Maximum Fluidized Bed Boiler Temperature Determination for Coal–Biomass Combustion Condition Through Ash Area Reduction Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Fuel Samples
2.2. Experimental Methods
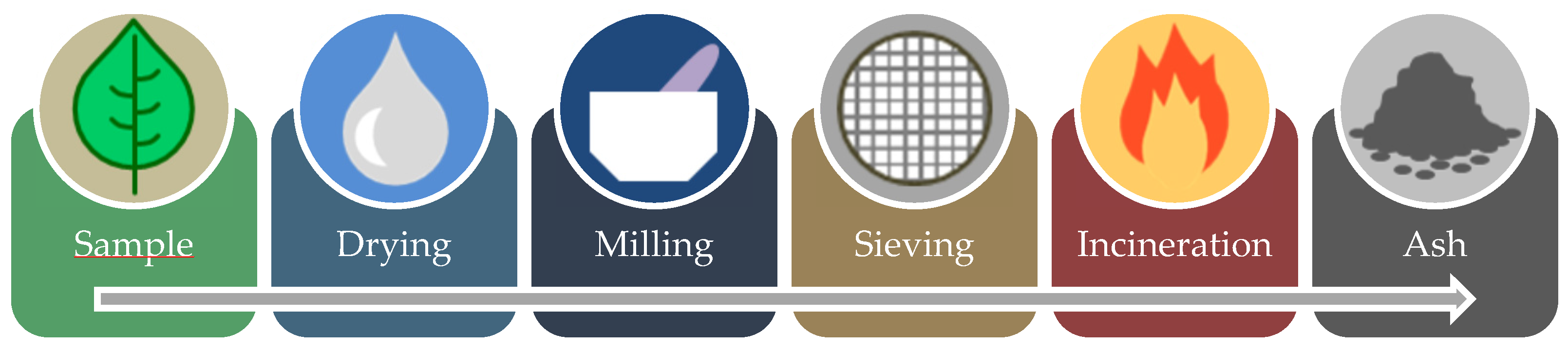
2.2.1. Standardization and Characterization Analysis
2.2.2. Simultaneous Thermal Analysis (STA)
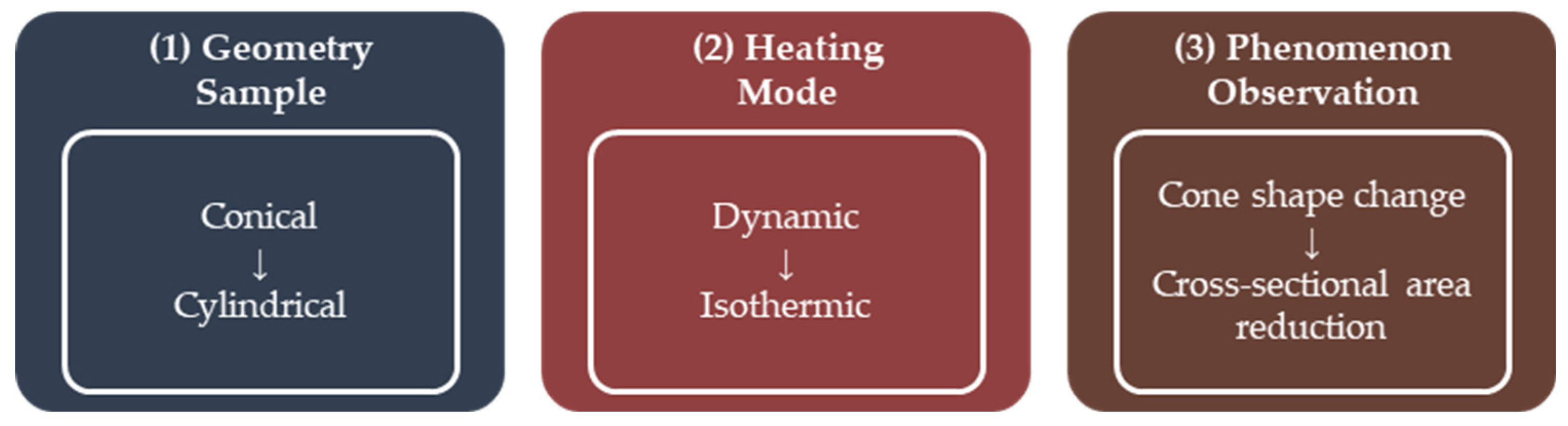
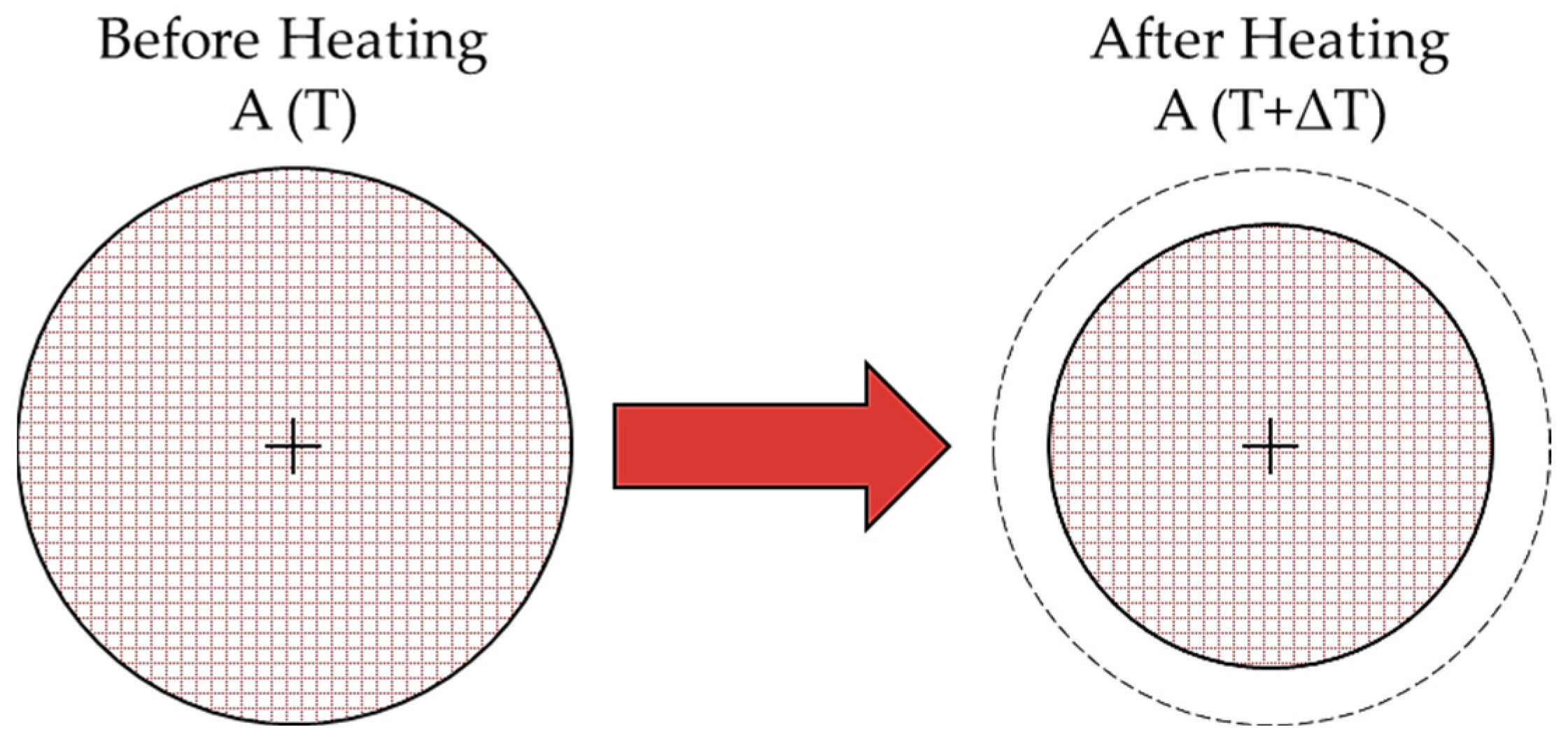
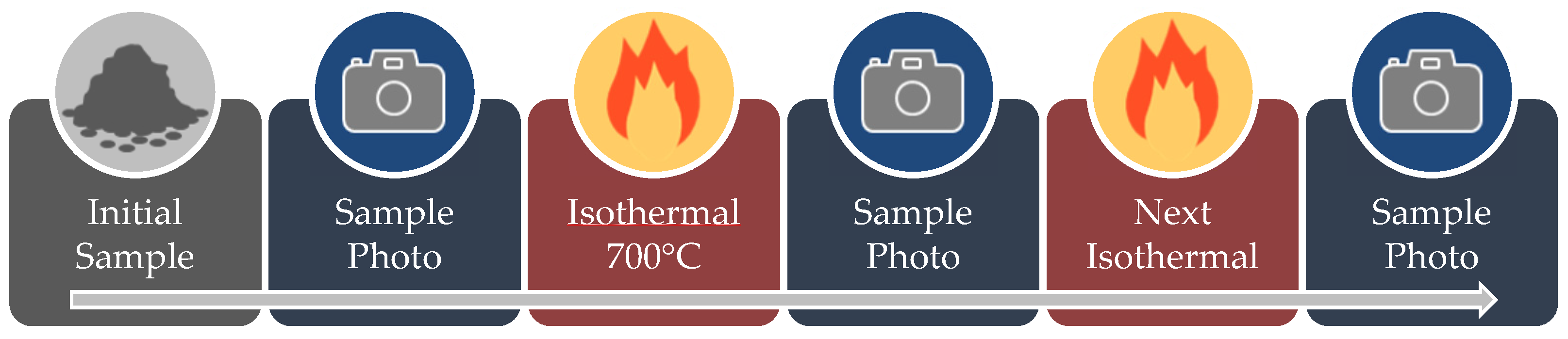
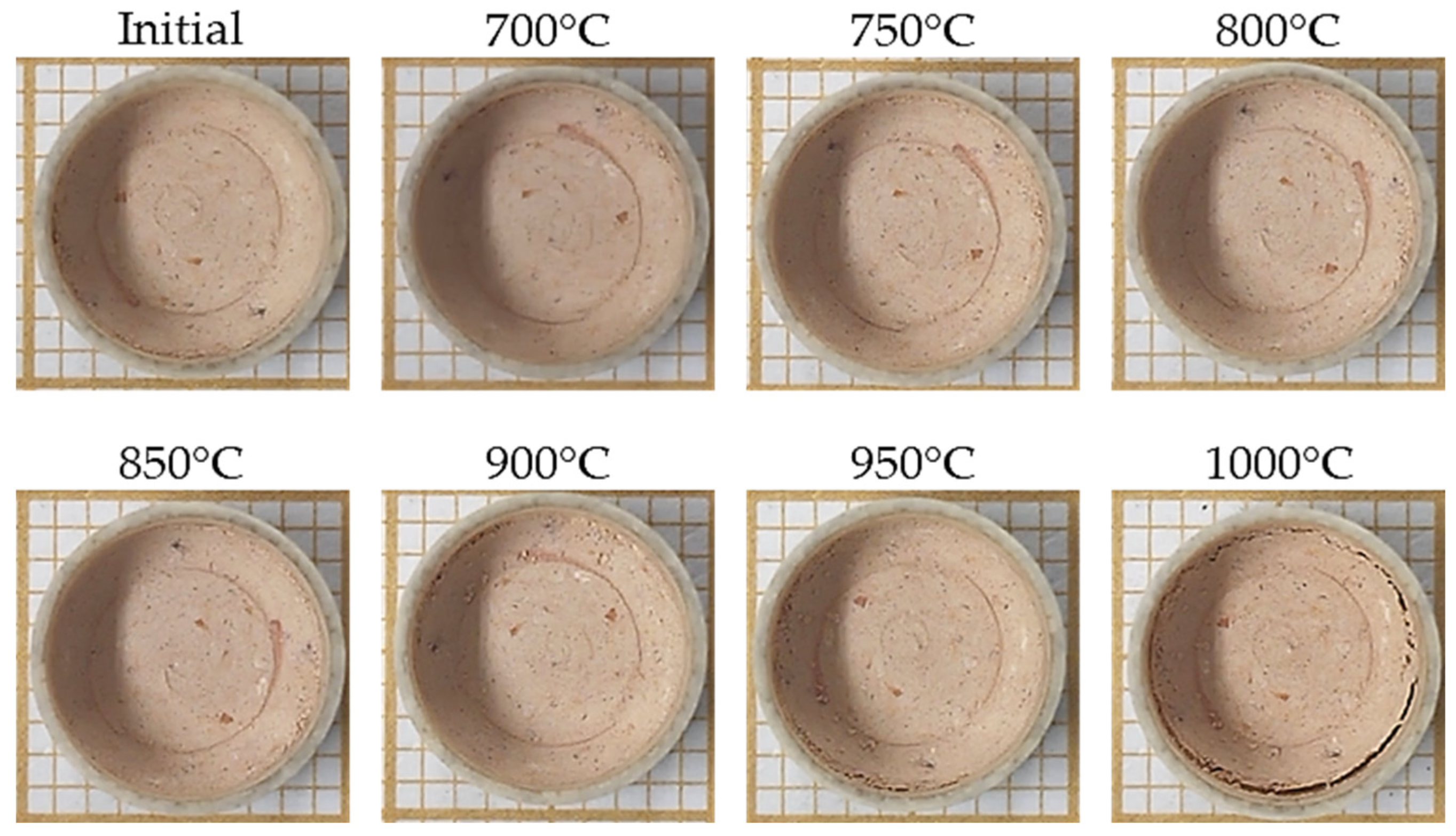
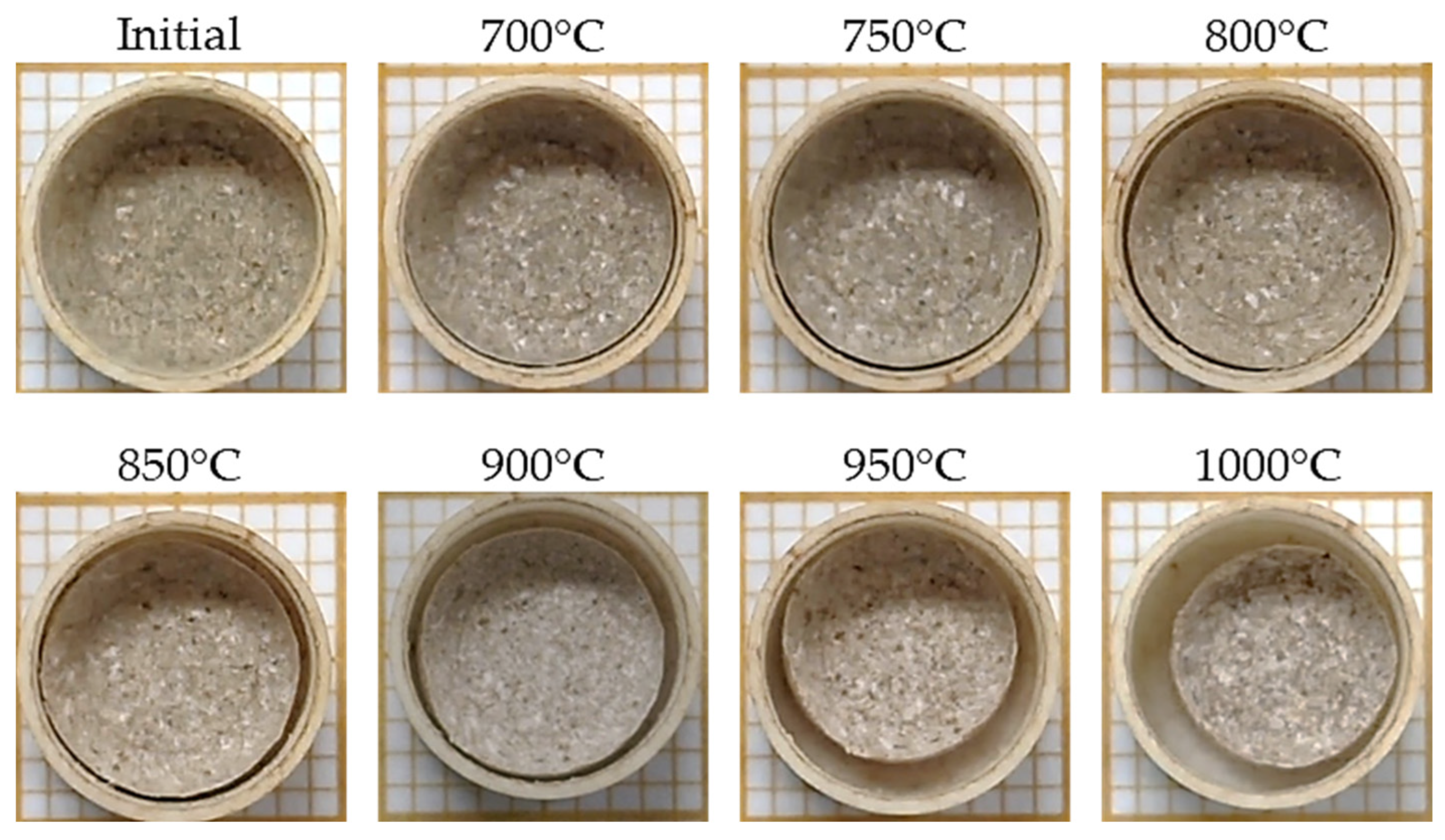
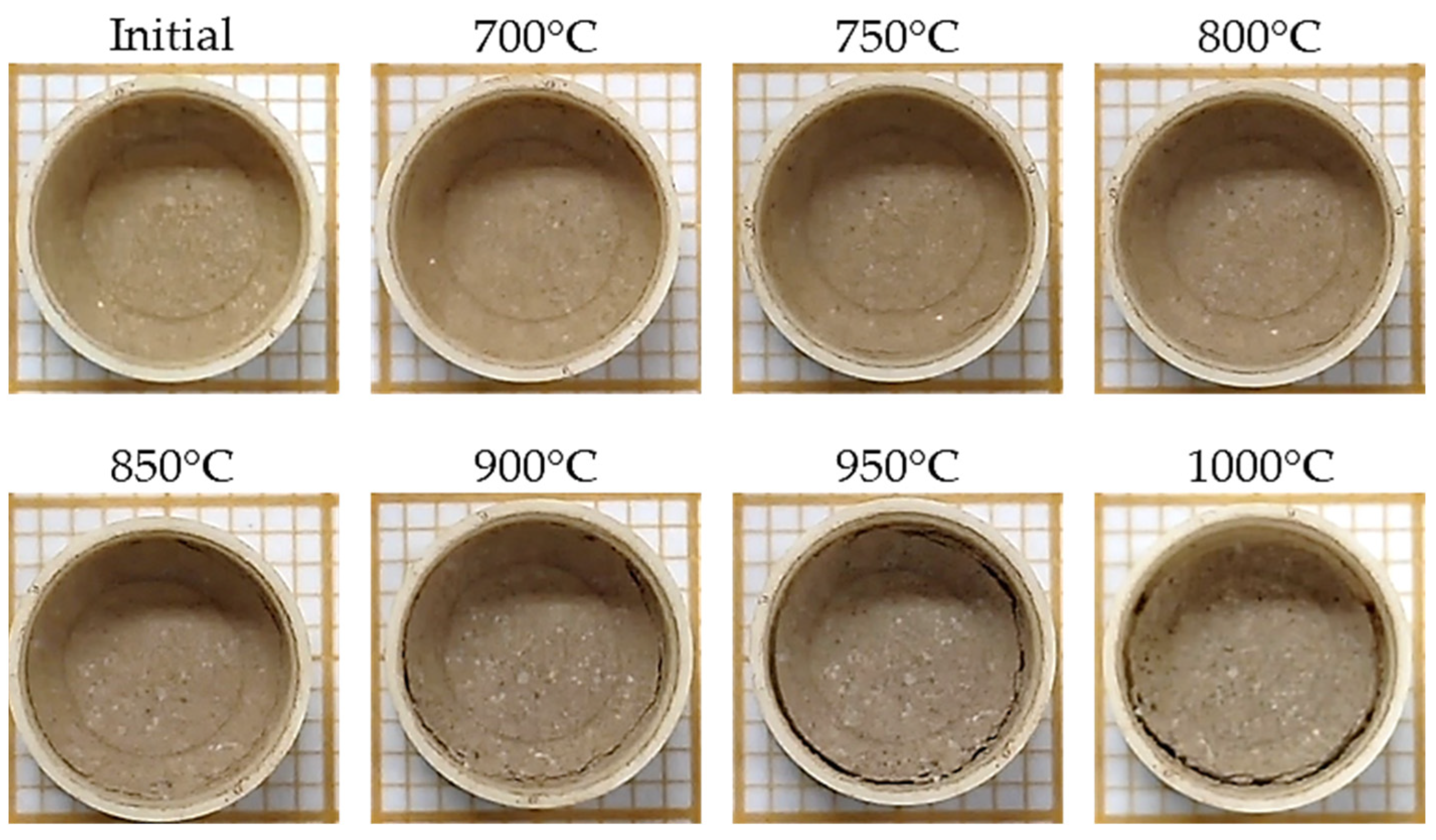
2.2.3. Sintering Analysis by Area Reduction Technique
3. Results
3.1. Ashes Chemical Composition
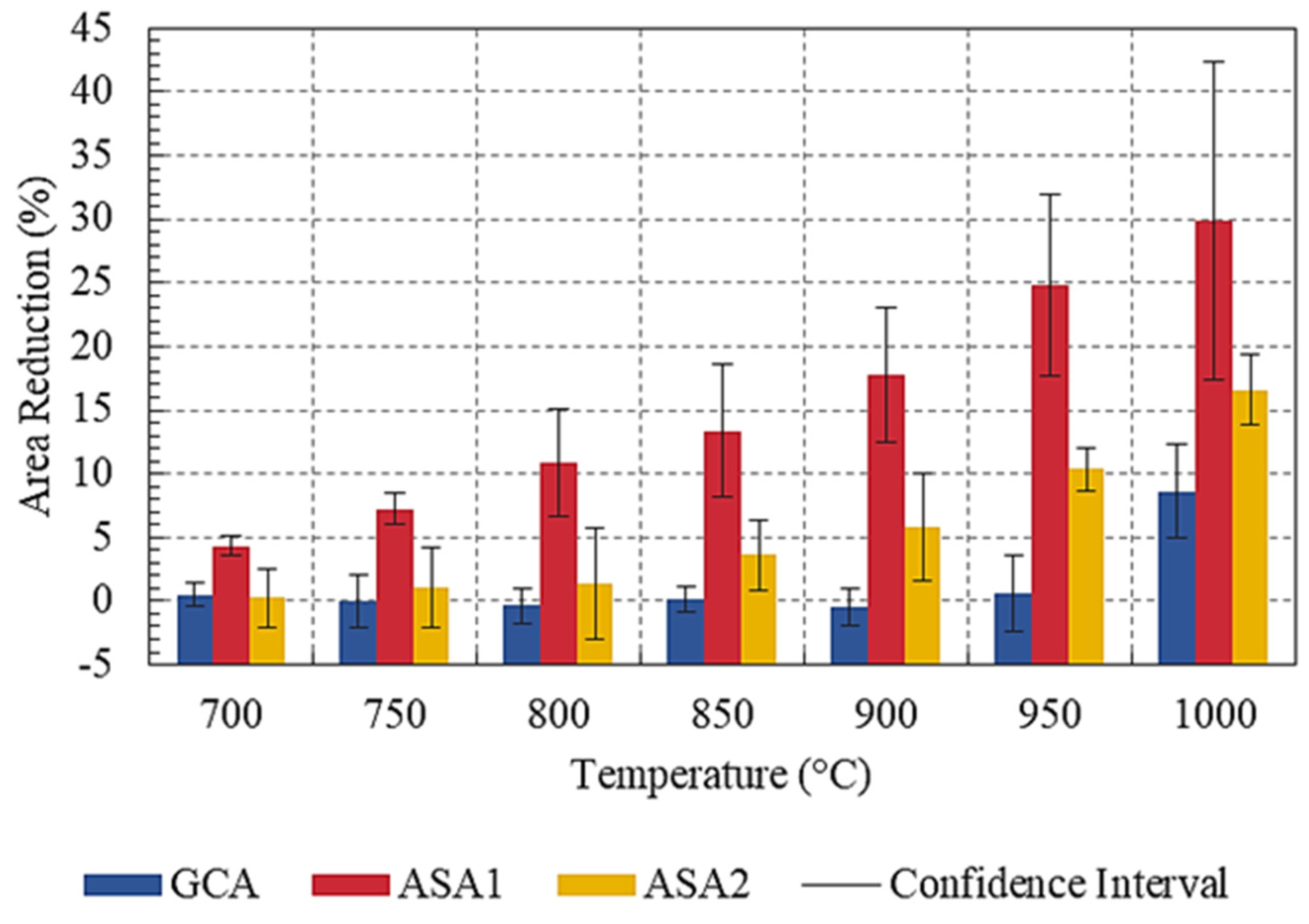
3.2. Sintering Analysis by Area Reduction
3.3. TG and DTA Analysis (STA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Sharma, R.K. Fluidized Bed Combustion: Technology for Efficient Utilization of Biomass Residues. Asian J. Eng. Appl. Technol. 2018, 7, 73–79. [Google Scholar] [CrossRef]
- Iannello, S.; Morrin, S.; Materazzi, M. Fluidized bed reactors for the thermochemical conversion of biomass and waste. KONA Powder Part. J. 2020, 37, 114–131. [Google Scholar] [CrossRef]
- Basu, P. Combustion and Gasification in Fluidized Bed, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2006; 466p. [Google Scholar]
- Liu, Q.; Zhong, W.; Yu, A.; Wang, C.H. Co-firing of coal and biomass under pressurized oxy-fuel combustion mode in a 10 kW fluidized bed: Nitrogen and sulfur pollutants. Chem. Eng. J. 2022, 450, 138401. [Google Scholar] [CrossRef]
- Bannon, D.; Deza, M.; Masoumi, M.; Estejab, B. Assessment of Irregular Biomass Particles Fluidization in Bubbling Fluidized Beds. Energies 2023, 16, 2051. [Google Scholar] [CrossRef]
- IBGE Brazilian Institute of Geography and Statistics (IBGE). Agricultural Production: Brazil and Federation Units. 2022. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/açaí-cultivo/br (accessed on 27 July 2024).
- Brasil. Centro de Tecnologia da Indústria Química e Têxtil do SENAI. Valorização de Resíduos da Indústria do Açaí: Oportunidades e Desafios; Centro de Tecnologia da Indústria Química e Têxtil do SENAI: Rio de Janeiro, Brazil, 2021; 14p. [Google Scholar]
- Soares, G.C.P.; Moreira, J.V.R.; Santos, F.H.B.; Guerra, D.R.S.; Nogueira, M.F.M. Combustion Process of Coal–Açai Seed Mixtures in a Circulating Fluidized Bed Boiler. Energies 2024, 17, 4635. [Google Scholar] [CrossRef]
- Moreira, J.; Carneiro, A.; Oliveira, D.; Santos, F.; Guerra, D.; Nogueira, M.; Rocha, H.; Charvet, F.; Tarelho, L. Thermochemical Properties for Valorization of Amazonian Biomass as Fuel. Energies 2022, 15, 7343. [Google Scholar] [CrossRef]
- Rosendahl, L. Biomass Combustion Science, Technology and Engineering; Woodhead Publishing: Sawston, UK, 2013; 315p. [Google Scholar]
- Furuvik, N.C.I.S.; Jaiswal, R.; Thapa, R.K.; Moldestad, B.M.E. CPFD model for prediction of flow behavior in an agglomerated fluidized bed gasifier. Int. J. Energy Prod. Manag. 2019, 4, 105–114. [Google Scholar] [CrossRef]
- Rojek, J.; Nosewicz, S.; Maździarz, M.; Kowalczyk, P.; Wawrzyk, K.; Lumelskyj, D. Modeling of a Sintering Process at Various Scales. Procedia Eng. 2017, 177, 263–270. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matovic, M.D.; Whalen, J.K. Exploring switchgrass and hardwood combustion on excess air and ash fouling/slagging potential: Laboratory combustion test and thermogravimetric kinetic analysis. Energy Convers. Manag. 2015, 97, 409–419. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N.; Abelha, P. Evaluation of slagging and fouling tendency during biomass co-firing with coal in a fluidized bed. Biomass-Bioenergy 2012, 39, 192–203. [Google Scholar] [CrossRef]
- Loo, S.V. The Handbook of Biomass Combustion and Co-Firing; Earthscan: Oxford, UK, 2008; 465p. [Google Scholar]
- Ruiz, I.S. Chapter 4: Coal Combustion. In Applied Coal Petrology: The Role Petrology in Coal Utilization; Academic Press: Cambridge, MA, USA, 2008; pp. 85–117. [Google Scholar]
- Vega-Nieva, D.J. Slagging and Fouling Risks Derived from the Combustion of Solid Biofuels. WIT Trans. State-Art Sci. Eng. 2015, 85, 137–147. [Google Scholar] [CrossRef]
- Lu, D.Y.; Tan, Y.; Duchesne, M.A.; McCalden, D. Potassium capture by ilmenite ore as the bed material during fluidized bed conversion. Fuel 2023, 335, 127008. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Lundberg, A.; Pettersson, H.; Wilewska-Bien, M.; Andersson, D. Investigation of ash sintering during combustion of agricultural residues and the effect of additives. Energy Fuels 2009, 23, 5655–5662. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.-U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Llorente, M.J.F.; Arocas, P.D.; Nebot, L.G.; García, J.E.C. The effect of the addition of chemical materials on the sintering of biomass ash. Fuel 2008, 87, 2651–2658. [Google Scholar] [CrossRef]
- Contreras-Trejo, J.C.; Vega-Nieva, D.J.; Heya, M.N.; Prieto-Ruíz, J.A.; Nava-Berúmen, C.A.; Carrillo-Parra, A. Sintering and Fusibility Risks of Pellet Ash from Different Sources at Different Combustion Temperatures. Energies 2022, 15, 5026. [Google Scholar] [CrossRef]
- Llorente, M.F.; Cuadrado, R.E.; Laplaza, J.M.; García, J.C. Combustion in bubbling fluidized bed with bed material of limestone to reduce the biomass ash agglomeration and sintering. Fuel 2006, 85, 2081–2092. [Google Scholar] [CrossRef]
- Du, S.; Yang, H.; Qian, K.; Wang, X.; Chen, H. Fusion and transformation properties of the inorganic components in biomass ash. Fuel 2014, 117, 1281–1287. [Google Scholar] [CrossRef]
- Rizvi, T.; Xing, P.; Pourkashanian, M.; Darvell, L.I.; Jones, J.M.; Nimmo, W. Prediction of biomass ash fusion behavior by the use of detailed characterization methods coupled with thermodynamic analysis. Fuel 2015, 141, 275–284. [Google Scholar] [CrossRef]
- Yan, T.; Kong, L.; Bai, J.; Bai, Z.; Li, W. Thermomechanical analysis of coal ash fusion behavior. Chem. Eng. Sci. 2016, 147, 74–82. [Google Scholar] [CrossRef]
- Ma, T.; Fan, C.; Hao, L.; Li, S.; Song, W.; Lin, W. Fusion characterization of biomass ash. Thermochim. Acta 2016, 638, 1–9. [Google Scholar] [CrossRef]
- Link, S.; Yrjas, P.; Lindberg, D.; Trikkel, A.; Mikli, V. Ash melting behavior of reed and woody fuels blends. Fuel 2022, 314, 123051. [Google Scholar] [CrossRef]
- Yongtie, C.; Wenming, Y.; Zhimin, Z.; Mingchen, X.; Boon, S.K.; Subbaiah, P. Modelling of ash deposition in biomass boilers: A review. Energy Procedia 2017, 143, 623–628. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behavior of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, G.B.; Jeon, C.H. Prediction of correlation between ash fusion temperature of ASTM and Thermo-Mechanical Analysis. Appl. Therm. Eng. 2017, 125, 1291–1299. [Google Scholar] [CrossRef]
- Lv, Y.; Niu, Y.; Wang, G.; Xu, L.; Lei, Y.; Hui, S.; Wang, D. Experimental and kinetic studies on ash fusion behavior: A high-precision acquisition method for ash fusion temperatures. Asia-Pac. J. Chem. Eng. 2021, 16, e2644. [Google Scholar] [CrossRef]
- Cai, Y.; Tay, K.; Zheng, Z.; Yang, W.; Wang, H.; Zeng, G.; Li, Z.; Boon, S.K.; Subbaiah, P. Modeling of ash formation and deposition processes in coal and biomass fired boilers: A comprehensive review. Appl. Energy 2018, 230, 1447–1544. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Koga, N.; Schick, C. Handbook of Thermal Analysis and Calorimetry: Recent Advances, Techniques and Applications; Elsevier: Amsterdam, The Netherlands, 2018; 860p. [Google Scholar]
- Wall, T.F.; Creelman, R.A.; Gupta, R.P.; Gupta, S.K.; Coin, C.; Lowe, A. Coal ash fusion temperatures-new characterization techniques, and implications for slagging and fouling. Prog. Energy Combust. Sci. 1998, 24, 345–353. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, R.P.; Bryant, G.W.; Juniper, L.; Wall, T.F. Thermomechanical Analysis and Alternative Ash Fusibility Temperatures; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Zhang, L.; Wang, J.; Song, X.; Bai, Y.; Yao, M.; Yu, G. Influence of biomass ash additive on fusion characteristics of high-silicon-aluminum coal ash. Fuel 2020, 282, 118876. [Google Scholar] [CrossRef]
- ABNT NBR 16508; Matérias Primas para Fundição–Carvão Mineral–Método de Ensaio para Determinação do Teor de Umidade. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brasil, 2017.
- CEN/TS 14774-1; Methods for Determination of Moisture Content–Oven Dry Method–Part 1: Total Moisture–Reference Method. European Committee for Standardization: Brussels, Belgium, 2004.
- ABNT NBR 16586; Matérias Primas para Fundição–Carvão Mineral–Métodos para Determinação do Teor de Cinzas. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brasil, 2017.
- CEN/TS 14775; Methods for Determination of Ash Content. European Committee for Standardization: Brussels, Belgium, 2004.
- Arvelakis, S.; Jensen, P.A.; Dam-Johansen, K. Simultaneous Thermal Analysis (STA) on Ash from High-Alkali Biomass. Energy Fuels 2004, 18, 1066–1076. [Google Scholar] [CrossRef]
- Magdziarz, A.; Gajek, M.; Nowak-Woźny, D.; Wilk, M. Mineral phase transformation of biomass ashes—Experimental and thermochemical calculations. Renew. Energy 2018, 128, 446–459. [Google Scholar] [CrossRef]
- Kaniowski, W.; Taler, J.; Wang, X.; Kalemba-Rec, I.; Gajek, M.; Mlonka-Mędrala, A.; Nowak-Woźny, D.; Magdziarz, A. Investigation of biomass, RDF and coal ash-related problems: Impact on metallic heat exchanger surfaces of boilers. Fuel 2022, 326, 125122. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Yang, T.; Raninger, B. Investigation of MSWI fly ash melting characteristic by DSC–DTA. Waste Manag. 2007, 27, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Angel, J.D.M.; Vásquez, T.G.P.; Junkes, J.A.; Hotza, D. Caracterização de cinza obtida por combustão de casca de arroz em reator de leito fluidizado. Quimica Nova 2009, 32, 1110–1114. [Google Scholar] [CrossRef]
- ASTM D1857/D1857M–24; Standard Test Method for Fusibility of Coal and Coke Ash. ASTM International: West Conshohocken, PA, USA, 2024.
- Chai, Y.; Fan, Y.; Wu, J.; Zhang, Y.; Luo, G.; Wang, Y. Research on the Ash Melting Characteristics of Blended Coal Based on DFT Calculations. ACS Omega 2021, 6, 22039–22046. [Google Scholar] [CrossRef]
- Putra, H.P.; Kuswa, F.M.; Ghazidin, H.; Darmawan, A.; Prabowo; Hariana. Slagging-fouling evaluation of empty fruit bunch and palm oil frond mixture with bituminous ash coal as co-firing fuel. Bioresour. Technol. Rep. 2023, 22, 101489. [Google Scholar] [CrossRef]
- Li, F.; Yu, B.; Wang, G.; Fan, H.; Wang, T.; Guo, M.; Fang, Y. Investigation on improve ash fusion temperature (AFT) of low-AFT coal by biomass addition. Fuel Process. Technol. 2019, 191, 11–19. [Google Scholar] [CrossRef]
- Gilbe, C.; Öhman, M.; Lindström, E.; Boström, D.; Backman, R.; Samuelsson, R.; Burvall, J. Slagging characteristics during residential combustion of biomass pellets. Energy Fuels 2008, 22, 3536–3543. [Google Scholar] [CrossRef]
- Öhman, M.; Nordin, A.; Hedman, H.; Jirjis, R. Reasons for slagging during stemwood pellet combustion and some measures for prevention. Biomass-Bioenergy 2004, 27, 597–605. [Google Scholar] [CrossRef]

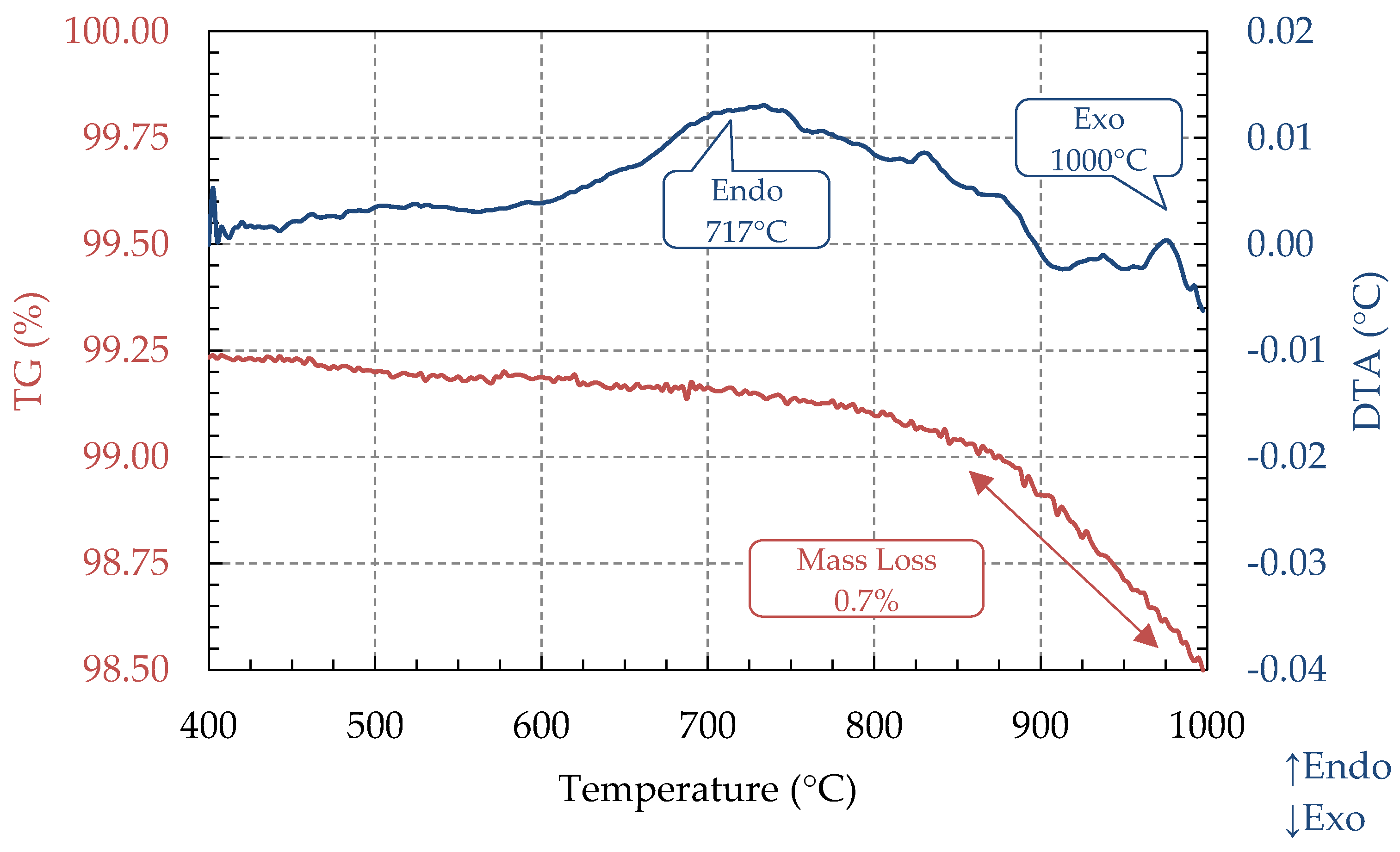
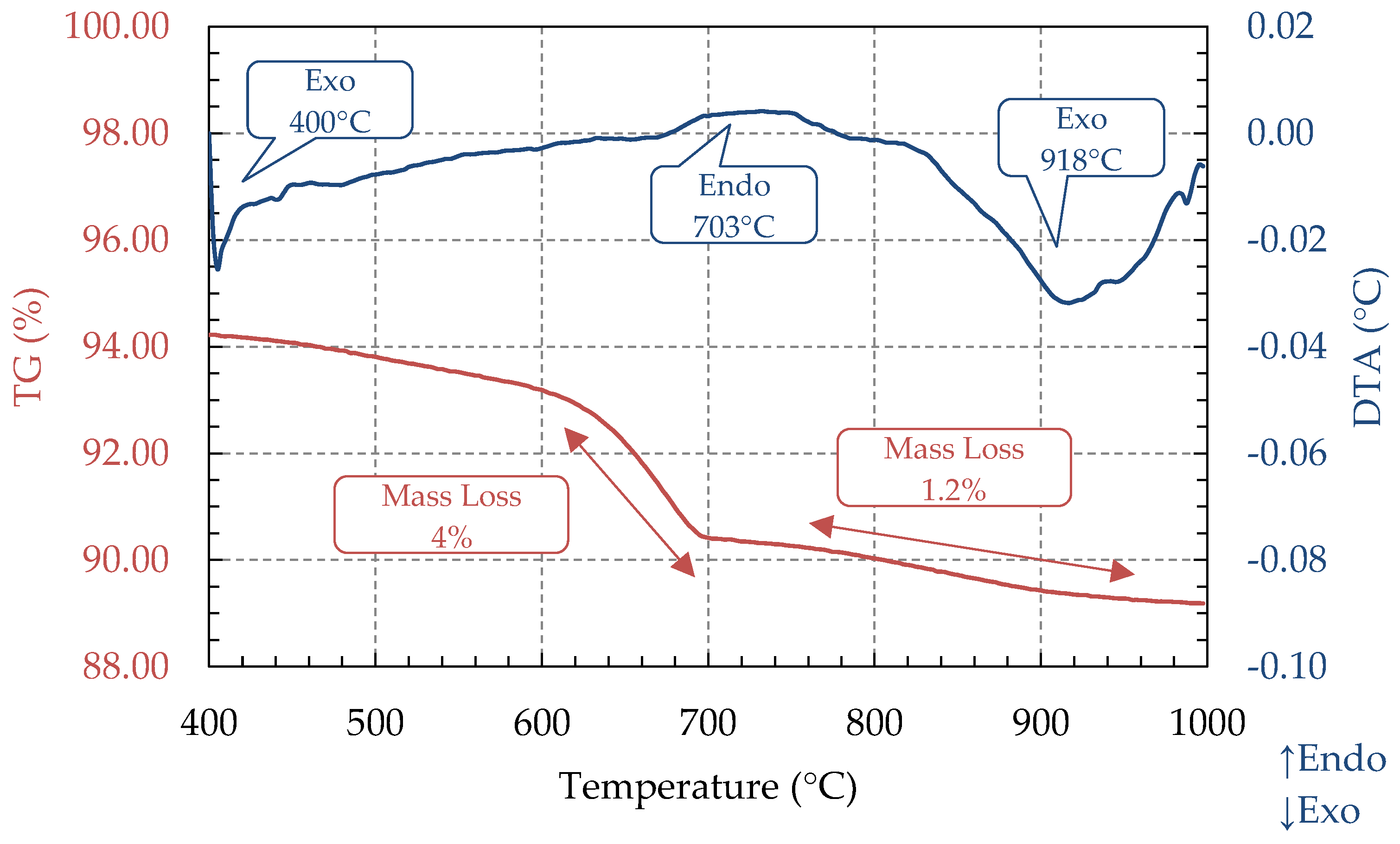
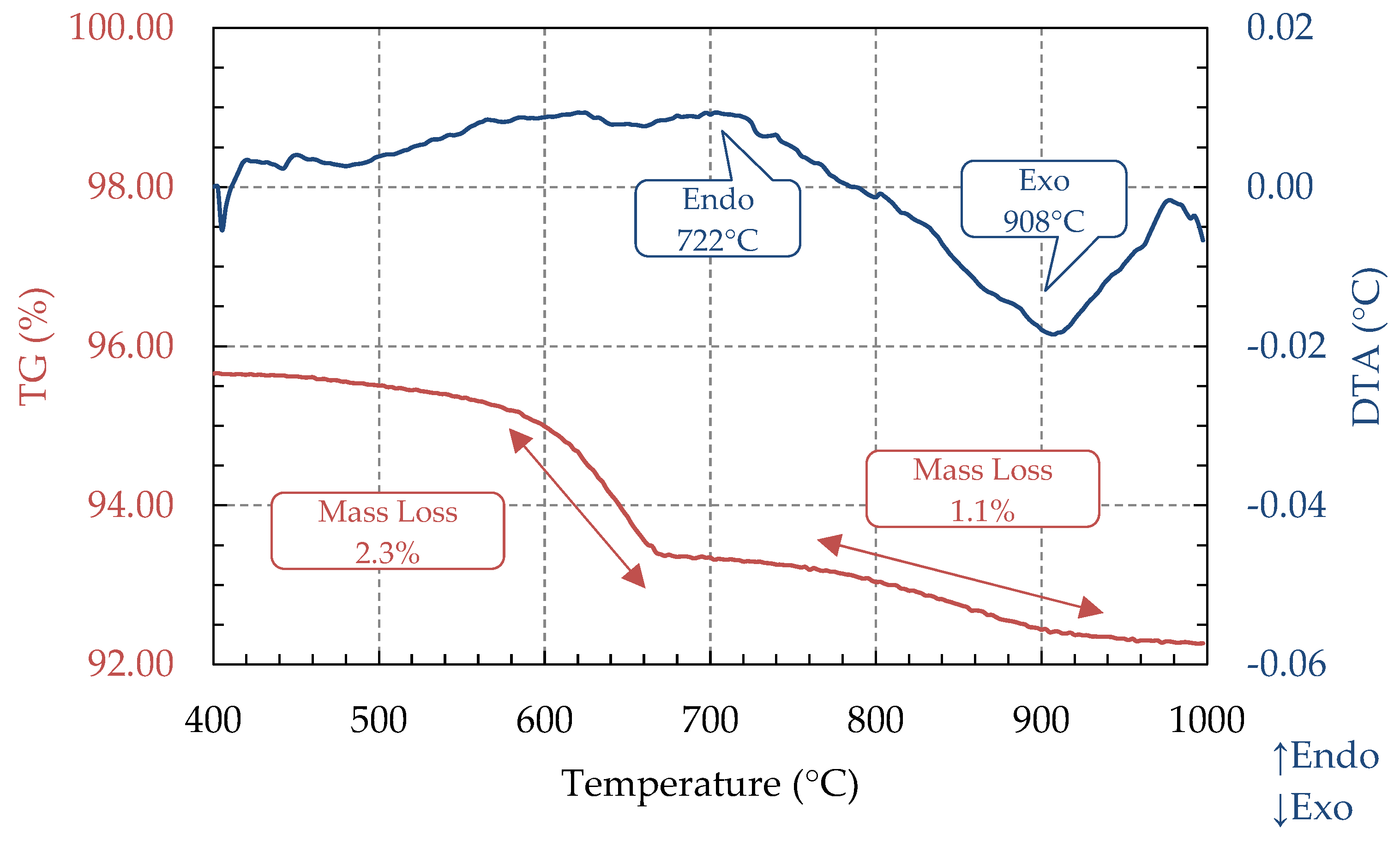
| Ash Sample | Code | Proximate Analysis (%, d.b) | HHV (MJ/kg) | Reference | ||
|---|---|---|---|---|---|---|
| Volatile | Fixed Carbon | Ash | ||||
| Glencore coal ash | GCA | 34.9 | 49.7 | 15.4 | 26.8 | [8] |
| Açai fiber seed ash | ASA1 | 77.6 | 21.6 | 0.8 | 19.5 | [8] |
| Açai fiber-free seed ash | ASA2 | 78.2 | 20.3 | 1.5 | 19.3 | This paper |
| Composition (%wt) | GCA | ASA1 | ASA2 |
|---|---|---|---|
| Al2O3 | 24.4 | 5.6 | 6.9 |
| CaO | 1.7 | 7.6 | 9.5 |
| Fe2O3 | 6.7 | 3.0 | 16.3 |
| K2O | 2.9 | 21.3 | 27.1 |
| MgO | 2.0 | 4.4 | 4.4 |
| MnO | - | 1.8 | 2.1 |
| Na2O | 1.4 | - | - |
| P2O5 | 0.2 | 10.1 | 11.0 |
| SiO2 | 57.1 | 41.0 | 16.0 |
| SO3 | 2.6 | 4.5 | 5.7 |
| TiO2 | 1.0 | 0.7 | 0.5 |
| ZnO | - | - | 0.5 |
| Temperature (°C) | 700 | 750 | 800 | 850 | 900 | 950 | 1000 |
|---|---|---|---|---|---|---|---|
| GCA | 0.5 ± 1.0 | −0.1 ± 2.1 | −0.4 ± 1.4 | 0.2 ± 1.0 | −0.5 ± 1.5 | 0.6 ± 3.0 | 8.6 ± 3.6 |
| ASA1 | 4.3 ± 0.8 | 7.3 ± 1.2 | 10.9 ± 4.2 | 13.4 ± 5.2 | 17.8 ± 5.3 | 24.8 ± 7.1 | 29.9 ± 12.5 |
| ASA2 | 0.3 ± 2.3 | 1.1 ± 3.2 | 1.3 ± 4.4 | 3.6 ± 2.8 | 5.8 ± 4.3 | 10.3 ± 1.7 | 16.6 ± 2.7 |
| Ash Sample | CGA | ASA1 | ASA2 |
|---|---|---|---|
| Deviation (mm2) | 0.2–0.5 | 0.1–0.3 | 0.1–0.4 |
| Confidence Interval (mm2) | 0.3–0.7 | 0.1–0.5 | 0.2–0.6 |
| Uncertain (mm2) | 5.4–5.9 | 4.7–6.2 | 5.2–6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.H.B.; Moreira, J.V.R.; Soares, G.C.P.; Carneiro, A.N.; Guerra, D.R.S.; Nogueira, M.F.M.; Tarelho, L.A.C. Maximum Fluidized Bed Boiler Temperature Determination for Coal–Biomass Combustion Condition Through Ash Area Reduction Technique. Energies 2025, 18, 1662. https://doi.org/10.3390/en18071662
Santos FHB, Moreira JVR, Soares GCP, Carneiro AN, Guerra DRS, Nogueira MFM, Tarelho LAC. Maximum Fluidized Bed Boiler Temperature Determination for Coal–Biomass Combustion Condition Through Ash Area Reduction Technique. Energies. 2025; 18(7):1662. https://doi.org/10.3390/en18071662
Chicago/Turabian StyleSantos, Fernando H. B., João V. R. Moreira, Gabriel C. P. Soares, Alan N. Carneiro, Danielle R. S. Guerra, Manoel F. M. Nogueira, and Luís A. C. Tarelho. 2025. "Maximum Fluidized Bed Boiler Temperature Determination for Coal–Biomass Combustion Condition Through Ash Area Reduction Technique" Energies 18, no. 7: 1662. https://doi.org/10.3390/en18071662
APA StyleSantos, F. H. B., Moreira, J. V. R., Soares, G. C. P., Carneiro, A. N., Guerra, D. R. S., Nogueira, M. F. M., & Tarelho, L. A. C. (2025). Maximum Fluidized Bed Boiler Temperature Determination for Coal–Biomass Combustion Condition Through Ash Area Reduction Technique. Energies, 18(7), 1662. https://doi.org/10.3390/en18071662








