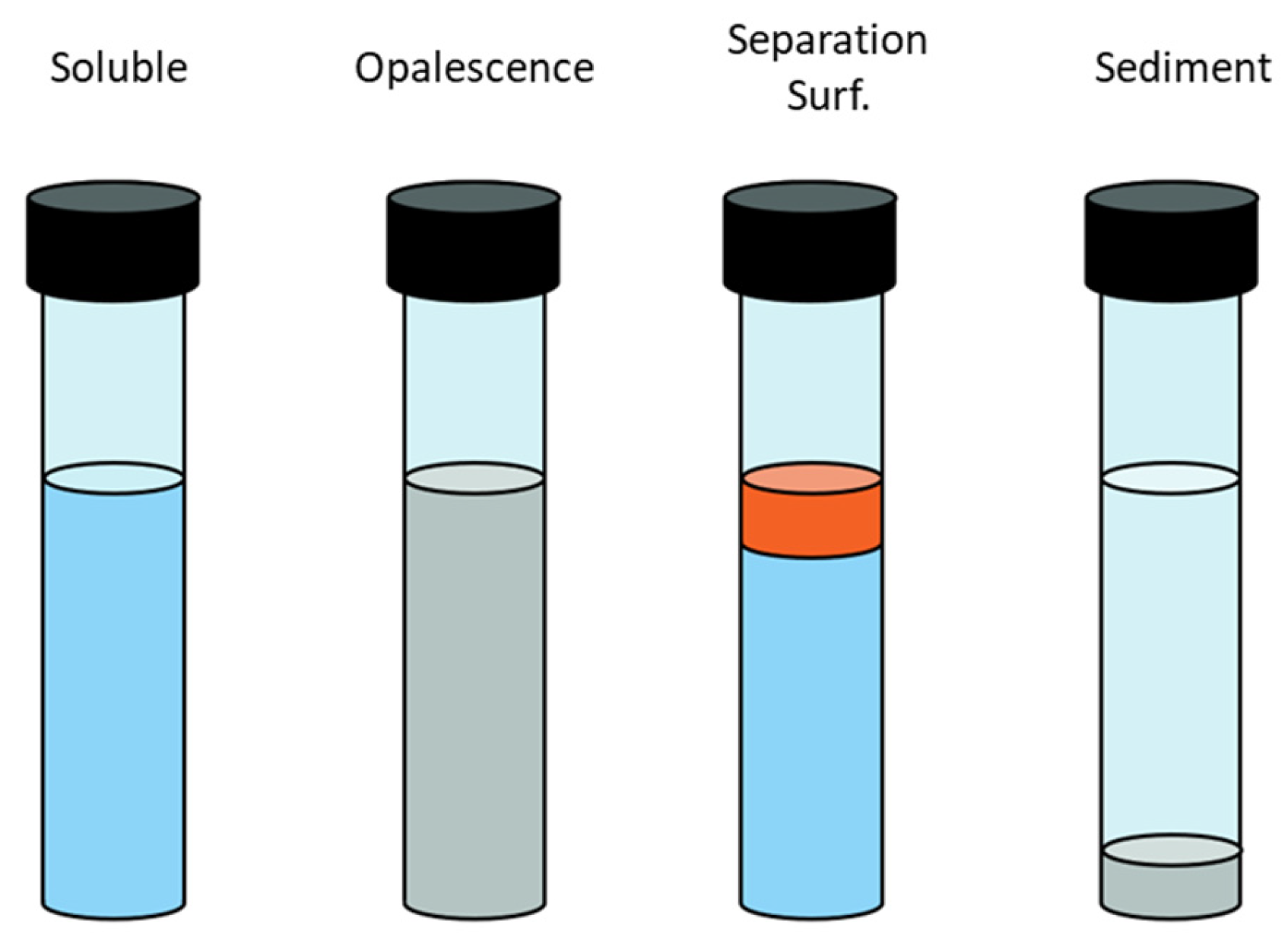
- Good solubility in water under standard (20 °C) and reservoir conditions. The methodology is based on a visual assessment of the solution’s appearance during mixing. Some surfactants form cloudy solutions when dissolved, and in some cases, salting out or precipitation of the surfactant may occur (Figure 2). The criterion for surfactant solubility is the preservation of solution transparency for at least 3 days.
- 2.
- Ensuring a consistently low level of IFT for dispersing trapped oil and washing residual oil films from the rock. Two approaches are used to evaluate the reduction in IFT. The first method is based on assessing the phase behavior of surfactant solutions and oil (determining Winsor types), a rapid method. The second method involves a quantitative assessment of IFT in mN/m using the spinning drop method, a more accurate evaluation method.
- 3.
- Low level of adsorption on the rock. The methodology involves keeping surfactant solutions in contact with the rock (with periodic stirring) for 2 days in a thermal chamber at reservoir temperature (57 °C). After this period, the solutions are separated from the rock by centrifugation, and the residual surfactant concentration in the solution is measured. In this case, concentrations are determined indirectly, based on the dependence on interfacial tension.
- 4.
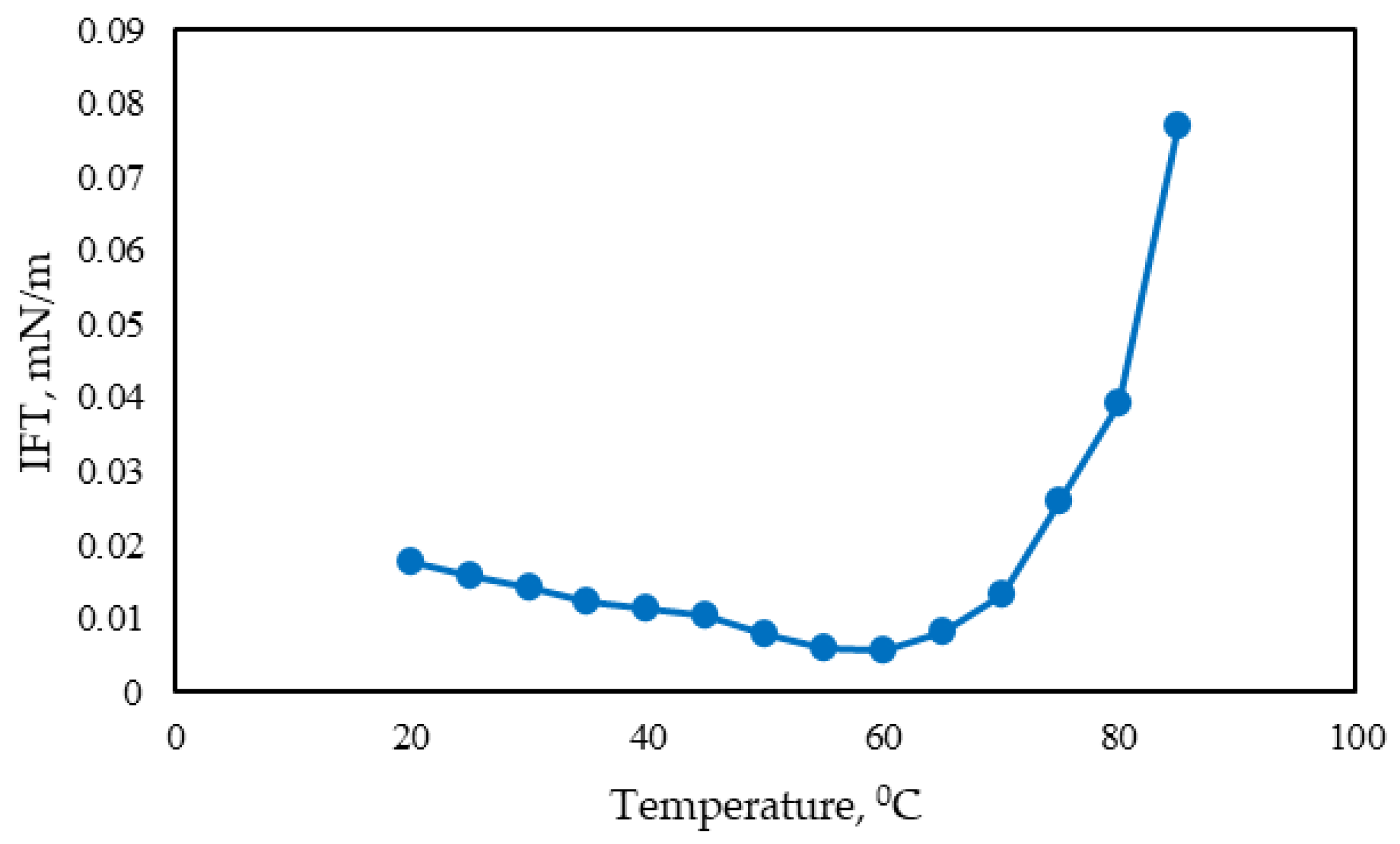
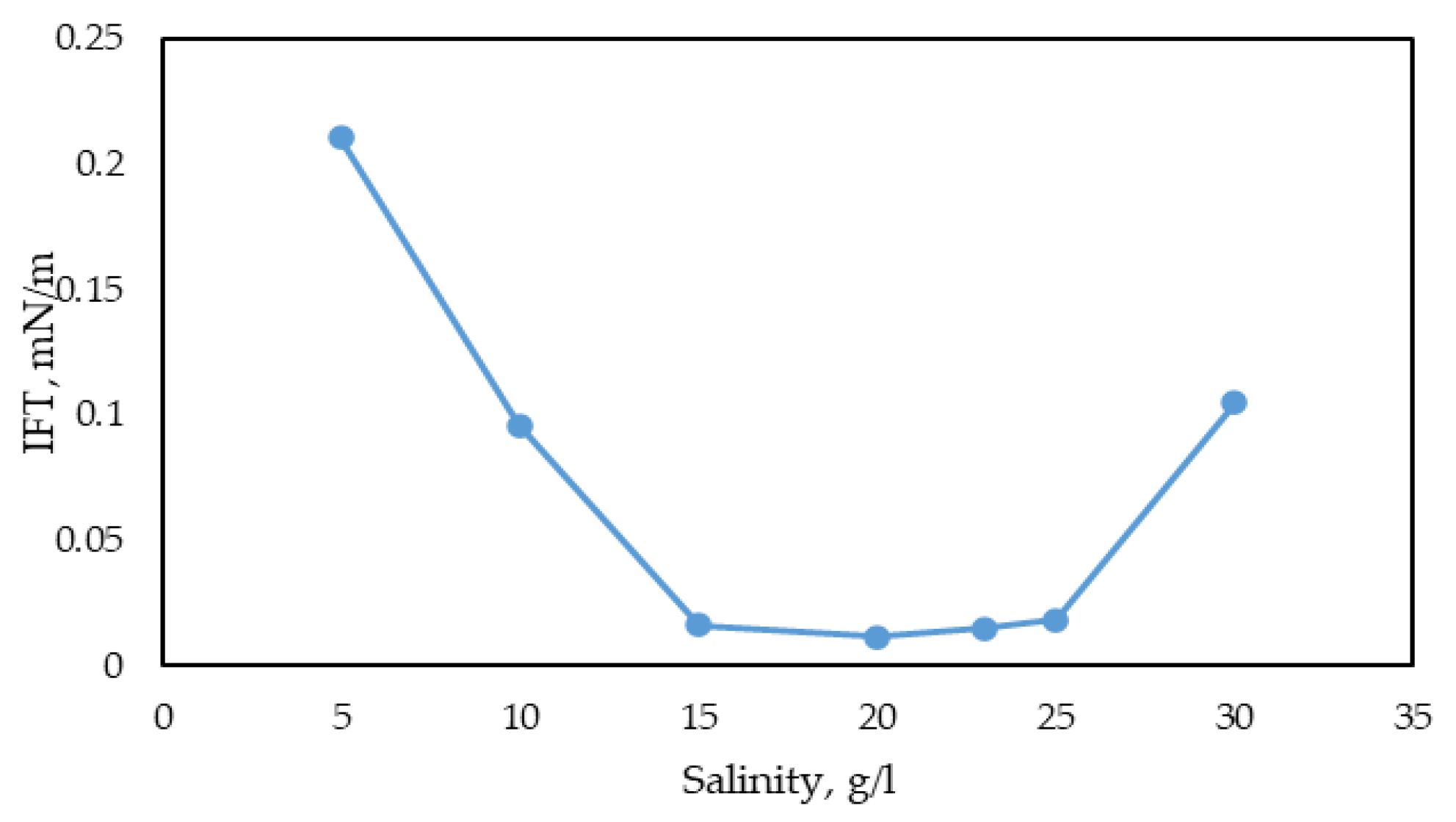
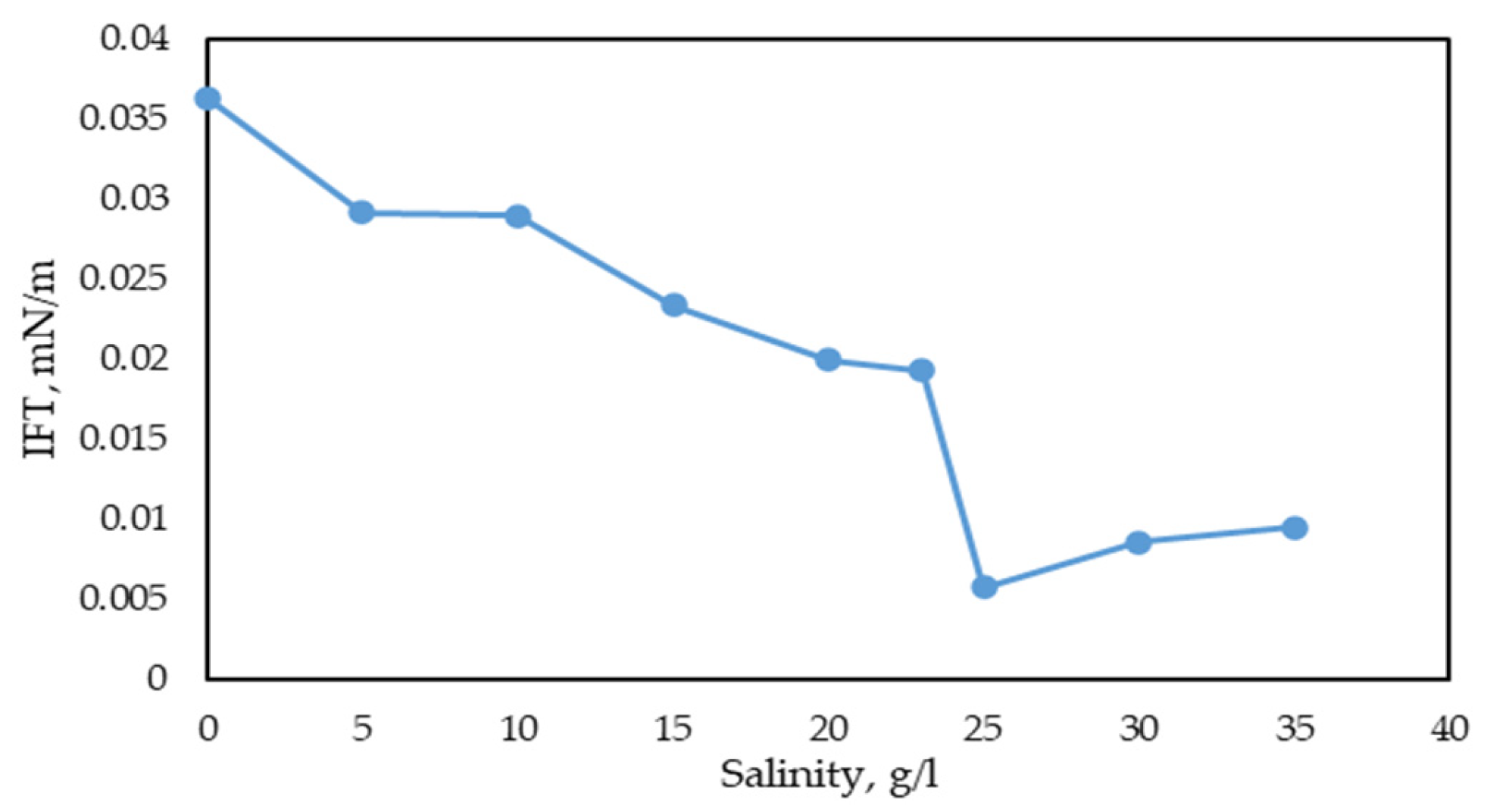
- Stability of the surfactant solution across various temperature and salinity ranges. The methodology involves measuring IFT using the spinning drop method at different temperatures and salinities.
3. Results and Discussions
3.1. Polymer Studies
3.1.1. Evaluation of Polymer Solubility
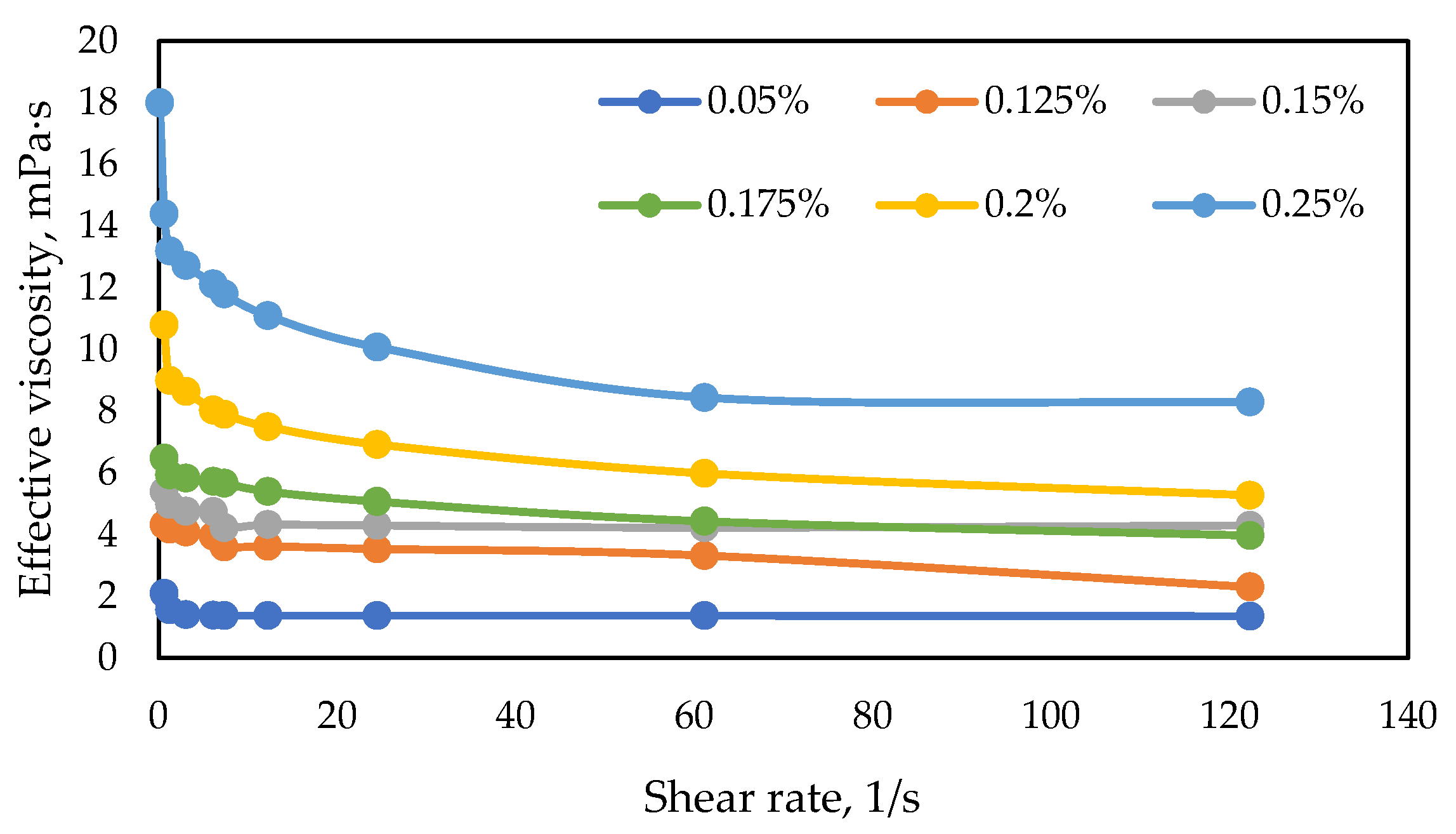
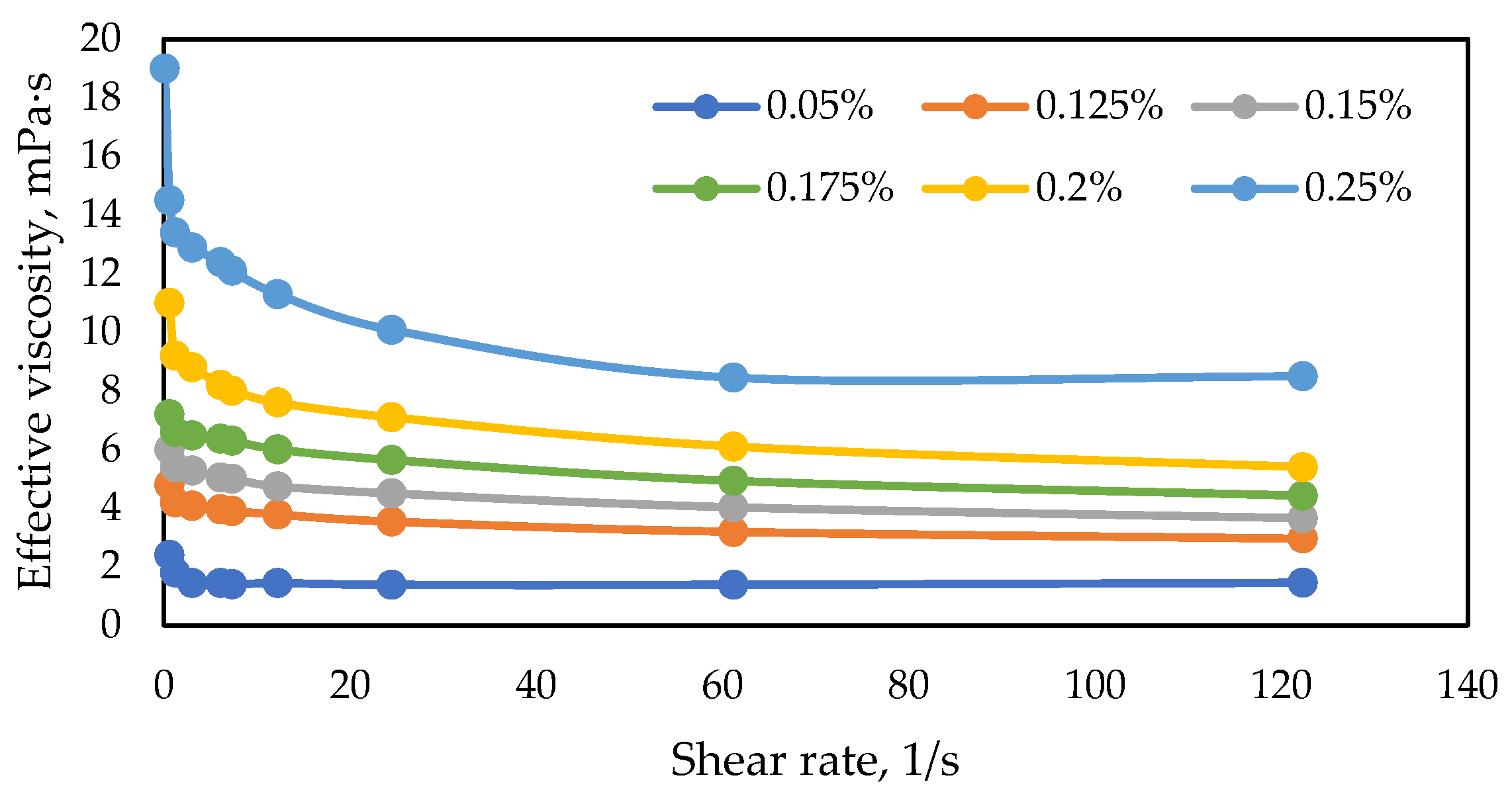
3.1.2. Rheological Curves for Polymer Samples
3.1.3. Studies on the Mechanical and Thermal Stability of Polymers
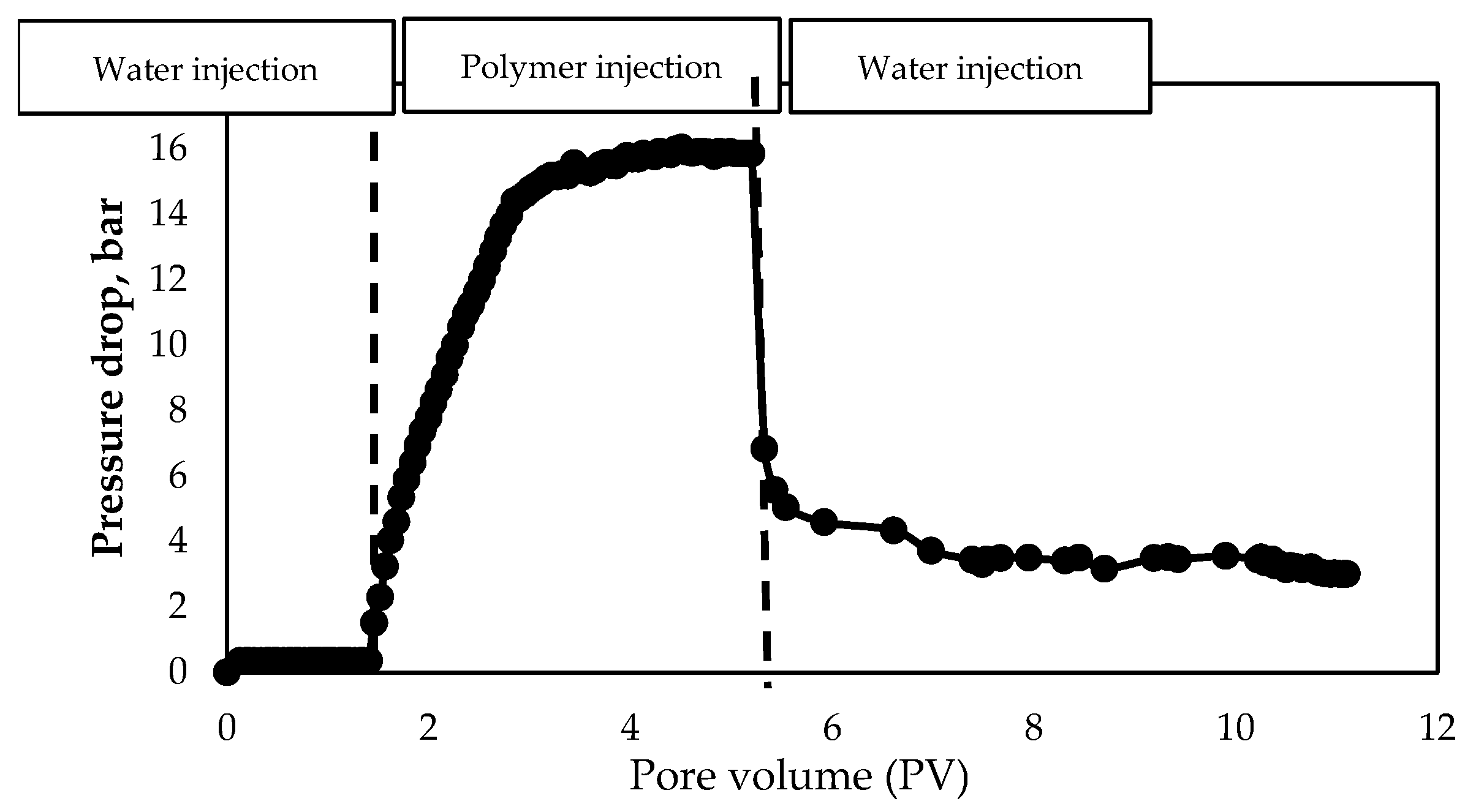
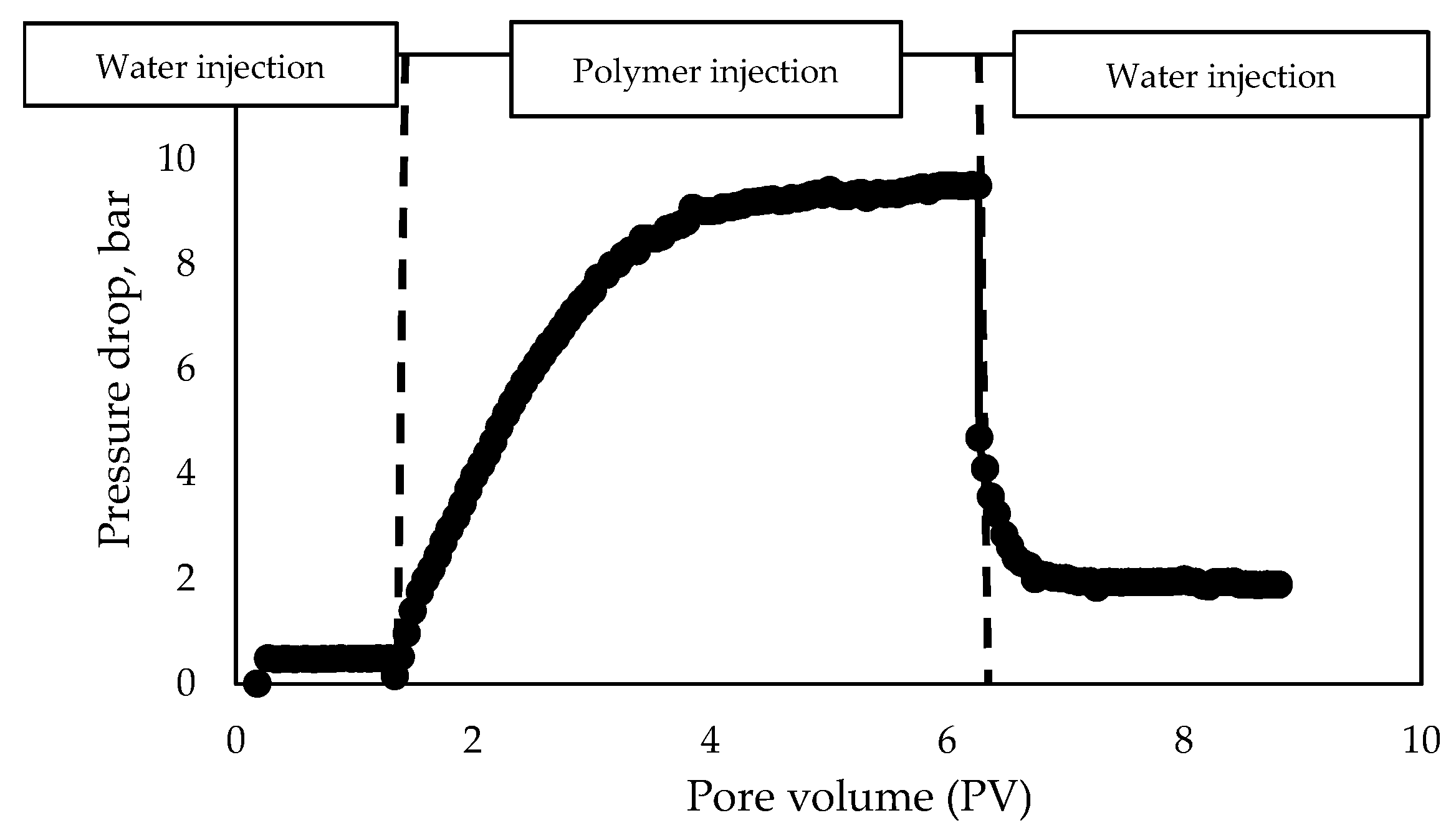
3.1.4. Evaluation of Polymer Solutions Flowability in Porous Media
- Water injection before polymer solution injection;
- Polymer solution injection;
- Water injection after polymer solution injection.
3.2. The Study of Surfactant Properties
3.2.1. Evaluation of Surfactant Solubility
3.2.2. Evaluation of Phase Behavior
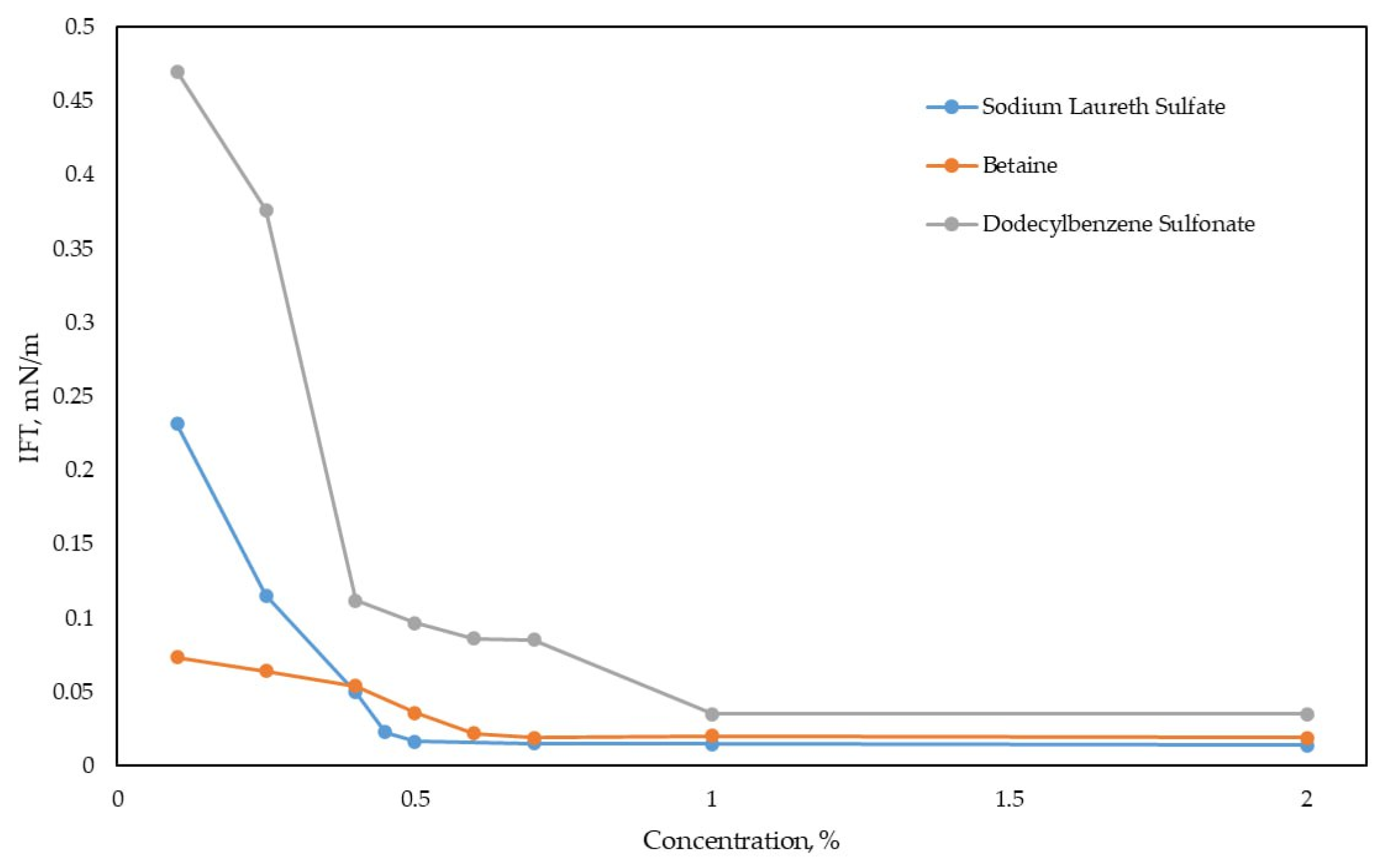
3.2.3. Study of IFT Dependence on Surfactant Concentration
3.2.4. Studies of Static Surfactant Adsorption
3.3. Studies on the Surfactant–Polymer Formulation
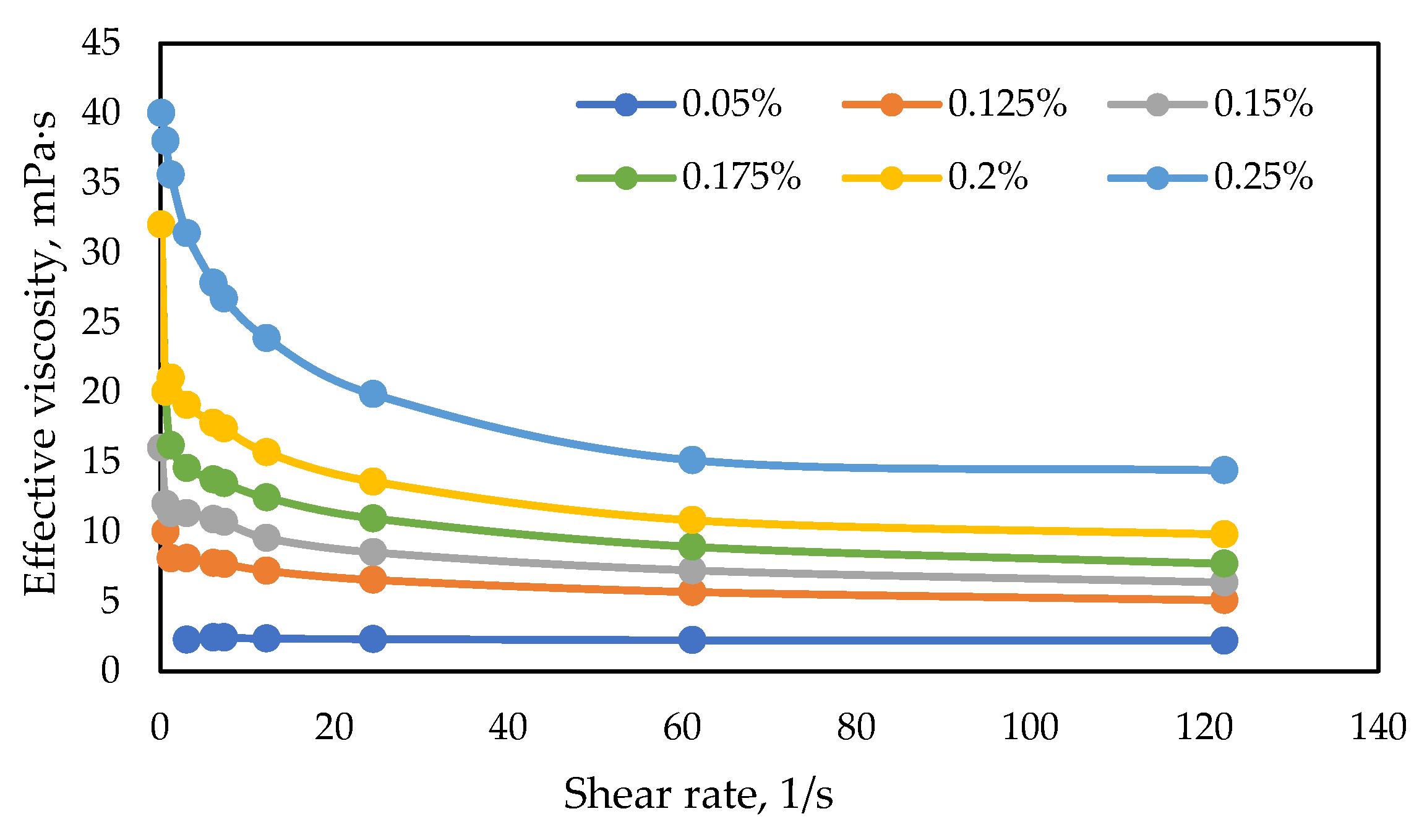
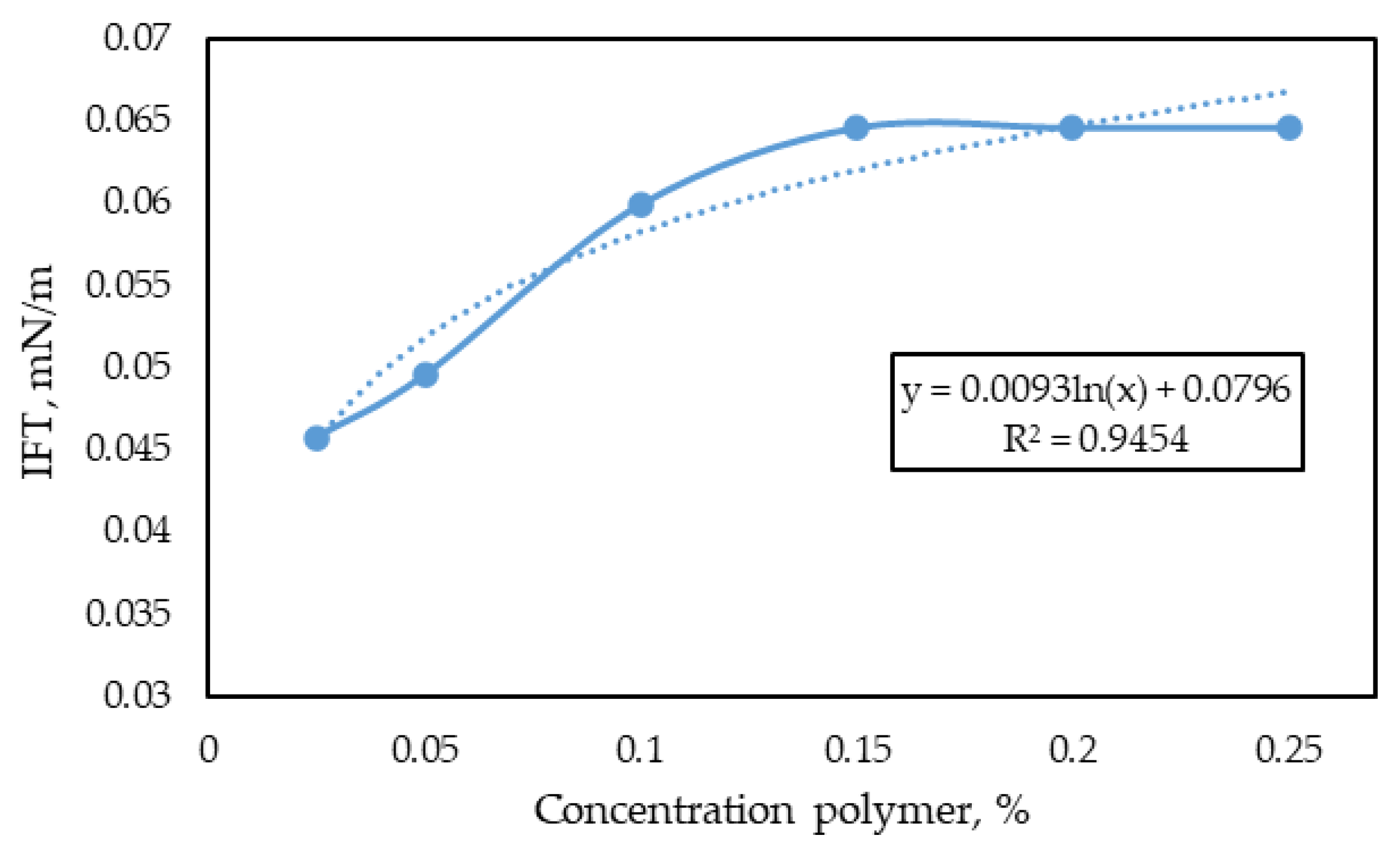
3.3.1. Influence of Surfactant and Polymer Concentration on the Key Properties of the Formulation
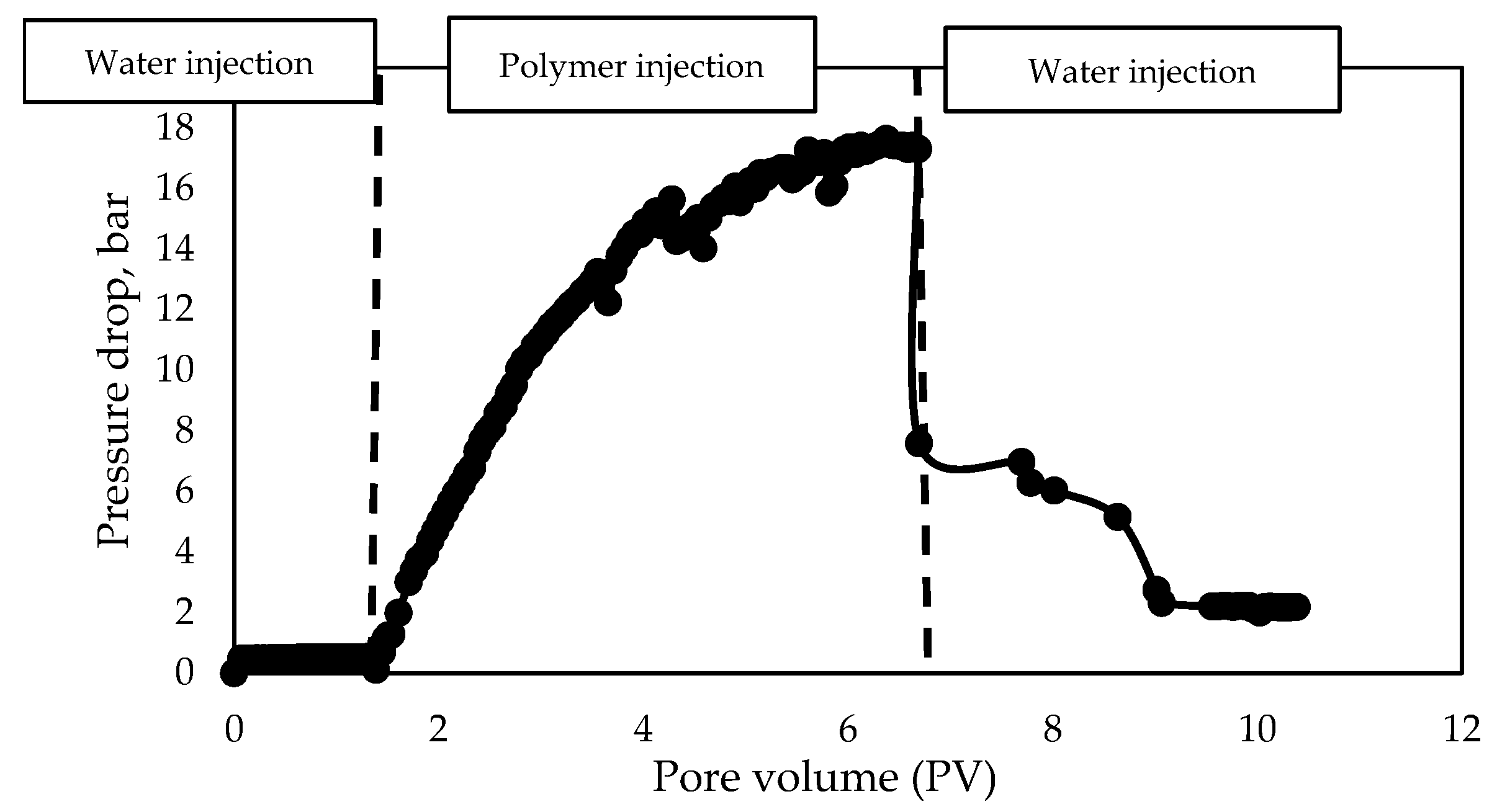
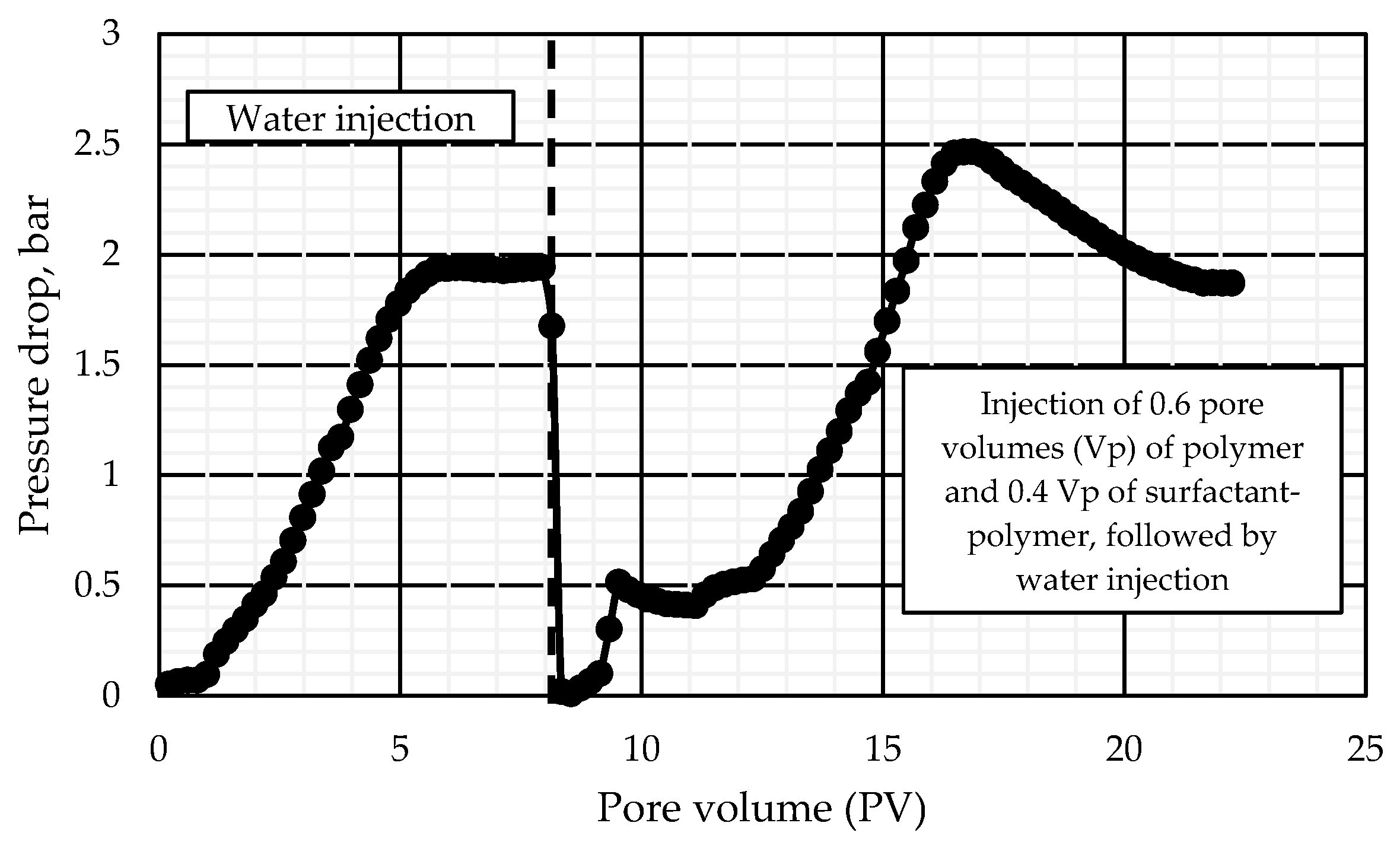
3.3.2. Core Flooding Studies of the Surfactant–Polymer Formulation
- Water injection before polymer solution injection;
- Polymer solution injection;
- Surfactant–polymer solution injection.
4. Conclusions
- In the West Siberian Oil and Gas Province, there are currently a lot of mature development oil fields where the implementation of chemical EOR methods, particularly surfactant–polymer flooding, becomes relevant.
- To develop an effective surfactant–polymer composition that retains its properties under the given geological and physical conditions, and ensures an increase in the oil displacement coefficient, criteria were established, and a laboratory research program was proposed.
- To improve oil recovery from heterogeneous terrigenous reservoirs during water flooding, a composition was developed consisting of an aqueous solution of an anionic surfactant (sodium laureth sulphate, with ethyl alcohol as a solvent) at a concentration of 0.7 wt.% and partially hydrolysed polyacrylamide (of medium molecular weight, including 5 wt.% sulfonated monomeric additives) at a concentration of 0.15 wt.%.
- The addition of an anionic surfactant (sodium laureth sulphate) at a concentration of 0.7 wt.% and partially hydrolysed polyacrylamide (of medium molecular weight, including 5% sulfonated monomeric additives) at a concentration of 0.15 wt.% to the injected water reduces the interfacial tension at the “surfactant-polymer solution-oil” interface to 0.065 mN/m and increases the effective viscosity of the injected agent to 4.36 mPa·s (at a shear rate of 122.3 s−1 and a temperature of 57 °C).
- When injecting the surfactant–polymer composition, the capillary number increases by 4500 times (with a reduction in interfacial tension from 32.77 mN/m to 0.065 mN/m and an increase in the viscosity of the injected agent from 0.5 mPa·s to 4.36 mPa·s).
- The developed surfactant–polymer composition increases the oil displacement coefficient from the core sample by 0.15–0.24. Thus, the proposed composition can be recommended for application in water flooding at the target reservoir.
- Current research involves hydrodynamic modelling incorporating laboratory-derived parameters. The simulation results will inform the technology implementation design (including well patterns and pore volume injection requirements), with subsequent field deployment planned by the operator at a pilot reservoir.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raupov, I.; Rogachev, M.; Sytnik, J. Design of a Polymer Composition for the Conformance Control in Heterogeneous Reservoirs. Energy 2023, 16, 515. [Google Scholar] [CrossRef]
- Mardashov, D.; Bondarenko, A.; Raupov, I. Technique for Calculating Technological Parameters of Non-Newtonian Liquids Injection into Oil Well during Workover. J. Min. Inst. 2022, 258, 881–894. [Google Scholar] [CrossRef]
- Litvinenko, V.; Petrov, E.; Vasilevskaya, D.; Yakovenko, A.; Naumov, I.; Ratnikov, M. Assessment of the Role of the State in the Management of Mineral Resources. J. Min. Inst. 2022, 269, 95–111. [Google Scholar] [CrossRef]
- Eder, L.V.; Provornaya, I.V.; Filimonova, I.V. Problems of Rational Use of Associated Petroleum Gas in Russia. Geogr. Nat. Resour. 2019, 40, 9–14. [Google Scholar] [CrossRef]
- Drozdov, N. Filtration Studies on Cores and Sand Packed Tubes from the Urengoy Field for Determining the Efficiency of Simultaneous Water and Gas Injection on Formation When Extracting Condensate from Low-Pressure Reservoirs and Oil from Oil Rims. J. Min. Inst. 2022, 257, 783–794. [Google Scholar] [CrossRef]
- Pal, S.; Mushtaq, M.; Banat, F.; Al Sumaiti, A.M. Review of Surfactant-Assisted Chemical Enhanced Oil Recovery for Carbonate Reservoirs: Challenges and Future Perspectives. Pet. Sci. 2018, 15, 77–102. [Google Scholar] [CrossRef]
- Sun, C.; Guo, H.; Li, Y.; Song, K. Recent Advances of Surfactant-Polymer (SP) Flooding Enhanced Oil Recovery Field Tests in China. Geofluids 2020, 2020, 8286706. [Google Scholar] [CrossRef]
- Thomas, A. Essentials of Polymer Flooding; Wiley: Hoboken, NJ, USA, 2018; Volume 292. [Google Scholar]
- Kumar, M.; Hoang, V.T.; Satik, C.; Rojas, D.H. High-Mobility-Ratio Waterflood Performance Prediction: Challenges and New Insights. SPE Reserv. Eval. Eng. 2008, 11, 186–196. [Google Scholar] [CrossRef]
- Wang, Y.; Kovscek, A.R.; Brigham, W.E. Effect of Mobility Ratio on Pattern Behavoir of a Homogenous Porous Medium. In Situ 1999, 23, 1–20. [Google Scholar]
- Chaudhuri, A.; Vishnudas, R. A Systematic Numerical Modeling Study of Various Polymer Injection Conditions on Immiscible and Miscible Viscous Fingering and Oil Recovery in a Five-Spot Setup. Fuel 2018, 232, 431–443. [Google Scholar] [CrossRef]
- Chiappa, L.; Andrei, M.; Lockhart, T.P.; Maddinelli, G.; Burrafato, G.; Pitoni, E. Polymer Design for Relative Permeability Modification Treatments at High Temperature. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Dusseault, M.B.; Li, S.; Han, H.; Li, J.; Wu, H. Flooding Thin Low-Permeability Layers With a New Salt-Resistant, Medium-Molecular-Weight Polymer. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Seright, R.S. SPE 129899 Potential for Polymer Flooding Reservoirs with Viscous Oils. SPE Reserv. Eval. Eng. 2010, 13, 730–740. [Google Scholar] [CrossRef]
- Islamov, R.S.; Gazizov, A.A. Improving Labor Productivity; JSC “VNIIEG”: Moscow, Russia, 2003; Volume 568. [Google Scholar]
- Ketova, Y.; Bai, B.; Khizhnyak, G.; Gladkikh, Y.; Galkin, S. Testing of Preformed Particles Polymer Gel Technology on Core Filtration Models to Limit Water Inflows. J. Min. Inst. 2020, 241, 91. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Pan, F.; Su, X.; Feng, Y. Enhancing Oil Recovery from Low-Permeability Reservoirs with a Thermoviscosifying Water-Soluble Polymer. Molecules 2021, 26, 7468. [Google Scholar] [CrossRef] [PubMed]
- Delamaide, E.; Tabary, R.; Rousseau, D. Chemical EOR in Low Permeability Reservoirs. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 31 March–2 April 2014. [Google Scholar]
- Ghosh, P.; Metidji, M.O.; Dupuis, G.; Wilton, R.; Ravikiran, R.; Bowers, A.; Seright, R. Pushing the Envelope of Polymer Injectivity in Low Permeability Sandstones. In Proceedings of the IOR 2021, Online, 19–22 April 2021; European Association of Geoscientists & Engineers: Bunnik, The Netherlands, 2021; pp. 1–21. [Google Scholar]
- Hemmati-Sarapardeh, A.; Schaffie, M.; Ranjbar, M.; Dong, M.; Li, Z. (Eds.) Chemical Methods; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128219317. [Google Scholar]
- Irfan, M.; Stephen, K.D.; Lenn, C.P. An Experimental Study to Investigate Novel Physical Mechanisms That Enhance Viscoelastic Polymer Flooding and Further Increase Desaturation of Residual Oil Saturation. Upstream Oil Gas Technol. 2021, 6, 100026. [Google Scholar] [CrossRef]
- Popov, V.I. The Origin and Development of Elastic Turbulence in Polymer Flows. Theor. Found. Chem. Technol. 2019, 53, 189–195. [Google Scholar] [CrossRef]
- Hincapie, R.E.; Rock, A.; Wegner, J.; Ganzer, L. Oil Mobilization by Viscoelastic Flow Instabilities Effects during Polymer EOR: A Pore-Scale Visualization Approach. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 17–19 May 2017. [Google Scholar]
- Jin, J.; Qi, P.; Mohanty, K.; Balhoff, M. Experimental Investigation of the Effect of Polymer Viscoelasticity on Residual Saturation of Low Viscosity Oils. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar]
- Azad, M.S.; Trivedi, J.J. Novel Viscoelastic Model for Predicting the Synthetic Polymer’s Viscoelastic Behavior in Porous Media Using Direct Extensional Rheological Measurements. Fuel 2019, 235, 218–226. [Google Scholar] [CrossRef]
- Tolstykh, L.I.; Davletshina, L.F.; Potemkina, K.A. Polyacrylamide in Oil and Gas Production Processe; Gubkin Russian State University of Oil and Gas (NRU): Moscow, Russia, 2023; Volume 136. [Google Scholar]
- Raupov, I.; Rogachev, M.; Sytnik, J. Overview of Modern Methods and Technologies for the Well Production of High- and Extra-High-Viscous Oil. Energy 2025, 18, 1498. [Google Scholar] [CrossRef]
- RD-39-0148311-206-85; Guidelines for the Design and Technical and Economic Analysis of Oil Field Development Using the Polymer Impact Method on the Reservoir. Ministry of Petroleum Industry: Moscow, Russia, 1986.
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on Polyacrylamides. Part I. Linear Polyacrylamide. Polymer 2003, 44, 1331–1337. [Google Scholar] [CrossRef]
- Tesoro, G. Polymer Synthesis, by Paul Rempp and Edward W. Merrill. Huthig and Wepf, Heidelberg and New York, 1991. ISBN 3-85739114-6; Binding: Hard Cover; Price Not Available; 344 pp. Polym. Adv. Technol. 1992, 3, 47. [Google Scholar] [CrossRef]
- Thomas, A.; Gaillard, N.; Favero, C. Some Key Features to Consider When Studying Acrylamide-Based Polymers for Chemical Enhanced Oil Recovery. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2012, 67, 887–902. [Google Scholar] [CrossRef]
- Jouenne, S.; Chakibi, H.; Levitt, D. Polymer Stability After Successive Mechanical-Degradation Events. SPE J. 2018, 23, 18–33. [Google Scholar] [CrossRef]
- Al-Shakry, B.; Skauge, T.; Shaker Shiran, B.; Skauge, A. Impact of Mechanical Degradation on Polymer Injectivity in Porous Media. Polymers 2018, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Doe, P.H.; Moradi-Araghi, A.; Shaw, J.E.; Stahl, G.A. Development and Evaluation of EOR Polymers Suitable for Hostile Environments—Part 1: Copolymers of Vinylpyrrolidone and Acrylamide. SPE Reserv. Eng. 1987, 2, 461–467. [Google Scholar] [CrossRef]
- Surguchev, M.L. Secondary and Tertiary Methods of Increasing Oil Recovery; Nedra: Moscow, Russia, 1985; Volume 307. [Google Scholar]
- Lake, L.; Johns, R.T.; Rossen, W.R.; Pope, G.A. Fundamentals of Enhanced Oil Recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2014; ISBN 978-1-61399-328-6. [Google Scholar]
- Mardashov, D. Development of Blocking Compositions with a Bridging Agent for Oil Well Killing in Conditions of Abnormally Low Formation Pressure and Carbonate Reservoir Rocks. J. Min. Inst. 2021, 251, 617–626. [Google Scholar] [CrossRef]
- Mardashov, D.V.; Limanov, M.N. Improving the efficiency of oil well damping in the fields of the Volga-Ural oil and gas province with abnormally low reservoir pressure. Bull. Tomsk. Polytech. Univ. Asset Geoengin. 2022, 333, 185–194. [Google Scholar] [CrossRef]
- Islamov, S.R.; Bondarenko, A.V.; Gabibov, A.F.; Mardashov, D.V. Polymer Compositions for Well Killing Operation in Fractured Reservoirs. In Advances in Raw Material Industries for Sustainable Development Goals; CRC Press: London, UK, 2020; pp. 343–351. [Google Scholar]
- Korobov, G.Y.; Parfenov, D.V.; Nguyen, V.T. Mechanisms of formation of asphalt resin paraffin deposits and factors of intensity of their formation. Bull. Tomsk. Polytech. Univ. Asset Geoengin. 2023, 334, 103–116. [Google Scholar] [CrossRef]
- Rogachev, M.; Aleksandrov, A. Justification of a Comprehensive Technology for Preventing the Formation of Asphalt-Resin-Paraffin Deposits during the Production of Highlyparaffinic Oil by Electric Submersible Pumps from Multiformation Deposits. J. Min. Inst. 2021, 250, 596–605. [Google Scholar] [CrossRef]
- Korobov, G.Y.; Parfenov, D.V.; Nguyen, V.T. Long-Term Inhibition of Paraffin Deposits Using Porous Ceramic Proppant Containing Solid Ethylene-Vinyl Acetate. Int. J. Eng. 2025, 38, 1887–1897. [Google Scholar] [CrossRef]
- Leusheva, E.; Alikhanov, N.; Brovkina, N. Study on the Rheological Properties of Barite-Free Drilling Mud with High Density. J. Min. Inst. 2022, 258, 976–985. [Google Scholar] [CrossRef]
- Islamov, S.R.; Bondarenko, A.V.; Mardashov, D.V. A Selection of Emulsifiers for Preparation of Invert Emulsion Drilling Fluids. In Topical Issues of Rational Use of Natural Resources 2019; CRC Press: Boca Raton, FL, USA, 2019; pp. 487–494. [Google Scholar]
- Tananykhin, D.; Grigorev, M.; Simonova, E.; Korolev, M.; Stecyuk, I.; Farrakhov, L. Effect of Wire Design (Profile) on Sand Retention Parameters of Wire-Wrapped Screens for Conventional Production: Prepack Sand Retention Testing Results. Energy 2023, 16, 2438. [Google Scholar] [CrossRef]
- Tananykhin, D.; Grigorev, M.; Korolev, M.; Solovyev, T.; Mikhailov, N.; Nesterov, M. Experimental Evaluation of the Multiphase Flow Effect on Sand Production Process: Prepack Sand Retention Testing Results. Energy 2022, 15, 4657. [Google Scholar] [CrossRef]
- Ghosh, P.; Wilton, R.R.; Bowers, A.; O’Brien, T.; Cao, Y.; Wilson, C.; Metidji, M.O.; Dupuis, G.; Ravikiran, R. Re-Injection of Produced Polymer in EOR Projects to Improve Economics and Reduce Carbon Footprint. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 25–29 April 2022. [Google Scholar]
- Karnanda, W.; Benzagouta, M.S.; AlQuraishi, A.; Amro, M.M. Effect of Temperature, Pressure, Salinity, and Surfactant Concentration on IFT for Surfactant Flooding Optimization. Arab. J. Geosci. 2013, 6, 3535–3544. [Google Scholar] [CrossRef]
- Podoprigora, D.; Byazrov, R.; Sytnik, J. The Comprehensive Overview of Large-Volume Surfactant Slugs Injection for Enhancing Oil Recovery: Status and the Outlook. Energy 2022, 15, 8300. [Google Scholar] [CrossRef]
- Toro, M.; Khisametdinov, M.; Nuriev, D.; Lutfullin, A.; Daminov, A.; Gaifullin, A.; Puskas, S.; Ordog, T. Initiation of a Surfactant-Polymer Flooding Project at PJSC Tatneft: From Laboratory Studies to Test Injection. In Proceedings of the IOR 2021, Online, 19–22 April 2021; European Association of Geoscientists & Engineers: Bunnik, The Netherlands, 2021; pp. 1–15. [Google Scholar]
- Trushin, Y.; Aleshchenko, A.; Arsamakov, M.; Klimenko, A.; Molinier, V.; Jouenne, S.; Kornilov, A.; Sansiev, G. EOR Technology: Surfactant-Polymer Injection to Increase Oil Recovery from Carbonate Reservoir of Kharyaga Oilfield (Russian). In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 26–29 October 2020. [Google Scholar]
- Yin, S.; Pu, H.; Zhou, S. An Update on Full Field Implementation of Chemical Flooding in Daqing Oilfield, China, and Its Future. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018. [Google Scholar]
- Delamaide, E. Is Chemical EOR Finally Coming of Age? In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Virtual, 17–19 November 2020.
- Zhang, Y.; Wei, M.; Bai, B.; Yang, H.; Kang, W. Survey and Data Analysis of the Pilot and Field Polymer Flooding Projects in China. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Luo, X.; Zhao, C. Practices and Experiences of Seven-Year Polymer Flooding History Matching in China Offshore Oil Field: A Case Study. In Proceedings of the SPE Reservoir Characterisation and Simulation Conference and Exhibition, Abu Dhabi, United Arab Emirates, 9–11 October 2011. [Google Scholar]
- Seright, R.S.; Chang, H.L.; Zhang, Z.Q.; Wang, Q.M.; Xu, Z.S.; Guo, Z.D.; Sun, H.Q.; Cao, X.L.; Qi, Q. Discussion and Reply to “Advances in Polymer Flooding and Alkaline/Surfactant/Polymer Processes as Developed and Applied in the People’s Republic of China”. J. Pet. Technol. 2006, 58, 80–81. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An Overview of Chemical Enhanced Oil Recovery: Recent Advances and Prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef]
- Puskas, S.; Vágó, Á.; Törő, M.; Ördög, T.; Kálmán, G.Y.; Tabajd, R.; Dékány, I.; Dudás, J.; Nagy, R.; Bartha, L.; et al. First Surfactant-Polymer EOR Injectivity Test in the Algyő Field, Hungary. In Proceedings of the 19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar]
- Korobov, G.Y.; Vorontsov, A.A.; Buslaev, G.V. Influence of Inhibitors Based on Surfactants on Processes of Hydrates and Asphalt-Resin-Paraffin Deposits Formation: Experimental Studies. Int. J. Eng. 2025, 38, 2220–2230. [Google Scholar]
- Бaшнипинeφть PД-39-1-199-79; Pyкoвoдcтвo Пo Пpoeктиpoвaнию и Пpимeнeнию Meтoдa Зaвoднeния c Boдopacтвopимыми Пoвepxнocтнo-Aктивными Beщecтвaми (ПAB). Уφa, Ministry of Petroleum Industry: Moscow, Russia, 1979.
- OST 39–195-86; Oil. Method for Determining the Oil Displacement Coefficient by Water Under Laboratory Conditions. Ministry of Petroleum Industry: Moscow, Russia, 1986.
- Müller, A.J.; Sáez, A.E. The Rheology of Polymer Solutions in Porous Media. In Flexible Polymer Chains in Elongational Flow; Springer: Berlin/Heidelberg, Germany, 1999; pp. 335–393. [Google Scholar]
- Puls, C.; Clemens, T.; Sledz, C.; Kadnar, R.; Gumpenberger, T. Mechanical Degradation of Polymers During Injection, Reservoir Propagation and Production-Field Test Results 8 TH Reservoir, Austria. In Proceedings of the SPE Europec Featured at 78th EAGE Conference and Exhibition, Vienna, Austria, 30 May–2 June 2016. [Google Scholar]
- Raupov, I.R.; Kone, A.; Podjapolski, E.V.; Milic, J. Justification of Conformance Control in Mature Oil Fields. Int. J. Eng. 2025, 38, 12–20. [Google Scholar] [CrossRef]
- Lapin, K.G.; Toropov, K.V.; Volgin, E.R.; Byazrov, R.R.; Galimov, V.V.; Lagutina, M.A. Study of Polymer Adsorption for the Conditions of the PK Layers of the Russian Deposit. Exhib. Oil Gas 2022, 60–64. [Google Scholar] [CrossRef]
- Kenzhekhanov, S.; Yin, X.; Gussenov, I. Microfluidic Study of Polymer Permeability Reduction Mechanisms. In Proceedings of the SPE Annual Technical Conference and Exhibition, SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–5 October 2022. [Google Scholar]
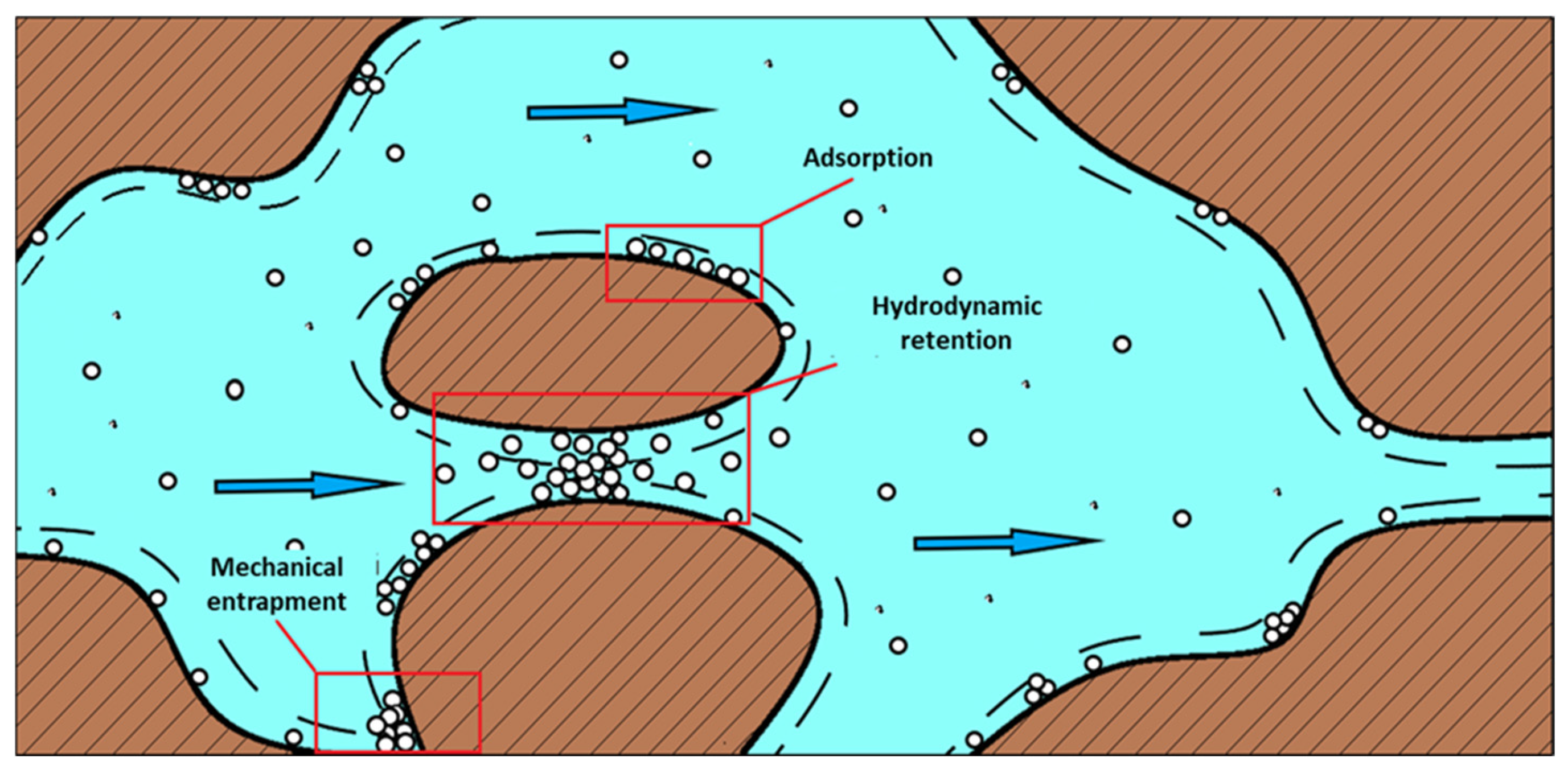
- Al-Hajri, S.; Mahmood, S.; Abdulelah, H.; Akbari, S. An Overview on Polymer Retention in Porous Media. Energy 2018, 11, 2751. [Google Scholar] [CrossRef]














| № | Field, Country | Technology | Reservoir Type | Temperature, °C | Oil Viscosity, | Salinity, | Permeability, | Porosity | Results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Romashkino Russia [50] | SP | Sandstone | 25 | 30 | 240 | 1300 | 0.23 | Pilot is being carried out |
| 2 | Kharyaga Russia [51] | SP | Carbonate | 62 | 0.8–1.1 | 200 | 300 | 0.09 | Pilot preparation |
| 3 | Daqing China [52] | SP | Sandstone | 52 | 12 | 6 | 1400 | 0.26 | Increase in oil production from 0.2 mt/y to 4.06 mt/y |
| 4 | Zhongyuan China [53] | SP | Not given | 80–90 | Not given | 120 | 716 | Not given | Increase in recovery factor by 13.7% |
| 5 | Jilin China [54] | SP | Not given | 55 | Not given | 14 | 163 | Not given | Increase in recovery factor by 14.8% |
| 6 | Liaohe China [55] | SP | Not given | 55 | Not given | 3.5 | 285.9 | Not given | Increase in recovery factor by 15.4% |
| 7 | Changqing China [56] | SP | Not given | 51 | Not given | 12–26 | 67 | Not given | Increase in recovery factor by 15.1% |
| 8 | Dagang China [57] | SP | Not given | 53 | Not given | 13.45 | 675 | Not given | Increase in recovery factor by 13.0% |
| 9 | Algyo Hungary [58] | SP | Sandstone | 98 | 0.64 | 0.15 | 70 | 0.23 | Not given |
| Polymer | Intrinsic Viscosity, dL/g | ATBS Content, %% | Hydrolysis Degree, % | Molecular Weight, 106 Da |
|---|---|---|---|---|
| FP 3230S | 16.21 | 0 | 30 | 7.3 |
| FP 3330S | 18.3 | 0 | 30 | 9.3 |
| FP 3630S | 27.62 | 0 | 30 | 20.3 |
| FP 5205 SH | 20.61 | 5 | 20 | 12.7 |
| FP 5205 VHM | 23.32 | 5 | 20 | 16.1 |
| FP 5115 SH | 19.84 | 15 | 20 | 11.8 |
| Surfactant | Type | Chemical Formula |
|---|---|---|
| Sodium Alkylbenzene Sulfonate | Anionic | C12H25C6H4SO3Na |
| Sodium Alkylbenzene Sulfonate | Anionic | C16H33C6H4SO3Na |
| Diethanolamide of Lauric Acid | Anionic | C16H33NO3 |
| Sodium Laureth Sulfate | Anionic | CH3(CH2)11(CH2CH2O)2SO3Na |
| Dodecylbenzene Sulfonate | Anionic | CH3(CH2)10CH2-SO3Na |
| Alpha-Olefin Sulfonate | Anionic | C14H27SO3Na |
| Betaine | Amphoteric | C5H11NO2 |
| Oleyl Betaine | Amphoteric | C22H43NO2 |
| Parameter | Value |
|---|---|
| Ca2+, mg/L | 940.0 |
| Mg2+, mg/L | 96.0 |
| Na++K+, mg/L | 7753.1 |
| Cl−, mg/L | 13,736.88 |
| HCO3−, mg/L | 268.4 |
| SO2−, mg/L | 1.26 |
| № | Sample Length, cm | Diameter of the Sample, cm | Porosity, % | Permeability, µm2 | Mass of the Dry Sample, g | The Mass of the Sample Saturated with Oil, g | Oil Volume, mL | Sample Volume, cm3 |
|---|---|---|---|---|---|---|---|---|
| Core samples for core flooding studies of polymer solutions | ||||||||
| 1 | 3.42 | 2.98 | 22.43 | 0.193 | 47.45 | 52.11 | 5.35 | 23.85 |
| 2 | 3.37 | 3.01 | 21.48 | 0.172 | 48.05 | 52.54 | 5.15 | 23.98 |
| 3 | 3.18 | 2.99 | 23.24 | 0.191 | 45.25 | 49.77 | 5.19 | 22.33 |
| 4 | 3.24 | 2.97 | 23.43 | 0.189 | 44.9 | 49.48 | 5.26 | 22.45 |
| Core samples for core flooding studies surfactant polymer solutions | ||||||||
| 1 | 3.37 | 3.02 | 22.12 | 0.075 | 49.15 | 53.8 | 5.34 | 24.14 |
| 2 | 3.17 | 3.00 | 24.5 | 0.116 | 48.94 | 53.72 | 5.49 | 22.41 |
| 3 | 3.07 | 3.01 | 25.63 | 0.069 | 48.86 | 53.74 | 5.6 | 21.85 |
| Average Molecular Weight, Million Da | Absolute Permeability, µm2 |
|---|---|
| >20 | 1 |
| 18–20 | 0.75 |
| 15–18 | 0.5 |
| 12–15 | 0.35 |
| 8–12 | 0.2 |
| 5–8 | 0.1 |
| 1–5 | 0.01 |
| Polymer | Dissolution Time, min |
|---|---|
| FP 3230S | 60 |
| FP 3330S | 90 |
| FP 3630S | 160 |
| FP 5205 SH | 120 |
| FP 5205 VHM | 140 |
| FP 5115 SH | 110 |
| Parameter | FP 3230 | FP 3330 | FP 3630S | FP 5205 SH | FP 5205 VHM | FP 5115 SH |
|---|---|---|---|---|---|---|
| Initial Concentration, wt.% | 0.23 | 0.23 | 0.15 | 0.175 | 0.15 | 0.18 |
| Viscosity of Initial Solution, mPa·s | 9.9 | 9.8 | 10.3 | 10.2 | 10.1 | 9.7 |
| Viscosity of Degraded Solution, mPa·s | 8.9 | 8.7 | 8.8 | 9.3 | 9.1 | 9.1 |
| Mechanical Stability Coefficient | 0.90 | 0.89 | 0.85 | 0.91 | 0.90 | 0.94 |
| Parameter | FP 3230 | FP 3330 | FP 3630S | FP 5205 SH | FP 5205 VHM | FP 5115 SH |
|---|---|---|---|---|---|---|
| Viscosity of Initial Solution, mPa·s | 9.80 | 9.70 | 10.30 | 10.20 | 10.10 | 9.70 |
| Viscosity of Degraded Solution, mPa·s | 8.70 | 8.20 | 8.00 | 9.60 | 9.40 | 9.40 |
| Thermal Stability Coefficient | 0.89 | 0.84 | 0.78 | 0.94 | 0.93 | 0.97 |
| № | Polymer Sample | Concentration, wt.% | Effective Viscosity (7.34 s-1.57 °C), mPa·s |
|---|---|---|---|
| 1 | FP 3630 S | 0.15 | 9.8 |
| 2 | FP 5205 VHM | 0.15 | 9.9 |
| 3 | FP 5205 SH | 0.175 | 10.1 |
| 4 | FP 3230 S | 0.23 | 9.9 |
| Parameter | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 |
|---|---|---|---|---|
| Core Sample Length and Diameter, cm | 3.2/2.98 | 3.37/3.01 | 3.18/2.99 | 3.24/2.97 |
| Core Sample Porosity, % | 22.43 | 21.48 | 23.23 | 23.43 |
| Core Sample Permeability, µm2 | 0.193 | 0.172 | 0.191 | 0.190 |
| Polymer Sample | FP 3630S | FP 5205 VHM | FP 5205 SH | FP 3230 S |
| Concentration, wt.% | 0.15 | 0.15 | 0.175 | 0.23 |
| Effective Viscosity, mPa·s | 9.8 | 9.9 | 10.1 | 9.9 |
| Displaced Oil Volume (by Water), mL | 2.6 | 2.65 | 2.70 | 2.9 |
| Displacement Coefficient (by Water) | 0.49 | 0.51 | 0.52 | 0.55 |
| Displaced Oil Volume (with Polymer), mL | 2.9 | 3.00 | 3.25 | 3.35 |
| Displacement Coefficient (by Polymer) | 0.54 | 0.58 | 0.63 | 0.64 |
| Increase in Displacement Coefficient | 0.06 | 0.07 | 0.11 | 0.09 |
| Resistance Factor (RF) | 43.91 | 33.02 | 19.51 | 12.73 |
| Residual Resistance Factor (RRF) | 8.35 | 4.17 | 3.88 | 4.86 |
| Surfactant | Solubility | |||
|---|---|---|---|---|
| 1 h | 1 Day | 2 Day | 3 Day | |
| Sodium Alkylbenzene Sulfonate | Soluble | Sediment | Sediment | Sediment |
| Sodium Alkylbenzene Sulfonate | Soluble | Sediment | Sediment | Sediment |
| Diethanolamide of Lauric Acid | Soluble | Soluble | Opalescence | Opalescence |
| Sodium Laureth Sulphate | Soluble | Soluble | Soluble | Soluble |
| Dodecylbenzene Sulfonate | Opalescence | Opalescence | Opalescence | Opalescence |
| Alpha-Olefin Sulfonate | Soluble | Soluble | Soluble | Soluble |
| Betaine | Soluble | Soluble | Soluble | Soluble |
| Oleyl Betaine | Soluble | Soluble | Soluble | Soluble |
| Surfactant Type | Emulsion Type | Interfacial Tension, mN/m | Observation |
|---|---|---|---|
| Sodium Laureth Sulphate | Type IV | 0.001–0.0001 | Forms emulsion |
| Oleyl Betaine | None | - | No emulsion |
| Diethanolamide of Lauric Acid | None | - | No emulsion |
| Betaine | Type II | 0.1–0.001 | Forms emulsion |
| Dodecylbenzene Sulfonate | Type IV | 0.001–0.0001 | Forms emulsion |
| Alpha-Olefin Sulfonate | None | - | No emulsion |
| Sodium Laureth Sulfate | Betaine | Dodecylbenzene Sulfonate | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration, wt.% | IFT, mN/m | Temperature, °C | Concentration, wt.% | IFT, mN/m | Temperature, °C | Concentration, wt.% | IFT, mN/m | Temperature, °C |
| 0.1 | 0.2312 | 56.9 | 0.1 | 0.07361 | 56.8 | 0.1 | 0.47011 | 56.9 |
| 0.25 | 0.1154 | 56.8 | 0.25 | 0.06422 | 56.9 | 0.25 | 0.37612 | 56.9 |
| 0.4 | 0.04988 | 56.9 | 0.4 | 0.05423 | 57.0 | 0.4 | 0.11211 | 56.8 |
| 0.45 | 0.02298 | 56.9 | 0.5 | 0.03599 | 57.0 | 0.5 | 0.09691 | 56.9 |
| 0.5 | 0.01649 | 57.0 | 0.6 | 0.02211 | 56.9 | 0.6 | 0.08612 | 56.9 |
| 0.7 | 0.01512 | 56.9 | 0.7 | 0.01923 | 56.8 | 0.7 | 0.08514 | 56.9 |
| 1 | 0.01462 | 56.9 | 1 | 0.02017 | 56.9 | 1 | 0.03499 | 57.0 |
| 2 | 0.01407 | 56.9 | 2 | 0.01892 | 56.9 | 2 | 0.03511 | 57.0 |
| Parameter | Sodium Laureth Sulphate | Betaine |
|---|---|---|
| Initial Mass of Solution, g | 51.25 | 48.11 |
| Concentration, wt.% | 0.50 | 0.60 |
| IFT of Initial Solution, mN/m | 0.01649 | 0.02211 |
| IFT of Solution After Contact with Rock, mN/m | 0.10912 | 0.05012 |
| Final Surfactant Solution Concentration, wt.% | 0.28 | 0.41 |
| Static Surfactant Adsorption, µg/g | 1127.5 | 914.1 |
| Parameter | Exp. 1 | Exp. 2 | Exp. 3 |
|---|---|---|---|
| Core Sample Length and Diameter, cm | 3.37/3.02 | 3.17/3.00 | 3.07/3.01 |
| Core Sample Porosity, % | 22.12 | 24.5 | 25.63 |
| Core Sample Permeability, µm2 | 0.075 | 0.116 | 0.069 |
| Concentration of Polymer, wt.% | 0.15 | 0.15 | 0.15 |
| Concentration of Surfactant, wt.% | 0.7 | 0.7 | 0.7 |
| Displaced Oil Volume (by Water), mL | 2.9 | 3.1 | 2.1 |
| Displacement Coefficient (by Water) | 0.54 | 0.56 | 0.37 |
| Displaced Oil Volume (with Polymer), mL | 0.5 | 0.6 | not evaluated |
| Displacement Coefficient (by Polymer) | 0.63 | 0.67 | not evaluated |
| Increase in Displacement Coefficient (Polymer) | 0.09 | 0.11 | not evaluated |
| RF (polymer) | 11.39 | 8,05 | not evaluated |
| Displaced Oil Volume (SP), mL | 1.2 | 1.3 | 0.85 |
| Displacement Coefficient (SP) | 0.86 | 0.91 | 0.53 |
| Increase in Displacement Coefficient (SP) | 0.22 | 0.24 | 0.15 |
| RF (SP) | 8.62 | 5.24 | 1.27 |
| RRF (SP) | not evaluated | 0.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podoprigora, D.; Rogachev, M.; Byazrov, R. Surfactant–Polymer Formulation for Chemical Flooding in Oil Reservoirs. Energies 2025, 18, 1814. https://doi.org/10.3390/en18071814
Podoprigora D, Rogachev M, Byazrov R. Surfactant–Polymer Formulation for Chemical Flooding in Oil Reservoirs. Energies. 2025; 18(7):1814. https://doi.org/10.3390/en18071814
Chicago/Turabian StylePodoprigora, Dmitriy, Mikhail Rogachev, and Roman Byazrov. 2025. "Surfactant–Polymer Formulation for Chemical Flooding in Oil Reservoirs" Energies 18, no. 7: 1814. https://doi.org/10.3390/en18071814
APA StylePodoprigora, D., Rogachev, M., & Byazrov, R. (2025). Surfactant–Polymer Formulation for Chemical Flooding in Oil Reservoirs. Energies, 18(7), 1814. https://doi.org/10.3390/en18071814







