Enhancing Biomethane Yield and Metabolic Pathways in Kitchen Waste Anaerobic Digestion Through Microbial Electrolysis Cell Integration
Abstract
1. Introduction
2. Materials and Methods
2.1. Kitchen Waste and Inoculum
2.2. Experimental Set Up and Design
2.3. Analytical Methods
2.4. Microbial Community and Metagenomic Sequencing Analysis
2.5. Statistical Analysis
3. Results and Discussion
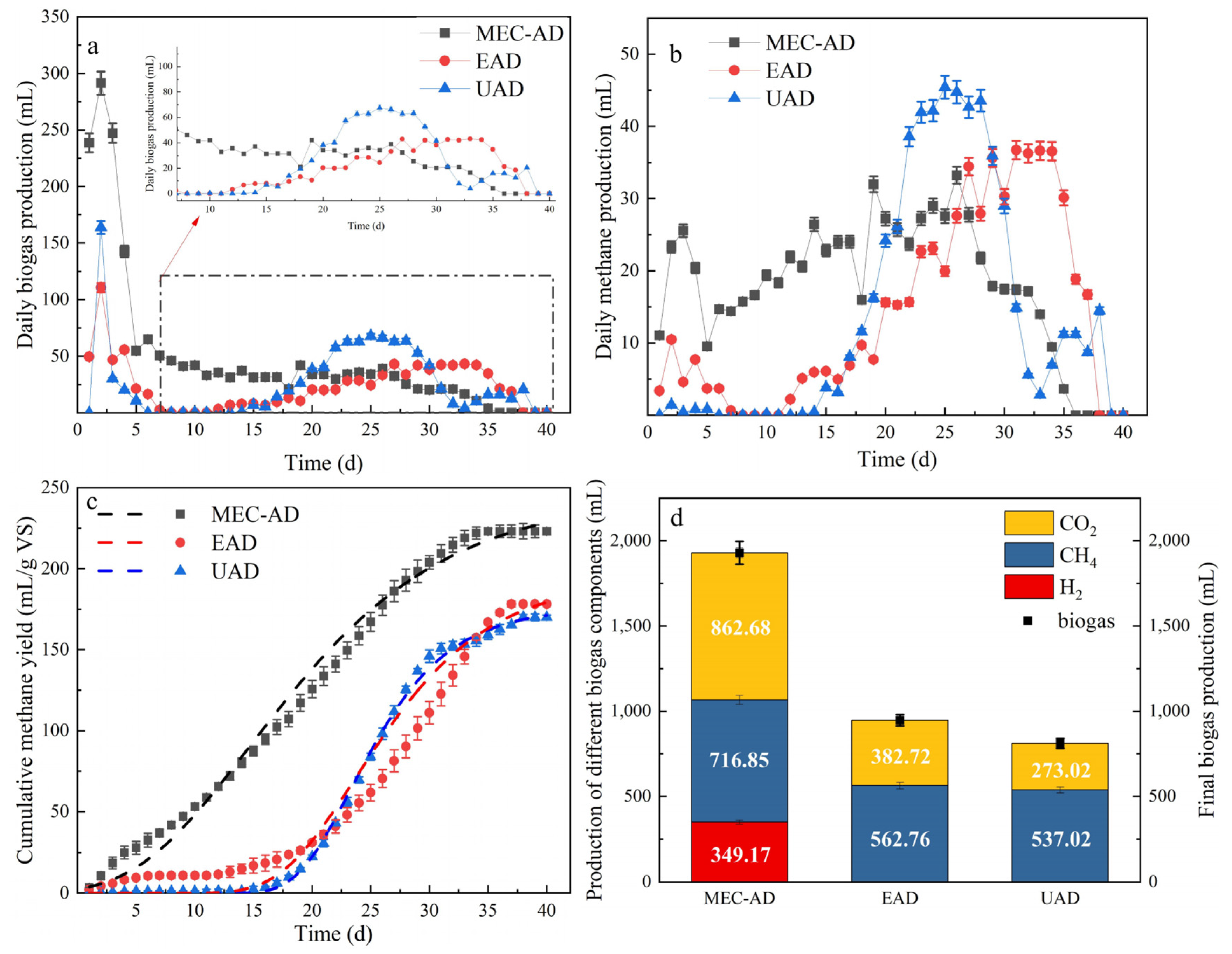
3.1. Biogas and Biomethane Production
3.1.1. Daily Biogas Production
3.1.2. Accumulative Biogas Yield
3.2. Substance Conversion
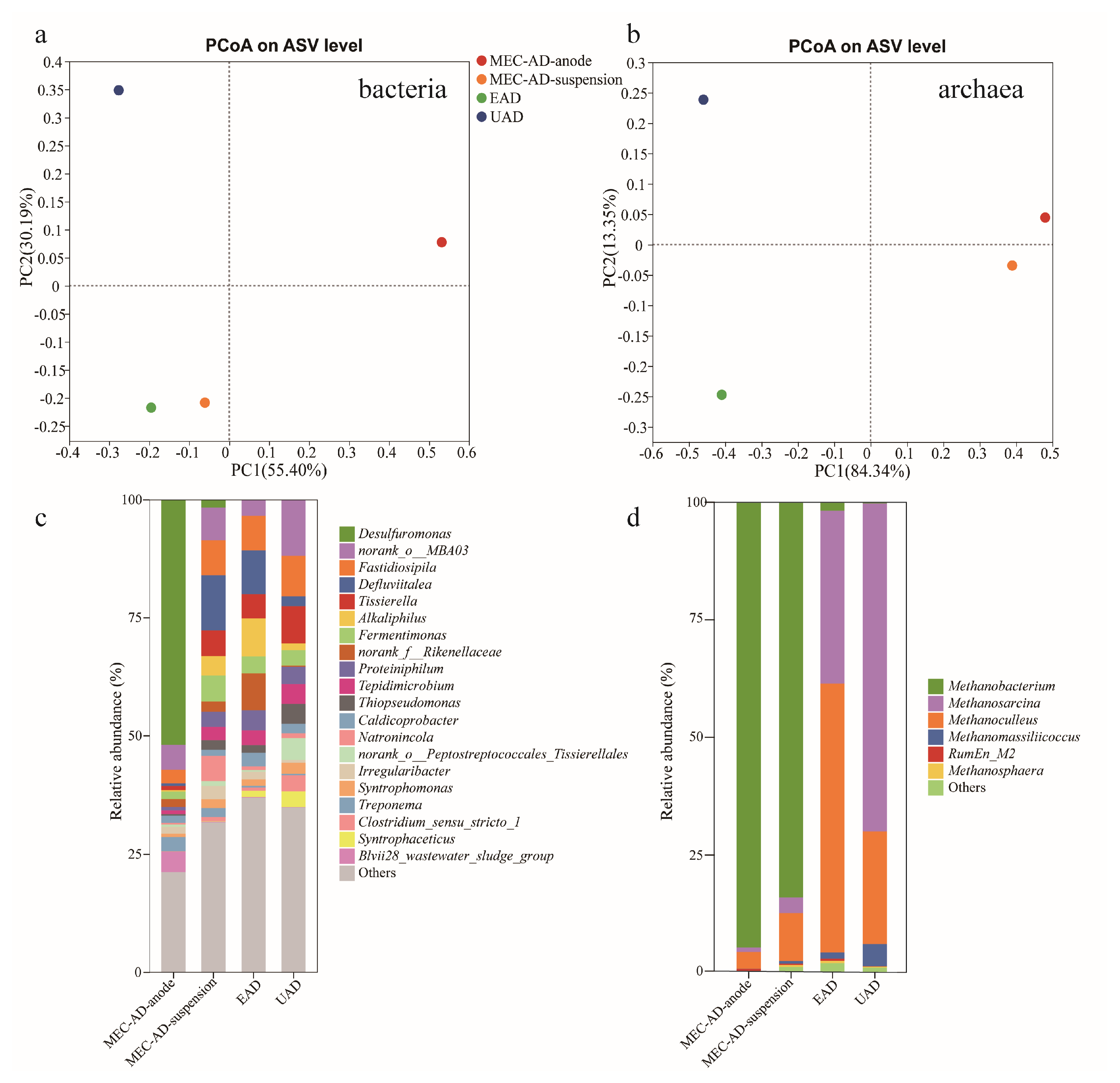
3.3. Microbiological Community Characteristics
3.3.1. Microbial Community Diversity
3.3.2. Microbial Community Structure
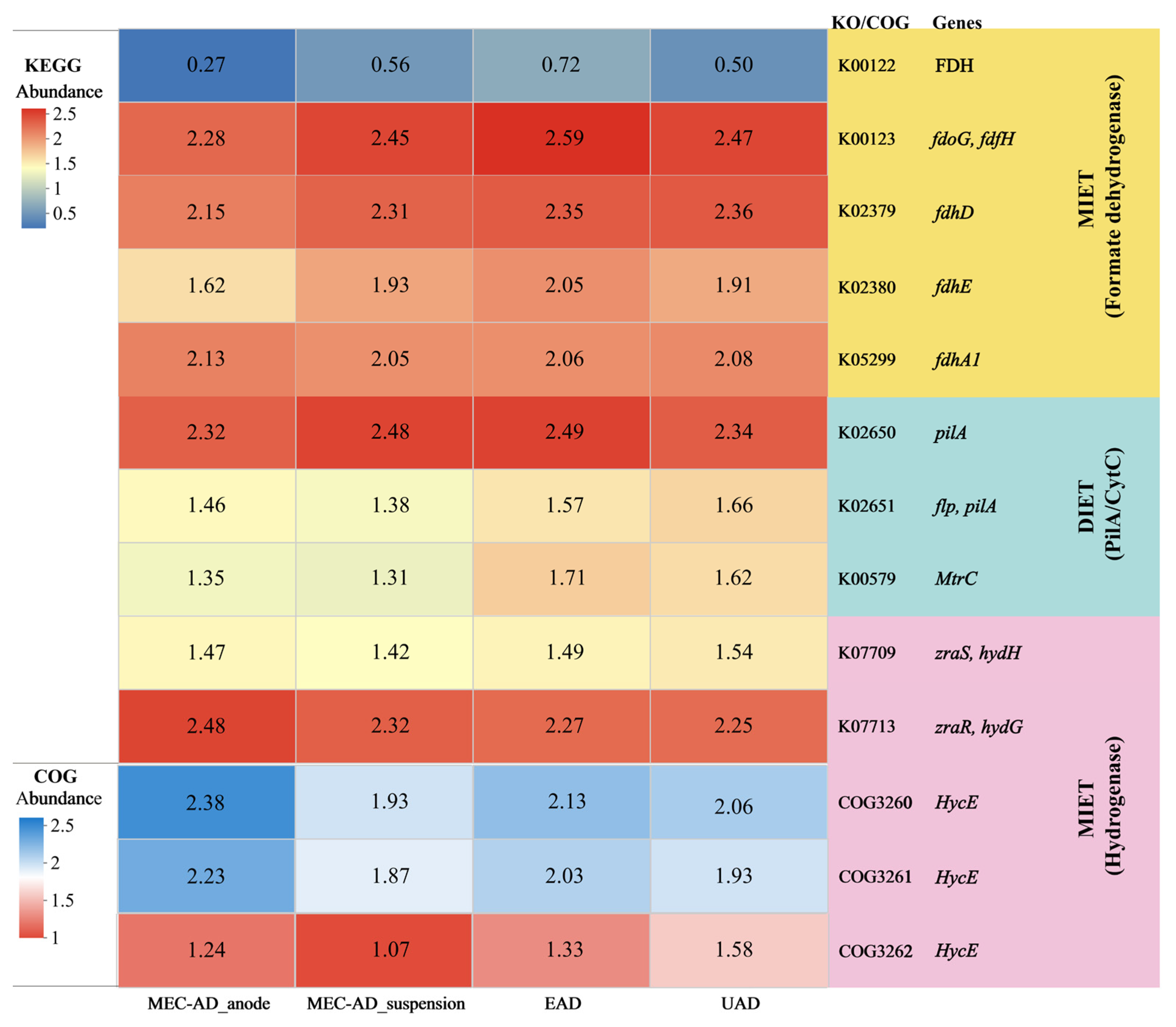
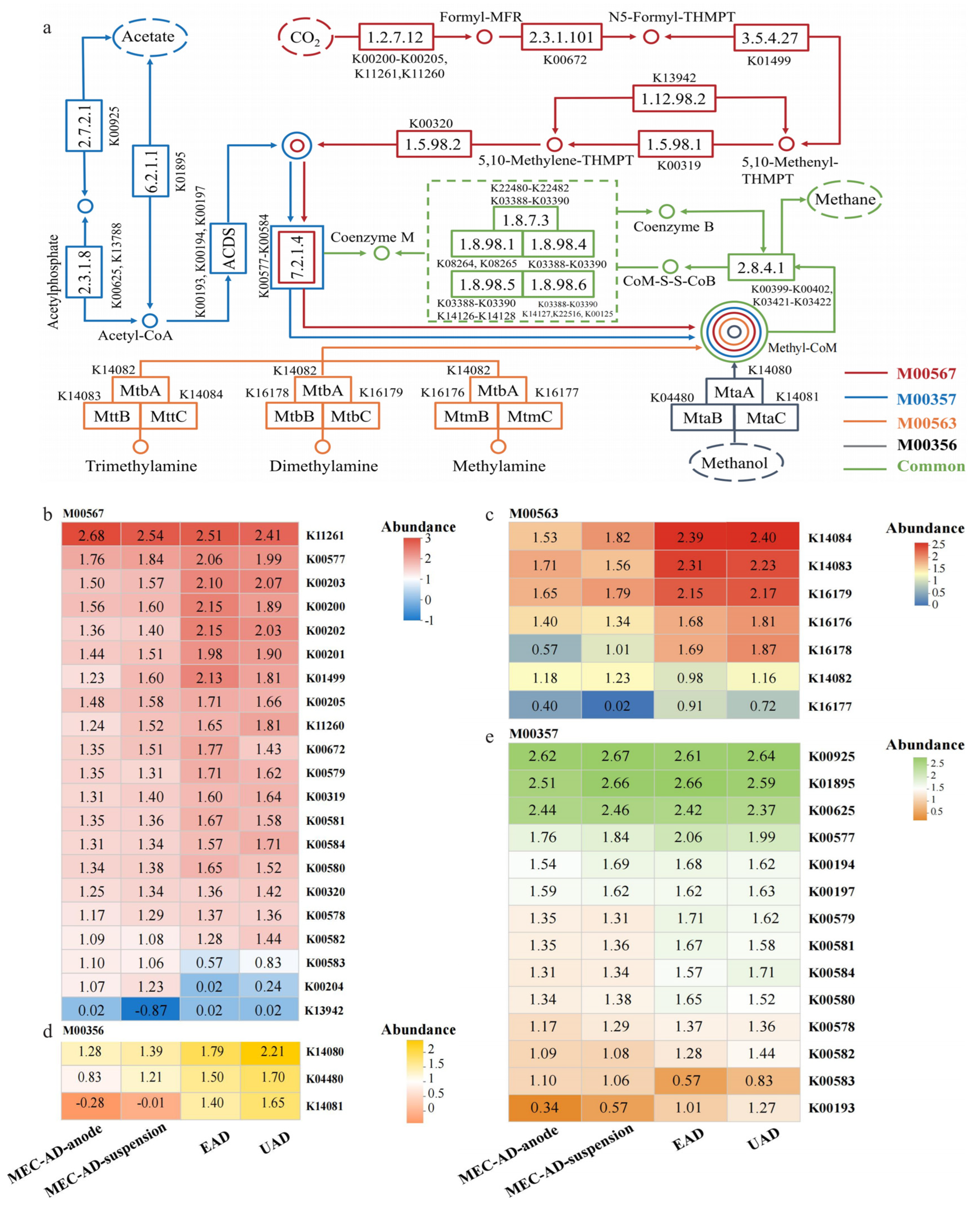
3.4. Crucial Metabolic Pathways and Macrogenomic Analysis
3.4.1. Interspecies Electron Transfer Syntrophic Metabolic Pathways
3.4.2. Methanogenic Metabolism Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, F.; Fu, N.; Wei, Q.; Liu, S.; Hu, Y.; Zhang, S.; Wang, X.; Peng, X.; Dai, H.; Wei, Y. Effect of Alkali Pretreatment Time on Kitchen Waste Anaerobic Digestion Performance Enhanced by Alkali Pretreatment Combined with Bentonite: Performance Enhancement, Microbial Community Structure, and Functional Gene Analysis. Environ. Sci. Pollut. Res. 2024, 31, 7167–7178. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Fu, Z.; Wang, Y.; Guan, D.; Xie, J.; Zhang, Q.; Liu, Q.; Wang, D.; Sun, Y. Metagenomic Approach Reveals the Mechanism of Calcium Oxide Improving Kitchen Waste Dry Anaerobic Digestion. Bioresour. Technol. 2023, 387, 129647. [Google Scholar] [CrossRef]
- Hu, F.; Fu, N.; Wei, Q.; Wang, X.; Pan, Z.; Hu, Y. The Potential Role of Iron-Carbon Micro-Electrolysis Materials in Curtailing Lag-Phase Stimulates Kitchen Waste Anaerobic Digestion at Different Solid Contents: Performance, Synergistic Effect and Microbial Response. J. Environ. Manag. 2024, 370, 122733. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Q.; Liu, Y.; Zhu, J.; Sun, F.; Cui, M.-H.; Liu, H.; Zhang, T.C.; Chen, C. Enhancing Degradation of Organic Matter in Microbial Electrolytic Cells Coupled with Anaerobic Digestion (MEC-AD) Systems by Carbon-Based Materials. Sci. Total Environ. 2023, 900, 165805. [Google Scholar] [CrossRef]
- Zou, L.; Wang, C.; Zhao, X.; Wu, K.; Liang, C.; Yin, F.; Yang, B.; Liu, J.; Yang, H.; Zhang, W. Enhanced Anaerobic Digestion of Swine Manure via a Coupled Microbial Electrolysis Cell. Bioresour. Technol. 2021, 340, 125619. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, S.; Jadhav, G.S.; Jadhav, D.A.; Ghangrekar, M.M.; Surampalli, R.Y. Electromethanogenic Reactor for Biogas Production Using Agricultural and Livestock Waste and Its Comparative Analysis with Biogas Plant: A Mini-Review. Biomass Bioenergy 2024, 185, 107246. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and Efficient Biohydrogen Production via Electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef]
- Kanellos, G.; Tremouli, A.; Arvanitakis, G.; Lyberatos, G. Boosting Methane Production and Raw Waste Activated Sludge Treatment in a Microbial Electrolysis Cell-Anaerobic Digestion (MEC-AD) System: The Effect of Organic Loading Rate. Bioelectrochemistry 2024, 155, 108555. [Google Scholar] [CrossRef]
- Choi, J.-M.; Lee, C.-Y. Bioelectrochemical Enhancement of Methane Production in Anaerobic Digestion of Food Waste. Int. J. Hydrogen Energy 2019, 44, 2081–2090. [Google Scholar] [CrossRef]
- Ao, T.-J.; Zhao, X.-Q.; Mehmood, M.A.; Wang, N.; Zhu, H.; Liu, C.-G.; Bai, F.-W. A Double-Chamber Microbial Electrolysis Cell Improved the Anaerobic Digestion Efficiency and Elucidated the Underlying Bio-Electrochemical Mechanism. Chem. Eng. J. 2023, 471, 144228. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Q.; Sun, C.; Zhu, Y.; Wang, Z.; Zhang, Y. Intermittent Voltage Induced Sludge Polarization to Enhance Anaerobic Digestion. Water Res. 2022, 224, 119071. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, C.; Pu, J.; Feng, Y.; Zhu, H.; Yang, M.; Xu, Z.; Zhang, Y.; Yang, L.; Luo, D.; et al. Intermittent Energization Improves Anaerobic Digestion of Microbial Electrolysis Cell-Assisted Nitrogen-Rich Sludge under Mesophilic and Thermophilic Conditions. J. Environ. Chem. Eng. 2024, 12, 111630. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Zhan, J.; Zhang, Y.; Zhao, Z.; Zhao, Z. Combining Metal-Microbe and Microbe-Microbe Dual Direct Electron Transfer on Fe(0)-Cathode of Bio-Electrochemical System to Enhance Anaerobic Digestion of Cellulose Wastewater. Chin. Chem. Lett. 2022, 33, 3106–3112. [Google Scholar] [CrossRef]
- Baek, G.; Saikaly, P.E.; Logan, B.E. Addition of a Carbon Fiber Brush Improves Anaerobic Digestion Compared to External Voltage Application. Water Res. 2021, 188, 116575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, M.; Mou, H.; An, Z.; Fu, H.; Su, X.; Chen, C.; Chen, J.; Lin, H.; Sun, F. Comparation of Mesophilic and Thermophilic Anaerobic Co-Digestion of Food Waste and Waste Activated Sludge Driven by Biochar Derived from Kitchen Waste. J. Clean. Prod. 2023, 408, 137123. [Google Scholar] [CrossRef]
- Zhang, S.; Guan, W.; Sun, H.; Zhao, P.; Wang, W.; Gao, M.; Sun, X.; Wang, Q. Intermittent Energization Improves Microbial Electrolysis Cell-Assisted Thermophilic Anaerobic Co-Digestion of Food Waste and Spent Mushroom Substance. Bioresour. Technol. 2023, 370, 128577. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, W.; Hou, J.; Qi, X.; Ye, M.; Jiang, N.; Meng, F.; Xi, B.; Li, M. Biogas Upgrading Performance and Underlying Mechanism in Microbial Electrolysis Cell and Anaerobic Digestion Integrated System. Bioresour. Technol. 2024, 400, 130683. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, X.; Wang, H.; Liu, X.; Gu, L. Insights into the Mitigative Effects of Microbial Electrolysis Cell on Anaerobic Digestion under High Ammonia Condition. Chem. Eng. J. 2023, 474, 145760. [Google Scholar] [CrossRef]
- Wang, A.; Liu, W.; Cheng, S.; Xing, D.; Zhou, J.; Logan, B.E. Source of Methane and Methods to Control Its Formation in Single Chamber Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2009, 34, 3653–3658. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, H.; Li, X. Anaerobic Bioconversion Efficiency of Rice Straw in Continuously Stirred Tank Reactor Systems Applying Longer Hydraulic Retention Time and Higher Load: One-Stage vs. Two-Stage. Bioresour. Technol. 2021, 321, 124206. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, X.; Liu, Y.; Jia, Z.; Cao, C.; Xiao, Q.; Du, J.; Kong, X.; Wu, X.; Chen, Z.; et al. Enhanced Anaerobic Digestion for Degradation of Swine Wastewater through a Fe/Ni-MOF Modified Microbial Electrolysis Cell. J. Clean. Prod. 2022, 380, 134773. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, Q.; Sun, C.; Wang, Z.; Jin, Z.; Zhu, Y.; Zhao, Z.; Zhang, Y. Additional Electric Field Alleviates Acidity Suppression in Anaerobic Digestion of Kitchen Wastes via Enriching Electro-Active Methanogens in Cathodic Biofilms. Water Res. 2022, 212, 118118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yuan, H.; Wang, H.; Li, X. Enhancing Biomethane Production from Corn Stover via Anaerobic Digestion Incorporated with Microbial Electrolysis Cell. Chin. J. Chem. Eng. 2025; in press. [Google Scholar] [CrossRef]
- Peng, C.; Wang, T.; Feng, Y.; Fan, X.; Niu, J.; Wang, J.; Gao, W.; Zhou, Y.; Hu, W.; Zhang, Q. Enhanced Hydrolysis and Methane Yield of Temperature-Phased Dewatered Sludge Anaerobic Digestion by Microbial Electrolysis Cell. Bioresour. Technol. 2024, 400, 130682. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A.; Kakde, A.; Liu, P.; Li, X. A Review on the Applications of Microbial Electrolysis Cells in Anaerobic Digestion. Bioresour. Technol. 2018, 255, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Cheng, J.; Li, H.; Yue, L.; Xia, R.; Zhou, J. Electron Transfer from Geobacter Sulfurreducens to Mixed Methanogens Improved Methane Production with Feedstock Gases of H2 and CO2. Bioresour. Technol. 2022, 347, 126680. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of pH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Yasser Farouk, R.; Li, L.; Wang, Y.; Li, Y.; Melak, S. Influence of Pretreatment and pH on the Enhancement of Hydrogen and Volatile Fatty Acids Production from Food Waste in the Semi-Continuously Running Reactor. Int. J. Hydrogen Energy 2020, 45, 3729–3738. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Pierra, M.; Carmona-Martínez, A.A.; Trably, E.; Godon, J.-J.; Bernet, N. Specific and Efficient Electrochemical Selection of Geoalkalibacter subterraneus and Desulfuromonas acetoxidans in High Current-Producing Biofilms. Bioelectrochemistry 2015, 106, 221–225. [Google Scholar] [CrossRef]
- Vikromvarasiri, N.; Koyama, M.; Kurniawan, W.; Pisutpaisal, N.; Nakasaki, K. Enhancing Methane Recovery by Intermittent Substrate Feeding and Microbial Community Response in Anaerobic Digestion of Glycerol. Renew. Energy 2023, 204, 106–113. [Google Scholar] [CrossRef]
- Sapireddy, V.; Katuri, K.P.; Muhammad, A.; Saikaly, P.E. Competition of Two Highly Specialized and Efficient Acetoclastic Electroactive Bacteria for Acetate in Biofilm Anode of Microbial Electrolysis Cell. Npj Biofilms Microbiomes 2021, 7, 47. [Google Scholar] [CrossRef]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-Reducing Microorganisms That Harvest Energy from Marine Sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Rimboud, M.; Etcheverry, L.; Barakat, M.; Achouak, W.; Bergel, A.; Délia, M.-L. Hypersaline Microbial Fuel Cell Equipped with an Oxygen-Reducing Microbial Cathode. Bioresour. Technol. 2021, 337, 125448. [Google Scholar] [CrossRef]
- Han, G.; Shin, S.G.; Cho, K.; Lee, J.; Kim, W.; Hwang, S. Temporal Variation in Bacterial and Methanogenic Communities of Three Full-Scale Anaerobic Digesters Treating Swine Wastewater. Environ. Sci. Pollut. Res. 2019, 26, 1217–1226. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Wang, P.; Li, K.; Su, Y.; Wu, D.; Xie, B. Potential of Biogas Residue Biochar Modified by Ferric Chloride for the Enhancement of Anaerobic Digestion of Food Waste. Bioresour. Technol. 2022, 360, 127530. [Google Scholar] [CrossRef]
- Xing, T.; Wang, Z.; Zhen, F.; Liu, H.; Wo, D.; Li, L.; Guo, Y.; Kong, X.; Sun, Y. Initial pH-Driven Production of Volatile Fatty Acid from Hybrid Pennisetum. Bioresour. Technol. 2022, 347, 126426. [Google Scholar] [CrossRef]
- Ao, T.; Chen, L.; Chen, Y.; Liu, X.; Wan, L.; Li, D. The Screening of Early Warning Indicators and Microbial Community of Chicken Manure Thermophilic Digestion at High Organic Loading Rate. Energy 2021, 224, 120201. [Google Scholar] [CrossRef]
- Kim, E.; Lee, J.; Han, G.; Hwang, S. Comprehensive Analysis of Microbial Communities in Full-Scale Mesophilic and Thermophilic Anaerobic Digesters Treating Food Waste-Recycling Wastewater. Bioresour. Technol. 2018, 259, 442–450. [Google Scholar] [CrossRef]
- Huang, S.; Shen, M.; Ren, Z.J.; Wu, H.; Yang, H.; Si, B.; Lin, J.; Liu, Z. Long-Term in Situ Bioelectrochemical Monitoring of Biohythane Process: Metabolic Interactions and Microbial Evolution. Bioresour. Technol. 2021, 332, 125119. [Google Scholar] [CrossRef]
- Feldewert, C.; Lang, K.; Brune, A. The Hydrogen Threshold of Obligately Methyl-Reducing Methanogens. FEMS Microbiol. Lett. 2020, 367, fnaa137. [Google Scholar] [CrossRef] [PubMed]
- Conway de Macario, E.; Macario, A.J.L. Methanogenic Archaea in Health and Disease: A Novel Paradigm of Microbial Pathogenesis. Int. J. Med. Microbiol. 2009, 299, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Ya-Hai, L.U. A Review of Interspecies Electron Transfer in Syntrophic-Methanogenic Associations. Microbiol. China 2015, 42, 920–927. [Google Scholar]
- Zhang, H.; Zhou, J.; Zhang, C.; Li, M. Interspecies Electron Transfer during Microbial Syntrophic Methanogenesis. Acta Microbiol. Sin. 2023, 63, 2047–2065. [Google Scholar] [CrossRef]
- Cai, C.; Li, L.; Hua, Y.; Liu, H.; Dai, X. Ferroferric Oxide Promotes Metabolism in Anaerolineae Other than Microbial Syntrophy in Anaerobic Methanogenesis of Antibiotic Fermentation Residue. Sci. Total Environ. 2021, 758, 143601. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Che, X.; Li, C.; Liang, D.; Zhou, H.; Liu, L.; Zhao, Z.; Zhang, Y. Biochar Stimulates Growth of Novel Species Capable of Direct Interspecies Electron Transfer in Anaerobic Digestion via Ethanol-Type Fermentation. Environ. Res. 2020, 189, 109983. [Google Scholar] [CrossRef]

| Kitchen Waste | Original Inoculum | Electrically Domesticated Inoculum | |
|---|---|---|---|
| TS (%) b | 67.64 ± 0.15 | 12.56 ± 0.10 | 6.00 ± 0.13 |
| VS (%) b | 59.64 ± 0.35 | 5.04 ± 0.13 | 3.10 ± 0.23 |
| Cellulose (%) c | 20.83 ± 2.04 | 1.87 ± 0.12 | 0.64 ± 0.05 |
| Hemicellulose (%) c | 16.54 ± 0.92 | 3.57 ± 0.74 | 1.31 ± 0.16 |
| Lignin (%) c | 11.01 ± 1.78 | 2.44 ± 0.16 | 1.11 ± 0.11 |
| pH | / | 7.82 ± 0.03 | 7.73 ± 0.05 |
| Modified Gompertz Model | |||||
|---|---|---|---|---|---|
| Reactors | H (mL CH4/g VS) | p (mL CH4/g VS) | a (d) | λ (mL CH4/(g VS·d)) | R2 |
| MEC-AD | 223.12 ± 2.14 | 245.45 ± 4.27 | 5.07 ± 0.15 | 9.40 ± 0.16 | 0.996 |
| EAD | 178.16 ± 1.72 | 194.34 ± 5.12 | 17.33 ± 0.47 | 11.11 ± 0.74 | 0.996 |
| UAD | 169.97 ± 0.98 | 176.08 ± 2.17 | 18.70 ± 0.14 | 13.92 ± 0.41 | 0.998 |
| Sample | Bacteria | Archaea | ||||||
|---|---|---|---|---|---|---|---|---|
| Chao | Shannon | Simpson | Coverage | Chao | Shannon | Simpson | Coverage | |
| MEC-AD_A | 1061.39 | 3.36 | 0.25 | 0.999 | 36.00 | 0.80 | 0.60 | 1.000 |
| MEC-AD_S | 1119.03 | 5.15 | 0.02 | 0.999 | 45.00 | 1.37 | 0.38 | 1.000 |
| EAD | 1196.21 | 5.02 | 0.02 | 1.000 | 76.00 | 1.95 | 0.23 | 1.000 |
| UAD | 1293.73 | 5.24 | 0.02 | 0.998 | 44.00 | 1.21 | 0.48 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wang, H.; Liu, R.; Yuan, H.; Li, X. Enhancing Biomethane Yield and Metabolic Pathways in Kitchen Waste Anaerobic Digestion Through Microbial Electrolysis Cell Integration. Energies 2025, 18, 1629. https://doi.org/10.3390/en18071629
Zhao Q, Wang H, Liu R, Yuan H, Li X. Enhancing Biomethane Yield and Metabolic Pathways in Kitchen Waste Anaerobic Digestion Through Microbial Electrolysis Cell Integration. Energies. 2025; 18(7):1629. https://doi.org/10.3390/en18071629
Chicago/Turabian StyleZhao, Qing, Heran Wang, Rufei Liu, Hairong Yuan, and Xiujin Li. 2025. "Enhancing Biomethane Yield and Metabolic Pathways in Kitchen Waste Anaerobic Digestion Through Microbial Electrolysis Cell Integration" Energies 18, no. 7: 1629. https://doi.org/10.3390/en18071629
APA StyleZhao, Q., Wang, H., Liu, R., Yuan, H., & Li, X. (2025). Enhancing Biomethane Yield and Metabolic Pathways in Kitchen Waste Anaerobic Digestion Through Microbial Electrolysis Cell Integration. Energies, 18(7), 1629. https://doi.org/10.3390/en18071629





