Abstract
Biogas production from lignocellulosic biomass, such as wheat and rapeseed straw, is an essential strategy for sustainable energy generation. However, the efficiency of anaerobic digestion depends on the physical characteristics of the substrate, particularly the particle size, which influences microbial accessibility and biogas yield. This study aims to optimize straw particle size for enhanced methane production by evaluating different fractionation levels. The straw was processed using a hammer mill and separated into three size fractions (2.4 mm, 1 mm) alongside non-separated and finely ground (2 mm) samples. The chemical composition was analyzed using X-ray fluorescence (XRF), and key parameters such as pH, dry matter (DM), and organic dry matter (ODM) were assessed. The results indicated that rapeseed straw had lower pH (6.05) and DM than wheat straw (7.01). Biogas yield analysis demonstrated that methane production varied with particle size. For rapeseed straw, non-separated samples achieved the highest methane yield (132.87 m3 Mg⁻1), whereas for wheat straw, methane yield decreased with increased fragmentation, with the highest yield observed for non-separated material (206.65 m3 Mg⁻1). The carbon-to-nitrogen (C/N) ratio was highest in rapeseed straw (153.82), potentially limiting microbial activity, while finer fractions had more balanced ratios. These findings highlight the importance of mechanical pretreatment in optimizing biogas production and provide insights into improving the efficiency of straw-based anaerobic digestion systems.
1. Introduction
Considering the growing demand for sustainable energy sources, increasing attention is being paid to biogas derived from lignocellulosic biomass, such as straw and agri-food waste. This type of feedstock is characterized by its significant availability and minimal impact on food markets, making it particularly attractive in the context of developing a low-emission economy [1]. At the same time, the efficient utilization of such biomass requires the continuous improvement of fermentation processes and the development of new techniques to enhance its degradability. It has to be also underlined that lignocellulosic materials (especially farmyard manure, of which Poland is the biggest producer in Europe with over 106 million tonnes per year) left on fields can generate some uncontrolled gas emissions during their decomposition [2,3].
The literature highlights the importance of biological, chemical, or physical pretreatments aimed at breaking down the structure of lignocellulosic fibers and increasing the availability of the carbohydrate fraction for fermentative microorganisms [4]. For example, milling, micronization, or thermal disintegration significantly improve biogas yield, although they often involve an additional energy input. On the other hand, chemical methods, such as acid or alkaline treatments, may lead to the formation of compounds that inhibit further degradation, necessitating precise control of process parameters. Some aggressive chemical compounds such as hydrogen peroxide can be also used to perform unconventional oxidation of the substrates [5].
One of the key aspects of efficient methane fermentation of lignocellulosic biomass is the selection of the optimal particle size of the substrate. Reducing particle size, e.g., through mechanical grinding, increases the surface-to-volume ratio, facilitating enzyme and microbial access to cellulose–hemicellulose fractions. In practice, this leads to a shorter hydrolysis phase and an increase in total methane production. However, there are limitations—particles that are too fine may cause technological issues, such as sludge compaction in the fermenter or hindered circulation [6]. Studies have shown that the optimal particle size may depend on the type of biomass—for wheat and rapeseed straw, selecting an appropriate degree of fragmentation is crucial to increasing the contact surface while avoiding rheological issues in the fermentation medium [7].
Many research centers are currently analyzing mechanical and thermal pretreatment processes for biomass, such as high-temperature extrusion, which allows for fiber structure breakdown with a relatively low energy input [8]. Such treatments significantly increase cellulose bioavailability and shorten the time required to reach maximum biogas production. However, the selection of pretreatment method, particle size reduction level, or processing time and temperature requires a detailed analysis of substrate specificity, process conditions, and economic costs. Studies on the use of energy crops, such as Silphium perfoliatum L., suggest that extrusion processing may improve the energy balance of fermentation. The results of Witaszek et al. [9] indicate that extruding this biomass at 175 °C yields up to 850.1 kWhe of electricity per ton of substrate, making it competitive with traditional fermentation feedstocks. Recently, there has also been growing interest in waste biomass, such as sorghum, which can be successfully cultivated in temperate climates, including regions located above 50° N latitude. Research indicates that sorghum waste biomass exhibits high biogas yield (437.76–494.67 m3·Mg⁻1 of fresh matter) and favorable electricity generation potential (1.00–1.12 MWh·Mg⁻1 of total solids), making it an attractive alternative substrate for biogas production [10].
All these efforts aim to enhance the potential of lignocellulosic biomass in methane fermentation processes, which could yield significant economic and environmental benefits. It should be emphasized that Poland is one of the EU countries with the greatest potential for biogas production [11,12]. However, only a small amount of the lignocellulosic substrates (including especially farmyard manure) is used for feeding biogas plants [13].
Given the crucial role of lignocellulosic biomass particle size in methane fermentation, it is essential to determine the optimal degree of fragmentation that maximizes biogas production while minimizing potential technological issues. The objective of this study is to evaluate the impact of wheat and rapeseed straw particle size on biogas and methane yield. The research considers various fraction separation levels and their influence on fermentation parameters such as the C/N ratio, pH, dry matter content, and organic matter concentration. The obtained results provide practical insights into optimizing the mechanical processing of straw, which may contribute to improving biogas production efficiency in anaerobic fermentation systems.
2. Materials and Methods
Wheat and rapeseed straw were shredded using a hammer mill type H-111/3 (Jaworzno, Poland) with an 8 mm perforated screen. The processed material was then separated using a grain cleaner (Petkus, K 294 A, Wutha-Farnroda, Germany) into three fractions using sieves with mesh sizes of 2.4 mm and 1 mm (Figure 1). Additionally, for further studies, both the unseparated material (MIX) and unseparated material were further ground in a cutting mill SM200 (Retsch, Haan, Germany) using a 2 mm sieve insert. The selected particle size ranges (<1 mm, 1–2.4 mm, and >2.4 mm) were based on the technical capabilities of the laboratory equipment used, which closely resembled the performance of industrial vibrating screens applied in biomass preprocessing. These fractions correspond to the practical size thresholds typically encountered in agricultural biogas plants and pilot-scale systems, where effective separation of fibrous material is necessary to ensure optimal fermentation conditions. Although a completely untreated raw straw sample was not included in the fermentation tests, the “MIX” samples—non-separated straw without additional fine milling—were used as reference materials to evaluate the impact of particle size fractionation. These samples reflect material that underwent minimal mechanical processing, simulating a baseline condition for practical application. Both types of straw underwent preliminary testing to determine the necessary parameters for estimating the sample size subjected to methane fermentation (inoculum-to-substrate ratio). The pH of the substrates was analyzed by weighing 20 g of the sample into an Erlenmeyer flask, adding water to reach a total weight of 200 g, and allowing the mixture to rest for 15 min. After this period, the pH value was measured using a CPC-411 pH meter. For this purpose, a laboratory dryer TIN-TF200 (PHOENIX Instrument, Berlin, Germany) was used to dry the straw at 105 °C for 24 h, and a muffle furnace L40/11 (Nabertherm, Bremen, Germany) was used to combust the samples at 550 °C for 3 h. The samples were analyzed for biogas yield according to standard methodologies (DIN 38414/S8 [14] and VDI 4630 [15]) [16,17]. The biogas yield study was conducted under standard methane fermentation conditions using a set of three-tank biofermenters [18]. Fermentation reactors with a capacity of 2 dm3 were first filled with inoculum (a dose of microorganisms from an operating biogas plant) and the shredded and separated straw samples [19]. The elemental analysis was conducted using an X-ray spectrophotometer with an SDD detector, utilizing GOLDD XRF Niton XL5 technology from Thermo Scientific (Waltham, MA, USA). The XRF analyzer operates based on measuring fluorescent (or secondary) X-ray radiation emitted by a sample excited by a primary X-ray source. Each element present in the sample generates a set of characteristic fluorescent X-ray emissions unique to that element. The emitted rays are captured by the detector, processed by a processor, and the final result provides the qualitative and quantitative composition of the sample. The carbon-to-nitrogen ratio was determined using a Flash 2000 instrument (Thermo Scientific, Waltham, MA, USA). The graphical abstract (Figure 2) presents the methodology of straw processing for biogas production, including shredding, material separation, additional milling, preliminary testing, and methane fermentation.
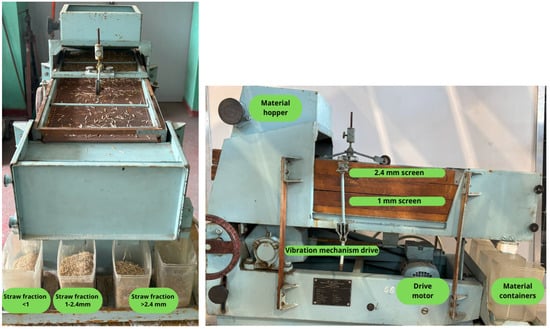
Figure 1.
Grain cleaner (Petkus).
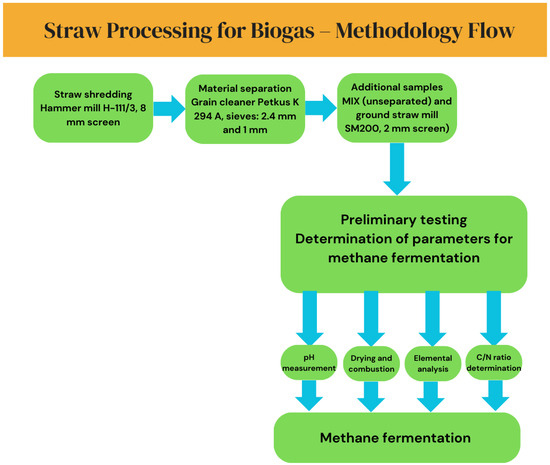
Figure 2.
Graphical abstract of the research methodology for straw processing in biogas production.
To assess the statistical significance of differences in methane and biogas yields across different particle size fractions, one-way analysis of variance (ANOVA) was performed using Statistica 13.3 software (StatSoft, Tulsa, OK, USA). The results were considered statistically significant at p < 0.05. Where appropriate, Tukey’s post hoc test was applied to determine which groups differed significantly from each other.
3. Results and Discussion
This section presents the relationships between the studied straws and their fractions. The results are presented in the form of tables and graphs. The substrate parameters were determined first. These parameters enabled the determination of the sample size necessary for biogas yield measurements. Table 1 below presents the substrate parameters. For both types of straw (rapeseed and wheat), the following parameters were analyzed: pH, dry matter, and organic dry matter.

Table 1.
Substrate parameters.
Table 1.
Substrate parameters.
| Substrate Parameters | |||
|---|---|---|---|
| Substrate | pH | Dry Mass (%) | Organic Dry Matter (%) |
| Rapeseed straw | 6.05 | 88.75 | 93.59 |
| Wheat straw | 7.01 | 90.10 | 96.18 |
Rapeseed straw exhibited a lower pH of 6.05, while wheat straw had a pH of 7.01. Additionally, rapeseed straw had lower dry matter and organic dry matter content. For rapeseed straw, these values were 88.75% dry matter and 93.59% organic dry matter, respectively. In the case of wheat straw, the values were 90.10% dry matter and 96.18% organic dry matter. The studied straws had slightly lower pH values compared with those examined by Figueiredo et al. and Halvarsson et al. [20,21]. According to these authors, the pH values were 6.8 for rapeseed straw and 7.5 for wheat straw. Similar organic dry matter values are reported by other authors. For wheat straw, the value is 95.35%, while for rapeseed straw, it is 92.15% [22,23].
Table 2 below presents the biogas yield results for the individual substrates. In the case of rapeseed straw, it was observed that non-separated straw had the highest methane content, while among the separated samples, the smallest particle size fraction exhibited the lowest methane content. For separated wheat straw, a decrease in methane content was observed with increasing substrate fragmentation, which was also noticeable in the non-separated samples. For both separated rapeseed and wheat straw, an increase in separation degree led to a significant rise in cumulative methane production and cumulative biogas yield across the entire study. A similar trend was observed in the non-separated samples. When converted to fresh matter, the highest values recorded for rapeseed straw were 132.87 m3 Mg−1 of cumulative methane and 281.34 m3 Mg−1 of cumulative biogas, whereas for wheat straw, the values reached 206.65 m3 Mg−1 of cumulative methane and 402.61 m3 Mg−1 of cumulative biogas. For rapeseed straw, these values were lower than those obtained in previous studies, where cumulative biogas production reached 349.98 m3 Mg−1 and cumulative methane 205.07 m3 Mg−1 [24]. In contrast, for wheat straw, the obtained values were higher than those reported by Kupryaniuk et al. [25], which were 197.18 m3 Mg−1 of methane and 374.70 m3 Mg−1 of biogas. For non-separated samples, both for rapeseed straw (167.82 m3 Mg−1 cumulative methane; 301.12 m3 Mg−1 cumulative biogas) and wheat straw (235.78 m3 Mg−1 cumulative methane; 441.88 m3 Mg−1 cumulative biogas), higher values were observed in the more fragmented samples. When converted to dry matter, for separated rapeseed straw, the highest values were 109.17 m3 Mg−1 cumulative methane and 202.80 m3 Mg−1 cumulative biogas, whereas for wheat straw, the maximum recorded values were 229.37 m3 Mg−1 cumulative methane and 446.36 m3 Mg−1 cumulative biogas. For non-separated rapeseed straw, these values were 189.10 m3 Mg−1 cumulative methane and 339.30 m3 Mg−1 cumulative biogas, which were lower than in the previous study by the authors (226.13 m3 Mg−1 methane and 385.93 m3 Mg−1 biogas). A similar pattern was observed for organic dry matter. For separated rapeseed straw, the highest biogas yield values were 159.98 m3 Mg−1 cumulative methane and 338.75 m3 Mg−1 cumulative biogas, while for non-separated samples, the values were 202.06 m3 Mg−1 cumulative methane and 362.56 m3 Mg−1 cumulative biogas. For separated wheat straw, the values reached 238.47 m3 Mg−1 cumulative methane and 464.09 m3 Mg−1 cumulative biogas, whereas for non-separated samples, they were 272.08 m3 Mg−1 cumulative methane and 509.91 m3 Mg−1 cumulative biogas.

Table 2.
Average biogas yield results.
Statistical analysis using one-way ANOVA revealed that the differences in cumulative methane and biogas production between particle size fractions were statistically significant (p < 0.05) for both wheat and rapeseed straw. The <1 mm fraction showed significantly higher methane yields compared with the >2.4 mm fraction. These results confirm the influence of particle size reduction on fermentation efficiency and support the observed trends in biogas potential across different substrate treatments.
Mechanical processing of lignocellulosic substrates, such as milling and extrusion, can significantly enhance methane fermentation efficiency by improving cellulose and hemicellulose accessibility to microorganisms. As demonstrated by Witaszek et al. [26], extrusion of maize silage at 175 °C resulted in a 12.4% increase in methane production, while fine milling accelerated the hydrolysis phase. In this study, the impact of the particle size of wheat and rapeseed straw on methane fermentation was evaluated using different fragmentation levels. Specifically, the samples were divided into fractions of <1 mm, 1–2.4 mm, and >2.4 mm. The selection of these ranges was based on previous research on the effect of fragmentation degree on fermentation efficiency. Additionally, mechanical pretreatment techniques based on industrial extrusion methods and conventional milling were applied, allowing an analysis of potential process intensification effects. The study revealed a similar trend, where smaller wheat straw fractions exhibited higher methane production compared with larger particles. During methane fermentation, hydrogen sulfide (H2S) can be produced, which negatively affects the quality of biogas. For example, for the rapeseed straw (MIX), the value was 277 ppm, and for the wheat straw (MIX) it was 125 ppm. Elevated levels of hydrogen sulfide significantly reduce the quality of biogas, increase corrosion risk in engines and pipelines, and may lead to additional maintenance costs. According to Ryckebosch et al. (2011) [27], H2S concentrations above 100–200 ppm require treatment before biogas can be used in combined heat and power (CHP) systems or injected into the gas grid. Several methods are available to mitigate H2S emissions. In situ techniques include the addition of iron salts (e.g., FeCl3), which react with H2S to form insoluble iron sulfides, as well as limited air dosing to promote the biological oxidation of H2S to elemental sulfur. Post-digestion methods involve adsorption on iron oxide pellets or activated carbon, chemical absorption using alkaline solutions (e.g., NaOH or Fe(OH)3), and biofiltration. For agricultural-scale biogas plants, dosing with iron compounds remains a cost-effective and widely applied strategy. Although detailed desulfurization was beyond the scope of this study, future research should consider H2S control techniques, particularly in the context of high-sulfur substrates such as rapeseed straw [27].
A simultaneous analysis of organic matter degradation was conducted. As shown in the table below, it was observed that the degree of organic matter decomposition increased with higher separation levels. For rapeseed straw, this increase ranged from 22.42% to 50.08% for the highest separation level. For separated wheat straw, the lowest organic matter degradation rate (50.60%) was higher than that of the highest separation level for rapeseed straw. The highest degradation rate (66.13%) was observed in the highest separation level of wheat straw. For wheat straw, the non-separated, finely ground samples exhibited a significantly higher organic matter degradation rate (71.19%), whereas for rapeseed straw, this value (49.46%) was lower than that observed in the highest separation level samples.
Table 3 below presents the results of high-temperature combustion measurements. This analysis allows for the determination of the percentage composition of carbon (C) and nitrogen (N). In the fermentation process, maintaining the appropriate carbon-to-nitrogen (C/N) ratio is crucial. If this ratio is too high (excess carbon and insufficient nitrogen), complete carbon conversion may not occur, preventing the full methane potential from being achieved. Conversely, an excess of nitrogen can lead to the formation of ammonia, which—even in low concentrations—inhibits bacterial growth and negatively affects the fermentation process.

Table 3.
Results of high-temperature combustion measurements.
The analysis of the composition of rapeseed and wheat straw, including the carbon-to-nitrogen ratio (C/N), dry matter content, and nitrogen (N) and carbon (C) content, is of significant importance in the context of biogas production. The C/N ratio is a key indicator of the efficiency of the anaerobic fermentation process, with the optimal ratio for biogas production typically ranging from 20:1 to 30:1.
For rapeseed straw, the highest C/N ratio of 153.82 in the mix indicates an excessive amount of carbon relative to nitrogen, which may limit the activity of fermentative microorganisms and reduce biogas production efficiency. Smaller fractions of rapeseed straw (>2.4 mm and 1–2.4 mm) have lower C/N values of 85.26 and 67.55, respectively, making them more suitable for fermentation. According to Luo et al. (2023) [28], the straw they examined had C/N values in the range of 52–54. The lowest ratio in rapeseed straw (<1 mm, 42.26) suggests that this fraction may better support the fermentation process.
Wheat straw is characterized by more favorable C/N values. The mixed wheat straw has a C/N ratio of 69.38, while finer fractions exhibit even lower ratios (ranging from 129.91 to 114.65), suggesting that they may be a better material for biogas production than some fractions of rapeseed straw. According to Cai (2024) [29], the C/N ratio falls within these values and is 97.56.
Nitrogen content is crucial for fermentation processes, as nitrogen supports the growth of microorganisms. Rapeseed straw exhibits a relatively low nitrogen content (0.31% in the mix and 0.47% in the >2.4 mm fraction), which may limit its efficiency as a substrate in biogas plants. However, in finer fractions, the nitrogen content increases to 0.59% and 0.90%, making them more valuable for the biogas process. In contrast, wheat straw, with a higher nitrogen content (up to 0.66% in the mix), may better support the development of fermentative microorganisms, promoting higher biogas production.
The average dry matter content in straw (approximately 95–96%) is beneficial, as a high dry matter content indicates lower water losses during fermentation, which enhances process efficiency. Carbon, as the primary energy source for microorganisms, is present in higher amounts in rapeseed straw (ranging from 37.98% to 48.00%), which can supply energy; however, an excessively high C/N ratio may hinder biogas processes. Wheat straw, with carbon levels ranging from 40.44% to 45.63%, can also serve as an efficient carbon source, particularly in finer fractions.
In conclusion, wheat straw, especially in finer fractions, appears to be a better substrate for biogas production compared with rapeseed straw due to its more favorable C/N ratio and higher nitrogen content. Smaller fractions of rapeseed straw may also constitute a valuable material, especially when combined with other nitrogen-rich substrates, which allows for optimal fermentation conditions. The selection of the appropriate material for biogas plants should consider these factors to maximize biogas production efficiency.
Alongside the fermentation process, elemental analyses of the straw were conducted. Table 4 below presents the results of the elemental analysis of both types of straw, categorized into individual fractions.

Table 4.
Results of the elemental analysis of rapeseed and wheat straw.
The results of the analyses concerning the elemental composition of different types of straw (rapeseed and wheat) in various particle size fractions provide valuable insights into their biogas potential and their impact on the fermentation process. Rapeseed straw in the MIX version exhibits relatively high concentrations of calcium (Ca: 4917 mg kg−1) and potassium (K: 16,358 mg kg−1), which promote microbial activity during fermentation. Additionally, the presence of silicon (Si: 22,197 mg kg−1) and sulfur (S: 1052 mg kg−1) may influence biochemical processes, and their presence in the substrate may affect biogas yield.
Rapeseed straw in the >2.4 mm fraction shows higher concentrations of chlorine (Cl: 13,825 mg kg−1) and sulfur (S: 9238 mg kg−1), as well as calcium (Ca: 9677 mg kg−1) and potassium (K: 29,193 mg kg−1). The higher content of these elements may enhance the efficiency of organic matter decomposition, although the elevated sulfur content may lead to hydrogen sulfide production during fermentation.
Rapeseed straw in the 1–2.4 mm fraction also contains high concentrations of calcium (Ca: 12,886 mg kg−1) and potassium (K: 28,909 mg kg−1), which can support microbial activity during organic matter decomposition. Furthermore, the phosphorus (P: 839 mg kg−1) and sulfur (S: 9383 mg kg−1) content facilitates further breakdown and biogas production.
For rapeseed straw <1 mm, the calcium (Ca: 25,983 mg kg−1) and potassium (K: 46,923 mg kg−1) content is significantly higher, promoting more efficient fermentation and increased biogas production. The presence of sulfur (S: 16,733 mg kg−1) and phosphorus (P: 905 mg kg−1) further enhances the efficiency of organic matter decomposition, leading to improved biogas yield.
Wheat straw in the MIX version exhibits high calcium (Ca: 19,904 mg kg−1) and potassium (K: 37,128 mg kg−1) content, which support microbial activity involved in organic matter degradation. The presence of sulfur (S: 15,175 mg kg−1) and phosphorus (P: 672 mg kg−1) also aid in decomposition and biogas production. Wheat straw in the <2.4 mm fraction contains slightly lower amounts of calcium (Ca: 3377 mg kg−1) and potassium (K: 16,793 mg kg−1), but the sulfur (S: 1340 mg kg−1) and phosphorus (P: 835 mg kg−1) content remains at a level conducive to efficient decomposition.
Wheat straw in the 1–2.4 mm fraction exhibits higher concentrations of calcium (Ca: 4667 mg kg−1) and potassium (K: 18,489 mg kg−1), which may positively affect microbial activity during fermentation. The sulfur (S: 1186 mg kg−1) and phosphorus (P: 813 mg kg−1) content also enhances the efficiency of the decomposition process and biogas production.
For wheat straw <1 mm, the calcium (Ca: 6523 mg kg−1) and potassium (K: 20,703 mg kg−1) content is the highest among the wheat straw samples, contributing to better biogas yield. The sulfur (S: 1360 mg kg−1) and phosphorus (P: 988 mg kg−1) content further support fermentation, enabling better processing of organic matter and achieving higher biogas production.
The elemental composition analysis of the examined wheat and rapeseed straw fractions indicates significant differences in the content of key elements that may influence the efficiency of anaerobic fermentation. It was found that calcium (Ca) and potassium (K) were present at the highest concentrations in the smallest fractions (<1 mm), which may promote anaerobic microbial activity and enhance biogas production. Similar results were obtained by Luo et al. [28], who indicated that high potassium concentrations improve enzymatic activity in fermentation processes.
The presence of sulfur (S) and chlorine (Cl) in large amounts in rapeseed straw (<1 mm) may contribute to increased hydrogen sulfide (H2S) production, posing a challenge for biogas plants due to the need for gas purification before utilization. Kupryaniuk et al. [24] demonstrated that high sulfur concentrations in rapeseed straw could inhibit methanogenic microbial activity, resulting in reduced biogas yield.
Wheat straw was characterized by a higher phosphorus (P) content, which plays a crucial role in the metabolism of microorganisms participating in fermentation. A high concentration of this element may contribute to improved fermentation stability and increased methane production. Studies by Cai et al. [29] suggest that enhanced phosphorus availability in organic substrates improves the growth of cellulose- and hemicellulose-hydrolyzing bacterial populations, thereby intensifying methane fermentation.
Additionally, the analysis revealed that the silicon (Si) content in wheat straw was significantly higher compared with rapeseed straw. According to Figueiredo et al. [20], silicon in lignocellulosic waste materials can hinder fermentation processes by limiting the availability of cellulose to hydrolytic enzymes. This may partially explain the differences in biogas production efficiency between wheat and rapeseed straw. The higher silica content in wheat straw could inhibit microbial access to fermentable carbohydrates, while the elevated sulfur and chlorine concentrations in rapeseed straw may contribute to the production of hydrogen sulfide, potentially inhibiting methanogenic activity [30]. Furthermore, variations in the carbon-to-nitrogen (C/N) ratio between the two straw types may affect microbial metabolism, with wheat straw exhibiting more balanced ratios conducive to fermentation. Additionally, differences in fiber composition, particularly the lignin content, could impact the biodegradability of each substrate, influencing overall methane yields [31]. These factors suggest that both the chemical and structural properties of straw play a crucial role in determining biogas production efficiency.
In summary, the elemental composition of the analyzed substrates indicates the necessity of proper selection and possible pretreatment before their use in methane fermentation. High potassium and calcium concentrations in finer straw fractions promote microbial activity; however, the presence of sulfur and chlorine in larger amounts may limit fermentation processes. These findings align with previous research on the influence of lignocellulosic substrate chemical composition on biogas production efficiency [21,23]. Furthermore, both the type of straw and its degree of fragmentation play a crucial role in biogas yield. Smaller particles (e.g., <1 mm) demonstrate higher efficiency in organic matter degradation, promoting biogas production. The presence of elements such as calcium, potassium, sulfur, and phosphorus is of significant importance for the fermentation process, affecting microbial activity and the efficiency of methane and biogas production.
Future research should focus on optimizing the mechanical and chemical pretreatment of straw to reduce the content of elements that negatively affect the fermentation process. Additionally, the potential for the co-fermentation of rapeseed straw with other substrates with a lower sulfur content should be explored to improve biogas yield. Finally, an analysis of the fiber content in both types of straw was conducted, including both MIX samples and individual fractions. The results of the fiber analysis are presented in Table 5.

Table 5.
Fiber analysis.
The analysis of the results revealed that the properties of substrates, such as rapeseed straw and wheat straw, vary depending on particle size and material type, which is crucial for their application in biogas plants. In the case of rapeseed straw, the values of parameters such as Acid Detergent Fiber (ADF), Neutral Detergent Fiber (NDF), and Crude Fiber decrease with the reduction in particle size. For the fraction < 1 mm, ADF averages 49.22%, NDF 59.52%, and Crude Fiber 79.40%. For the fraction > 2.4 mm, these values are significantly higher, reaching 61.52%, 80.99%, and 111.26%, respectively. This indicates that smaller particles are more susceptible to degradation, making them more favorable for fermentation processes.
A similar trend was observed for wheat straw. ADF and NDF values decrease from 52.84% and 82.47% for the >2.4 mm fraction to 45.95% and 77.07% for the <1 mm fraction. Crude Fiber also decreases from 43.28% to 33.14%. Smaller wheat straw particles, similar to rapeseed straw, exhibit a higher accessibility for microorganisms, increasing their efficiency in biogas processes.
Comparing both types of straw, rapeseed straw exhibits higher ADF, NDF, and Crude Fiber values across all analyzed fractions. The average ADF values for rapeseed straw range from 49.22% to 61.52%, while for wheat straw, they vary from 45.95% to 52.84%. Similarly, NDF in rapeseed straw ranges from 59.52% to 80.99%, whereas in wheat straw, it ranges from 77.07% to 87.09%. Crude Fiber in rapeseed straw is also higher (47.61–111.26%) compared with wheat straw (33.14–43.28%), suggesting that rapeseed straw, especially in larger fractions, is more challenging to decompose than wheat straw.
Standard deviations indicate the stability and homogeneity of measurements, ensuring the reliability of the results. An exception is Crude Fiber for rapeseed straw >2.4 mm, where the deviation is 7.841, possibly indicating greater variability within this fraction.
The fiber fraction analysis in wheat and rapeseed straw highlights significant differences in detergent fiber (ADF, NDF) and Crude Fiber content across different fractions. Compared with wheat straw, rapeseed straw has a higher lignocellulosic fraction content, which limits its biodegradability and makes its effective utilization in methane fermentation more difficult. As indicated by Karthikeyan et al. [32], the lignocellulosic structure of biomass serves as a key barrier restricting the access of hydrolytic enzymes to cellulose and hemicellulose, which, without proper pretreatment, can significantly reduce biogas yield.
The particle size analysis showed that finer fractions (<1 mm) had lower ADF and NDF content, suggesting greater accessibility of cellulose and hemicellulose for anaerobic microorganisms. Similar results were obtained by Klimiuk et al. [33], who demonstrated that reducing the lignin content in lignocellulosic substrates leads to better cellulose and hemicellulose utilization, thereby increasing fermentation efficiency.
Another factor influencing the degradation of rapeseed and wheat straw is lignin content. Studies by Weiland et al. [34] have shown that during anaerobic fermentation, lignin does not degrade to the same extent as cellulose and hemicellulose, which limits substrate bioavailability and may reduce methane production. However, it is worth noting that the lignin content in the smallest fractions was lower, suggesting that smaller particles may be more susceptible to decomposition in the fermentation process.
One way to improve the efficiency of lignocellulosic biomass conversion into biogas is through mechanical pretreatment, such as extrusion or milling into smaller fractions. As noted by Karimipour-Fard et al. [8], the use of an industrial pretreatment process can increase biogas production by up to 190% by improving the structural accessibility of the substrate for microorganisms.
A wide range of pretreatment techniques have been developed to enhance the biodegradability of lignocellulosic biomass. These include chemical (alkaline, acid, oxidative), thermal (steam explosion, hydrothermal), biological (fungal or microbial), and hybrid approaches. Compared with these, mechanical pretreatment (e.g., milling, extrusion) is simpler and more accessible for decentralized biogas plants but may not provide the same degree of structural disruption.
Alkaline pretreatment using NaOH or ammonia is particularly effective in lignin removal, which enhances enzymatic accessibility, but poses concerns related to chemical residues and effluent treatment [35]. Thermal pretreatment, such as hydrothermal and steam explosion, has been shown to significantly improve methane yields, as reported for pigeon pea stalks and wheat straw [36], though at the expense of higher energy input and process complexity. Biological pretreatment using fungi or rumen-derived microbes offers an environmentally friendly alternative, capable of degrading lignin with minimal inhibitor formation [37,38], yet typically requires longer residence times and strict process control.
Twin-screw extrusion, a scalable mechanical method, has demonstrated high potential for improving biomethane yields with reduced energy input, especially under high-solids conditions [8]. Nevertheless, as highlighted in a comprehensive review by Chevalier et al. (2025), the energy efficiency and net biogas gain from extrusion vary widely, emphasizing the need for further optimization [39].
The selection of pretreatment technology must therefore consider multiple factors, including the biomass type, scale of operation, energy efficiency, environmental impact, and cost-effectiveness. Mechanical pretreatments such as milling and extrusion remain attractive for on-farm or semi-industrial applications due to their simplicity, continuity, and potential for integration into existing feedstock preparation lines.
In the context of alternative biomass sources for methane fermentation, an interesting solution could be the use of other cellulose- and hemicellulose-rich plants, such as industrial hemp. Research by Michal et al. [40] has shown that hemp biomass can be an effective substrate for biogas production, achieving yields of 309–316 L CH4 kg−1 of dry organic matter, making it competitive with traditional substrates such as wheat and rapeseed straw.
Rapeseed straw is characterized by a relatively high C/N ratio and significant lignin content, which limits its biodegradability and reduces the efficiency of anaerobic digestion. One promising strategy to overcome these limitations is co-digestion with nitrogen-rich substrates, such as animal manure or food industry waste. Co-digestion has been shown to improve nutrient balance, enhance microbial activity, and reduce the accumulation of inhibitory compounds such as ammonia or hydrogen sulfide.
Gaballah et al. (2020) [41] demonstrated that the co-digestion of pretreated rapeseed straw with cattle manure significantly enhanced methane yield, achieving up to 305.7 mL CH4 g VS−1, which was 77.84% higher than untreated straw and up to 59% higher than the mono-digestion of cattle manure alone. These results confirm the synergistic effect of co-digestion in improving substrate accessibility and microbial activity.
Cai et al. (2024) [29] showed that combining wheat straw with chicken manure not only adjusted the C/N ratio to an optimal level for composting, but also positively influenced microbial community composition and reduced hydrogen sulfide emissions. Although this study focused on composting, the principles regarding microbial stimulation and sulfur transformation are equally applicable to anaerobic digestion.
Similar conclusions were drawn by Abbasi-Riyakhuni et al. (2025) [42], who proposed a biorefinery approach for rapeseed straw, combining pretreatment and co-digestion pathways. Their findings indicated that the solid fraction of pretreated straw produced up to 308 mL CH4 g VS−1, confirming that appropriate thermal processing combined with co-fermentation is key to improving rapeseed straw valorization in biogas systems.
Overall, these studies provide strong evidence that co-digestion is an effective strategy for optimizing the anaerobic digestion of recalcitrant lignocellulosic substrates such as rapeseed straw. Incorporating nitrogen-rich co-substrates facilitates nutrient balance, improves biodegradability, and enhances methane yields.
In conclusion, fiber fraction analysis revealed that smaller biomass particles are more susceptible to biodegradation, which may result in higher biogas production. The application of mechanical pretreatment, as well as the optimization of fermentation methods, can significantly enhance the efficiency of biogas production from rapeseed and wheat straw. Further research is needed on various pretreatment methods and the use of alternative biomass sources to improve the stability and efficiency of fermentation processes [36]. Additionally, smaller particles (<1 mm) of both straw types are more favorable for biogas processes due to lower ADF, NDF, and Crude Fiber values, facilitating their biodegradation. Compared with rapeseed straw, wheat straw shows greater susceptibility to decomposition, making it a more efficient substrate. Larger fractions (>2.4 mm) require additional grinding before application in biogas plants to improve their digestibility and enhance fermentation efficiency. Selecting the appropriate mechanical treatment, such as milling, is crucial for optimizing substrates in biogas processes.
4. Conclusions
The conducted research demonstrated the significant impact of wheat and rapeseed straw particle size on the efficiency of methane fermentation. In particular, smaller particles (<1 mm) exhibited higher biodegradability, which translated into greater biogas yield. In contrast, larger particles (>2.4 mm) showed lower methane production due to the limited availability of cellulose and hemicellulose for fermentative microorganisms.
The results indicate the following:
- 1.
- Wheat straw exhibited a greater fermentation potential than rapeseed straw, particularly in finer fractions. The higher cellulose content and more favorable C/N ratio in wheat straw facilitated more efficient biogas production.
- 2.
- The highest methane and biogas yield was obtained from wheat straw samples with the smallest particle size (<1 mm), confirming that mechanical pretreatment is crucial for improving the structural accessibility of lignocellulosic biomass.
- 3.
- Although rapeseed straw had a lower methane yield, it can still serve as a valuable substrate in biogas plants, especially when combined with other nitrogen-rich materials, which could improve the nutrient balance and optimize the fermentation process.
- 4.
- Mechanical treatments such as milling and extrusion can significantly enhance methane fermentation efficiency, aligning with previous studies on the use of grinding techniques and biodegradation process intensification.
Future research should focus on optimizing mechanical pretreatment methods and exploring the potential for co-fermentation of rapeseed straw with other substrates to reduce the impact of a high C/N ratio and mitigate the formation of gases that inhibit the fermentation process. The obtained results provide valuable insights for the biogas sector and may contribute to increasing the efficiency of lignocellulosic biomass utilization in biogas production, which is of significant importance from both economic and environmental perspectives. Although finer particle sizes resulted in increased methane yield, the benefits should be considered in the context of the energy consumption required for additional mechanical processing. In practical applications, the improved biogas output must be weighed against the energy and operational costs of fine milling. Future research should include an energy balance and economic feasibility assessment to determine whether the gains in methane production justify the additional input.
Author Contributions
Conceptualization, K.W., K.K. and W.C.; data curation, K.W. and K.K.; formal analysis, K.W. and K.K.; investigation, K.W. and W.C.; methodology, K.W. and K.K.; project administration, K.W.; resources, K.W. and K.K.; software, K.W., J.K. and J.P.; supervision, W.C.; validation, K.W., J.K. and J.P.; visualization, K.W. and K.K.; writing—original draft, K.W., K.K., J.K., J.P. and W.C.; writing—review and editing, K.W., K.K., J.K., J.P. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Claas-Stiftung, grant number 6/2023/F.CLASS, as part of the project titled “Development of a technology for the management of waste biomass from agricultural production through methane fermentation and pellet production (incineration)”.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to Jacek Dach from the Department of Biosystems Engineering, Poznań University of Life Sciences, for his valuable support and insightful comments during the research and manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A Review on Enhanced Biogas Production from Anaerobic Digestion of Lignocellulosic Biomass by Different Enhancement Techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- Sołowiej, P.; Pochwatka, P.; Wawrzyniak, A.; Łapiński, K.; Lewicki, A.; Dach, J. The Effect of Heat Removal during Thermophilic Phase on Energetic Aspects of Biowaste Composting Process. Energies 2021, 14, 1183. [Google Scholar] [CrossRef]
- Sitienei, R.; Qi, Z.; Grant, B.; Obi-Njoku, O.; Vanderzaag, A.; Boh, M.Y.; Clark, O.G.; Price, G.; Madramootoo, C.; Zhang, T.; et al. A New Framework for Simulating C Decomposition and Emissions from Land Applied Biosolids and Manures Using the Denitrification and Decomposition Model. Sci. Total Environ. 2025, 969, 178913. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Vivekanand, V. Biological Treatment of Lignocellulosic Biomass by Curvularia Lunata for Biogas Production. Bioresour. Technol. 2020, 306, 123151. [Google Scholar] [CrossRef]
- Jóźwiakowski, K.; Marzec, M.; Fiedurek, J.; Kamińska, A.; Gajewska, M.; Wojciechowska, E.; Wu, S.; Dach, J.; Marczuk, A.; Kowlaczyk-Juśko, A. Application of H2O2 to Optimize Ammonium Removal from Domestic Wastewater. Sep. Purif. Technol. 2017, 173, 357–363. [Google Scholar] [CrossRef]
- Momoh, O.L.Y.; Ouki, S. Development of a Novel Fractal-like Kinetic Model for Elucidating the Effect of Particle Size on the Mechanism of Hydrolysis and Biogas Yield from Ligno-Cellulosic Biomass. Renew. Energy 2018, 118, 71–83. [Google Scholar] [CrossRef]
- Naegele, H.-J.; Mönch-Tegeder, M.; Haag, N.L.; Oechsner, H. Effect of Substrate Pretreatment on Particle Size Distribution in a Full-Scale Research Biogas Plant. Bioresour. Technol. 2014, 172, 396–402. [Google Scholar] [CrossRef]
- Karimipour-Fard, P.; Chio, C.; Brunone, A.; Marway, H.; Thompson, M.; Abdehagh, N.; Qin, W.; Yang, T.C. Lignocellulosic Biomass Pretreatment: Industrial Oriented High-Solid Twin-Screw Extrusion Method to Improve Biogas Production from Forestry Biomass Resources. Bioresour. Technol. 2024, 393, 130000. [Google Scholar] [CrossRef]
- Witaszek, K.; Herkowiak, M.; Pilarska, A.A.; Czekała, W. Methods of Handling the Cup Plant (Silphium perfoliatum L.) for Energy Production. Energies 2022, 15, 1897. [Google Scholar] [CrossRef]
- Czekała, W.; Frankowski, J.; Sieracka, D.; Pochwatka, P.; Kowalczyk-Juśko, A.; Witaszek, K.; Dudnyk, A.; Zielińska, A.; Wisła-Świder, A.; Dach, J. The Energy Efficiency Analysis of Sorghum Waste Biomass Grown in a Temperate Climate. Energy 2025, 320, 135433. [Google Scholar] [CrossRef]
- Kozłowski, K.; Dach, J.; Lewicki, A.; Malińska, K.; do Carmo, I.E.P.; Czekała, W. Potential of Biogas Production from Animal Manure in Poland. Arch. Environ. Prot. 2023, 45, 99–108. [Google Scholar] [CrossRef]
- Thamizhakaran Stanley, J.; Thanarasu, A.; Senthil Kumar, P.; Periyasamy, K.; Raghunandhakumar, S.; Periyaraman, P.; Devaraj, K.; Dhanasekaran, A.; Subramanian, S. Potential Pre-Treatment of Lignocellulosic Biomass for the Enhancement of Biomethane Production through Anaerobic Digestion—A Review. Fuel 2022, 318, 123593. [Google Scholar] [CrossRef]
- Marks, S.; Dach, J.; Fernandez Morales, F.; Mazurkiewicz, J.; Pochwatka, P.; Gierz, Ł. New Trends in Substrates and Biogas Systems in Poland. J. Ecol. Eng. 2020, 21, 19–25. [Google Scholar] [CrossRef]
- DIN 38414-8; Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung; Schlamm und Sedimente (Gruppe S); Bestimmung des Faulverhaltens (S 8). Beuth Verlag GmbH: Berlin, Germany, 1985.
- VDI 4630; Vergärung Organischer Stoffe Substratcharakterisierung, Probenahme, Stoffdatenerhebung, Gärversuche. VDI Verein Deutscher Ingenieure: Düsseldorf, Germany, 2016.
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Witaszek, K.; Piekutowska, M.; Idzior-Haufa, M.; Wawrzyniak, A. Degree of Biomass Conversion in the Integrated Production of Bioethanol and Biogas. Energies 2021, 14, 7763. [Google Scholar] [CrossRef]
- Kintl, A.; Huňady, I.; Sobotková, J.; Vítěz, T.; Brtnický, M.; Vejražka, K.; Elbl, J. Data on the Effect of Co-Fermentation of Maize and Leguminous Crops on Biogas Production, Methane Production and Methane Content in Biogas. Data Brief 2024, 56, 110842. [Google Scholar] [CrossRef]
- Czekała, W. Concept of In-Oil Project Based on Bioconversion of By-Products from Food Processing Industry. J. Ecol. Eng. 2017, 18, 180–185. [Google Scholar] [CrossRef][Green Version]
- Mazurkiewicz, J. Loss of Energy and Economic Potential of a Biogas Plant Fed with Cow Manure Due to Storage Time. Energies 2023, 16, 6686. [Google Scholar] [CrossRef]
- Figueiredo, A.F.; Brede, M.; Heller, J.; Redzepovic, L.; Illi, L.; Weichgrebe, D. Anaerobic Digestion of Hemp and Flax Straw and Shives and Rapeseed Straw by the Ruminal Microbiota. Bioenergy Res. 2023, 17, 700–709. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Processing of Wheat Straw Materials for Production of Medium Density Fiberboard (MDF). In Proceedings of the 59th Appita Annual Conference and Exhibition: Incorporating the 13th ISWFPC (International Symposium on Wood, Fibre and Pulping Chemistry), Auckland, New Zealand, 16–19 May 2005. [Google Scholar]
- Franz Gouertoumbo, W.; Alhaj Hamoud, Y.; Guo, X.; Shaghaleh, H.; Ali Adam Hamad, A.; Elsadek, E. Wheat Straw Burial Enhances the Root Physiology, Productivity, and Water Utilization Efficiency of Rice under Alternative Wetting and Drying Irrigation. Sustainability 2022, 14, 16394. [Google Scholar] [CrossRef]
- Szwarc, D.; Głowacka, K. Increasing the Biogas Potential of Rapeseed Straw Using Pulsed Electric Field Pre-Treatment. Energies 2021, 14, 8307. [Google Scholar] [CrossRef]
- Kupryaniuk, K.; Oniszczuk, T.; Combrzyński, M.; Lisiecka, K.; Janczak, D. Influence of Modification of the Plasticizing System on the Extrusion-Cooking Process and Selected Physicochemical Properties of Rapeseed and Buckwheat Straws. Materials 2022, 15, 5039. [Google Scholar] [CrossRef] [PubMed]
- Kupryaniuk, K.; Oniszczuk, T.; Combrzyński, M.; Matwijczuk, A.; Pulka, J. Physical and Thermal Modification of Selected Lignocellulosic Raw Materials. Int. Agrophys. 2023, 37, 141–149. [Google Scholar] [CrossRef]
- Witaszek, K.; Pilarski, K.; Niedbała, G.; Pilarska, A.A.; Herkowiak, M. Energy Efficiency of Comminution and Extrusion of Maize Substrates Subjected to Methane Fermentation. Energies 2020, 13, 1887. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for Transformation of Biogas to Biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Tang, A. Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions. Energies 2023, 16, 4232. [Google Scholar] [CrossRef]
- Cai, S.; Ma, Y.; Bao, Z.; Yang, Z.; Niu, X.; Meng, Q.; Qin, D.; Wang, Y.; Wan, J.; Guo, X. The Impacts of the C/N Ratio on Hydrogen Sulfide Emission and Microbial Community Characteristics during Chicken Manure Composting with Wheat Straw. Agriculture 2024, 14, 948. [Google Scholar] [CrossRef]
- Cui, S.; Hu, D.; Chen, Z.; Wang, Y.; Yan, J.; Zhuang, S.; Jiang, B.; Ge, H.; Wang, Z.; Zhang, P. A Novel Anaerobic Membrane Bioreactor with Magnetotactic Bacteria for Organic Sulfur Pesticide Wastewater Treatment: Improvement of Enzyme Activities, Refractory Pollutants Removal and Methane Yield. Chem. Eng. J. 2025, 509, 161397. [Google Scholar] [CrossRef]
- Zhong, Y.; Ragauskas, A.J.; Zheng, Y.; Meng, X.; Zhou, Y.; Lin, Y. A Review on the Pretreatment of Straw Biomass by Using Biogas Slurry. Process Saf. Environ. Prot. 2025, 195, 106843. [Google Scholar] [CrossRef]
- Karthikeyan, P.K.; Bandulasena, H.C.H.; Radu, T. A Comparative Analysis of Pre-Treatment Technologies for Enhanced Biogas Production from Anaerobic Digestion of Lignocellulosic Waste. Ind. Crop. Prod. 2024, 215, 118591. [Google Scholar] [CrossRef]
- Klimiuk, E.; Pokój, T.; Budzyński, W.; Dubis, B. Theoretical and Observed Biogas Production from Plant Biomass of Different Fibre Contents. Bioresour. Technol. 2010, 101, 9527–9535. [Google Scholar] [CrossRef]
- Weiland, K.; Alge, K.; Mautner, A.; Bauer, A.; Bismarck, A. Horse Manure as Resource for Biogas and Nanolignocellulosic Fibres. Bioresour. Technol. 2023, 372, 128688. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pretreatment of Lignocellulosic Agricultural Waste for Delignification, Rapid Hydrolysis, and Enhanced Biogas Production: A Review. J. Indian Chem. Soc. 2021, 98, 100147. [Google Scholar] [CrossRef]
- Sathish, T.; Muthukumar, K.; Saravanan, R.; Giri, J. Optimized Thermal Pretreatment for Lignocellulosic Biomass of Pigeon Pea Stalks to Augment Quality and Quantity of Biogas Production. Int. J. Thermofluids 2024, 24, 100911. [Google Scholar] [CrossRef]
- Tamilselvan, R.; Immanuel Selwynraj, A. Enhancing Biogas Generation from Lignocellulosic Biomass through Biological Pretreatment: Exploring the Role of Ruminant Microbes and Anaerobic Fungi. Anaerobe 2024, 85, 102815. [Google Scholar] [CrossRef]
- Kovács, E.; Szűcs, C.; Farkas, A.; Szuhaj, M.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Pretreatment of Lignocellulosic Biogas Substrates by Filamentous Fungi. J. Biotechnol. 2022, 360, 160–170. [Google Scholar] [CrossRef]
- Chevalier, A.; Evon, P.; Monlau, F.; Vandenbossche, V.; Sambusiti, C. Extrusion as a Pretreatment for Lignocellulosic Agricultural Byproducts to Improve Biogas Production: A Review. Bioresour. Technol. Rep. 2025, 29, 102063. [Google Scholar] [CrossRef]
- Michal, P.; Svehla, P.; Malik, M.; Kaplan, L.; Hanc, A.; Tlustos, P. Production of Biogas from the Industrial Hemp Variety, Tiborszállási. Environ. Technol. Innov. 2023, 31, 103185. [Google Scholar] [CrossRef]
- Gaballah, E.S.; Abomohra, A.E.-F.; Xu, C.; Elsayed, M.; Abdelkader, T.K.; Lin, J.; Yuan, Q. Enhancement of Biogas Production from Rape Straw Using Different Co-Pretreatment Techniques and Anaerobic Co-Digestion with Cattle Manure. Bioresour. Technol. 2020, 309, 123311. [Google Scholar] [CrossRef]
- Abbasi-Riyakhuni, M.; Hashemi, S.S.; Denayer, J.F.M.; Aghbashlo, M.; Tabatabaei, M.; Karimi, K. Integrated Biorefining of Rapeseed Straw for Ethanol, Biogas, and Mycoprotein Production. Fuel 2025, 382, 133751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).