Sulfur Emission Dependence on Various Factors During Biomass Combustion
Abstract
1. Introduction
- Stoichiometric ratio/oxygen partial pressure.
- The sulfur content of fuel.
- Alkaline/earth–alkaline content of the ash.
- Temperature.
- Residence time.
2. Materials and Methods
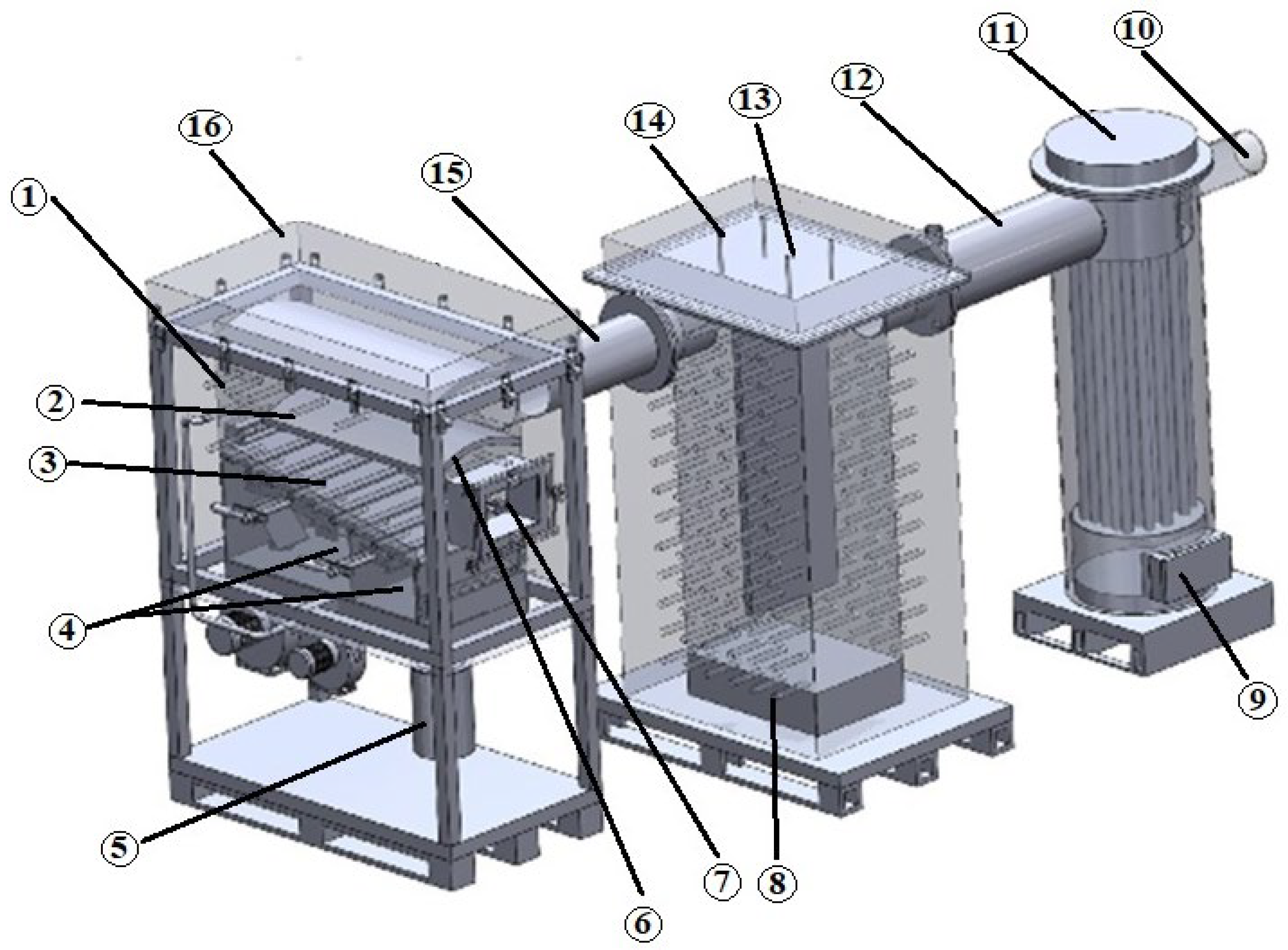
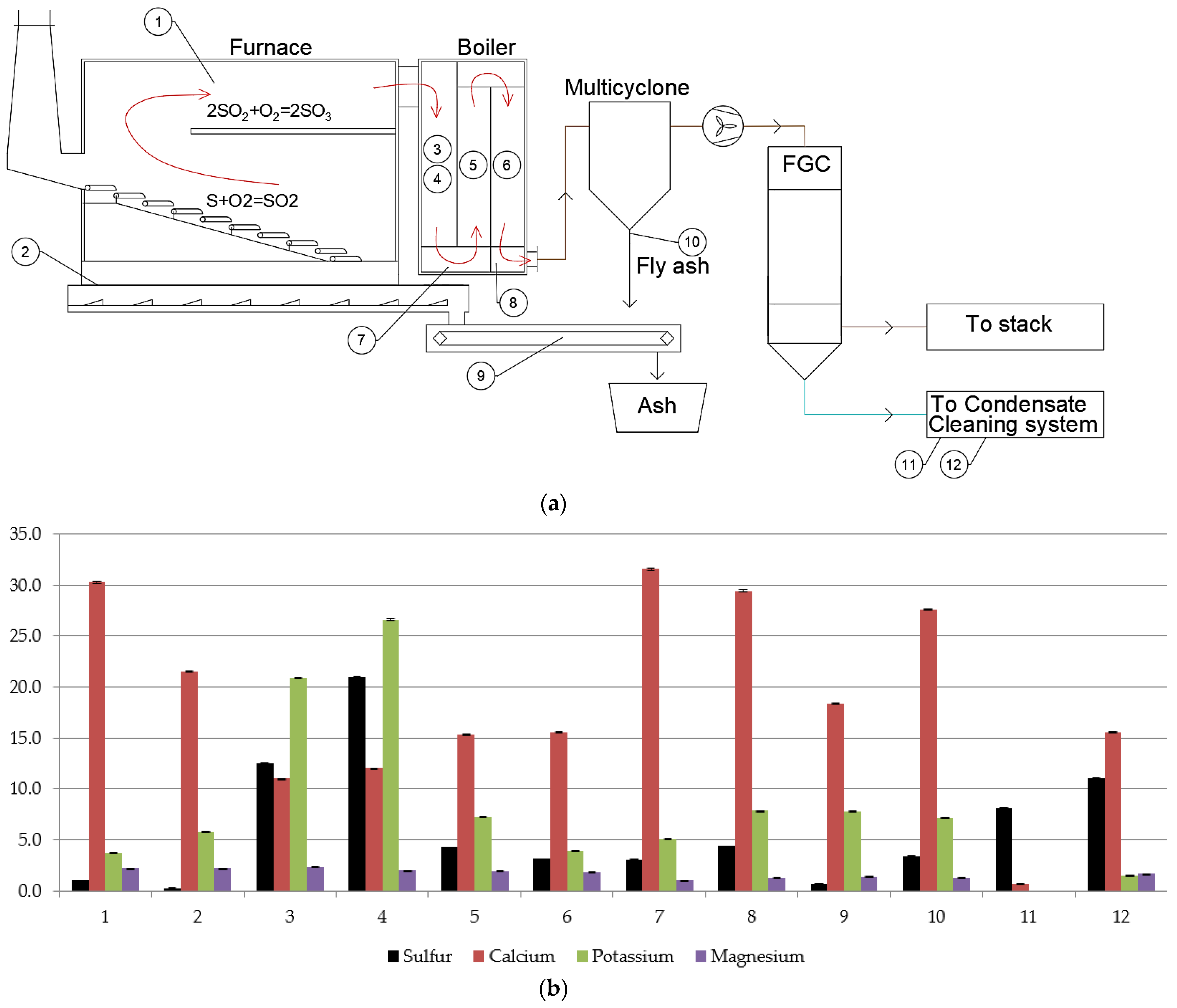
2.1. Description of the Experiments Using Experimental Stands
2.2. Experimental Fuel
2.3. Description of the Measuring Equipment
3. Results
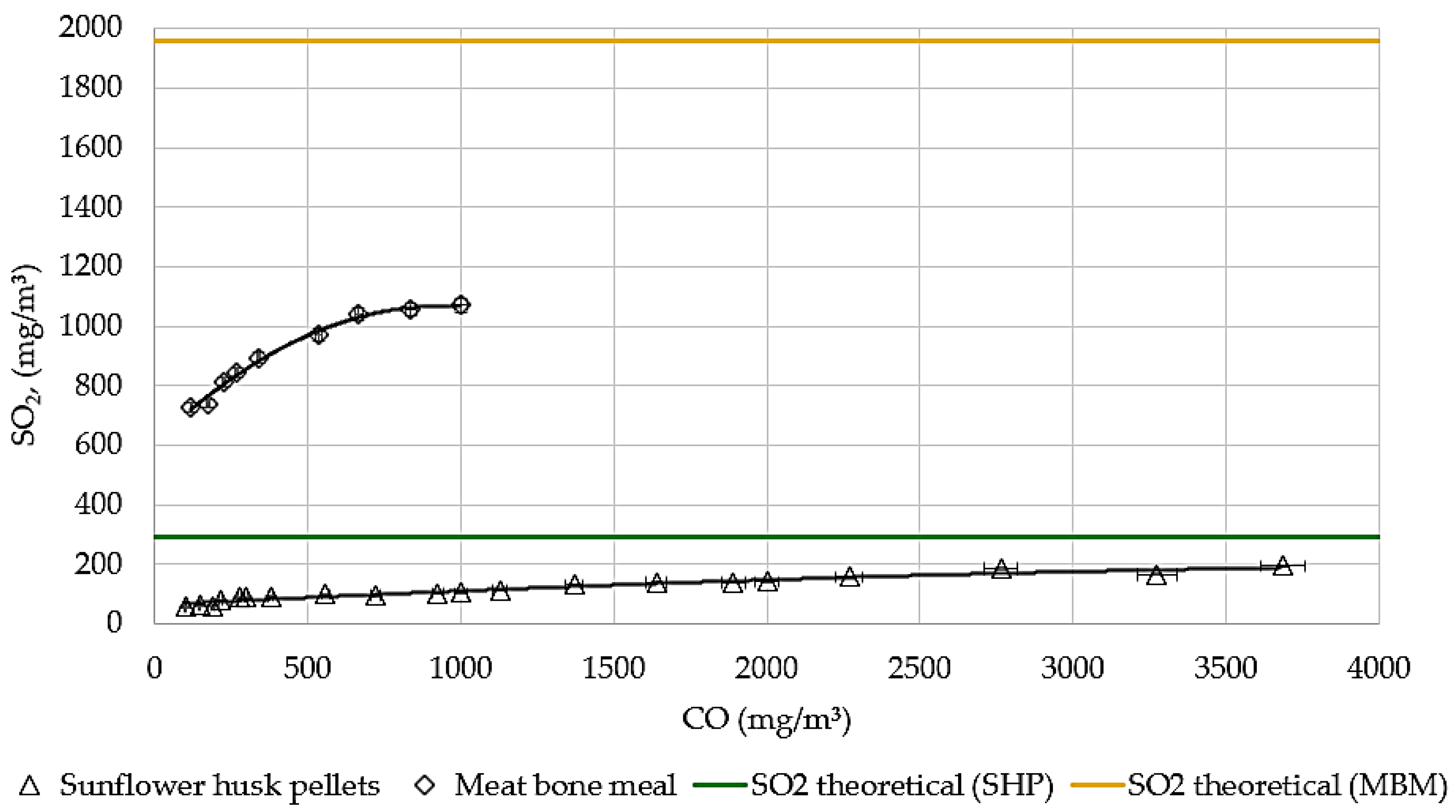
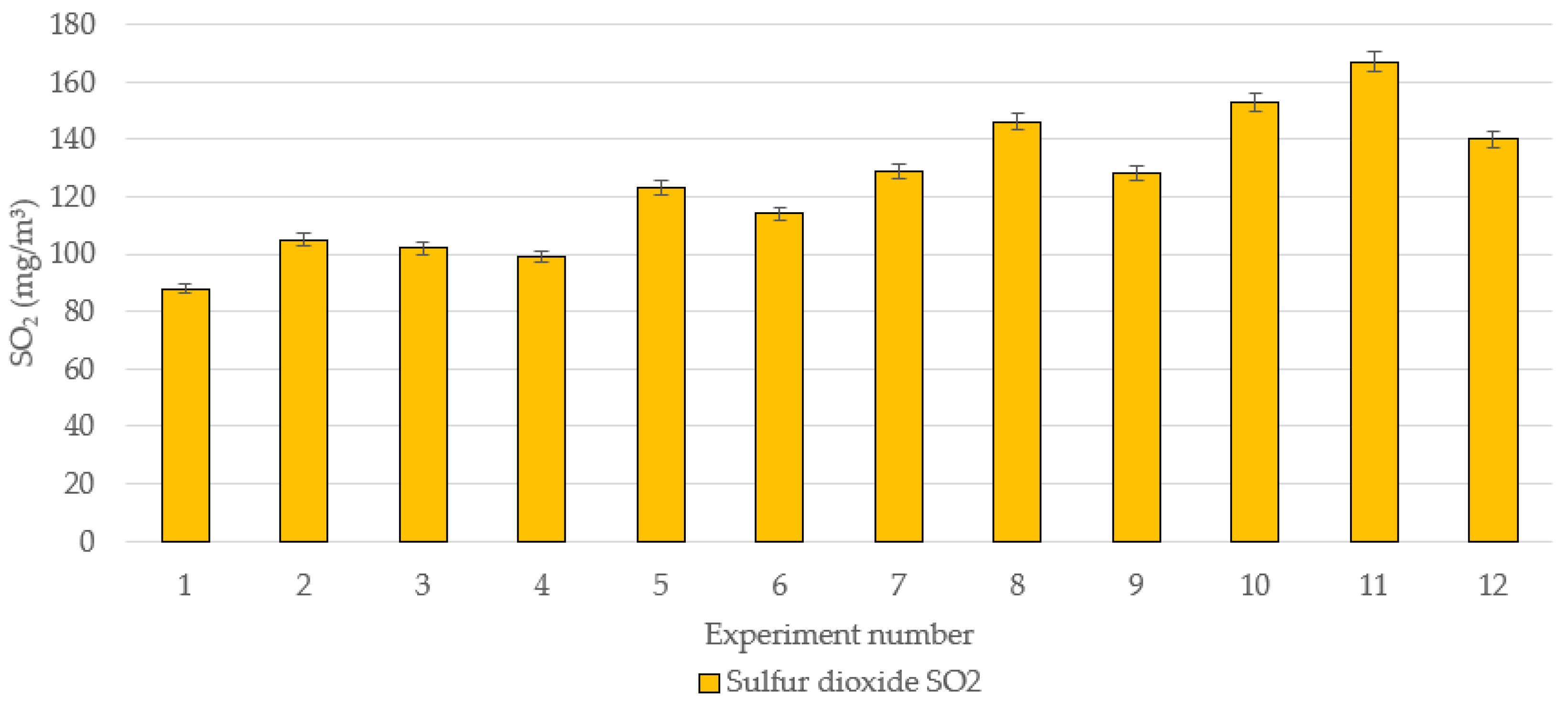
3.1. Theoretical and Experimentally Measured Values
3.2. The Influence of Combustion Completeness on Sulfur Compounds Emissions
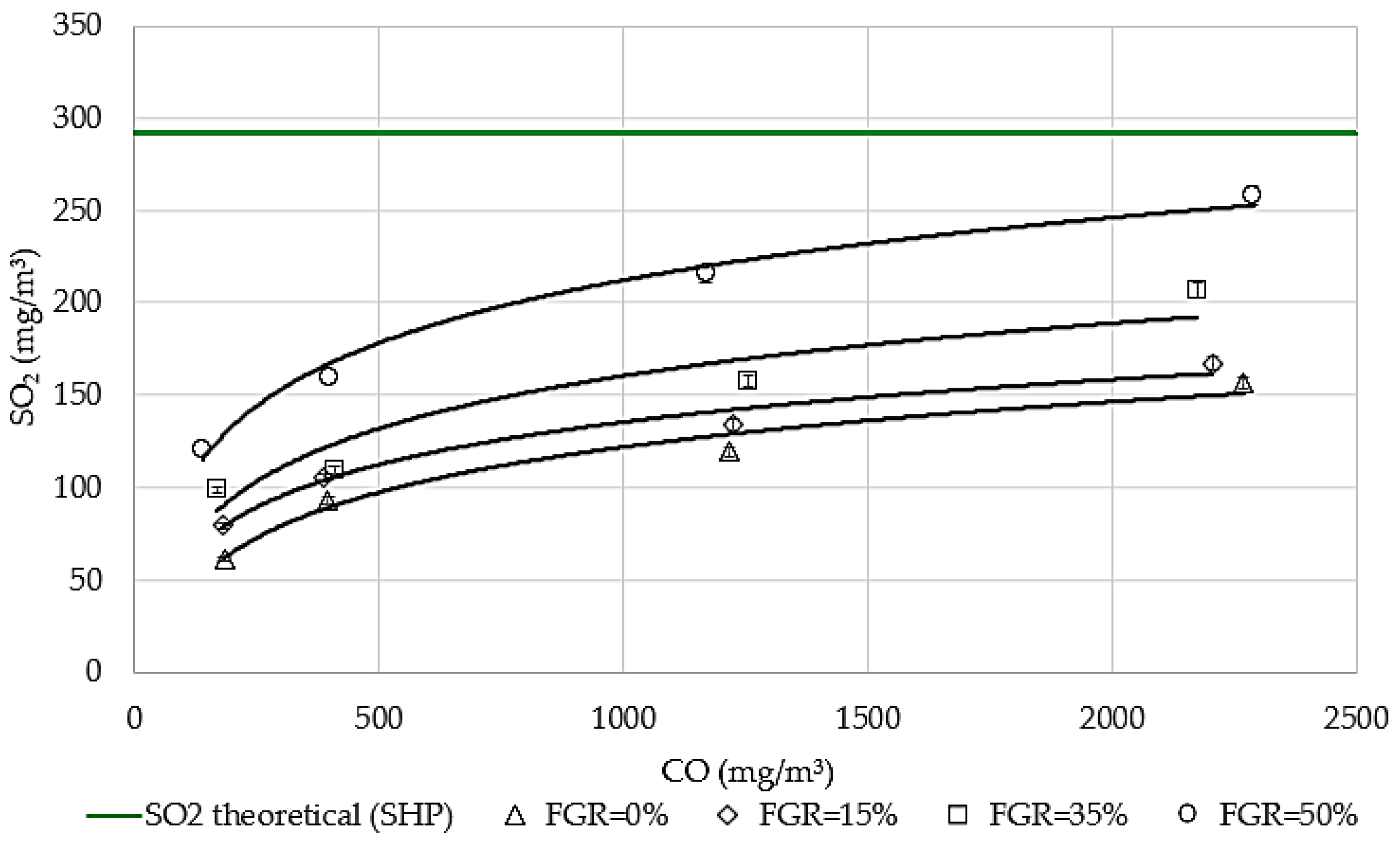
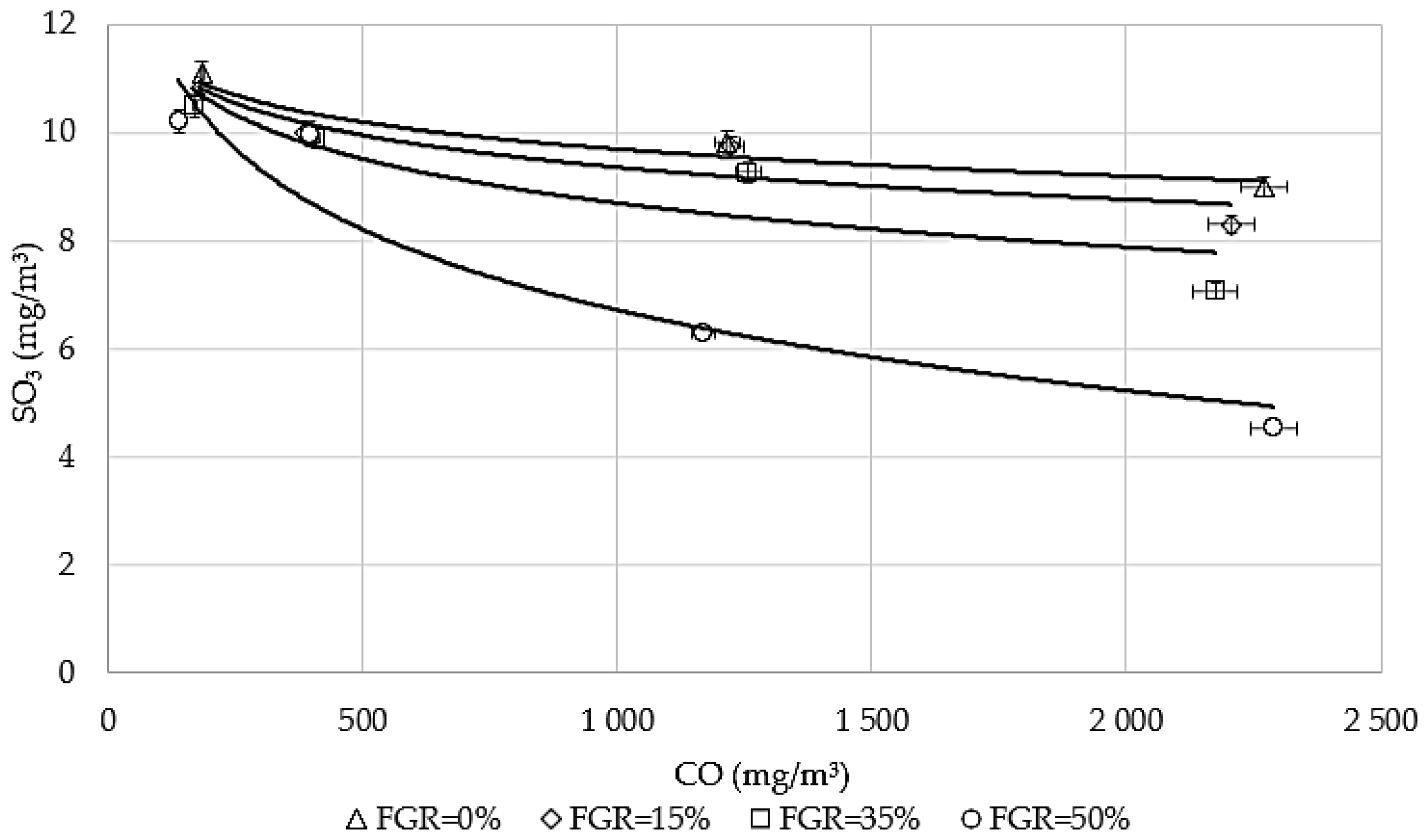
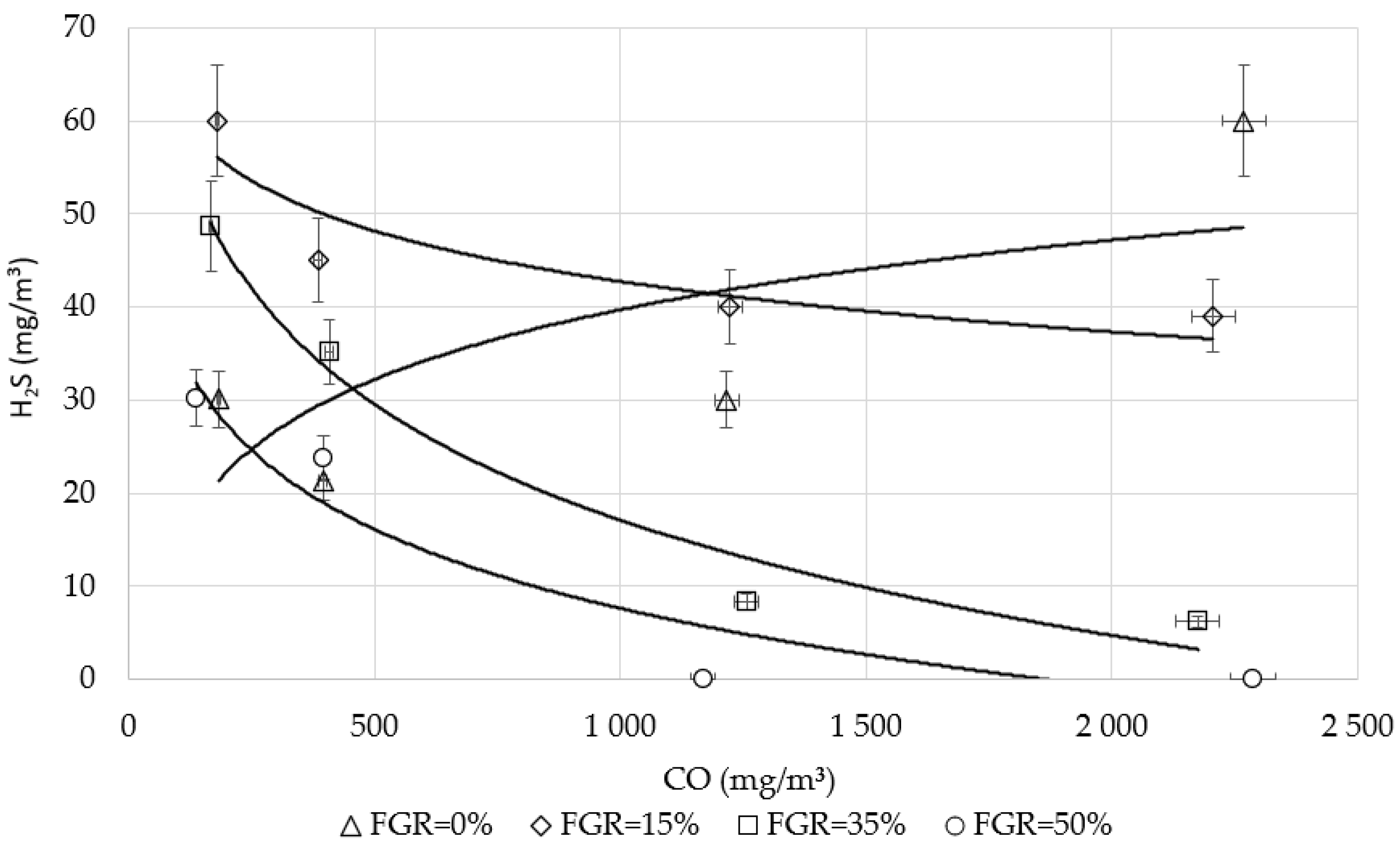
3.3. The Influence of CO on Sulfur Emissions Under Flue Gas Recirculation
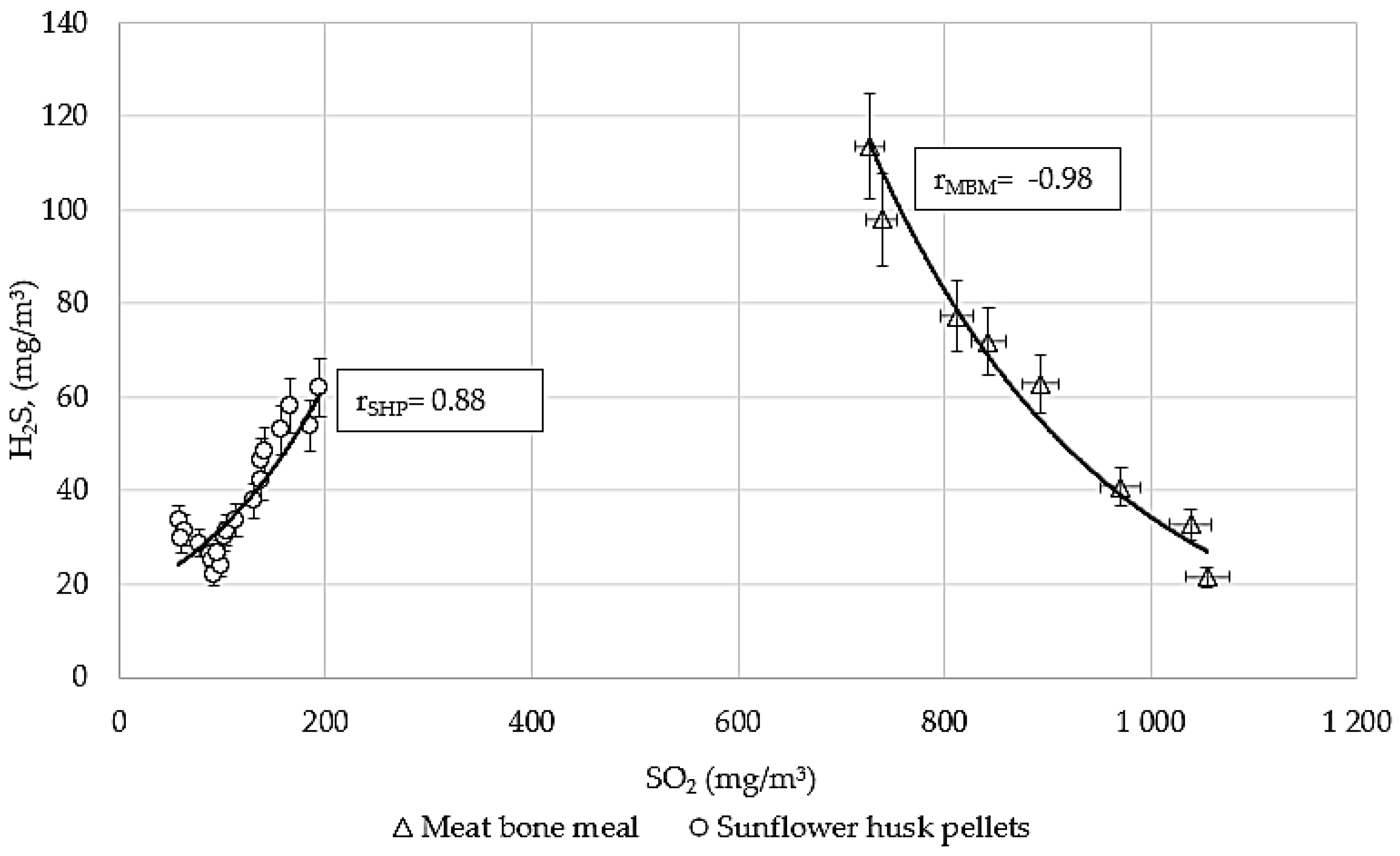
3.4. The Correlation Between SO2 and H2S
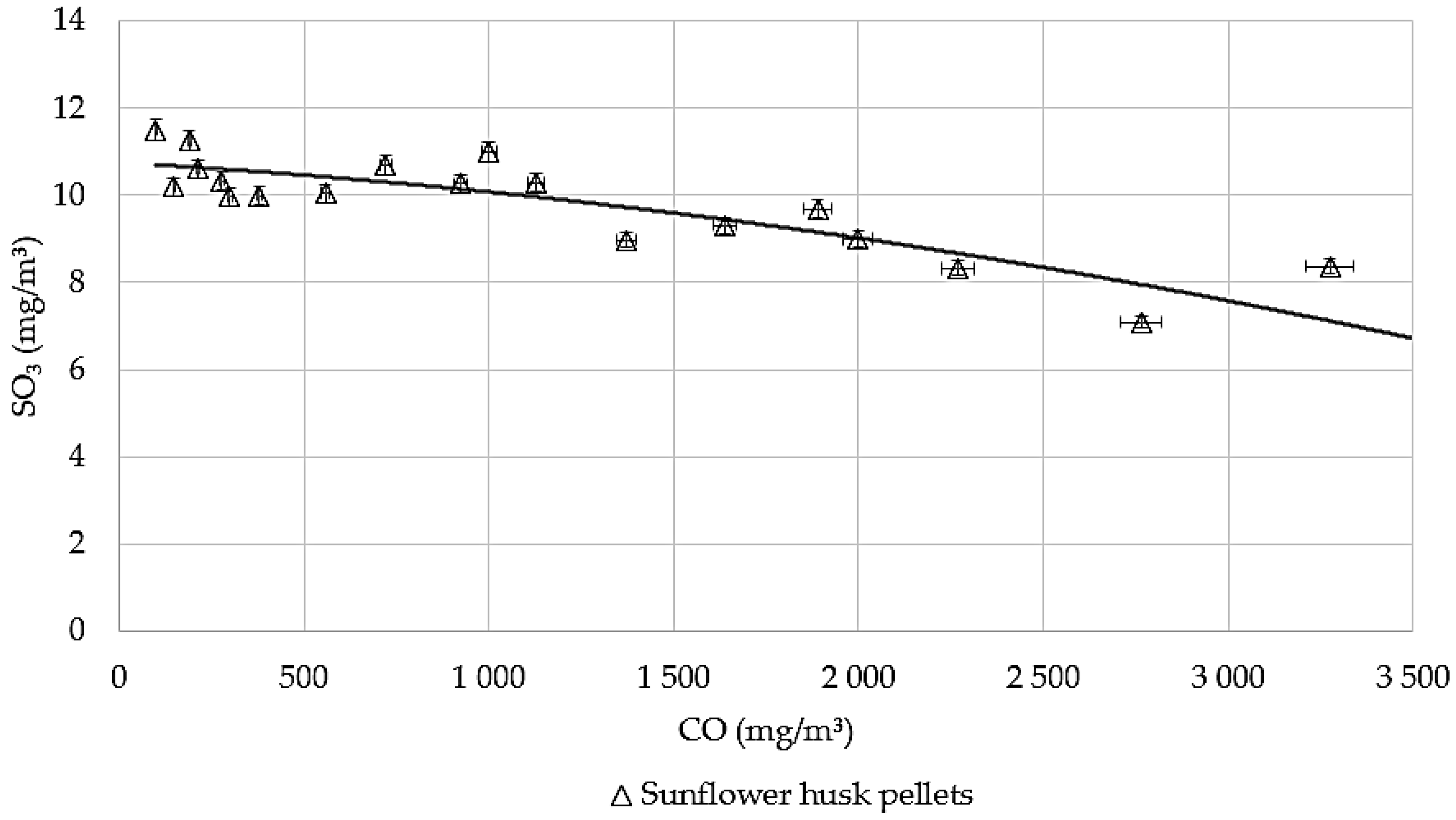
3.5. The Correlation Between SO2 and SO3
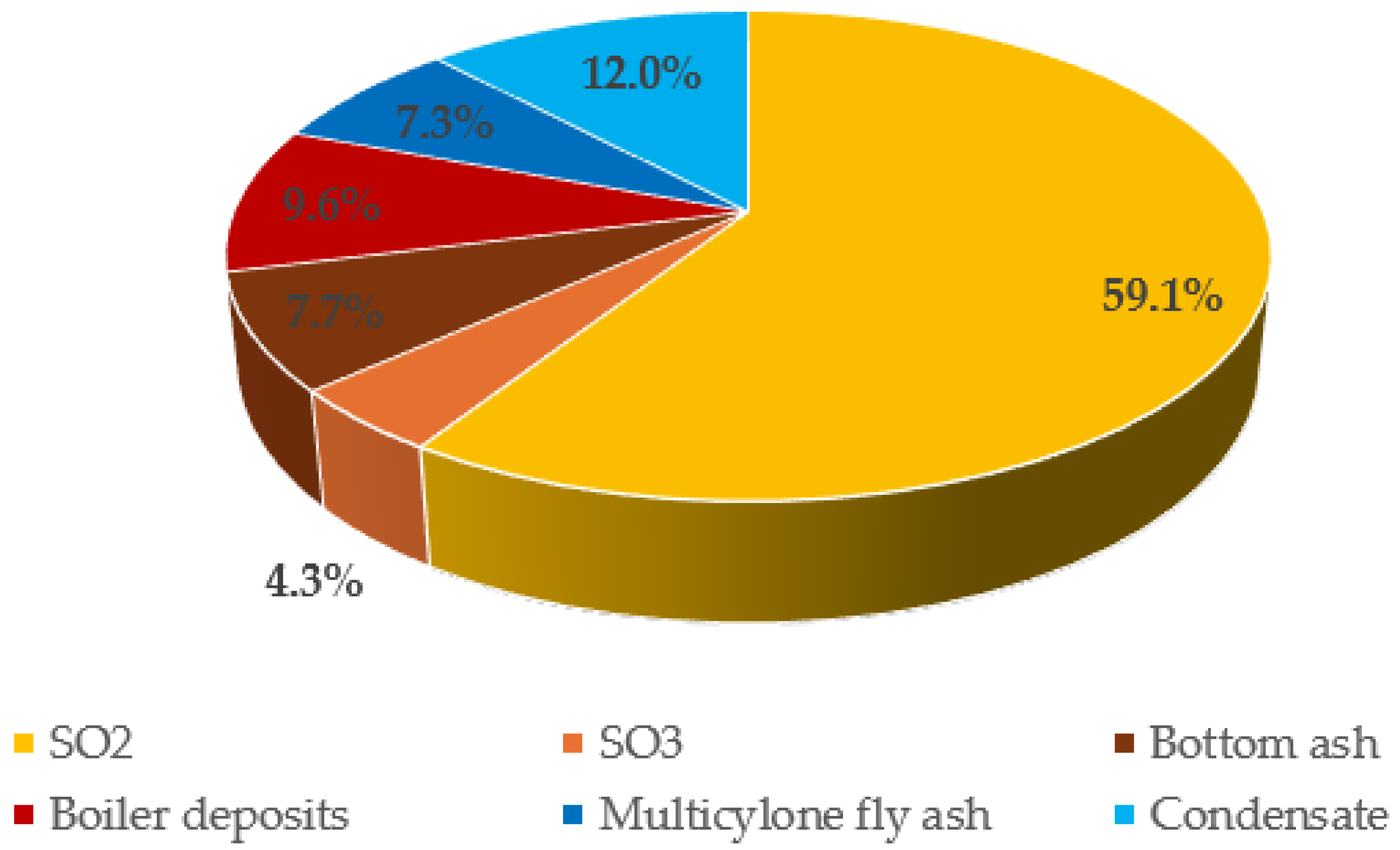
3.6. Sulfur Content in Flue Ash Deposits
3.7. Effect of CaO Introduction on SO2 Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kar, T.; Keles, S. Environmental impacts of biomass combustion for heating and electricity generation. J. Eng. Res. Appl. Sci. 2016, 5, 458–465. [Google Scholar]
- Directive (EU) 2015/2193 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32015L2193 (accessed on 2 December 2024).
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). Available online: https://eur-lex.europa.eu/legal-content/LT/TXT/?uri=CELEX:32010L0075 (accessed on 28 February 2025).
- Coykendall, L.H. Formation and Control of Sulfur Oxides in Boilers. J. Air Pollut. Control Assoc. 1962, 12, 567–591. [Google Scholar] [CrossRef]
- Jaworowski, R.J.; Mack, S.S. Evaluation of Methods for Measurement of S03/H2S04 in Flue Gas. J. Air Pollut. Control Assoc. 1979, 29, 43–46. [Google Scholar] [CrossRef]
- Spörl, R.; Maier, J.; Scheffknecht, G. Sulfur Oxide Emissions from Dust-fired Oxy-fuel Combustion of Coal. Energy Procedia 2013, 37, 1435–1447. [Google Scholar] [CrossRef]
- Musademba, D.; Simbi, D.; Kuipa, P. Trends in the control of NOx and SOx combustion emissions: Implications to the design of fluidised bed combustion operations. Chin. J. Mech. Eng. 2015, 231, 349–358. [Google Scholar] [CrossRef]
- Tang, R.; Liu, Q.; Zhong, W.; Lian, G.; Yu, H. Experimental Study of SO2 Emission and Sulfur Conversion Characteristics of Pressurized Oxy-Fuel Co-combustion of Coal and Biomass. Energy Fuels 2020, 34, 16693–16704. [Google Scholar] [CrossRef]
- Roy, Y.; Lefsrud, M.; Orsat, V.; Filion, F.; Bouchard, J.; Nguyen, Q.; Dion, L.M.; Glover, A.; Madadian, E.; Lee, C.P. Biomass combustion for green house carbon dioxide enrichment. Biomass Bioenergy 2014, 66, 186–196. [Google Scholar] [CrossRef]
- Løj, L.H. Gas Phase Sulfur, Chlorine and Potassium Chemistry in Biomass Combustion. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2007. [Google Scholar]
- Lu, J.; Zhou, Z.; Zhang, H.; Yang, Z. Influenced factors study and evaluation for SO2/SO3 conversion rate in SCR process. Fuel 2019, 245, 528–533. [Google Scholar] [CrossRef]
- Carpenella, S.; Cecere, D.; Giacomazzi, E.; Quaranta, I.; Sorrentino, G.; Sabia, P.; Battista Ariemma, G.; Ragucci, R. Large Eddy Simulation of Hydrogen/Air MILD combustion in a cyclonic burner. Appl. Therm. Eng. 2024, 244, 122733. [Google Scholar] [CrossRef]
- Fang, L.; Bos, W.J.T. An EDQNM study of the dissipation rate in isotropic non-equilibrium turbulence. J. Turbul. 2023, 24, 217–234. [Google Scholar] [CrossRef]
- Sartor, K.; Restivo, Y.; Ngendakumana, P.; Dewallef, P. Prediction of SOx and NOx emissions from a medium size biomass boiler. Biomass Bioenergy 2014, 65, 91–100. [Google Scholar] [CrossRef]
- Ciukaj, S.; Pronobis, M. Dew point of the flue gas of boilers co-firing biomass with coal. Chem. Process Eng. 2013, 34, 101–108. [Google Scholar] [CrossRef]
- Oviedo, M.D.; Mendoza, J.M.; German, S.S.; Rhenals-Julio, J.D. Effect of biomass addition on SOx, NOx, and CO2 emissions during co-firing of pulverized coal. J. Southwest Jiaotong Univ. 2024, 59, 87–97. [Google Scholar] [CrossRef]
- Córdoba, P. Status of Flue Gas Desulfurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Galanopoulos, C.; Yan, J.; Li, H.; Liu, L. Impacts of acidic gas components on combustion of contaminated biomass fuels. Biomass Bioenergy 2018, 111, 263–277. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Miller, C.A.; Erickson, C.; Jambhekar, R. Emissions of Sulfur Trioxide from Coal-Fired Power Plants. J. Air Waste Manag. Assoc. 2012, 54, 750–762. [Google Scholar] [CrossRef]
- Zewdie, D.F.; Bizualem, Y.D.; Nurie, A.G. A review on removal CO2, SO2, and H2S from fue gases using zeolite based adsorbents. Discov. Appl. Sci. 2024, 6, 331. [Google Scholar] [CrossRef]
- Koech, L.; Rutto, H.; Lerotholi, L. Spray drying absorption for desulphurization: A review of recent developments. Clean Technol. Environ. Policy 2021, 23, 1665–1686. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Hou, Y.; Wu, W. Capture of Acidic Gases from Flue Gas by Deep Eutectic Solvents. Processes 2021, 9, 1268. [Google Scholar] [CrossRef]
- Sher, F.; Pans, M.A.; Afilaka, D.T.; Sun, C.; Liu, H. Experimental investigation of woody and non-woody biomass combustion in a bubbling fluidised bed combustor focusing on gaseous emissions and temperature profiles. Energy 2017, 141, 2069–2080. [Google Scholar] [CrossRef]
- Li, S.; Xu, T.; Sun, P.; Zhou, Q.; Tan, H.; Hui, S. NOx and SOx emissions of a high sulfur self-retention coal during air-staged combustion. Fuel 2008, 87, 723–731. [Google Scholar] [CrossRef]
- Khan, W.; Gibbs, B. High temperature desulfurization by fine limestone during staged fluidized-bed combustion. Can. J. Chem. Eng. 2000, 78, 1102–1110. [Google Scholar] [CrossRef]
- Shirai, H.; Ikeda, M.; Aramaki, H. Characteristics of hydrogen sulfide formation in pulverized coal combustion. Fuel 2013, 114, 114–119. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Yang, W.; Blasiak, W. Effects of Flue Gas Internal Recirculation on NOx and SOx Emissions in a Co-Firing Boiler. Int. J. Clean Coal Energy 2013, 2, 13–21. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Q.; Luo, Z.; Eddings, E.; Ring, T.; Li, S.; Lin, J.; Xue, S.; Han, L.; Xie, G. Experimental and numerical investigation on sulfur transformation in pressurized oxy-fuel combustion of pulverized coal. Appl. Energy 2019, 253, 113542. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Zhao, J.; Liu, B.; Wang, T.; Wang, P.; Mao, H. Control of NOx emissions by air staging in small- and medium-scale biomass pellet boilers. Environ. Sci. Pollut. Res. 2019, 26, 9717–9729. [Google Scholar] [CrossRef]
- Lupiáñez, C.; Guedea, I.; Bolea, I.; Díez, L.I.; Romeo, L.M. Experimental study of SO2 and NOx emissions in fluidized bed oxy-fuel combustion. Fuel Process. Technol. 2013, 106, 587–594. [Google Scholar] [CrossRef]
- Jančauskas, A.; Buinevičius, K. Combination of Primary Measures on Flue Gas Emissions in Grate-Firing Biofuel Boiler. Energies 2021, 14, 793. [Google Scholar] [CrossRef]
- Shahzad, K.; Saleem, M.; Ghauri, M.; Akhtar, J.; Ali, N.; Akhtar, N.A. Emissions of NOx, SO2, and CO from co-combustion of wheat straw and coal under fast fluidized bed condition. Combust. Sci. Technol. 2015, 187, 1079–1092. [Google Scholar] [CrossRef]
- Ahn, J.; Overacker, D.; Okerlund, R.; Fry, A.; Eddings, E. SO3 Formation During Oxy-Coal Combustion. Int. J. Greenh. Gas Control 2011, 5, 127–135. [Google Scholar] [CrossRef]
- Zhang, Z.; Qinda, Z.; Hao, R.; Hongzhou, H.; Yang, F.; Mao, X.; Mao, Y.; Zhao, P. Combustion behavior, emission characteristics of SO2, SO3 and NO, and in situ control of SO2 and NO during the co-combustion of anthracite and dried sawdust sludge. Sci. Total Environ. 2019, 646, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Pałaszynska, K.; Juszczak, M. Gaseous emissions during agricultural biomass combustion in a 50 kW moving step grate boiler. Chem. Process Eng. 2018, 39, 197–208. [Google Scholar] [CrossRef]
- Jančauskas, A.; Buinevičius, K. Grate-Firing Boilers Grate Movement Impact onto NOx, SO2 Emissions. Mechanika 2020, 26, 503–510. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- EN ISO 16994:2016; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/70097.html (accessed on 1 December 2024).
- EN ISO 18134-2:2024; Solid Biofuels–Determination of Moisture Content—Part 2: Simplified Method. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/86024.html (accessed on 1 December 2024).
- EN ISO 18125:2017; Solid Biofuels—Determination of Calorific Value. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/61517.html (accessed on 1 December 2024).
- Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Available online: https://phyllis.nl/Browse/Standard/ECN-Phyllis#meat%20bon%20meal (accessed on 18 December 2024).
- Cai, J.; Li, D.; Chen, D.; Li, Z. NOx and H2S formation in the reductive zone of air-staged combustion of pulverized blended coals. Front. Energy 2020, 15, 4–13. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Åmand, L.-E.; Leckner, B. Potassium, Chlorine, and Sulfur in Ash, Particles, Deposits, and Corrosion during Wood Combustion in a Circulating Fluidized-Bed Boiler. Energy Fuels 2007, 21, 71–81. [Google Scholar] [CrossRef]
- Fleig, D.; Andersson, K.; Normann, F.; Johnsson, F. SO3 formation under oxyfuel combustion conditions. Ind. Eng. Chem. Res. 2011, 50, 8505–8514. [Google Scholar] [CrossRef]
- Baxter, L.; Miles, T.R.; Miles, T.R., Jr.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The behavior of inorganic material in biomass-fired power boilers: Field and laboratory experiences. Fuel Process. Technol. 1996, 54, 47–78. [Google Scholar] [CrossRef]
- Chen, L.; Bhattacharya, S. Sulfur Emission from Victorian Brown Coal Under Pyrolysis, Oxy-Fuel Combustion and Gasification Conditions. Environ. Sci. Technol. 2013, 47, 1729–1734. [Google Scholar] [CrossRef]
- Sarbassov, Y.; Duan, L.; Manovic, V.; Anthony, E.J. Sulfur trioxide formation/emissions in coal-fired air- and oxy-fuel combustion processes: A review. Greenh. Gases Sci. Technol. 2018, 8, 402–428. [Google Scholar] [CrossRef]
- Castaldi, F.J. Aqueous Behavior of Elements in a Flue Gas Desulfurization Sludge Disposal Site. 1. Waste Water Treatment. Water Encycl. 2005, 1, 848–853. [Google Scholar] [CrossRef]
- Gollmer, C.; Siegmund, T.; Weigel, V.; Kaltschmitt, M. Comparative Analysis of Primary and Secondary Emission Mitigation Measures for Small-Scale Wood Chip Combustion. Energies 2024, 17, 4403. [Google Scholar] [CrossRef]
- Van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008; p. 465. [Google Scholar]





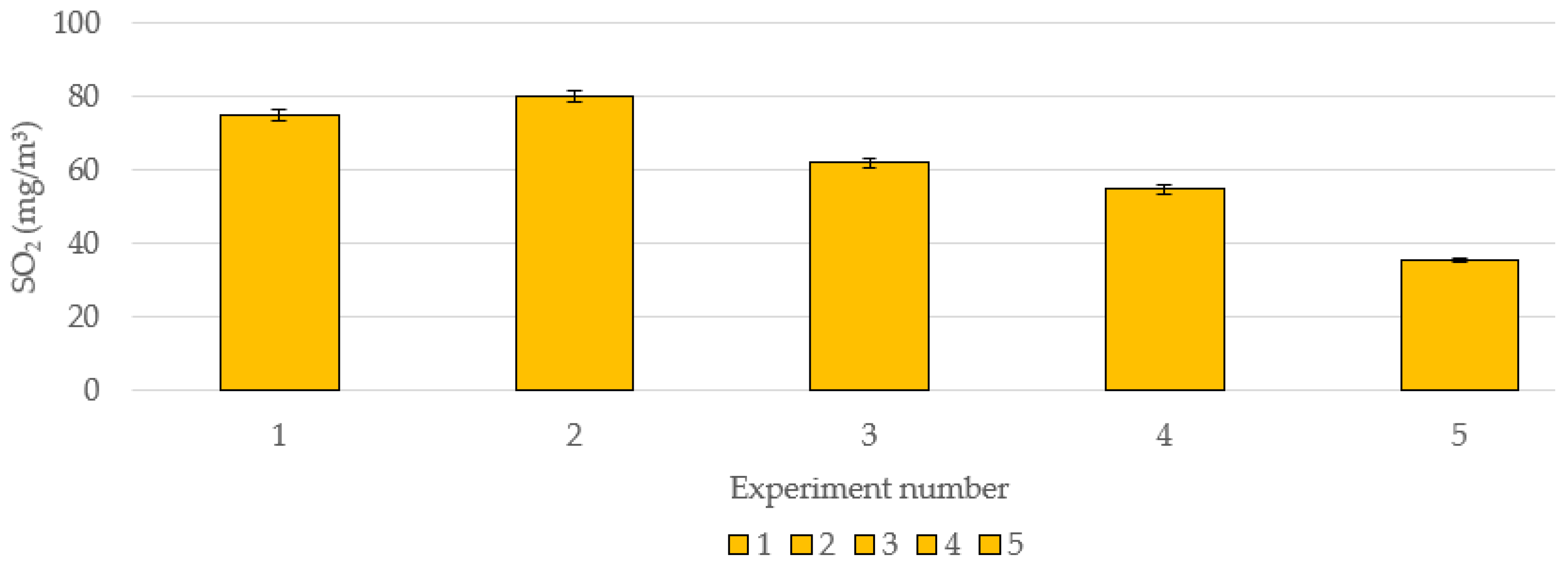
| Fuel | Moisture | Sulfur (S) | Ash | Lower Calorific Value |
|---|---|---|---|---|
| % | % | % | kJ/kg | |
| Sunflower husk pellets | 8.6 | 0.095 | 2.9 | 15,366 |
| Woodchips | 45 | 0.02 | 1.23 | 10,197 |
| Meat bone meal | 2.9 | 0.63 | 22.0 | 20,972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jomantas, G.; Buinevičius, K.; Šereika, J. Sulfur Emission Dependence on Various Factors During Biomass Combustion. Energies 2025, 18, 1701. https://doi.org/10.3390/en18071701
Jomantas G, Buinevičius K, Šereika J. Sulfur Emission Dependence on Various Factors During Biomass Combustion. Energies. 2025; 18(7):1701. https://doi.org/10.3390/en18071701
Chicago/Turabian StyleJomantas, Giedrius, Kęstutis Buinevičius, and Justas Šereika. 2025. "Sulfur Emission Dependence on Various Factors During Biomass Combustion" Energies 18, no. 7: 1701. https://doi.org/10.3390/en18071701
APA StyleJomantas, G., Buinevičius, K., & Šereika, J. (2025). Sulfur Emission Dependence on Various Factors During Biomass Combustion. Energies, 18(7), 1701. https://doi.org/10.3390/en18071701






