Vacuum Processability of Self-Assembled Monolayers and Their Chemical Interaction with Perovskite Interfaces
Abstract
1. Introduction
2. Background
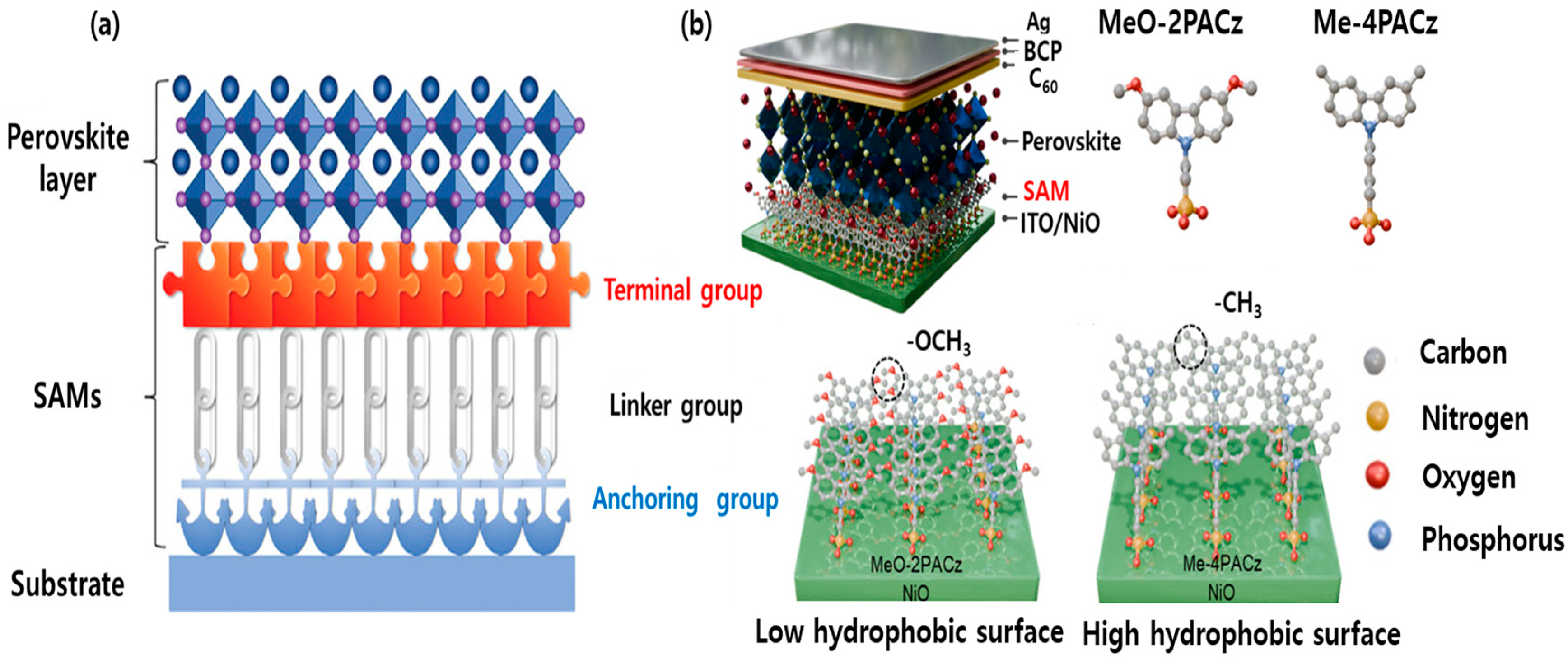
2.1. Structural Components of SAM
2.2. Binding Modes of SAM
2.3. Processing of SAMs
3. Promising for Vacuum Deposition of SAM
4. Importance of Interaction Between SAM and Perovskite
4.1. Surface Contact Behavior Between SAM and Perovskite
4.2. Perovskite Crystallization Depending on SAM
4.3. Defect Passivation by SAM
5. Perspective
- Vacuum deposition processability: Evaporation techniques for SAMs and perovskites have demonstrated significant potential in enhancing the performance of perovskite solar cells. Vacuum-deposited SAMs enable precise molecular ordering and uniform thin layers formation with physical adsorption. Therefore, it is suitable as a preceding process for vacuum-processed perovskite deposition. Despite these advantages, the SAM vacuum deposition process remains underexplored, requiring further investigation to address several critical challenges. In particular, the thermal stability of SAMs is a major concern, as the SAM layer tends to decompose at high temperatures. Therefore, studies focusing on enhancing the thermal stability of SAMs are essential. Additionally, due to the formation of weaker physisorption compared to solution-based processing, post-deposition treatments are essential to improve the bonding strength.
- Functional group interaction with perovskite: The interaction between the terminal group of the SAM and the perovskite directly influences the perovskite’s lattice structure, crystallization process, and surface properties, which are key to enhancing the performance of perovskite-based devices. These interactions, which include coordination bonding, van der Waals forces, and electrostatic interactions, enable SAMs to integrate into the perovskite lattice structure. This incorporation can modify the perovskite’s surface wettability and lattice crystallinity, affecting key factors like crystallization and defect passivation. Despite these advantages, the mechanisms underlying the enhancement of perovskite crystallinity, contact behavior, and passivation effects induced by the SAM layer remain poorly understood. Specifically, the interaction between the SAM layer and perovskite varies depending on the functional groups in the SAM. Therefore, developing functional groups that can enhance perovskite device performance is essential.
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, I.B.; Kim, Y.J.; Kim, D.Y.; Jang, S.Y. A Thiophene Based Dopant-Free Hole-Transport Polymer for Efficient and Stable Perovskite Solar Cells. Macromol. Res. 2022, 30, 391–396. [Google Scholar] [CrossRef]
- Park, J.Y.; Jang, J.W.; Shen, X.; Jang, J.H.; Kwak, S.L.; Choi, H.; Lee, B.R.; Hwang, D.H. Fluorene- and Arylamine-Based Photo-Crosslinkable Hole Transporting Polymer for Solution-Processed Perovskite and Organic Light-Emitting Diodes. Macromol. Res. 2023, 31, 721–732. [Google Scholar] [CrossRef]
- Arivunithi, V.M.; Park, H.Y.; Reddy, S.S.; Do, Y.; Park, H.; Shin, E.S.; Noh, Y.Y.; Song, M.; Jin, S.H. Introducing an Organic Hole Transporting Material as a Bilayer to Improve the Efficiency and Stability of Perovskite Solar Cells. Macromol. Res. 2021, 29, 149–156. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, D.Y.; Yang, S.Y. Surface Induced Porous Morphological Transition of the Organic Self-Assembled Monolayer Hybridized Polyelectrolyte Thin Films. Macromol. Res. 2013, 21, 127–129. [Google Scholar] [CrossRef]
- Sery, A.A.; Abd El-Samad, A.E.; Mostafa, R.S.; Zeenelabden, H.H.; Elseman, A.M.; Sajid, S.; Rashad, M.M.; El-Aasser, M. Toward High-Performance Carbon-Based Perovskite Solar Cells. Sol. Energy 2025, 287, 113261. [Google Scholar] [CrossRef]
- Liu, H.-S.; Huan, C.-M.; Xiao, X.-D.; Bi, Z.-N.; Lu, Y.; Qi, S.; Zhan, Y.-J.; Xu, X.-Q.; Xu, G. Research Progress of All-Inorganic Perovskite Solar Cells. Adv. New Renew. Energy 2019, 7, 142–148. [Google Scholar]
- Yuan, S.-Y.; Li, Z.-Z.; Wang, Y.-T.; Zhao, H. CsPbI3 All-Inorganic Perovskite Solar Cells: Development Status and Theoretical Prediction. J. Solid State Chem. 2024, 336, 124780. [Google Scholar] [CrossRef]
- Afre, R.A.; Pugliese, D. Perovskite Solar Cells: A Review of the Latest Advances in Materials, Fabrication Techniques, and Stability Enhancement Strategies. Micromachines 2024, 15, 192. [Google Scholar] [CrossRef]
- Goni, L.K.M.O.; Abdur, R.; Hossain, M.; Chowdhury, S.; Shahinuzzaman, M.; Shaikh, M.A.A.; Jamal, M.S. Exploring the Impact of Halide Composition on Stability and Power Conversion Efficiency in All-Inorganic Perovskite Solar Cells. J. Mater. Sci. 2024, 59, 18279–18315. [Google Scholar]
- Sharif, R.; Khalid, A.; Ahmad, S.W.; Rehman, A.; Qutab, H.G.; Akhtar, H.H.; Mahmood, K.; Afzal, S.; Saleem, F. A Comprehensive Review of the Current Progresses and Material Advances in Perovskite Solar Cells. Nanoscale Adv. 2023, 5, 3803–3833. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, J.; Song, J.; Cao, J.; Zhou, P.; Xu, X.; Zhou, Q.; Li, G.; Tu, Y.; Chu, L.; et al. Synchronous Modulation of Hole-Selective Self-Assembled Monolayer and Buried Interface for Inverted Perovskite Solar Cells. Cell Rep. Phys. Sci. 2024, 5, 101992. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, S.J.; Byeon, S.E.; He, X.; Yoon, H.J. Self-Assembled Monolayers as Interface Engineering Nanomaterials in Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2002606. [Google Scholar] [CrossRef]
- Park, S.M.; Wei, M.; Lempesis, N.; Yu, W.; Hossain, T.; Agosta, L.; Carnevali, V.; Atapattu, H.R.; Serles, P.; Eickemeyer, F.T.; et al. Low-Loss Contacts on Textured Substrates for Inverted Perovskite Solar Cells. Nature 2023, 624, 289–294. [Google Scholar] [CrossRef]
- Yu, X.; Sun, X.; Zhu, Z.; Li, Z. Stabilization Strategies of Buried Interface for Efficient SAM-Based Inverted Perovskite Solar Cells. Angew. Chem.-Int. Ed. 2024, 64, e202419608. [Google Scholar] [CrossRef]
- Er-Raji, O.; Lange, S.; Hartwig, C.E.; Prasetio, A.; Bivour, M.; Hermle, M.; Turek, M.; De Wolf, S.; Glunz, S.W.; Borchert, J.; et al. Tuning Self-Assembly of Hole-Selective Monolayers for Reproducible Perovskite/Silicon Tandem Solar Cells. Small Methods 2025, 2401758. [Google Scholar] [CrossRef]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-Assembled Monolayers in Organic Electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.R.; Shao, J.Y.; Zhong, Y.W. Self-Assembled Monolayers as Hole-Transporting Materials for Inverted Perovskite Solar Cells. Mol. Syst. Des. Eng. 2023, 8, 1440–1455. [Google Scholar] [CrossRef]
- Yi, Z.; Li, X.; Xiong, Y.; Shen, G.; Zhang, W.; Huang, Y.; Jiang, Q.; Ng, X.R.; Luo, Y.; Zheng, J.; et al. Self-assembled Monolayers (SAMs) in Inverted Perovskite Solar Cells and Their Tandem Photovoltaics Application. Interdiscip. Mater. 2024, 3, 203–244. [Google Scholar] [CrossRef]
- Yeo, D.; Shin, J.; Kim, D.; Jaung, J.Y.; Jung, I.H. Self-Assembled Monolayer-Based Hole-Transporting Materials for Perovskite Solar Cells. Nanomaterials 2024, 14, 175. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Xiao, W.; Chen, R.; Sun, Z.; Zhang, Y.; Lei, X.; Hu, S.; Kober-Czerny, M.; Wang, J.; et al. Buried Interface Molecular Hybrid for Inverted Perovskite Solar Cells. Nature 2024, 632, 536–542. [Google Scholar] [CrossRef]
- Choi, K.; Choi, H.; Min, J.; Kim, T.; Kim, D.; Son, S.Y.; Kim, G.W.; Choi, J.; Park, T. A Short Review on Interface Engineering of Perovskite Solar Cells: A Self-Assembled Monolayer and Its Roles. Sol. RRL 2020, 4, 1900251. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Wu, Y. Advantages and Challenges of Self-Assembled Monolayer as a Hole-Selective Contact for Perovskite Solar Cells. Mater. Futures 2023, 2, 012105. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Z.; Li, Q.; Yang, C.; Zhan, S.; Jia, X.; Li, C.; Islam, M.R.; Wang, Z.; Yue, S.; et al. Comprehensively Evaluating Feasibility of Self-Assembled Materials Applied to Hole Transport Layer for Commercializing Perovskite Solar Cells. Mater. Res. Bull. 2023, 165, 112327. [Google Scholar] [CrossRef]
- Kim, G.; Kim, H.; Kim, M.; Sin, J.; Kim, M.; Kim, J.; Zhou, H.; Kang, S.H.; Oh, H.M.; Yang, J.Y. Enhancing Surface Modification and Carrier Extraction in Inverted Perovskite Solar Cells via Self-Assembled Monolayers. Nanomaterials 2024, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Shen, Z.; Shen, Y.; Yan, G.; Wang, Y.; Han, Q.; Han, L. Reinforcing Self-Assembly of Hole Transport Molecules for Stable Inverted Perovskite Solar Cells. Science 2024, 383, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, M.; Qi, F.; Lin, F.R.; Jen, A.K.Y. Self-Assembled Monolayers for Interfacial Engineering in Solution-Processed Thin-Film Electronic Devices: Design, Fabrication, and Applications. Chem. Rev. 2024, 124, 2138–2204. [Google Scholar] [CrossRef]
- Takhellambam, D.; Castriotta, L.A.; Zanotti, G.; Mancini, L.; Raglione, V.; Mattioli, G.; Paci, B.; Generosi, A.; Guaragno, M.; Campanari, V.; et al. Enhancing Hole Transfer in Perovskite Solar Cell with Self-Assembled Monolayer by Introducing [1]Benzothieno [3,2-b][1]Benzothiophene Interlayer. Sol. RRL 2023, 7, 2300658. [Google Scholar] [CrossRef]
- Levine, I.; Al-Ashouri, A.; Musiienko, A.; Hempel, H.; Magomedov, A.; Drevilkauskaite, A.; Getautis, V.; Menzel, D.; Hinrichs, K.; Unold, T.; et al. Charge Transfer Rates and Electron Trapping at Buried Interfaces of Perovskite Solar Cells. Joule 2021, 5, 2915–2933. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Magomedov, A.; Roß, M.; Jošt, M.; Talaikis, M.; Chistiakova, G.; Bertram, T.; Márquez, J.A.; Köhnen, E.; Kasparavičius, E.; et al. Conformal Monolayer Contacts with Lossless Interfaces for Perovskite Single Junction and Monolithic Tandem Solar Cells. Energy Environ. Sci. 2019, 12, 3356–3369. [Google Scholar] [CrossRef]
- Pitaro, M.; Alonso, J.E.S.; Di Mario, L.; Romero, D.G.; Tran, K.; Kardula, J.; Zaharia, T.; Johansson, M.B.; Johansson, E.M.J.; Chiechi, R.C.; et al. Tuning the Surface Energy of Hole Transport Layers Based on Carbazole Self-Assembled Monolayers for Highly Efficient Sn/Pb Perovskite Solar Cells. Adv. Funct. Mater. 2023, 34, 2306571. [Google Scholar] [CrossRef]
- Peng, C.; Huang, H.; Liu, W.; Zhang, Z.; Xu, Y.; Chen, S.; Li, S.; Du, S.; Wang, S.; He, Z.; et al. Reinforcement of Carbazole-Based Self-Assembled Monolayers in Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2025, 17, 10745–10754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, W.; Duan, Y.; Sun, R.; Li, Y.; Xie, Z.; Xu, D.; Wu, M.; Wang, Y.; Li, H.; et al. Glycol Monomethyl Ether-Substituted Carbazolyl Hole-Transporting Material for Stable Inverted Perovskite Solar Cells with Efficiency of 25.52%. Angew. Chem.-Int. Ed. 2024, 63, e202403068. [Google Scholar] [CrossRef]
- Pitaro, M.; Alonso, J.S.; Di Mario, L.; Romero, D.G.; Tran, K.; Zaharia, T.; Johansson, M.B.; Johansson, E.M.J.; Loi, M.A. A Carbazole-Based Self-Assembled Monolayer as the Hole Transport Layer for Efficient and Stable Cs0.25FA0.75Sn0.5Pb0.5I3 Solar Cells. J. Mater. Chem. A 2023, 11, 11755–11766. [Google Scholar] [CrossRef]
- Li, W.; Martínez-Ferrero, E.; Palomares, E. Self-Assembled Molecules as Selective Contacts for Efficient and Stable Perovskite Solar Cells. Mater. Chem. Front. 2023, 8, 681–699. [Google Scholar] [CrossRef]
- Huang, S.; Liang, C.; Lin, Z. Application of PACz-Based Self-Assembled Monolayer Materials in Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2024, 16, 64424–64446. [Google Scholar] [CrossRef]
- Peng, H.; Zheng, W.; Kim, G.Y.; Lee, J.W. Self-Assembled Monolayers as Hole-Selective Contacts in Inverted Perovskite Solar Cells: A Review. Korean J. Chem. Eng. 2024, 41, 3717–3735. [Google Scholar] [CrossRef]
- Kasparavičius, E.; Franckevičius, M.; Driukas, S.; Gulbinas, V. Charge Carrier Dynamics at the Perovskite Interface with Self-Assembled Monolayers. ACS Appl. Mater. Interfaces 2024, 16, 59477–59487. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.G. Materials and Methods for Interface Engineering toward Stable and Efficient Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Liu, S.C.; Lin, C.T.; Fan, B.; Li, Y.; Gao, H.; Liu, M.; Lin, F.R.; Jen, A.K.Y. Durable Organic Photovoltaics Enabled by a Morphology-Stabilizing Hole-Selective Self-Assembled Monolayer. Adv. Energy Mater. 2024, 14, 2303354. [Google Scholar] [CrossRef]
- Li, W.; Li, T.; Tong, Y.; Li, Y.; Wang, H.; Qi, H.; Wang, K.; Wang, H. Reducing Nonradiative Losses of Air-Processed Perovskite Films via Interface Modification for Bright and Efficient Light Emitting Diodes. Adv. Funct. Mater. 2024, 34, 2311133. [Google Scholar] [CrossRef]
- Shih, C.J.; Chen, Y.S.; Luo, D.; Yu, C.W.; Chen, K.H.; Murokinas, G.; Huang, Y.C.; Li, C.F.; Huang, Y.C.; Liu, S.W. Exploring Buried Interface in All-Vapor-Deposited Perovskite Photovoltaics. Sol. Energy 2024, 280, 112872. [Google Scholar] [CrossRef]
- Lee, K.C.; Kim, S.; Son, J.; Kong, J.; Yang, C. A Brief Review on Self-Assembled Monolayers in Organic Solar Cells: Progress, Challenges, and Future Prospects. ACS Appl. Electron. Mater. 2025, 7, 946–963. [Google Scholar] [CrossRef]
- Diercks, A.; Petry, J.; Feeney, T.; Singh, R.; Zhao, T.; Hu, H.; Li, Y.; Paetzold, U.W.; Fassl, P. Sequential Evaporation of Inverted FAPbI3 Perovskite Solar Cells—Impact of Substrate on Crystallization and Film Formation. ACS Energy Lett. 2025, 10, 1165–1173. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.; Hu, W. Recent Progress of Interface Self-Assembled Monolayers Engineering Organic Optoelectronic Devices. DeCarbon 2024, 3, 100035. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Lin, F.R.; Li, Z.; Yang, S.; Jen, A.K.Y. Self-Assembled Monolayers as Emerging Hole-Selective Layers Enable High-Performance Thin-Film Solar Cells. Innovation 2023, 4, 100369. [Google Scholar] [CrossRef]
- Ren, Z.; Cui, Z.; Shi, X.; Wang, L.; Dou, Y.; Wang, F.; Lin, H.; Yan, H.; Chen, S. Poly(Carbazole Phosphonic Acid) as a Versatile Hole-Transporting Material for p-i-n Perovskite Solar Cells and Modules. Joule 2023, 7, 2894–2904. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, K.; Franco, L.R.; Wu, J.; Öhrström, L.; Liu, X.; Gumbo, M.; Ozório, M.S.; Araujo, C.M.; Zhang, G.; et al. Effects of Alkyl Spacer Length in Carbazole-Based Self-Assembled Monolayer Materials on Molecular Conformation and Organic Solar Cell Performance. Adv. Sci. 2024, 12, 2410277. [Google Scholar] [CrossRef]
- Han, F.; Tu, Z.; Wan, Z.; Luo, J.; Xia, J.; Hao, G.; Yi, Y.; Wang, R.; Jia, C. Effect of Functional Group Position Change of Pyridinesulfonic Acid as Interface-Modified Layer on Perovskite Solar Cell. Appl. Surf. Sci. 2018, 462, 517–525. [Google Scholar] [CrossRef]
- Arkan, E.; Unal, M.; Yalcin, E.; Arkan, M.Z.Y.; Yurtdas, S.; Can, M.; Tozlu, C.; Demic, S. Influence of End Groups Variation of Self Assembled Monolayers on Performance of Planar Perovskite Solar Cells by Interface Regulation. Mater. Sci. Semicond. Process. 2021, 123, 105514. [Google Scholar] [CrossRef]
- Arkan, E.; Yalcin, E.; Unal, M.; Arkan, M.Z.Y.; Can, M.; Tozlu, C.; Demic, S. Effect of Functional Groups of Self Assembled Monolayer Molecules on the Performance of Inverted Perovskite Solar Cell. Mater. Chem. Phys. 2020, 254, 123435. [Google Scholar] [CrossRef]
- Dai, Z.; Yadavalli, S.K.; Chen, M.; Abbaspourtamijani, A.; Qi, Y.; Padture, N.P. Interfacial Toughening with Self-Assembled Monolayers Enhances Perovskite Solar Cell Reliability. Science 2021, 372, 618–622. [Google Scholar] [CrossRef]
- Anizelli, H.; David, T.W.; Tyagi, P.; Laureto, E.; Kettle, J. Enhancing the Stability of Perovskite Solar Cells through Functionalisation of Metal Oxide Transport Layers with Self-Assembled Monolayers. Sol. Energy 2020, 203, 157–163. [Google Scholar] [CrossRef]
- Suo, J.; Yang, B.; Bogachuk, D.; Boschloo, G.; Hagfeldt, A. The Dual Use of SAM Molecules for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2024, 15, 2400205. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.J.; Lee, S.H.; Kang, Y.J.; Kwon, S.N.; Kim, D.H.; Na, S.I. Mixed Self-Assembled Hole-Transport Monolayer Enables Simultaneous Improvement of Efficiency and Stability of Perovskite Solar Cells. Sol. RRL 2024, 8, 2400067. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Li, Y.; Gong, L.; Yuan, Z.; Liang, L.; Chen, J.; Ganesan, P.; Zhang, Y.; Ma, J.; et al. Deciphering the Impact of Aromatic Linkers in Self-Assembled Monolayers on the Performance of Monolithic Perovskite/Si Tandem Photovoltaic. Angew. Chem.-Int. Ed. 2024, 64, e202420585. [Google Scholar] [CrossRef]
- Lenaers, S.; Lammar, S.; Krishna, A.; Stacchini, V.; Cardeynaels, T.; Penxten, H.; Weijtens, C.; Verhage, M.; Ruttens, B.; Maes, W.; et al. Pyrene-Based Self-Assembled Monolayer with Improved Surface Coverage and Energy Level Alignment for Perovskite Solar Cells. Adv. Funct. Mater. 2024, 2411922. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhang, H.; Yan, W.; Li, Y.; Liang, L.; Yu, W.; Yu, X.; Wang, Y.; Yang, Y.; et al. Fully Aromatic Self-Assembled Hole-Selective Layer toward Efficient Inverted Wide-Bandgap Perovskite Solar Cells with Ultraviolet Resistance. Angew. Chem.-Int. Ed. 2024, 63, e202315281. [Google Scholar] [CrossRef]
- Galvis, C.E.P.; Ruiz, D.A.G.; Martínez-Ferrero, E.; Palomares, E. Challenges in the Design and Synthesis of Self-Assembling Molecules as Selective Contacts in Perovskite Solar Cells. Chem. Sci. 2023, 15, 1534–1556. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Liu, M.; Fu, H.; Qi, F.; Lin, F.R.; Walsh, A.; Jen, A.K.Y. A Hole-Selective Self-Assembled Monolayer for Both Efficient Perovskite and Organic Solar Cells. Langmuir 2024, 40, 4772–4778. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y.; Du, M.; Cao, Y.; Ren, X.; Zhang, L.; Wang, H.; Zhao, S.; Wang, K.; Liu, S. Self-Assembled Amphiphilic Monolayer for Efficient and Stable Wide-Bandgap Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2202802. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, R.; Tang, Y.; Su, Z.; Hu, S.; Zhang, X.; Zhang, J.; Zhao, J.; Xue, Y.; Gao, X.; et al. Anchoring Charge Selective Self-Assembled Monolayers for Tin–Lead Perovskite Solar Cells. Adv. Mater. 2024, 36, e2312264. [Google Scholar] [CrossRef]
- Baek, M.G.; Shin, J.E.; Park, S.G. Differences in ITO Interface Characteristics Change According to the Formation of Aromatic-Ring and Aliphatic Self-Assembled Monolayers. Crystals 2021, 11, 26. [Google Scholar] [CrossRef]
- Hotchkiss, P.J.; Jones, S.C.; Paniagua, S.A.; Sharma, A.; Kippelen, B.; Armstrong, N.R.; Marder, S.R. The Modification of Indium Tin Oxide with Phosphonic Acids: Mechanism of Binding, Tuning of Surface Properties, and Potential for Use in Organic Electronic Applications. Acc. Chem. Res. 2012, 45, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kwon, H.; Kim, E.; Kim, D.W.; Son, H.J.; Kim, D.H. Interfacial Engineering of a ZnO Electron Transporting Layer Using Self-Assembled Monolayers for High Performance and Stable Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 2105–2113. [Google Scholar] [CrossRef]
- Li, E.; Liu, C.; Lin, H.; Xu, X.; Liu, S.; Zhang, S.; Yu, M.; Cao, X.M.; Wu, Y.; Zhu, W.H. Bonding Strength Regulates Anchoring-Based Self-Assembly Monolayers for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2103847. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Y.J. Physisorption and Chemisorption of a Self-Assembled Monolayer by the Quartz Crystal Microbalance. Langmuir 2014, 30, 9637–9642. [Google Scholar] [CrossRef]
- Dai, Z.; You, S.; Chakraborty, D.; Li, S.; Zhang, Y.; Ranka, A.; Barlow, S.; Berry, J.J.; Marder, S.R.; Guo, P.; et al. Connecting Interfacial Mechanical Adhesion, Efficiency, and Operational Stability in High Performance Inverted Perovskite Solar Cells. ACS Energy Lett. 2024, 9, 1880–1887. [Google Scholar] [CrossRef]
- Mingorance, A.; Xie, H.; Kim, H.S.; Wang, Z.; Balsells, M.; Morales-Melgares, A.; Domingo, N.; Kazuteru, N.; Tress, W.; Fraxedas, J.; et al. Interfacial Engineering of Metal Oxides for Highly Stable Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800367. [Google Scholar] [CrossRef]
- Kreuzer, H.J.; Wang, R.L.C.; Grunze, M. Hydroxide Ion Adsorption on Self-Assembled Monolayers. J. Am. Chem. Soc. 2003, 125, 8384–8389. [Google Scholar] [CrossRef]
- Fakult, D.T.; Universit, F. Hierarchical Assemblies of Core-Shell Metal Oxide Nanomaterials via Amide Coupling Reactions Hierarchische Anordnungen von Kern-Schale Metalloxid Nanomaterialien Über Amid-Kupplungsreaktionen. Ph.D. Thesis, University of Erlangen-Nuremberg, Erlangen, Germany, 2022. [Google Scholar]
- Zhang, S.; Wu, R.; Mu, C.; Wang, Y.; Han, L.; Wu, Y.; Zhu, W.H. Conjugated Self-Assembled Monolayer as Stable Hole-Selective Contact for Inverted Perovskite Solar Cells. ACS Mater. Lett. 2022, 4, 1976–1983. [Google Scholar] [CrossRef]
- Wang, G.; Chen, K.; Cheng, L.; Wang, D.; Meng, F.; Xiang, W. Application of Hole-Selective Self-Assembled Monolayers in Inverted Perovskite Solar Cells. Sol. RRL 2024, 8, 2300996. [Google Scholar] [CrossRef]
- Lim, M.S.; Feng, K.; Chen, X.; Wu, N.; Raman, A.; Nightingale, J.; Gawalt, E.S.; Korakakis, D.; Hornak, L.A.; Timperman, A.T. Adsorption and Desorption of Stearic Acid Self-Assembled Monolayers on Aluminum Oxide. Langmuir 2007, 23, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Chen, Y.; Zhang, H.; Zhang, S.T.; Zhang, Z.; Lin, F.; Liang, L.; Gong, L.; Hao, H.; et al. Enhancing Efficiency of Industrially-Compatible Monolithic Perovskite/Silicon Tandem Solar Cells with Dually-Mixed Self-Assembled Monolayers. Adv. Funct. Mater. 2024, 34, 2407805. [Google Scholar] [CrossRef]
- Feeney, T.; Petry, J.; Torche, A.; Hauschild, D.; Hacene, B.; Wansorra, C.; Diercks, A.; Ernst, M.; Weinhardt, L.; Heske, C.; et al. Understanding and Exploiting Interfacial Interactions between Phosphonic Acid Functional Groups and Co-Evaporated Perovskites. Matter 2024, 7, 2066–2090. [Google Scholar] [CrossRef]
- Abate, S.Y.; Huang, D.C.; Tao, Y.T. Surface Modification of TiO2 Layer with Phosphonic Acid Monolayer in Perovskite Solar Cells: Effect of Chain Length and Terminal Functional Group. Org. Electron. 2020, 78, 105583. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Chen, M.; Zhao, Y.; Lin, X.; Qin, Z.; Wang, Y.; Han, L. Constructing a Stable and Efficient Buried Heterojunction via Halogen Bonding for Inverted Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2203250. [Google Scholar] [CrossRef]
- Hung, C.M.; Mai, C.L.; Wu, C.C.; Chen, B.H.; Lu, C.H.; Chu, C.C.; Wang, M.C.; Yang, S.D.; Chen, H.C.; Yeh, C.Y.; et al. Self-Assembled Monolayers of Bi-Functionalized Porphyrins: A Novel Class of Hole-Layer-Coordinating Perovskites and Indium Tin Oxide in Inverted Solar Cells. Angew. Chem.-Int. Ed. 2023, 62, e202309831. [Google Scholar] [CrossRef]
- Xu, Y.; Musgrave, C.B. A DFT Study of the Al2O3 Atomic Layer Deposition on SAMs: Effect of SAM Termination. Chem. Mater. 2004, 16, 646–653. [Google Scholar] [CrossRef]
- Fürer, S.O.; Rietwyk, K.J.; Pulvirenti, F.; McMeekin, D.P.; Surmiak, M.A.; Raga, S.R.; Mao, W.; Lin, X.; Hora, Y.; Wang, J.; et al. Naphthalene-Imide Self-Assembled Monolayers as a Surface Modification of ITO for Improved Thermal Stability of Perovskite Solar Cells. ACS Appl. Energy Mater. 2023, 6, 667–677. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, T.; Pu, X.; He, X.; Xiao, M.; Chen, H.; Zhuang, L.; Wei, Q.; Loi, H.L.; Guo, P.; et al. Co-Self-Assembled Monolayers Modified NiOx for Stable Inverted Perovskite Solar Cells. Adv. Mater. 2024, 36, e2311970. [Google Scholar] [CrossRef]
- Zhang, C.; Das, S.; Sakurai, N.; Imaizumi, T.; Sanjayan, S.; Shoji, Y.; Fukushima, T.; Zharnikov, M. Phosphonic Acid Anchored Tripodal Molecular Films on Indium Tin Oxide. Phys. Chem. Chem. Phys. 2024, 26, 11360–11369. [Google Scholar] [CrossRef] [PubMed]
- Fournier, O.; Bapaume, C.D.; Messou, D.; Bouttemy, M.; Schulz, P.; Ozanam, F.; Lombez, L.; Schneider, N.; Rousset, J. Chemical Passivation with Phosphonic Acid Derivatives of ZnO Deposited by Atomic Layer Deposition and Its Influence on the Halide Perovskite Interface. ACS Appl. Energy Mater. 2021, 4, 5787–5797. [Google Scholar] [CrossRef]
- Lange, I.; Reiter, S.; Pätzel, M.; Zykov, A.; Nefedov, A.; Hildebrandt, J.; Hecht, S.; Kowarik, S.; Wöll, C.; Heimel, G.; et al. Tuning the Work Function of Polar Zinc Oxide Surfaces Using Modified Phosphonic Acid Self-Assembled Monolayers. Adv. Funct. Mater. 2014, 24, 7014–7024. [Google Scholar] [CrossRef]
- Wu, T.; Mariotti, S.; Ji, P.; Ono, L.K.; Guo, T.; Rabehi, I.N.; Yuan, S.; Zhang, J.; Ding, C.; Guo, Z.; et al. Self-Assembled Monolayer Hole-Selective Contact for Up-Scalable and Cost-Effective Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2316500. [Google Scholar] [CrossRef]
- Song, D.; Narra, S.; Li, M.Y.; Lin, J.S.; Diau, E.W.G. Interfacial Engineering with a Hole-Selective Self-Assembled Monolayer for Tin Perovskite Solar Cells via a Two-Step Fabrication. ACS Energy Lett. 2021, 6, 4179–4186. [Google Scholar] [CrossRef]
- Ullah, A.; Park, K.H.; Nguyen, H.D.; Siddique, Y.; Shah, S.F.A.; Tran, H.; Park, S.; Lee, S.I.; Lee, K.K.; Han, C.H.; et al. Novel Phenothiazine-Based Self-Assembled Monolayer as a Hole Selective Contact for Highly Efficient and Stable p-i-n Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2103175. [Google Scholar] [CrossRef]
- Xie, L.; Chen, J.; Vashishtha, P.; Zhao, X.; Shin, G.S.; Mhaisalkar, S.G.; Park, N.G. Importance of Functional Groups in Cross-Linking Methoxysilane Additives for High-Efficiency and Stable Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 2192–2200. [Google Scholar] [CrossRef]
- Guo, H.; Liu, C.; Hu, H.; Zhang, S.; Ji, X.; Cao, X.M.; Ning, Z.; Zhu, W.H.; Tian, H.; Wu, Y. Neglected Acidity Pitfall: Boric Acid-Anchoring Hole-Selective Contact for Perovskite Solar Cells. Natl. Sci. Rev. 2023, 10, nwad057. [Google Scholar] [CrossRef]
- Cassella, E.J.; Spooner, E.L.K.; Thornber, T.; O’Kane, M.E.; Catley, T.E.; Bishop, J.E.; Smith, J.A.; Game, O.S.; Lidzey, D.G. Gas-Assisted Spray Coating of Perovskite Solar Cells Incorporating Sprayed Self-Assembled Monolayers. Adv. Sci. 2022, 9, 2104848. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Zhang, Y.; Chen, M.; Liu, T.; Xiao, C.; Gao, D.; Patel, J.B.; Kuciauskas, D.; Magomedov, A.; et al. Co-Deposition of Hole-Selective Contact and Absorber for Improving the Processability of Perovskite Solar Cells. Nat. Energy 2023, 8, 462–472. [Google Scholar] [CrossRef]
- Zhan, L.; Zhang, S.; Li, Z.; Li, W.; Zhang, H.; He, J.; Ji, X.; Liu, S.; Yu, F.; Wang, S.; et al. Anchorable Polymers Enabling Ultra-Thin and Robust Hole-Transporting Layers for High-Efficiency Inverted Perovskite Solar Cells. Angew. Chem.-Int. Ed. 2025, 64, 202422571. [Google Scholar] [CrossRef]
- Chen, T.P.; Lin, C.W.; Li, S.S.; Tsai, Y.H.; Wen, C.Y.; Lin, W.J.; Hsiao, F.M.; Chiu, Y.P.; Tsukagoshi, K.; Osada, M.; et al. Self-Assembly Atomic Stacking Transport Layer of 2D Layered Titania for Perovskite Solar Cells with Extended UV Stability. Adv. Energy Mater. 2018, 8, 1701722. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, M.; Zhang, Y.; Wang, Z.; Sun, Z.; Zhang, W.; Xiong, Y.; Zong, X.; Wang, Y.; Liang, M. Asymmetric Modification of Carbazole Based Self-Assembled Monolayers by Hybrid Strategy for Inverted Perovskite Solar Cells. Angew. Chem.-Int. Ed. 2024, 64, e202416188. [Google Scholar] [CrossRef]
- Bi, H.; Liu, J.; Wang, L.; Liu, T.; Zhang, Z.; Shen, Q.; Hayase, S. Selective Contact Self-Assembled Molecules for High-Performance Perovskite Solar Cells. eScience 2025, 5, 100329. [Google Scholar] [CrossRef]
- Pauls, A.; Dunnum, A.; Martin, A.; Huth, A.; Du, C.; Thuo, M. Correcting Edge Defects in Self-Assembled Monolayers through Thermal Annealing. ChemPhysChem 2024, 25, e202400626. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, C.; Lei, H.; Zheng, X.; Qin, P.; Xiong, L.; Zhao, X.; Yan, Y.; Fang, G. Interface Engineering in Planar Perovskite Solar Cells: Energy Level Alignment, Perovskite Morphology Control and High Performance Achievement. J. Mater. Chem. A 2017, 5, 1658–1666. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.S.; Park, J.; Lee, J.W.; Kim, K. Opportunities and Challenges for Perovskite Solar Cells Based on Vacuum Thermal Evaporation. Adv. Mater. Technol. 2023, 8, 2200928. [Google Scholar] [CrossRef]
- Gaur, N.; Misra, M.; Hossain, K.; Kabra, D. Improved Thermally Activated Delayed Fluorescence-Based Electroluminescent Devices Using Vacuum-Processed Carbazole-Based Self-Assembled Monolayers. ACS Energy Lett. 2024, 9, 1056–1062. [Google Scholar] [CrossRef]
- Du, P.; Wang, L.; Li, J.; Luo, J.; Ma, Y.; Tang, J.; Zhai, T. Thermal Evaporation for Halide Perovskite Optoelectronics: Fundamentals, Progress, and Outlook. Adv. Opt. Mater. 2022, 10, 2101770. [Google Scholar] [CrossRef]
- Choi, Y.; Koo, D.; Jeong, M.; Jeong, G.; Lee, J.; Lee, B.; Choi, K.J.; Yang, C.; Park, H. Toward All-Vacuum-Processable Perovskite Solar Cells with High Efficiency, Stability, and Scalability Enabled by Fluorinated Spiro-OMeTAD through Thermal Evaporation. Sol. RRL 2021, 5, 2100415. [Google Scholar] [CrossRef]
- Wang, Z.; Lyu, M.; Zhang, B.W.; Xiao, M.; Zhang, C.; Han, E.Q.; Wang, L. Thermally Evaporated Metal Halide Perovskites and Their Analogues: Film Fabrication, Applications and Beyond. Small Methods 2024, 9, e2301633. [Google Scholar] [CrossRef]
- Bae, S.R.; Heo, D.Y.; Kim, S.Y. Recent Progress of Perovskite Devices Fabricated Using Thermal Evaporation Method: Perspective and Outlook. Mater. Today Adv. 2022, 14, 100232. [Google Scholar] [CrossRef]
- Kosasih, F.U.; Erdenebileg, E.; Mathews, N.; Mhaisalkar, S.G.; Bruno, A. Thermal Evaporation and Hybrid Deposition of Perovskite Solar Cells and Mini-Modules. Joule 2022, 6, 2692–2734. [Google Scholar] [CrossRef]
- Yuan, Q.; Lohmann, K.B.; Oliver, R.D.J.; Ramadan, A.J.; Yan, S.; Ball, J.M.; Christoforo, M.G.; Noel, N.K.; Snaith, H.J.; Herz, L.M.; et al. Thermally Stable Perovskite Solar Cells by All-Vacuum Deposition. ACS Appl. Mater. Interfaces 2023, 15, 772–781. [Google Scholar] [CrossRef]
- Farag, A.; Feeney, T.; Hossain, I.M.; Schackmar, F.; Fassl, P.; Küster, K.; Bäuerle, R.; Ruiz-Preciado, M.A.; Hentschel, M.; Ritzer, D.B.; et al. Evaporated Self-Assembled Monolayer Hole Transport Layers: Lossless Interfaces in p-i-n Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2203982. [Google Scholar] [CrossRef]
- Chen, B.; Peng, C.; Guo, R.; He, Z.; Sun, L.; Zhang, F.; He, X.; Zeng, H.; Wang, L. Dual-Function Self-Assembled Molecules as Hole-Transport Layers for Thermally Evaporated High-Efficiency Blue Perovskite Light-Emitting Diodes. Adv. Mater. 2024, 37, e2411451. [Google Scholar] [CrossRef] [PubMed]
- Kore, B.P.; Er-Raji, O.; Fischer, O.; Callies, A.; Schultz-Wittmann, O.; Schulze, P.S.C.; Bivour, M.; De Wolf, S.; Glunz, S.W.; Borchert, J. Efficient Fully Textured Perovskite Silicon Tandems with Thermally Evaporated Hole Transporting Materials. Energy Environ. Sci. 2024, 18, 354–366. [Google Scholar] [CrossRef]
- Mariotti, S.; Rabehi, I.N.; Zhang, C.; Huo, X.; Zhang, J.; Ji, P.; Wu, T.; Li, T.; Yuan, S.; Liu, X.; et al. Unraveling the Morphological and Energetic Properties of 2PACz Self-Assembled Monolayers Fabricated With Upscaling Deposition Methods. Energy Environ. Mater. 2024, 8, e12825. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; He, B.; Zheng, R.; Wang, Y.; Chen, S. An Energy Level Alignment Study of 2PACz Molecule on Perovskite Device-Related Interfaces by Vacuum Deposition. Adv. Energy Sustain. Res. 2025, 2400336. [Google Scholar] [CrossRef]
- JIANG, W.; Wang, D.; Shang, W.; Li, Y.; Zeng, J.; Zhu, P.; Zhang, B.; Mei, L.; Chen, X.-K.; Xu, Z.-X.; et al. Spin-coated and Vacuum-processed Hole-extracting Self-assembled Multilayers with H-aggregation for High-performance Inverted Perovskite Solar Cells. Angew. Chemie Int. Ed. 2024, 63, e202411730. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Wang, X.; Gao, Y.; Hou, T.A.; Teng, X.; Wang, H.; Chen, W.; Gao, S.; Li, X.; et al. Complementary Self-Assembled Monolayers Enabling Improved Energy Level Alignment in Inverted Perovskite Solar Cells. J. Energy Chem. 2025, 104, 136–145. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Marčinskas, M.; Kasparavičius, E.; Malinauskas, T.; Palmstrom, A.; Getautis, V.; Albrecht, S.; McGehee, M.D.; Magomedov, A. Wettability Improvement of a Carbazole-Based Hole-Selective Monolayer for Reproducible Perovskite Solar Cells. ACS Energy Lett. 2023, 8, 898–900. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, F.; Wang, X.; Chen, R.; Zhang, H.; Zhan, L.; Jiang, X.; Li, Y.; Ji, X.; Liu, S.; et al. Minimizing Buried Interfacial Defects for Efficient Inverted Perovskite Solar Cells. Science 2023, 380, 404–409. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.J.; Kim, D.H.; Noh, Y.J.; Kwon, S.N.; Kim, D.H.; Na, S.I. Vacuum-Assisted Deposition of Highly Hydrophobic Self-Assembled Monolayer for High-Efficiency Perovskite Solar Cells. Sol. RRL 2024, 8, 2400170. [Google Scholar] [CrossRef]
- Li, T.; Wang, K.; Tong, Y.; Qi, H.; Yue, S.; Li, W.; Wang, H. In Situ Dehydration Condensation of Self-Assembled Molecules Enables Stabilization of CsPbI3 Perovskites for Efficient Photovoltaics. Adv. Funct. Mater. 2024, 34, 2409621. [Google Scholar] [CrossRef]
- Wagstaffe, M.; Thomas, A.G.; Jackman, M.J.; Torres-Molina, M.; Syres, K.L.; Handrup, K. An Experimental Investigation of the Adsorption of a Phosphonic Acid on the Anatase TiO2(101) Surface. J. Phys. Chem. C 2016, 120, 1693–1700. [Google Scholar] [CrossRef]
- Shao, S.; Loi, M.A. The Role of the Interfaces in Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7, 1901469. [Google Scholar]
- Zhang, J.; Yang, J.; Dai, R.; Sheng, W.; Su, Y.; Zhong, Y.; Li, X.; Tan, L.; Chen, Y. Elimination of Interfacial Lattice Mismatch and Detrimental Reaction by Self-Assembled Layer Dual-Passivation for Efficient and Stable Inverted Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2103674. [Google Scholar] [CrossRef]
- Mann, D.S.; Patil, P.; Kwon, S.N.; Na, S.I. Enhanced Performance of P-i-n Perovskite Solar Cell via Defect Passivation of Nickel Oxide/Perovskite Interface with Self-Assembled Monolayer. Appl. Surf. Sci. 2021, 560, 149973. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Subbiah, A.S.; Eswaran, M.K.; Howells, C.T.; Babayigit, A.; De Bastiani, M.; Yengel, E.; Liu, J.; Furlan, F.; Harrison, G.T.; et al. Scaling-up Perovskite Solar Cells on Hydrophobic Surfaces. Nano Energy 2021, 81, 105633. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Zhang, M.; Hameiri, Z.; Liu, X.; Bai, Y.; Hao, X. PTAA as Efficient Hole Transport Materials in Perovskite Solar Cells: A Review. Sol. RRL 2022, 6, 2200234. [Google Scholar] [CrossRef]
- Xu, J.; Dai, J.; Dong, H.; Li, P.; Chen, J.; Zhu, X.; Wang, Z.; Jiao, B.; Hou, X.; Li, J.; et al. Surface-Tension Release in PTAA-Based Inverted Perovskite Solar Cells. Org. Electron. 2022, 100, 106378. [Google Scholar] [CrossRef]
- Kuan, C.H.; Luo, G.S.; Narra, S.; Maity, S.; Hiramatsu, H.; Tsai, Y.W.; Lin, J.M.; Hou, C.H.; Shyue, J.J.; Diau, E.W.-G. How Can a Hydrophobic Polymer PTAA Serve as a Hole- Transport Layer for an Inverted Tin Perovskite Solar Cell? Chem. Eng. J. 2022, 450, 138037. [Google Scholar] [CrossRef]
- Scarfato, P.; Schiavone, N.; Rossi, G.; Incarnato, L. An Easy Route to Wettability Changes of Polyethylene Terephthalate-Silicon Oxide Substrate Films for High Barrier Applications, Surface-Modified with a Self-Assembled Monolayer of Fluoroalkylsilanes. Polymers 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, T. Improving the Performance Parameters of Organic Field-Effect Transistors via Alkyl Chain Length of Boronic Acid Self-Assembled Monolayers. J. Mater. Sci. Mater. Electron. 2024, 35, 966. [Google Scholar] [CrossRef]
- Zhao, Y.; Luan, X.; Han, L.; Wang, Y. Post-Assembled Alkylphosphonic Acids for Efficient and Stable Inverted Perovskite Solar Cells. Adv. Funct. Mater. 2024, 34, 2405646. [Google Scholar] [CrossRef]
- Pylnev, M.; Barbisan, A.M.; Wei, T.C. Effect of Wettability of Substrate on Metal Halide Perovskite Growth. Appl. Surf. Sci. 2021, 541, 148559. [Google Scholar] [CrossRef]
- Li, E.; Bi, E.; Wu, Y.; Zhang, W.; Li, L.; Chen, H.; Han, L.; Tian, H.; Zhu, W.H. Synergistic Coassembly of Highly Wettable and Uniform Hole-Extraction Monolayers for Scaling-up Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1909509. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, M.; Han, T.; Wang, Y.; Luo, X.; Chai, W.; Xi, H.; Zhou, L.; Chen, D.; Zhang, J.; et al. Homogeneous Crystallization of MA-Free, Wide-Bandgap Perovskite Films via Self-Assembled Monolayer Capping for Laminated Silicon/Perovskite Tandem Solar Cells. Chem. Eng. J. 2024, 500, 156798. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, X.; Zhang, J.; Yuan, W.; Qu, D.; Chen, Y.; He, L.; Wang, H.; Yang, G.; Zhang, W.; et al. Manipulating the Crystallization Kinetics of Halide Perovskites for Large-Area Solar Modules. Commun. Mater. 2024, 5, 131. [Google Scholar] [CrossRef]
- Ali, F.; Roldán-Carmona, C.; Sohail, M.; Nazeeruddin, M.K. Applications of Self-Assembled Monolayers for Perovskite Solar Cells Interface Engineering to Address Efficiency and Stability. Adv. Energy Mater. 2020, 10, 2002989. [Google Scholar] [CrossRef]
- Li, M.; Sun, R.; Chang, J.; Dong, J.; Tian, Q.; Wang, H.; Li, Z.; Yang, P.; Shi, H.; Yang, C.; et al. Orientated Crystallization of FA-Based Perovskite via Hydrogen-Bonded Polymer Network for Efficient and Stable Solar Cells. Nat. Commun. 2023, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, Y.; Li, F.; Lin, F.R.; Jen, A.K.Y. Hole-Selective Contact with Molecularly Tailorable Reactivity for Passivating High-Performing Inverted Perovskite Solar Cells. CCS Chem. 2024, 6, 1654–1661. [Google Scholar] [CrossRef]
- Chen, M.; Kapil, G.; Wang, L.; Sahamir, S.R.; Baranwal, A.K.; Nishimura, K.; Sanehira, Y.; Zhang, Z.; Kamarudin, M.A.; Shen, Q.; et al. High Performance Wide Bandgap Lead-Free Perovskite Solar Cells by Monolayer Engineering. Chem. Eng. J. 2022, 436, 135196. [Google Scholar] [CrossRef]
- Zeng, J.; Bi, L.; Cheng, Y.; Xu, B.; Jen, A.K.Y. Self-Assembled Monolayer Enabling Improved Buried Interfaces in Blade-Coated Perovskite Solar Cells for High Efficiency and Stability. Nano Res. Energy 2022, 1, e9120004. [Google Scholar] [CrossRef]
- Zuo, L.; Gu, Z.; Ye, T.; Fu, W.; Wu, G.; Li, H.; Chen, H. Enhanced Photovoltaic Performance of CH3NH3PbI3 Perovskite Solar Cells through Interfacial Engineering Using Self-Assembling Monolayer. J. Am. Chem. Soc. 2015, 137, 2674–2679. [Google Scholar] [CrossRef]
- Huang, Z.; Ge, X.; Liu, Z.; Shi, B.; Wang, P.; Zhao, Y.; Zhang, X. Highly Efficient Blade-Coated 1.67 EV p-i-n Perovskite Solar Cells Enabled by a Hybrid Self-Assembled Monolayer and Surface Passivation. ACS Appl. Energy Mater. 2024, 7, 11683–11690. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.; Li, Y.; An, Y.; Yao, Z.; Fan, B.; Qi, F.; Liu, K.; Yip, H.L.; Lin, F.R.; et al. Defect-Passivating and Stable Benzothiophene-Based Self-Assembled Monolayer for High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 2024, 14, 2303742. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, M.; Li, Y.; Lin, F.R.; Jen, A.K.Y. Rational Molecular Design of Multifunctional Self-Assembled Monolayers for Efficient Hole Selection and Buried Interface Passivation in Inverted Perovskite Solar Cells. Chem. Sci. 2024, 15, 2778–2785. [Google Scholar] [CrossRef]
- Alghamdi, A.R.M.; Yanagida, M.; Shirai, Y.; Andersson, G.G.; Miyano, K. Surface Passivation of Sputtered NiOx Using a SAM Interface Layer to Enhance the Performance of Perovskite Solar Cells. ACS Omega 2022, 7, 12147–12157. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in Perovskite-Halides and Their Effects in Solar Cells. Nat. Energy 2016, 1, 16149. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Mosconi, E.; De Angelis, F. Formation of Surface Defects Dominates Ion Migration in Lead-Halide Perovskites. ACS Energy Lett. 2019, 4, 779–785. [Google Scholar] [CrossRef]
- Naguib, A.; Elseman, A.M.; Ishak, E.A.; El-Gaby, M.S.A. Novel Hole Transport Materials of Pyrogallol-Sulfonamide Hybrid: Synthesis, Optical, Electrochemical Properties and Molecular Modelling for Perovskite Solar Cells. Mater. Renew. Sustain. Energy 2025, 14, 3. [Google Scholar] [CrossRef]
- Fadl, A.M.M.; Elseman, A.M.; El-Adasy, A.B.A.A.M.; Rashad, M.M.; El-Gaby, M.S.A. Passivator Materials Based on the Benzothiazole-Sulfonamide Hybrid: Synthesis, Optical, Electrochemical Properties, and Molecular Modeling for Perovskite Solar Cells. Mater. Res. Bull. 2025, 182, 113151. [Google Scholar] [CrossRef]
- Cassella, E.J.; Spooner, E.L.K.; Smith, J.A.; Thornber, T.; O’Kane, M.E.; Oliver, R.D.J.; Catley, T.E.; Choudhary, S.; Wood, C.J.; Hammond, D.B.; et al. Binary Solvent System Used to Fabricate Fully Annealing-Free Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2203468. [Google Scholar] [CrossRef]
- Zhu, T.; Su, J.; Labat, F.; Ciofini, I.; Pauporté, T. Interfacial Engineering through Chloride-Functionalized Self-Assembled Monolayers for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 744–752. [Google Scholar] [CrossRef]
- Tan, Q.; Li, Z.; Luo, G.; Zhang, X.; Che, B.; Chen, G.; Gao, H.; He, D.; Ma, G.; Wang, J.; et al. Inverted Perovskite Solar Cells Using Dimethylacridine-Based Dopants. Nature 2023, 620, 545–551. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Yun, S.; Irshad, Z.; Lee, W.; Kim, M.; Lim, J.; Kim, J. Vacuum Processability of Self-Assembled Monolayers and Their Chemical Interaction with Perovskite Interfaces. Energies 2025, 18, 1782. https://doi.org/10.3390/en18071782
Han H, Yun S, Irshad Z, Lee W, Kim M, Lim J, Kim J. Vacuum Processability of Self-Assembled Monolayers and Their Chemical Interaction with Perovskite Interfaces. Energies. 2025; 18(7):1782. https://doi.org/10.3390/en18071782
Chicago/Turabian StyleHan, Hyeji, Siwon Yun, Zobia Irshad, Wonjong Lee, Min Kim, Jongchul Lim, and Jinseck Kim. 2025. "Vacuum Processability of Self-Assembled Monolayers and Their Chemical Interaction with Perovskite Interfaces" Energies 18, no. 7: 1782. https://doi.org/10.3390/en18071782
APA StyleHan, H., Yun, S., Irshad, Z., Lee, W., Kim, M., Lim, J., & Kim, J. (2025). Vacuum Processability of Self-Assembled Monolayers and Their Chemical Interaction with Perovskite Interfaces. Energies, 18(7), 1782. https://doi.org/10.3390/en18071782





