Abstract
Hydrogen production via water splitting is a crucial strategy for addressing the global energy crisis and promoting sustainable energy solutions. This review systematically examines water-splitting mechanisms, with a focus on photocatalytic and electrochemical methods. It provides in-depth discussions on charge transfer, reaction kinetics, and key processes such as the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). Various electrode synthesis techniques, including hydrothermal methods, chemical vapor deposition (CVD), pulsed laser deposition (PLD), and radio frequency sputtering (RF), are reviewed for their advantages and limitations. The role of carbon-based materials such as graphene, biochar, and graphitic carbon nitride (g-C3N4) in photocatalytic and photoelectrochemical (PEC) water splitting is also highlighted. Their exceptional conductivity, tunable band structures, and surface functionalities contribute to efficient charge separation and enhanced light absorption. Further, advancements in heterojunctions, doped systems, and hybrid composites are explored for their ability to improve photocatalytic and PEC performance by minimizing charge recombination, optimizing electronic structures, and increasing active sites for hydrogen and oxygen evolution reactions. Key challenges, including material stability, cost, scalability, and solar spectrum utilization, are critically analyzed, along with emerging strategies such as novel synthesis approaches and sustainable material development. By integrating water splitting mechanisms, electrode synthesis techniques, and advancements in carbon-based materials, this review provides a comprehensive perspective on sustainable hydrogen production, bridging previously isolated research domains.
1. Introduction
Recent studies have revealed that the world population is increasing daily and is expected to reach approximately 10 billion by the end of 2050. This growth rate is estimated to be around 22% by that time [1]. This surge in population is projected to drive a corresponding rise in global energy demand. Currently, about 80% of the global energy requirements are met through the use of fossil fuels. However, fossil fuels significantly contribute to greenhouse gas emissions, which pose significant threats to ecosystems and human health [2]. To mitigate dependence on fossil fuels, researchers are actively investigating alternative energy solutions, with a particular focus on renewable sources [3].
Hydrogen, recognized as a clean and sustainable fuel, has emerged as a key contender in the quest for eco-friendly energy. With no harmful environmental impacts and a high calorific value, hydrogen holds immense promise as a potential energy source for the future [4]. The applications of hydrogen span a wide range of industries as depicted in Figure 1. It powers hydrogen vehicles through fuel cells, helps convert CO2 into synthetic fuels, and is used in upgrading oil and biomass [5]. In the production of ammonia for fertilizers, hydrogen reacts with nitrogen (N2), playing a key role in agriculture. It is also used in metal refining, providing the high-energy environment required for industrial processes. Furthermore, hydrogen can be distributed via gas infrastructure for heating and is employed in various other industrial uses. This interconnectedness underscores hydrogen potential in driving future energy systems across power generation, storage, transportation, and industry.
There are various methods, such as steam methane reforming, partial oxidation of methane, autothermal reforming, plasmolysis of hydrocarbons, and water splitting to produce hydrogen [6]. Each method has its unique advantages and challenges, which largely depend on the specific processes and feedstocks used for hydrogen production.
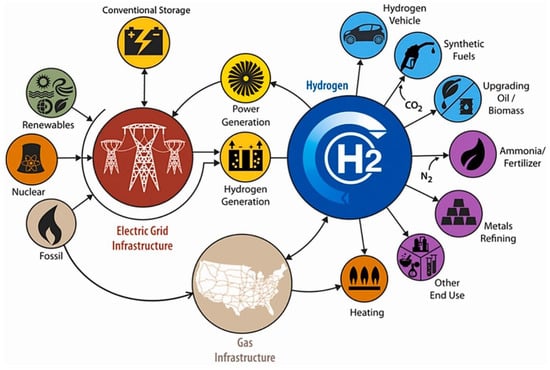
Figure 1.
Applications of hydrogen from domestic to industrial levels, spanning energy generation, storage, transportation, heating, synthetic fuels, ammonia production, and metals refining. Reprinted with permission from [7].
Steam methane reforming is one of the most widely used techniques, requiring a temperature range of 800–1000 °C. This method offers a high conversion rate of methane, around 85%, to produce hydrogen. However, the main drawback of this process is the emission of CO2, which poses another threat to natural resources and the environment by increasing atmospheric temperatures [8]. Another method, known as partial oxidation of methane, operates at similar temperatures to steam methane reforming and can convert feedstock into approximately 90% hydrogen [9]. However, CO production during this process presents a challenge, as CO removal technologies complicate industrial implementation, limiting its widespread adoption despite its potential. Hydrogen can also be produced through biological methods. Unlike methane reforming, this approach does not require high temperatures. It can convert around 90% of the feedstock into hydrogen. This method offers a promising eco-friendly alternative, as no harmful gases such as CO2 or CO are emitted during the process. However, it is relatively slow, and the microorganisms involved require very specific conditions, which makes scaling it for industrial use challenging [10,11]. Hydrogen can also be produced through metal corrosion [12]. In this regard, aluminum is considered a suitable candidate for hydrogen production due to its fundamental properties, such as low density (2.7 g/cm3), a highly negative standard electrode potential (−1660 mV), and low atomic mass [13]. Under ideal conditions, one gram of aluminum can generate approximately 1360 mL of hydrogen at standard conditions. However, the formation of a passivating oxide layer in water significantly reduces the hydrogen yield from aluminum. Additionally, factors such as temperature, molarity, and the acidic or basic nature of the medium during the corrosion process influence hydrogen production [14]. Therefore, to maximize hydrogen generation from metal corrosion, particularly with aluminum, optimizing the experimental conditions is essential.
Hydrogen production through water splitting is an attractive and environmentally friendly method, as it utilizes renewable resources such as water, thereby avoiding environmental concerns [5]. Importantly, no byproduct gases such as CO2 or CO are emitted during the water splitting process, further emphasizing its eco-friendliness. Water splitting encompasses various categories (Figure 2), including electrochemical, photoelectrochemical (PEC), photocatalytic, photobiological, and thermal decomposition methods, each contributing to efficient hydrogen production and the transition toward sustainable energy sources [6,7]. Electrolysis, though efficient, accounts for only 4% of global hydrogen production due to high costs, but advancements in renewable electricity are reducing these expenses. By 2035, “green hydrogen” (produced via renewable-powered electrolysis) is expected to become more cost-effective than “blue hydrogen” (derived from fossil fuels with carbon capture) [1].
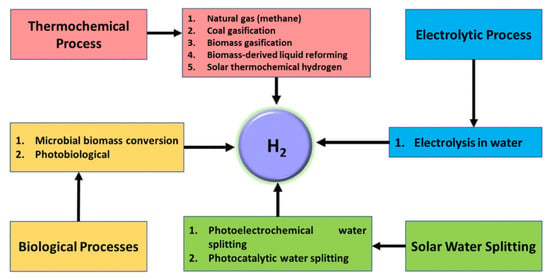
Figure 2.
Hydrogen production through various water splitting methods. Reprinted with permission from [15].
Photoelectrochemical (PEC) water splitting is a highly promising method for sustainable hydrogen production, leveraging solar energy to drive water dissociation into hydrogen and oxygen gases. This process is advantageous as it eliminates reliance on fossil fuels, minimizes carbon emissions, and operates under ambient conditions, making it more energy-efficient and environmentally friendly. Moreover, the use of low-cost and carbon-based materials in PEC systems reduces production expenses, enhancing the feasibility of large-scale implementation [8]. However, several challenges must be addressed to optimize its potential. One of the primary limitations is the development of efficient and stable photoelectrode materials capable of prolonged operation under solar irradiation without significant degradation. The efficiency of PEC material is often hindered by poor charge carrier mobility and rapid recombination of photogenerated electron–hole pairs, necessitating the incorporation of co-catalysts, heterostructures, or surface modifications to enhance performance [6]. Despite these hurdles, PEC water splitting remains a highly attractive approach for renewable hydrogen generation, aligning with global efforts to achieve carbon neutrality and sustainable energy security.
To date, various review articles have highlighted hydrogen production from water. However, these articles often focus on specific domains. For instance, Alnarabiji et al. [1] reviewed the synthesis of electrode materials for hydrogen production, while some studies, such as Mishra et al., discuss the role of carbon-based materials like biochar in hydrogen production [2]. Similarly, Jiang et al. [3] reviewed the role of carbon nitride in hydrogen production, and Bora et al. [4] explored the fundamentals of water splitting for hydrogen production. In contrast to the existing literature, our review uniquely integrates three key areas: water-splitting mechanisms, electrode synthesis techniques, and advancements in carbon-based materials. It offers a holistic perspective on sustainable hydrogen production and bridges previously isolated research domains.
2. Water Splitting Mechanism
2.1. Photocatalytic Water Splitting
Photocatalytic water splitting has garnered significant attention as a sustainable method for hydrogen production, an essential energy carrier crucial for tackling global energy and environmental challenges [16]. This process harnesses sunlight to decompose water into hydrogen and oxygen using semiconductor photocatalysts such as TiO2, CdS, or ZnO [17]. The fundamental mechanism of photocatalytic water splitting involves the ability of semiconductor materials to absorb photons, generating electron–hole pairs that drive redox reactions, resulting in the formation of H2 and O2, as illustrated in Figure 3. The overall reaction can be expressed as 2H2O → 2H2 + O2, with two half-reactions: water oxidation at the valence band (producing O2) and proton reduction at the conduction band (producing H2) [18]. A critical requirement for effective photocatalysts is that their conduction band edge must be more negative than the hydrogen reduction potential (0 V vs. NHE), while their valence band edge must be more positive than the OER potential (+1.23 V vs. NHE) to ensure sufficient energy for these reactions [19].
To efficiently absorb sunlight, an ideal photocatalyst should have a suitable bandgap, typically between 1.5 eV and 2.5 eV. While many semiconductors like TiO2, ZnO, and CdS have been extensively studied, they often require modifications to optimize their bandgaps for enhanced solar absorption. A major challenge lies in minimizing charge carrier recombination during the transition from the valence to the conduction band. Once charge separation occurs, photogenerated electrons must migrate to the photocatalyst surface for water reduction, while holes facilitate water oxidation [20]. Materials with a high surface area and abundant active sites are essential for improved interaction with water molecules, thereby enhancing reaction efficiency, as illustrated in Figure 4.
Several types of photocatalysts have been developed to address these challenges, including metal oxides, sulfides, perovskites, and two-dimensional materials. Titanium dioxide (TiO2) is particularly valued for its stability, non-toxicity, and abundance; however, its large bandgap (3.2 eV) restricts its efficiency in the visible light region, which constitutes the majority of the solar spectrum [21]. Strategies to overcome this limitation include doping TiO2 with elements like nitrogen or combining it with other materials such as silver to extend its absorption into the visible range [22]. In contrast, cadmium sulfide (CdS) has a narrower bandgap (2.4 eV), making it more effective at absorbing visible light, but it is susceptible to photocorrosion. Recent research focuses on stabilizing CdS by integrating it with metal nanoparticles or co-catalysts [23].
Emerging materials such as perovskites and two-dimensional substances like graphene and g-C3N4 exhibit great promise due to their tunable bandgaps and excellent charge transport properties [24]. Heterojunctions, which combine different semiconductors, enhance charge separation and reduce recombination by creating a built-in electric field, significantly improving photocatalytic performance. Notable examples of these heterojunctions include TiO2/CdS and BiVO4/WO3 [25].
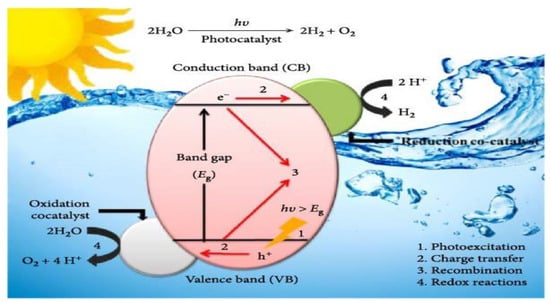
Figure 3.
Mechanism of photocatalytic water splitting. Under light irradiation (hv ≥ Eg), the semiconductor generates electron–hole pairs. Electrons in the conduction band reduce protons (H⁺) to form hydrogen (H2), while holes in the valence band oxidize water (H2O) to produce oxygen (O2). Reprinted with permission from [26].
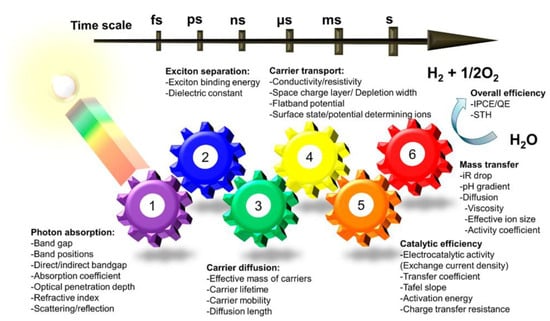
Figure 4.
Key parameters involved in the photocatalytic process. Efficient overall photocatalytic water splitting relies on the optimization of all six interconnected factors depicted. Reprinted with permission from [20].
2.2. Photoelectrochemical Reactions
PEC reactions, as initially described by Pleskov [27], are a specific type of photochemical process in which the reaction occurs due to the flow of current through an external circuit. In PEC water splitting, photoexcitation at the photo anode generates electrons, which are transferred through the external circuit to the cathode. At the photo anode, OER takes place, producing oxygen (O2), while at the cathode, the HER simultaneously generates hydrogen (H2).
In photocatalytic and PEC systems, the primary step in heterogeneous catalysis is the generation of electron–hole pairs within the semiconductor. These semiconductors absorb photons with energy equal to or greater than their band gap, leading to the formation of charge carriers [28,29,30,31,32]. Efficient charge separation and mobility are crucial for sustaining redox reactions. Once generated, electrons and holes follow different pathways, including migration to the semiconductor surface, bulk transport, or recombination at surface and bulk sites. In PEC processes, enhanced charge mobility facilitates rapid charge transfer and minimizes recombination losses, thereby increasing their availability at active sites and improving overall reaction kinetics. However, charge recombination remains a major bottleneck, as it reduces the number of charge carriers available for chemical transformations [33].
The lifetime of mobile charge carriers plays a critical role in rectifying, photo-conducting, and regulating transistor behavior. Moreover, photocurrent density and quantum efficiency are directly influenced by charge mobility. A higher photocurrent indicates efficient charge collection at the electrodes, driven by faster charge transport [34]. However, photocatalytic performance can be hindered by space charge effects, where accumulated charges at interfaces impede carrier transport in low-mobility materials.
Materials with higher charge mobility generally require lower overpotentials, resulting in improved energy conversion efficiency. To enhance charge carrier mobility and minimize recombination losses, several strategies have been employed, including elemental doping [35], heterojunction formation [36], and defect engineering [37]. These approaches enable better charge separation and transport, ultimately improving photocatalytic and PEC system performance.
2.2.1. Hydrogen Evolution Reaction
The HER is a critical electrochemical process in water splitting, responsible for generating hydrogen gas at the cathode. This process involves multiple steps, beginning with the adsorption of protons or water molecules onto the catalyst surface. The first step, known as the Volmer step, involves the reduction in protons or water molecules to form adsorbed hydrogen atoms [38]. These hydrogen atoms can either combine chemically in the Tafel step or react electrochemically with another proton and electron in the Heyrovsky step to produce hydrogen gas [39,40], as illustrated in Figure 5.
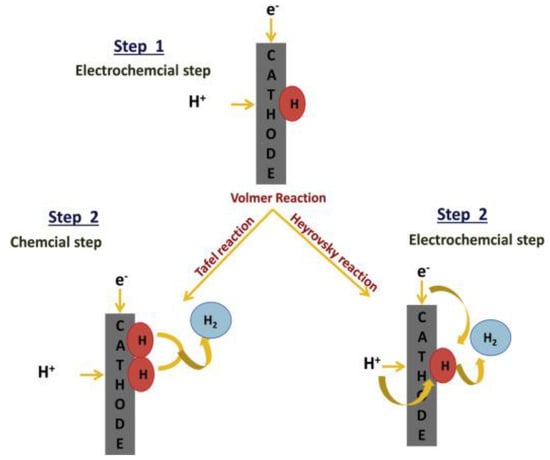
Figure 5.
Schematic of the HER mechanism, illustrating the Volmer step (proton adsorption), followed by the Tafel or Heyrovsky steps, leading to H2 release via the Volmer–Tafel and Volmer–Heyrovsky pathways on the electrocatalyst surface. Reprinted with permission from [41].
The overall HER can occur in either acidic or alkaline media, represented by the following reactions:
HER can occur in acidic, basic, or neutral media, but its efficiency depends heavily on the electrocatalyst and solution of pH. In acidic conditions, protons (H⁺) are readily available for reduction, accelerating the reaction. In alkaline environments, water molecules are reduced instead of protons, which slows the process but can reduce corrosion. Recent studies demonstrated that HER could be pH independent. The NiCuMo medium entropy alloy electrocatalyst exhibited highly efficient HER, with overpotentials of ~63 mV in 1 M KOH and ~115 mV in pH 7 buffer at a current density of 100 mA cm−2. This performance highlights its pH universality and enhanced surface reactivity for water and hydrogen adsorption/desorption [42].
The overpotential for HER, denoted as , is calculated as the difference between the applied potential and the thermodynamic potential .
The thermodynamic potential is 0 V versus the reversible hydrogen electrode (RHE) under standard conditions. For example, if the applied potential is −0.25 V vs. RHE, the overpotential would be:
A lower overpotential indicates a more efficient catalyst. The choice of catalyst is critical for optimizing HER efficiency. Platinum (Pt) is the most effective catalyst due to its near-zero hydrogen binding energy, allowing hydrogen to easily adsorb and desorb. However, due to the high cost of Pt, researchers are investigating alternative materials. These include transition metal alloys, metal oxides, and carbon-based compounds, which aim to achieve comparable performance at a lower cost. For example, Kinjal et al. recently developed Cu2CoSnS4 as a working electrode to study HER across a broad range of pH levels. Their results were promising, showing a turnover frequency (TOF) of 1.70 s−1 in acidic media, 0.84 s−1 in basic conditions, and 0.61 s−1 in neutral environments at an overpotential of −250 mV vs. RHE. A low overpotential of ~233 mV was observed at a current density of 10 mA/cm2 in 0.5 M H2SO4. The electrode also demonstrated excellent stability, sustaining hydrogen production for over 40 h with a steady current density of 200 mA/cm2 [43]. HER efficiency relies on balancing hydrogen adsorption and desorption. If hydrogen binds too strongly to the catalyst, it cannot easily form H2 gas; if it binds too weakly, effective adsorption does not occur. As shown in Figure 6a, noble metals outperform other pure metals in HER due to their near-ideal hydrogen binding energy, facilitating a thermodynamically favorable transition from reactants to products [44].
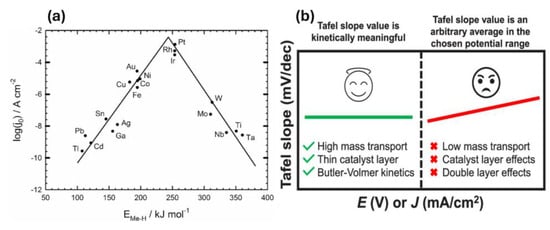
Figure 6.
(a) Relationship between hydrogen binding energy (EM−H) and current density (log(j)) for various metals, showing superior HER performance for noble metals like Pt and Rh. (b) Tafel slope significance in HER kinetics, where lower slopes indicate efficient kinetics, and higher slopes reflect mass transport and catalyst layer effects. Reprinted with permission from [44,45].
HER kinetics can be further evaluated through Tafel slope analysis, which provides insights into the reaction mechanism (Figure 6b). The Tafel equation is given by:
Here, η is the overpotential, j is the current density, a is the Tafel intercept, and b is the Tafel slope. A lower Tafel slope indicates faster reaction kinetics, meaning the catalyst generates hydrogen more efficiently with less energy [45]. In summary, improving HER performance involves designing catalysts with optimal hydrogen binding energy, enhancing the active sites for hydrogen adsorption, and ensuring long-term stability under operational conditions.
2.2.2. Oxygen Evolution Reaction
The OER is a key process in electrochemical water splitting, where water molecules are oxidized to produce oxygen gas at the anode. This multi-step reaction involves the transfer of four electrons and is considered more complex and sluggish compared to the HER [46]. The efficiency of OER heavily depends on the electrocatalyst used, and the overpotential required to drive the reaction is a fundamental factor in evaluating catalyst performance. OER can occur in both acidic and basic environments, though the reaction mechanism differs slightly depending on the medium. In acidic solutions, water molecules are oxidized, while in alkaline conditions, hydroxide ions are involved. The overall OER can be represented as follows:
Or
Regardless of the medium, the reaction proceeds through several intermediate stages, including the formation of oxygen-containing species such as hydroxyl groups (*OH) and oxygen atoms (*O), which eventually combine to form molecular oxygen (O2). Caiwu et al. reported the electrodeposition of hydrous, amorphous iridium oxide films on fluorine-doped tin oxide substrates [47]. Their study revealed that the active species involved in the reaction, Ir4ˣ⁺–*O, exhibit stronger binding in alkaline environments compared to acidic ones at low surface coverage. However, repulsive interactions between these species are more pronounced in alkaline electrolytes. Catalysts play a fundamental role in enhancing OER efficiency by lowering the overpotential. The overpotential is calculated as the difference between the applied potential and the thermodynamic potential .
The thermodynamic potential for OER is 1.23 V vs. the reversible hydrogen electrode (RHE) under standard conditions. For example, if the applied potential is 1.50 V, the overpotential would be
A lower overpotential indicates a more efficient catalyst. Noble metal oxides, such as ruthenium dioxide (RuO2) and iridium dioxide (IrO2), are the most effective OER catalysts, particularly in acidic environments. These materials demonstrate high catalytic activity and stability but are costly, limiting their widespread use [48]. As a result, researchers are exploring more affordable alternatives, including transition metal oxides, hydroxides, and layered double hydroxides (LDHs), to improve OER efficiency [49]. LDHs have garnered considerable interest due to their unique layer-stacking structure, adjustable chemical composition, and a broad range of anion exchange possibilities. In particular, transition metal LDHs (TM-LDHs), characterized by distinctive oxygen vacancy (Ov) defects, represent a promising class of nanomaterials that could replace precious metal catalysts [50]. This potential stems from their exceptional physicochemical properties, which allow for modifications to the electronic and lattice structures of the catalyst. The introduction of oxygen vacancies facilitates the optimization of surface desorption and adsorption reactions, enhancing the efficiency of both OER and HER during water splitting [51].
The OER mechanism (Figure 7) typically follows either the electrochemical oxide pathway or the adsorbate evolution mechanism, depending on the catalyst material. For many transition metals, the formation of intermediate species, such as *O, *OH, and *OOH, on the catalyst surface is crucial. The free energy associated with the adsorption and desorption of these intermediates dictates the reaction rate. Ideally, catalysts should have intermediate binding energies for these species to achieve optimal performance. OER kinetics are commonly evaluated using Tafel slope analysis, similar to HER, where a lower Tafel slope indicates faster reaction kinetics [52]. However, due to the complex nature of OER, determining the reaction mechanism based solely on Tafel analysis is challenging. Nevertheless, optimizing catalyst design, increasing active site density, and ensuring stability under operational conditions are critical research areas for making OER more efficient and cost-effective for practical applications such as water electrolysis and renewable energy storage [53].
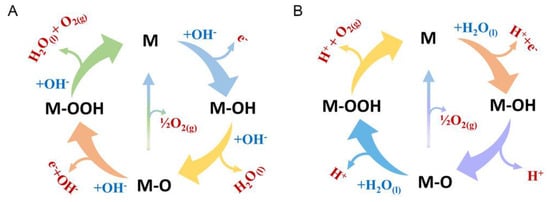
Figure 7.
Schematic illustration of the OER mechanism on a metal (M) catalyst surface in (A) alkaline and (B) acidic media, showing the formation of M–OH, M–O, and M–OOH intermediates, with O2 evolution and catalyst regeneration in both conditions. Reprinted with permission from [54].
The efficiency of water splitting is primarily dictated by the kinetics of the OER and HER. Both reactions are inherently slow due to high overpotential and activation energy constraints, necessitating the use of catalysts to enhance reaction rates [55,56,57]. Among the two, OER exhibits slower kinetics because it involves a four-electron transfer process with a significant activation energy barrier. The typical adsorbate evolution mechanism of OER follows these steps:
This reaction typically proceeds through intermediate species, with the rate-determining step depending on the catalyst material. Most catalysts, particularly metal oxides, exhibit the highest kinetic barrier during O–O bond formation [58]. The Tafel slope provides valuable insight into the reaction mechanism and the rate-determining step of the catalyst. A Tafel value of 120 mV/dec indicates sluggish kinetics with high activation energy, where electron transfer is the limiting step. A value of 60 mV/dec suggests that the chemical rearrangement of intermediates dominates the process, while 40 mV/dec signifies a fast electron transfer mechanism [59].
The HER is a multi-step process involving the adsorption and desorption of hydrogen intermediates on the electrode surface. It primarily follows two common mechanisms: the Volmer–Heyrovsky and the Volmer–Tafel pathways [60,61]. In an alkaline medium, the reaction proceeds as follows:
The Tafel slope is an important parameter for determining the dominant step in HER. A Tafel slope of 120 mV/dec indicates that the Volmer step is the rate-determining step, suggesting limited charge transfer capability and making it the slowest step in the reaction [62]. In contrast, Tafel slopes of 40 mV/dec and 30 mV/dec correspond to the Heyrovsky and Tafel steps, respectively. A lower Tafel slope signifies improved reaction kinetics; the Heyrovsky step indicates that electrochemical desorption controls the reaction, while the Tafel step represents a fast hydrogen evolution process where hydrogen atoms efficiently combine. To enhance catalytic performance in water splitting, it is essential to optimize charge transfer and consider the mechanistic insights of HER catalysts [63].
3. Electrode Synthesis Method for Water Splitting
The cost of hydrogen production through water splitting is significantly influenced by the electrodes used in the process. In the past, expensive precious metals such as platinum (Pt) and palladium (Pd) were commonly employed for electrode fabrication in water splitting. Over the last three decades, the prices of Pt and Pd have risen dramatically, from USD 12.3 and 2.8 per gram to USD 34.1 and 70.1 per gram, respectively [64]. To address this cost challenge, various affordable materials, including metal oxides, metals, and polymers such as TiO2, V2O5, Ni, and graphene oxide, have been investigated as alternative electrode materials to lower the overall cost of hydrogen production through water splitting [65,66,67]. Additionally, electrode synthesis plays a pivotal role in advancing water splitting technologies, particularly for sustainable hydrogen production. The performance of the water splitting process heavily depends on the properties of electrode materials, including their conductivity, catalytic activity, stability, and surface area [68]. Advanced synthesis techniques such as hydrothermal methods, chemical vapor deposition (CVD), radio frequency (RF) techniques, and atomic layer deposition (ALD) have facilitated the development of highly efficient and durable electrodes. These methods allow for precise control over the material’s structure, morphology, and composition, enabling the creation of tailored surfaces that optimize the OER and HER.
3.1. Chemical Methods
3.1.1. Hydrothermal Fabrication
The hydrothermal method is widely used for synthesizing catalysts by selecting appropriate precursors and controlling specific conditions like temperature. This technique can produce catalysts with various morphologies, such as nanotubes and nanowires. It was first introduced by Schafhäutl et al. [69]. The synthesis conditions, including temperature, solvent type, precursor-to-solvent ratio, and reaction time, significantly affect the catalyst yield. While this method can yield catalysts with diverse morphologies, a major drawback is the difficulty of observing the reaction process, as it is conducted in a sealed vessel. Despite this limitation, the hydrothermal method has advantages: it is simple, cost-effective, and capable of producing catalysts with varied morphologies. This is especially important since catalyst morphology plays a key role in the efficiency of water splitting for hydrogen production. Recently, Iffat et al. [65] employed the hydrothermal method to synthesize multilayered Ti3C2/V2O5 hybrid nanostructures for evaluating their HER performance. The resulting nanostructures exhibited excellent HER and OER efficiencies, with overpotentials of 90 mV and 240 mV, respectively, at a current density of 10 mA cm−2. The catalyst demonstrated long-term stability, maintaining minimal current density loss over 24 h. Similarly, Liu et al. demonstrated the utility of the hydrothermal method in synthesizing coreshell MoO2/α-Mo2C heterojunctions, achieving overpotentials of 100 mV and 152 mV at 10 mA cm−2 in acidic and alkaline media, respectively [70]. Overall, the hydrothermal method remains a valuable approach for preparing catalysts for hydrogen production via water splitting. However, the choice of precursor, temperature range, and reaction time are crucial factors. The literature suggests that reaction times can vary from several hours to days, depending on the desired efficiency of the catalyst [71].
3.1.2. Electrochemical Deposition
Electrochemical deposition is a widely used method for synthesizing catalytic materials for the HER and OER. This technique involves applying a voltage field to deposit materials from an electrolyte onto an electrode. Variations in the applied voltage can influence the growth of ions on the electrode surface, ultimately impacting the electrode efficiency in water splitting for hydrogen production [72]. One key advantage of electrochemical deposition is its versatility, allowing for the deposition of various metals, metal oxides, and polymers as electrode materials by dissolving these substances in the electrolyte solution. Additionally, the thickness of the electrode can be precisely controlled by adjusting the applied voltage [64]. However, several factors, including the pH of the electrolyte, deposition time, current density, and solvent temperature, affect the quality of electrode formation. Optimizing these parameters is important for achieving high electrode efficiency in both HER and OER. Nevertheless, challenges such as surface roughness and poor long-term stability due to internal stress remain significant.
Electrodeposition time is another main factor that influences electrode performance. It directly affects the thickness of the electrode, which, in turn, impacts its efficiency in water splitting. Electrodeposition times can range from a few minutes to over 90 min, depending on the material being used. For instance, Boukhouietev et al. [66] deposited Ni metal onto a Cu substrate with a deposition time of 10 min, while Masatoshi et al. [73] used the same Cu substrate but opted for a 90 min deposition time to deposit Cu metal.
The pH of the solution is also an important parameter in the electrodeposition process, as it can alter the electrode’s morphology and thus its performance in HER and OER. For example, Sengupta et al. adjusted the pH to create a porous nickel electrode, demonstrating how pH manipulation can significantly influence electrode properties [74].
Additionally, HER efficiency can be affected by the temperature during the electrodeposition process. Wangping’s study highlights the critical impact of temperature on the electrodeposition of iridium–cobalt (Ir–Co) thin films, used as electrocatalysts for water splitting [75]. Ir–Co films were deposited onto copper foam electrodes in an alkaline environment, and HER performance was evaluated by adjusting catalyst loading and solution temperature. The study showed that increasing the temperature from 20 to 40 °C enhanced catalytic performance, but further temperature increases resulted in performance decline. The calculated thermal activation energy of ~13.9 kJ mol−1 emphasizes the importance of optimizing temperature to achieve maximum electrocatalytic efficiency.
The optimal precursor concentration is also a key factor in the electrodeposition process. The concentration of the precursor directly affects the structural properties of the electrode, which can range from micro- to nanostructures, depending on the concentration used. By adjusting the precursor concentration, it is possible to tune the nucleation and growth processes, thereby influencing the electrode’s morphology. A lower precursor concentration leads to reduced nucleation growth, which can, in turn, lower the overpotential, resulting in enhanced performance for both the OER and HER [76].
3.2. Physical Method
Chemical vapor deposition (CVD) can be employed to deposit electrode materials for water splitting. This physical method produces electrodes in the form of uniform thin films. However, several factors, including the precursor type, substrate nature, distance between the precursor and substrate, substrate temperature, and the use of carrier gases such as argon or nitrogen, can influence the electrode’s thickness, ultimately affecting its water-splitting properties [77]. For example, Sangchai et al. used CVD to deposit three-dimensional graphene oxide onto a nickel substrate to evaluate HER performance in acidic media [67]. The electrode prepared via CVD demonstrated excellent HER activity, with an overpotential of 83.2 mV at a current density of 10 mA cm−2. Similarly, Zhenhuan et al. reported the deposition of ternary metal phosphide on graphene-supported nickel foam to evaluate both HER and OER properties [78]. The resulting electrode exhibited an overpotential of 84 mV and a Tafel slope of 78 mV dec−1.
Atomic layer deposition (ALD) offers exceptional precision in controlling the thickness and uniformity of electrodes for water splitting applications. By depositing materials one atomic layer at a time, ALD enables the development of nanostructured electrodes with enhanced surface area and tailored properties [79]. This nanostructuring improves light absorption, charge transport, and stability in water-splitting devices. Proper conditioning of ALD catalysts is essential to achieve optimal surface area and catalytic activity. The fine-tuning of process variables makes ALD adaptable for various material systems, and continuous advancements in ALD technology have the potential to further enhance the efficiency and performance of nanostructured water-splitting devices. Additionally, the low-temperature deposition of thin films through ALD makes it particularly attractive for these applications [80].
For instance, Siowwoon et al. [81] used Al2O3, SiO2, and TiO2 precursors in ALD to deposit three-dimensional thin films on carbon electrodes to investigate their PEC properties. The study found that ALD-coated electrodes demonstrated improved OER and HER performance. However, it was also observed that varying the number of ALD cycles affected these properties. Therefore, optimizing experimental conditions, such as the number of cycles, is crucial for achieving highly efficient electrodes for water splitting. Moreover, ALD can be used to deposit binary and ternary electrode materials, which often exhibit higher water-splitting efficiency compared to their individual counterparts. For example, Haydous et al. [82] used ALD to deposit Co-Pi/Al2O3 over CaNbO2N to evaluate OER performance. The ternary composite Co-Pi/Al2O3 exhibited superior OER performance, attributed to the reduction in charge carrier recombination and surface roughness.
Another physical method for depositing electrodes for water splitting is radio frequency (RF) sputtering, which offers a faster deposition process compared to Atomic Layer Deposition (ALD) due to the shorter time required to form thin films. However, several key parameters in RF sputtering need careful optimization, including RF power, substrate temperature, precursor-to-substrate distance, deposition time, and the type of inert gases used. These factors directly influence the water splitting performance of the deposited thin-film electrodes. For example, Somnath et al. [83] investigated the deposition of tungsten sulfide thin films at varying RF power levels, ranging from 80 to 180 watts. Their findings revealed that the electrode deposited at 150 watts exhibited the best performance, with a lower Tafel slope of ~0.374 V, highlighting improved efficiency. The study also showed that changing the RF power impacted the crystallinity of the tungsten sulfide electrode, with 150 watts yielding optimal results. In another study, Miroslava et al. [84] explored the effect of substrate temperature on electrodes made from Mo, V, Ni, and Co. The results indicated that a substrate temperature of 800 °C provided the best water splitting properties, with significantly lower overpotential. These findings suggest that optimizing RF sputtering parameters, particularly RF power and substrate temperature, is crucial for enhancing the efficiency of electrodes in water splitting applications.
The water-splitting efficiency of electrodes can be further optimized by adjusting the basal plane and crystal structure of the deposited material. Pulsed laser deposition (PLD) plays a key role in this optimization [85]. By altering the substrate temperature during PLD, the basal plane of the electrode can be modified, leading to enhanced water-splitting performance. For instance, Grigoriev et al. [86] reported the fabrication of MoSeₓ/Mo electrodes using PLD, varying the substrate temperature from room temperature to 250 °C. The study showed that the MoSeₓ/Mo electrode deposited at 250 °C in an argon gas environment demonstrated the best HER performance, with a Tafel slope of approximately 86 mV/dec. These results highlight the significance of fine-tuning PLD parameters, such as temperature and gas conditions, to achieve maximum water-splitting efficiency.
The key challenges in electrode synthesis for HER and OER include controlling surface roughness and internal stress, which can lead to poor long-term stability. Additionally, precise optimization of electrocatalytic parameters, such as pH, deposition time, current density, and temperature, is crucial for achieving enhanced water-splitting performance under optimal conditions. As discussed above, each electrode fabrication method has its advantages and disadvantages. Therefore, selecting the appropriate method and optimizing the experimental conditions for electrode synthesis is essential. Table 1 provides a summary of the various electrode synthesis methods for water splitting, highlighting their respective advantages and disadvantages.

Table 1.
Electrode deposition methods for water splitting: advantages and disadvantages.
4. Carbon-Based Material for Water Splitting
Carbon-based materials have shown significant potential for enhancing water splitting due to their superior properties, including exceptional conductivity, tunable surface characteristics, and structural versatility. The structure and morphology of the catalyst play a fundamental role in optimizing charge transfer, catalytic efficiency, and light absorption [87]. Further, doping, defects and vacancy engineering of carbon-based materials can be used to modulate electronic properties for improved water splitting performance [88]. Furthermore, constructing heterostructures by integrating carbon-based materials with semiconductors, metal oxides, or sulfides facilitates charge separation and transfer, reducing recombination losses and enhancing photocatalytic performance [89]. The synergistic interaction between carbon matrices and active sites not only stabilizes catalysts under operational conditions but also boosts the efficiency of hydrogen and oxygen evolution reactions.
4.1. Graphene and Its Derivatives
Graphene is a form of carbon characterized by a unique geometric arrangement of carbon atoms in a hexagonal structure. This configuration makes graphene a highly prominent material for various advanced applications, such as photocatalytic water-splitting for hydrogen production (Figure 8). Graphene boasts high thermal conductivity (~4000 W/m·K) [90], remarkable mechanical strength of around 130 GPa, superior electronic properties, a high specific surface area and surface defects. However, these properties of graphene depend on its number of layers and functional groups. These functional groups further determine the nature of the graphene, such as graphene oxide and reduced graphene oxide. There are various physical and chemical methods to synthesize graphene and its derivatives, as depicted in Figure 9. A large amount of oxygen functional groups transforms graphene into graphene oxide, which has different optical and mechanical properties compared to pristine graphene. These functional groups can be further minimized by various methods such as high- and low-temperature reduction, resulting in reduced graphene oxide [91]. However, it is important that during the reduction process and the removal of functional groups, there should be minimal distortion of the aromatic carbon atoms. This preservation is necessary for maintaining the graphene-based material efficiency in photocatalytic water-splitting applications.
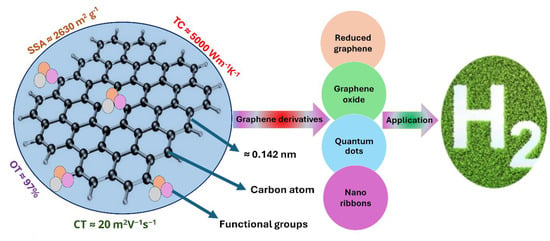
Figure 8.
Properties of graphene and its composites for their applications in sustainable green hydrogen production.
Karthik et al. [92] recently investigated the role of functional groups present in graphene for electrocatalytic water splitting, focusing on OER and HER. The study demonstrates that partially reduced graphene oxide (GO), achieved through different methods, significantly enhances the electrocatalytic performance for water splitting, specifically towards HER. They employed three different methods UV, IR, and MW to reduce the functional groups in graphene. Among the samples, the microwave-reduced GO (M-GO) showed superior HER activity with the lowest overpotential and a Tafel slope of 141.3 mV/dec, indicating efficient reaction kinetics. Furthermore, M-GO exhibited the minimum overpotential of 365.4 mV, compared to U-GO (415.2 mV) and I-GO (397.1 mV). Moreover, M-GO demonstrated excellent durability and stability, retaining 91.6% of its performance after 50 h. This improvement is attributed to the synergistic interaction between sp2 and sp3 carbons, increased surface area, and efficient ion adsorption dynamics at the electrode interface.
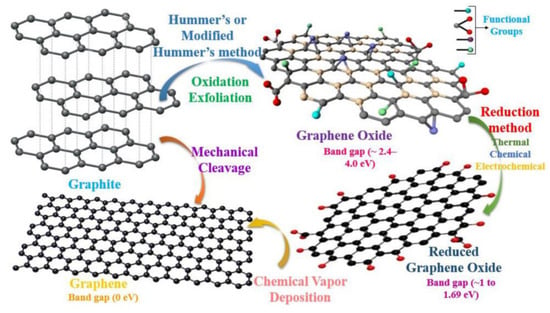
Figure 9.
The reduction in functional groups of graphene through physical methods such as chemical vapor deposition and chemical methods such as the oxidation process. Reprinted with permission from [93].
It is also important to note that the ratio of graphene and its derivatives is crucial when forming composites with metals, metal oxides, or polymers. An excess of graphene and its derivatives relative to other materials can compromise its optoelectronic properties, blocking the active sites in the composites and thereby reducing photocatalytic water-splitting efficiency. For example, if the amount of graphene exceeds the optimal level in the composites, it can hinder light penetration, negatively affecting the photocatalytic water-splitting process. Furthermore, increased π-π stacking interactions in graphene can lead to irreversible agglomeration. This agglomeration reflects light and further obstructs light penetration, thereby impairing the photocatalytic water-splitting process.
Wang et al. [94] explored the effect of reduced graphene oxide (rGO) at different weight percentages, ranging from 0.5% to 5%, with TiO2 and Pt nanoparticle composites. They investigated this for photocatalytic hydrogen production under solar light using triethanolamine as a sacrificial reagent, as shown in Figure 10. The study revealed that adding Pt and rGO significantly enhances TiO2 efficiency. Pure TiO2 showed limited H2 production, but loading Pt increased the HER rate to 216.8 μmol h−1 g−1. The incorporation of rGO further boosted performance, with 2 wt% rGO achieving the highest rate of 1075.68 μmol h−1 g−1, 81 times higher than pure TiO2 and 5 times higher than the Pt-loaded sample. However, excessive rGO (3% to 5%) decreased efficiency due to increased electron–hole recombination and light scattering. Thus, 2 wt% rGO is the optimal composition for hydrogen production.
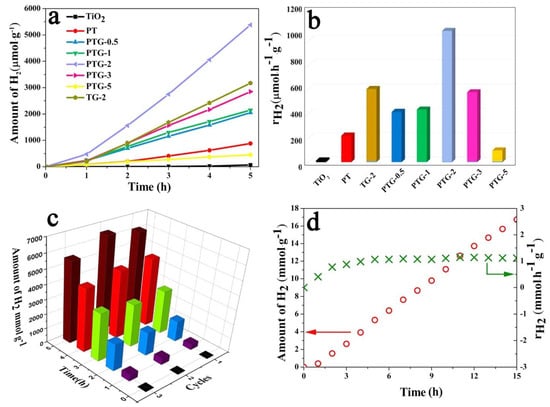
Figure 10.
(a) The amount of H2 produced over time by prepared TiO2 and Pt nanoparticle composites at various weight percentages of rGO. (b) Rate of hydrogen production under the same experimental conditions. (c) Cyclic stability of hydrogen production. (d) Cumulative amount of hydrogen produced over an extended period at 2 wt% rGO. Reprinted with permission from [94].
Graphene and its derivatives can also be combined with other carbon materials, such as C3N4, to support water-splitting hydrogen. Hafeez et al. [95] investigated the effect of different ratios of graphene oxide (GO) ranging from 0.5 to 2 wt% with TiO2 and C3N4 for water-splitting hydrogen production. They concluded that increasing the GO ratio from 0.5 to 1 wt% in the composites enhanced water-splitting hydrogen production, reaching a maximum of 23,143 μmol g−1h−1. However, when the GO ratio increased from 1 wt% to 2 wt%, the water-splitting hydrogen production efficiency decreased. This indicates that GO enhances hydrogen production by reducing charge recombination in TiO2/C3N4 composites. However, beyond the optimal GO value, it starts decreasing the efficiency of TiO2/C3N4 by hindering and reflecting light penetration.
Li et al. [96] synthesized nitrogen and sulfur co-doped graphene for OER and HER. In this study, it was concluded that the specific surface area and surface defects in the prepared graphene composites are the main factors responsible for enhancing OER and HER. However, controlling defects such as di-vacancy and zigzag was challenging. The resultant composites achieved an overpotential of 281 mV vs. RHE at 10 mA·cm2 in 1 M KOH, surpassing many metal-free catalysts. This simple, low-cost method demonstrates the potential for both carbon material synthesis and hydrogen production.
Reduced graphene oxide electrocatalytic electrodes with MoO3 were synthesized by Alotaibi et al. [97] for OER and HER at various annealing temperatures ranging from 600 °C to 1000 °C. The optimal annealing condition was observed at 1000 °C, yielding the HER at a potential of 35 mV with a current density of 10 mA/cm2 and stability of 14 h. The OER achieved a maximum at a potential of 1.39 V with a current density of 10 mA/cm2. Overall, the three-dimensional porous structure of the prepared electrodes makes them ideal candidates for water splitting.
Louis et al. [98] used reduced graphene oxide to support the ZnO electrode through the hydrothermal method to enhance the water-splitting ability of pristine ZnO. The reduced graphene oxide improved the photoelectronic water-splitting efficiency of ZnO by 2.7-fold by enhancing the charge separation ratio. However, it was observed that the ratio of reduced graphene oxide to ZnO was crucial, with the optimal amount being around 0.5 wt% GO with ZnO to achieve the maximum HER rate. This resulted in a Tafel slope of 101 mV/dec, compared to 149 mV/dec for pristine ZnO, as presented in Figure 11.
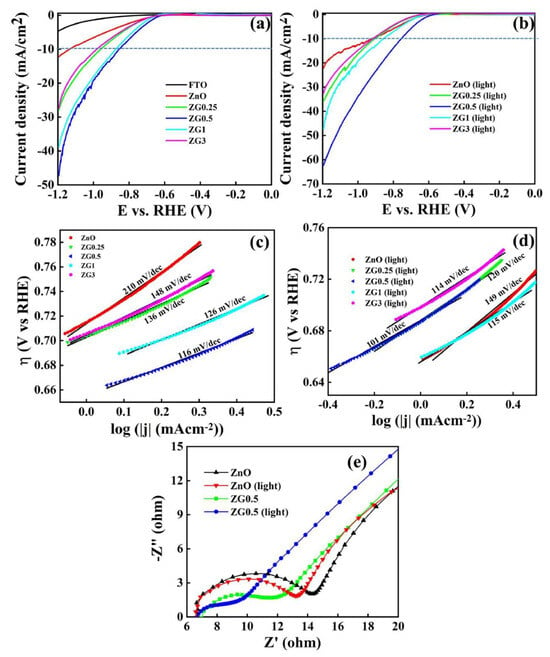
Figure 11.
The current density (j) as a function of potential with the scan rate of 5 mV/s (a) in dark conditions and (b) under illumination. Corresponding Tafel plots for ZnO and ZnO/rGO hybrids: (c) in the absence of light, and (d) under light exposure. (e) Nyquist impedance plots for ZnO and ZG0.5, comparing measurements taken with and without light. Reprinted with permission from [98].
The water-splitting efficiency of graphene-based binary nanocomposites can be further enhanced by converting them into ternary nanocomposites. In this regard, Younas et al. [99] used the wet chemical route to prepare ZnO nanowires supported with copper oxide and reduced graphene oxide. The ternary nanocomposite exhibited excellent HER and OER performance compared to binary nanocomposites. The HER reached a maximum at −358 mV, while the OER peaked at 1.678 V. It was noted that the pH was fixed at 14 to achieve the maximum values for both HER and OER.
4.2. Biochar for Water Splitting
Biochar has garnered significant interest due to its naturally abundant supply, porous structure, and substantial surface area, making it an attractive option for catalytic support [100,101]. Biochar can be derived from cost-effective natural sources such as forest waste, food waste, and seaweed [102]. Biochar typically contains approximately 60 to 70 percent carbon as its primary constituent, with the remainder consisting of oxygen, hydrogen, and nitrogen as secondary elements. However, the specific composition of these elements can vary depending on the choice of feedstock as depicted in Figure 12. For instance, biochar derived from agricultural feedstock tends to exhibit a higher carbon content [103,104]. Moreover, biochar contains various functional groups, including C–O, C=O, and –OH [105]. These functional groups play a crucial role in influencing the catalytic performance of biochar by providing active sites during catalytic reactions. It is worth noting that the ratio of functional groups can be altered by adjusting the pyrolysis temperature. The literature indicated that when the pyrolysis temperature exceeds 750 °C, there is a significant reduction in the abundance of functional groups within the biochar [106]. Therefore, the selection process of feedstock should be approached with utmost care, as some unwanted contents can enhance energy consumption and the use of chemicals in biochar production, potentially leaving a negative impact on the environment [107,108]. Therefore, the characteristics of biochar are subject to influence by diverse factors, including the specific thermal treatment and the selection of biochar feedstock [109]. Furthermore, recent research has highlighted that while biochar has been utilized on a micro scale for photocatalytic water treatment, the potential of photocatalytic water splitting remains largely unexplored. Also, biochar on a nanoscale level can significantly enhance reactivity and provide a higher specific surface area, both of which are highly desirable for improving photocatalytic hydrogen production [110].
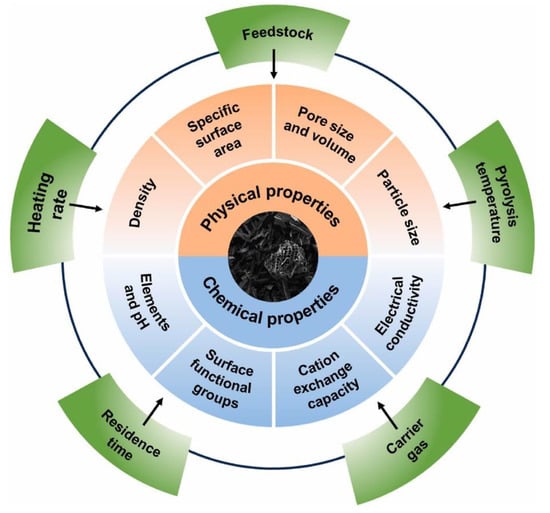
Figure 12.
Influence of feedstock and synthesis conditions on the physicochemical properties of biochar for water splitting applications. Reprinted with permission from [111].
The inherent properties of pristine biochar have their limitations. These limitations include a limited adsorption capacity, a narrow adsorption range, and a relatively low specific surface area [112,113]. To overcome these limitations, the properties of pristine biochar can be refined by combining it with nanomaterials. This combination allows for the fine-tuning of biochar’s properties and enhances its performance.
Nanomaterials hold significant importance in water splitting hydrogen production due to their capability to manipulate properties through band gap engineering, augment specific surface area, and facilitate heterojunction formation [114]. Therefore, the combination of biochar with metals and metal oxides offers the potential to fine-tune the band gap, enhance light absorption, and increase the specific surface area. Numerous nanomaterials are currently being employed to bolster water splitting hydrogen production.
For example, Mingze et al. [115] conducted a study on the enhancement of heterojunction photocatalysts by modifying TiO2 with biochar for the purpose of photocatalytic water splitting and hydrogen production. The findings revealed a significant increase in hydrogen evolution, with a rate of 159.7 μmol·h−1, surpassing that of pure TiO2 by a remarkable 11.5 times (13.9 μmol·h−1). This improvement can be attributed to the development of an S-scheme heterojunction between biochar-modified TiO2, specifically involving the anatase and rutile phases. This configuration facilitated the efficient transfer of photogenerated carriers across the anatase–rutile interface, thereby enhancing the photocatalytic activity. Deng et al. [116] successfully synthesized nano-composites consisting of biochar supported by Zn0.2Cd0.8S. These prepared nanocomposites underwent testing for photocatalytic activity when exposed to solar irradiation. Remarkably, the photocatalytic hydrogen production efficiency demonstrated a staggering increase, surpassing that of pristine biochar by 24.5 folds higher. Additionally, it was observed that the molar ratio of Zn0.2Cd0.8S. played a critical role in the composition, with an optimal ratio identified at 0.4. The composites with Zn0.2Cd0.8S at 0.4 mmol in biochar aerogels also exhibited the highest quantum efficiency, around 9.37%, with the lowest reduction potential of −0.86 V and the maximum charge separation ratio as depicted in Figure 13. In short, at 0.4 mmol, the nanocomposites demonstrated superior charge separation, lower charge transfer resistance, higher current density, and longer charge carrier lifetimes.
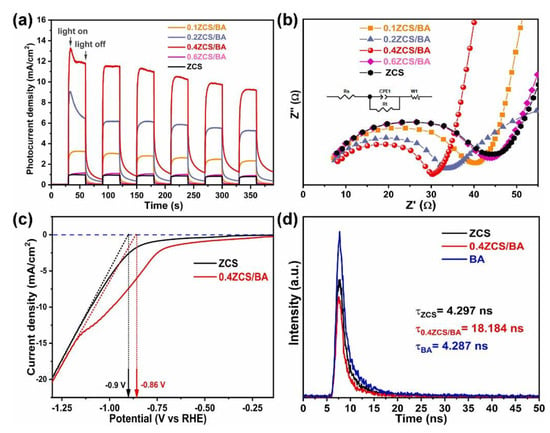
Figure 13.
Performance and characteristics of Zn0.2Cd0.8S with Biochar for hydrogen production: (a) photocurrent density, (b) Nyquist plots, (c) linear sweep voltammetry, and (d) time-resolved photoluminescence. Reprinted with permission from [116].
Chonghai et al. [117] investigated the utilization of porous biomass-derived CdS nanoparticles composites for photocatalytic water splitting. The results showed that the HERefficiency reached 14.67 mmol∙g−1∙h−1 for the optimized CdS/PBC-3 composite under simulated solar irradiation. This performance represents a significant improvement, being 43.1 times higher than that observed with pure CdS nanoparticles.
4.3. Graphitic Carbon Nitride for Water Splitting
Graphitic carbon nitride (g-C3N4) has been a subject of research for nearly two centuries. However, its recognition as a valuable photocatalyst only gained momentum after Antonietti et al. published a groundbreaking study in 2009, highlighting its effectiveness in water splitting [118]. Since then, g-C3N4, also known as melon-based carbon nitride, has emerged as a prominent material in energy research due to its affordability, high oxidation resistance (up to 500 °C), and tunable optical, chemical, and catalytic properties [119].
The photocatalytic performance of g-C3N4 can be significantly enhanced by modifying its structural dimensions. Saikat et al. [120] demonstrated this by preparing 2D- g-C3N4 through chemical exfoliation, which exhibited a lower overpotential of 108 mV and a current density of 10 mA cm−2 in a 0.1 M KOH solution (Figure 14). In comparison, bulk g-C3N4 had a higher overpotential of 170 mV. Furthermore, the reduced Tafel slope of 24 mV dec−1 for 2D- g-C3N4, compared to 31 mV dec−1 for its bulk form, indicates superior OER properties in the 2D structure.
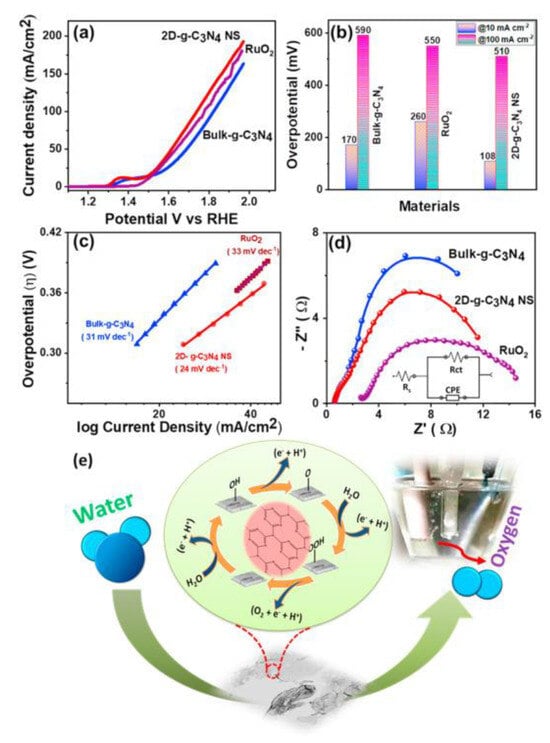
Figure 14.
(a) Potential versus current density curves for the 2D- g-C3N4 catalysts, (b) Tafel plots illustrating the reaction kinetics, (c) comparison of overpotential values for different catalysts, (d) electrochemical impedance spectroscopy results, and (e) proposed OER mechanism. Reprinted with permission from [120].
In addition to structural modifications, combining g-C3N4 with other nanomaterials has proven effective for water splitting. The ratio of g-C3N4 to these materials plays an important role in optimizing performance. Fan et al. [121] developed g-C3N4/Co3O4 composites by varying the mass ratio of g-C3N4 (from 0.1 to 0.67). They found that a ratio of 0.2 yielded the highest OER performance under identical conditions compared to pure g-C3N4. The composite achieved a low Tafel slope of 54 mV dec−1, a higher current density of 10 mA cm−2, and a lower overpotential of 627 mV.
Further enhancements in the water-splitting ability of g-C3N4 can be achieved by incorporating it into ternary nanocomposites. The structure of the dopant material is key to boosting efficiency. For instance, Pan et al. [122] developed a MnOx/CdS/Pt/g-C3N4 hollow core–shell heterojunction that exhibited an 80-fold increase in HER efficiency compared to single CdS. This composite produced ~1303.39 μmol∙g−1∙h−1 of hydrogen and 641.60 μmol∙g−1∙h−1 of oxygen, with enhanced stability and efficiency attributed to Pt nanoparticles facilitating electron transfer, Mn ions aiding hole-related processes, and the hollow structure providing more active sites.
g-C3N4 can also be combined with metal oxides and metals for greater efficiency in both OER and HER. Amal et al. [123] doped nickel and manganese into ZnO/g-C3N4, producing a composition that demonstrated excellent OER activity with an overpotential of 380 mV and HER activity with an overpotential of 288 mV at 10 mA cm−2. The low Tafel slope of 72 mV dec−1 highlighted its improved catalytic efficiency.
Similarly, Aleena et al. [124] incorporated cuprous oxide (Cu2O) into g-C3N4 to improve HER performance. The Cu2O/g-C3N4 composite exhibited a high specific surface area of 68.32 m2 g−1 and a pore size of 0.7 nm, leading to a low overpotential of 148.7 mV and a Tafel slope of around 30 mV dec−1. The increased surface area and optimized pore size were identified as key factors contributing to the improved HER activity.
The combination of g-C3N4 with other materials enhances both HER and OER performance, making it attractive for water-splitting applications. For example, Subramanian et al. [125] synthesized g-C3N4/chlorocobaloxime composites, which exhibited enhanced HER and OER activity. The HER activity showed an overpotential of 173 mV at −10 mA cm−2, while the OER activity had an overpotential of 303 mV. Among the composites, g-C3N4/C2 displayed the highest efficiency, with Tafel slopes of 55 mV dec−1 for HER and 114 mV dec−1 for OER, outperforming other materials. Despite the potential of g-C3N4 for photocatalytic water splitting and hydrogen production, several critical challenges need to be addressed to maximize its efficiency. These challenges primarily revolve around its limited charge separation and recombination issues, which hinder its overall performance, as demonstrated in studies like those by Saikat et al. [120] and Fan et al. [121]. To overcome these obstacles, there is a need for more precise modifications, such as selective doping with heteroatoms or combining g-C3N4 with other nanomaterials, as highlighted by Amal et al. [123] and Aleena et al. [124]. Structural enhancements, such as the formation of hollow core–shell nanocomposites, have been shown to drastically improve both HER and OER performance. Therefore, future work must focus on optimizing synthesis methods to improve crystallinity and stability, enhancing the g-C3N4 catalytic activity through the careful selection of nitrogen precursors and structural configurations. Moreover, there is a pressing need to explore not only HER but also OER aligning with the broader goal of sustainable energy solutions.
5. Challenges and Future Directions for Sustainable Hydrogen Production Using Photocatalytic and Electrochemical Methods
Green hydrogen production through water splitting is a cornerstone of sustainable energy strategies, addressing the urgent need for decarbonization. Among the available approaches, photocatalytic and electrochemical water splitting are two promising techniques that leverage renewable energy sources such as sunlight and water. However, each method faces distinct challenges that must be addressed to achieve large-scale implementation.
5.1. Challenges and Future Directions in Photocatalysis
Photocatalytic water splitting is limited by several key challenges, including low charge separation efficiency, rapid charge carrier recombination, and limited visible light absorption. These factors significantly hinder the overall efficiency of hydrogen generation. One approach to overcoming these limitations is the development of heterostructures, where carbon-based materials such as graphene, biochar, and carbon nitride (C3N4) are integrated with semiconductors to improve charge transfer and light absorption.
Furthermore, doping strategies can be employed to enhance visible light utilization by modifying the electronic structure of photocatalysts. Defect engineering and the incorporation of co-catalysts (e.g., graphene derivatives or biochar) can also create additional active sites, improving the hydrogen evolution reaction (HER) efficiency. Furthermore, optimizing band alignments in photocatalytic systems can facilitate better charge carrier migration, reducing recombination losses.
5.2. Challenges and Future Directions in Electrocatalysis
Electrochemical water splitting, while more efficient under controlled conditions, faces challenges related to catalyst durability, cost-effectiveness, and scalability. Traditional electrocatalysts, such as noble metals, are expensive and suffer from degradation over extended use. The development of carbon-based electrocatalysts, including graphene-based materials and biochar, presents a viable solution by offering high conductivity, large surface area, and tunable electronic properties.
One critical aspect of improving electrochemical performance is electrode design. By optimizing electrode surface morphology and pore structure, charge transfer kinetics can be enhanced, leading to improved catalytic activity. Also, scalable and standardized fabrication techniques must be established to ensure reproducibility and facilitate the transition from laboratory research to industrial applications.
Another key challenge is ensuring long-term operational stability. The incorporation of protective layers or hybrid structures that combine carbon-based materials with transition metal catalysts can significantly enhance the longevity and robustness of electrochemical systems. Furthermore, modular reactor designs can be developed to streamline integration into existing hydrogen production infrastructure.
5.3. Pathway to Large-Scale Implementation
The scalable synthesis of carbon-based materials is crucial for reducing production costs and enabling widespread adoption. Sustainable biochar production methods and cost-effective graphene fabrication techniques offer promising solutions for commercial viability. Also, the development of standardized testing protocols will allow for better performance comparisons across different catalytic systems.
By systematically addressing these challenges, photocatalytic and electrochemical water splitting technologies can progress from fundamental research to industrial-scale hydrogen production. The continued integration of carbon-based materials with advanced catalyst designs will play a pivotal role in ensuring the sustainability, efficiency, and economic feasibility of hydrogen generation. Ultimately, these advancements will contribute to a cleaner and more sustainable energy future.
Author Contributions
Conceptualization, H.I. and A.J.; methodology, A.J.; investigation H.I. and A.J.; data curation, A.J.; software, A.J.; validation, A.J. and H.I.; writing—original draft, A.J.; writing—review and editing, H.I.; visualization, A.J.; supervision, H.I.; funding acquisition, H.I.; resources, H.I.; project administration, H.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC DG: RGPIN-2024-04760), Canada Foundation for Innovation (CFI JELF: 37758), and the VPR Discretionary Fund at the U of R, which are gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barokh, H.; Siavashi, M. Pore-scale direct numerical simulation of steam methane reforming (SMR) for hydrogen production in open-cell porous catalytic foam. Int. J. Hydrog. Energy 2024, 83, 1294–1308. [Google Scholar] [CrossRef]
- Jeje, S.O.; Marazani, T.; Obiko, J.O.; Shongwe, M.B. Advancing the hydrogen production economy: A comprehensive review of technologies, sustainability, and future prospects. Int. J. Hydrog. Energy 2024, 78, 642–661. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- Tüysüz, H. Alkaline Water Electrolysis for Green Hydrogen Production. Acc. Chem. Res. 2024, 57, 558–567. [Google Scholar] [CrossRef]
- Otto, M.; Chagoya, K.L.; Blair, R.G.; Hick, S.M.; Kapat, J.S. Optimal hydrogen carrier: Holistic evaluation of hydrogen storage and transportation concepts for power generation, aviation, and transportation. J. Energy Storage 2022, 55, 105714. [Google Scholar] [CrossRef]
- Tahir, M.B.; Batool, A. Chapter 3—Recent development in sustainable technologies for clean hydrogen evolution: Current scenario and future perspectives. In Sustainable Materials and Green Processing for Energy Conversion; Cheong, K.Y., Apblett, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–130. [Google Scholar]
- Shahid, M.U.; Najam, T.; Helal, M.H.; Hossain, I.; El-Bahy, S.M.; El-Bahy, Z.M.; Rehman, A.u.; Shah, S.S.A.; Nazir, M.A. Transition metal chalcogenides and phosphides for photocatalytic H2 generation via water splitting: A critical review. Int. J. Hydrog. Energy 2024, 62, 1113–1138. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Abdelouahed, L.; Kourdourli, F.; Taouk, B.; Estel, L. Energy evaluation of processes for the production of hydrogen from biomass biodigestion under Aspen Plus. In Computer Aided Chemical Engineering; Montastruc, L., Negny, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 51, pp. 163–168. [Google Scholar]
- Ma, X.; Tao, H. Chapter 6—Cost–benefit analysis of waste-to-biohydrogen systems. In Waste to Renewable Biohydrogen; Zhang, Q., He, C., Ren, J., Goodsite, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 123–141. [Google Scholar]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrog. Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Han, C.; Cai, T.; Cui, Y.; Xing, W.; Zhi, C. Corrosion of metallic anodes in aqueous batteries. Energy Environ. Sci. 2025, 18, 2050–2094. [Google Scholar] [CrossRef]
- Coronel-García, M.A.; Salazar-Barrera, J.G.; Malpica-Maldonado, J.J.; Martínez-Salazar, A.L.; Melo-Banda, J.A. Hydrogen production by aluminum corrosion in aqueous hydrochloric acid solution promoted by sodium molybdate dihydrate. Int. J. Hydrog. Energy 2020, 45, 13693–13701. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, K. An overview on activation of aluminium-water reaction for enhanced hydrogen production. J. Alloys Compd. 2020, 835, 155189. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Nishioka, S.; Osterloh, F.E.; Wang, X.; Mallouk, T.E.; Maeda, K. Photocatalytic water splitting. Nat. Rev. Methods Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Rosman, N.N.; Yunus, R.M.; Shah, N.R.A.M.; Shah, R.M.; Arifin, K.; Minggu, L.J.; Ludin, N.A. An overview of co-catalysts on metal oxides for photocatalytic water splitting. Int. J. Energy Res. 2022, 46, 11596–11619. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Mohammed, Y.; Hafeez, H.Y.; Mohammed, J.; Suleiman, A.B.; Ndikilar, C.E.; Idris, M.G. Hydrogen production via photocatalytic water splitting using spinel ferrite-based photocatalysts: Recent and future perspectives. Next Energy 2024, 4, 100145. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Hussain, I. Unlocking the potential of TiO2-based photocatalysts for green hydrogen energy through water-splitting: Recent advances, future perspectives and techno feasibility assessment. Int. J. Hydrog. Energy 2024, 59, 958–981. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef]
- Sharma, K.; Hasija, V.; Malhotra, M.; Verma, P.K.; Parwaz Khan, A.A.; Thakur, S.; Van Le, Q.; Phan Quang, H.H.; Nguyen, V.-H.; Singh, P.; et al. A review of CdS-based S-scheme for photocatalytic water splitting: Synthetic strategy and identification techniques. Int. J. Hydrog. Energy 2024, 52, 804–818. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Ajmal, Z.; Abd El-Gawad, H.H.; Ghernaout, D.; Al-Hadeethi, Y.; Raza, S.; Orooji, Y. Advances/Scope and prospects of g-C3N4 derived fascinating photocatalyst as a leading route towards solar energy adaption. J. Clean. Prod. 2024, 438, 140568. [Google Scholar] [CrossRef]
- Ashfaq, Z.; Iqbal, T.; Ali, H.; Eldin, S.M.; Mahtab Alam, M.; Al-Harbi, F.F.; Arshad, M.; Galal, A.M. Review of different CdS/TiO2 and WO3/ g-C3N4 composite based photocatalyst for hydrogen production. Arab. J. Chem. 2023, 16, 105024. [Google Scholar] [CrossRef]
- Keshipour, S.; Hadidi, M.; Gholipour, O. A Review on Hydrogen Generation by Photo-, Electro-, and Photoelectro-Catalysts Based on Chitosan, Chitin, Cellulose, and Carbon Materials Obtained from These Biopolymers. Adv. Polym. Technol. 2023, 2023, 8835940. [Google Scholar] [CrossRef]
- Wijayatha, K.G.U. 5—Photoelectrochemical cells for hydrogen generation. In Functional Materials for Sustainable Energy Applications; Kilner, J.A., Skinner, S.J., Irvine, S.J.C., Edwards, P.P., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 91–146e. [Google Scholar]
- Zhang, L.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 263–276. [Google Scholar]
- Mansoor, M.A.; Ehsan, M.A.; McKee, V.; Huang, N.-M.; Ebadi, M.; Arifin, Z.; Basirun, W.J.; Mazhar, M. Hexagonal structured Zn (1−x) Cd x O solid solution thin films: Synthesis, characterization and applications in photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5284–5292. [Google Scholar] [CrossRef]
- Mansoor, M.; Huang, N.; McKee, V.; Peiris, T.N.; Wijayantha, K.; Arifin, Z.; Misran, M.; Mazhar, M. Single phased MnZnO3 solid solution thin films for solar energy harvesting applications. Sol. Energy Mater. Sol. Cells 2015, 137, 258–264. [Google Scholar]
- Daraz, U.; Ansari, T.M.; Arain, S.A.; Mansoor, M.A.; Mazhar, M. Study of solvent effect on structural and photoconductive behavior of ternary chalcogenides InBiS3-In2S3-Bi2S3 composite thin films deposited via AACVD. Main Group Met. Chem. 2019, 42, 102–112. [Google Scholar]
- Naeem, R.; Shakir, S.; Sharif, S.; Afzal, S.; Bashir, S.; Mansoor, M.A. The photoelectrochemically enhanced oxygen evolution reaction via thin films of novel (1:2:1) SnO-Mn2O3-TiO2 hybrid nanotubes. Surf. Interfaces 2024, 46, 104034. [Google Scholar]
- Kang, J.; Ghule, B.G.; Gyeong, S.G.; Ha, S.-J.; Jang, J.-H. Alleviating Charge Recombination Caused by Unfavorable interaction of P and Sn in Hematite for Photoelectrochemical Water Oxidation. ACS Catal. 2024, 14, 10355–10364. [Google Scholar]
- Marriam, F.; Arshad, A.; Munawar, K.; Mansoor, M.A.; Ebadi, M.; Naeem, R. Synergistic Approach of TiO2@ MnZnO3 Heterostructure for Efficient Photoelectrochemical Water Splitting. J. Electrochem. Soc. 2024, 171, 096501. [Google Scholar] [CrossRef]
- Dong, G.; Yan, L.; Bi, Y. Advanced oxygen evolution reaction catalysts for solar-driven photoelectrochemical water splitting. J. Mater. Chem. A 2023, 11, 3888–3903. [Google Scholar] [CrossRef]
- Balapure, A.; Dutta, J.R.; Ganesan, R. Recent advances in semiconductor heterojunctions: A detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Anantharaj, S. Hydrogen evolution reaction on Pt and Ru in alkali with volmer-step promotors and electronic structure modulators. Curr. Opin. Electrochem. 2022, 33, 100961. [Google Scholar] [CrossRef]
- Xu, W.; Tang, X.-Q.; Zhang, Z.; Wang, Y.-R.; Luo, J.-L.; Bulugu, I.; Yin, W.-J.; Tang, X.; Shen, Y. Experimental and computational insight into the role of cobalt and phosphorus in boosting Volmer and Heyrovsky steps of Ni in alkaline hydrogen evolution reaction. Fuel 2024, 358, 130174. [Google Scholar] [CrossRef]
- Manohar, A.; Suvarna, T.; Chintagumpala, K.; Ubaidullah, M.; Mameda, N.; Kim, K.H. Exploring progress in binary and ternary nanocomposites for photoelectrochemical water splitting: A comprehensive review. Coord. Chem. Rev. 2025, 522, 216180. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Veerubhotla, R.; Pandit, S.; Das, D. Chapter 5.7—Biohydrogen Production Using Microbial Electrolysis Cell: Recent Advances and Future Prospects. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 843–869. [Google Scholar]
- Zhang, B.; Yao, J.; Liu, J.; Zhang, T.; Wan, H.; Wang, H. Reducing the pH dependence of hydrogen evolution kinetics via surface reactivity diversity in medium-entropy alloys. EES Catal. 2023, 1, 1017–1024. [Google Scholar] [CrossRef]
- Joshi, K.K.; Pataniya, P.M.; Bhadu, G.R.; Sumesh, C.K. Cu2CoSnS4 electrocatalyst embedded paper working electrodes for efficient, stable, pH universal, and large-current-density hydrogen evolution reaction. Int. J. Hydrog. Energy 2024, 49, 829–842. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- van der Heijden, O.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.M. Tafel Slope Plot as a Tool to Analyze Electrocatalytic Reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef]
- Singha Roy, S.; Nagappan, S.; Satheesan, A.K.; Karmakar, A.; Kundu, S. Surface-Enhanced Raman Scattering Coupled with In Situ Raman Spectroscopy for the Detection of the OER Mechanism: A Mini-Review. J. Phys. Chem. C 2024, 128, 13634–13650. [Google Scholar] [CrossRef]
- Liang, C.; Katayama, Y.; Tao, Y.; Morinaga, A.; Moss, B.; Celorrio, V.; Ryan, M.; Stephens, I.E.L.; Durrant, J.R.; Rao, R.R. Role of Electrolyte pH on Water Oxidation for Iridium Oxides. J. Am. Chem. Soc. 2024, 146, 8928–8938. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jung, W. Recent advances in doped ruthenium oxides as high-efficiency electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 15506–15521. [Google Scholar] [CrossRef]
- Wang, H.; Sun, F.; Qi, J.; Zhang, D.; Sun, H.; Wang, Q.; Li, Z.; Wu, Y.A.; Hu, Z.; Wang, B. Recent progress on layered double hydroxides: Comprehensive regulation for enhanced oxygen evolution reaction. Mater. Today Energy 2022, 27, 101036. [Google Scholar] [CrossRef]
- Chen, K.; Dao, V.; Yadav, S.; Lee, I.-H. Progress and perspective of transition metal layered double hydroxides with oxygen vacancies for enhancing water splitting applications: A review. J. Environ. Chem. Eng. 2024, 12, 113773. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Yuan, Y.; Guo, Z.; Chen, G.; Yang, J.; Bao, Q.; Guo, L.; Chen, C. Transition metal-based layered double hydroxides and their derivatives for efficient oxygen evolution reaction. Int. J. Hydrog. Energy 2024, 63, 918–936. [Google Scholar] [CrossRef]
- Aghamohammadi, P.; Hüner, B.; Altıncı, O.C.; Akgul, E.T.; Teymur, B.; Simsek, U.B.; Demir, M. Recent advances in the electrocatalytic applications (HER, OER, ORR, water splitting) of transition metal borides (MBenes) materials. Int. J. Hydrog. Energy 2024, 87, 179–198. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, J.Z.; Qumar, U.; Zaheer, A.; Babar, Z.U.D.; Iannotti, V.; Cassinese, A. Strategies for robust electrocatalytic activity of 2D materials: ORR, OER, HER, and CO2RR. Mater. Today Adv. 2024, 22, 100488. [Google Scholar] [CrossRef]
- Ismail, N.; Qin, F.; Fang, C.; Liu, D.; Liu, B.; Liu, X.; Wu, Z.-l.; Chen, Z.; Chen, W. Electrocatalytic acidic oxygen evolution reaction: From nanocrystals to single atoms. Aggregate 2021, 2, e106. [Google Scholar] [CrossRef]
- Liu, C.; Qian, J.; Ye, Y.; Zhou, H.; Sun, C.-J.; Sheehan, C.; Zhang, Z.; Wan, G.; Liu, Y.-S.; Guo, J. Oxygen evolution reaction over catalytic single-site Co in a well-defined brookite TiO2 nanorod surface. Nat. Catal. 2021, 4, 36–45. [Google Scholar]
- Ehsan, M.A.; Aftab, F.; Younas, M.; Mansoor, M.A.; Ahmed, S. Graphite sheet-supported bimetallic RhNi thin film alloys for enhanced and durable hydrogen evolution in acidic environments. Int. J. Hydrog. Energy 2024, 69, 411–420. [Google Scholar]
- Bibi, H.; Mansoor, M.A.; Asghar, M.A.; Ahmad, Z.; Numan, A.; Haider, A. Facile hydrothermal synthesis of highly durable binary and ternary cobalt nickel copper oxides for high-performance oxygen evolution reaction. Int. J. Hydrog. Energy 2024, 107, 369–377. [Google Scholar]
- Guo, T.; Li, L.; Wang, Z. Recent development and future perspectives of amorphous transition metal-based electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2022, 12, 2200827. [Google Scholar]
- Antipin, D.; Risch, M. Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism. Electrochem. Sci. Adv. 2023, 3, e2100213. [Google Scholar]
- Bao, F.; Kemppainen, E.; Dorbandt, I.; Bors, R.; Xi, F.; Schlatmann, R.; van de Krol, R.; Calnan, S. Understanding the hydrogen evolution reaction kinetics of electrodeposited nickel-molybdenum in acidic, near-neutral, and alkaline conditions. ChemElectroChem 2021, 8, 195–208. [Google Scholar] [CrossRef]
- Long, G.-f.; Wan, K.; Liu, M.-y.; Liang, Z.-x.; Piao, J.-h.; Tsiakaras, P. Active sites and mechanism on nitrogen-doped carbon catalyst for hydrogen evolution reaction. J. Catal. 2017, 348, 151–159. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X.; Eikerling, M. The rate-determining term of electrocatalytic reactions with first-order kinetics. Electrochim. Acta 2021, 393, 139019. [Google Scholar] [CrossRef]
- Wan, C.; Ling, Y.; Wang, S.; Pu, H.; Huang, Y.; Duan, X. Unraveling and Resolving the Inconsistencies in Tafel Analysis for Hydrogen Evolution Reactions. ACS Cent. Sci. 2024, 10, 658–665. [Google Scholar]
- Alnarabiji, M.S.; Tsang, S.C.E.; Mahadi, A.H. Advances in electrode synthesis and fabrication for electrochemical water splitting. Fuel 2024, 357, 129741. [Google Scholar] [CrossRef]
- Ashraf, I.; Ahmad, S.; Nazir, F.; Dastan, D.; Shi, Z.; Garmestani, H.; Iqbal, M. Hydrothermal synthesis and water splitting application of d-Ti3C2 MXene/V2O5 hybrid nanostructures as an efficient bifunctional catalyst. Int. J. Hydrog. Energy 2022, 47, 27383–27396. [Google Scholar] [CrossRef]
- Boukhouiete, A.; Boumendjel, S.; SOBHI, N.-E.-H. Effect of current density on the microstructure and morphology of the electrodepositednickel coatings. Turk. J. Chem. 2021, 45, 1599–1608. [Google Scholar] [PubMed]
- Sarawutanukul, S.; Phattharasupakun, N.; Sawangphruk, M. 3D CVD graphene oxide-coated Ni foam as carbo- and electro-catalyst towards hydrogen evolution reaction in acidic solution: In situ electrochemical gas chromatography. Carbon 2019, 151, 109–119. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- O’Hare, D. Hydrothermal Synthesis. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3989–3992. [Google Scholar]
- Liu, Y.; Huang, B.; Xie, Z. Hydrothermal synthesis of core-shell MoO2/α-Mo2C heterojunction as high performance electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2018, 427, 693–701. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Kim, Y.; Jun, S.E.; Lee, G.; Nam, S.; Jang, H.W.; Park, S.H.; Kwon, K.C. Recent Advances in Water-Splitting Electrocatalysts Based on Electrodeposition. Materials 2023, 16, 3044. [Google Scholar] [CrossRef]
- Sakairi, M.; Goto, Y.; Fushimi, K.; Kikuchi, T.; Takahashi, H. Fabrication of Cu Micro-rods with Co-axial Dual Capillary Solution Flow Type Droplet Cell and Electrodeposition with the Cell. Electrochemistry 2010, 78, 118–121. [Google Scholar] [CrossRef]
- Sengupta, S.; Patra, A.; Jena, S.; Das, K.; Das, S. A Study on the Effect of Electrodeposition Parameters on the Morphology of Porous Nickel Electrodeposits. Metall. Mater. Trans. A 2018, 49, 920–937. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Johannes, N. Electrodeposition of Ir–Co thin films on copper foam as high-performance electrocatalysts for efficient water splitting in alkaline medium. Int. J. Hydrog. Energy 2021, 46, 609–621. [Google Scholar]
- Milchev, A. Electrocrystallization: Fundamentals of Nucleation and Growth; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Koshy, S.S.; Rath, J.; Kiani, A. Fabrication of binder-less metal electrodes for electrochemical water splitting—A review. Heliyon 2024, 10, e37188. [Google Scholar] [CrossRef]
- Zhao, Z.; Schipper, D.E.; Leitner, A.P.; Thirumalai, H.; Chen, J.-H.; Xie, L.; Qin, F.; Alam, M.K.; Grabow, L.C.; Chen, S.; et al. Bifunctional metal phosphide FeMnP films from single source metal organic chemical vapor deposition for efficient overall water splitting. Nano Energy 2017, 39, 444–453. [Google Scholar] [CrossRef]
- Sivagurunathan, A.T.; Adhikari, S.; Kim, D.-H. Strategies and implications of atomic layer deposition in photoelectrochemical water splitting: Recent advances and prospects. Nano Energy 2021, 83, 105802. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Seenivasan, S.; Jung, H.; Han, J.W.; Kim, D.-H. Atomic layer deposition-triggered hierarchical core/shell stable bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 21132–21141. [Google Scholar] [CrossRef]
- Ng, S.; Sanna, M.; Redondo, E.; Pumera, M. Engineering 3D-printed carbon structures with atomic layer deposition coatings as photoelectrocatalysts for water splitting. J. Mater. Chem. A 2024, 12, 396–404. [Google Scholar] [CrossRef]
- Haydous, F.; Si, W.; Guzenko, V.A.; Waag, F.; Pomjakushina, E.; El Kazzi, M.; Sévery, L.; Wokaun, A.; Pergolesi, D.; Lippert, T. Improved Photoelectrochemical Water Splitting of CaNbO2N Photoanodes by CoPi Photodeposition and Surface Passivation. J. Phys. Chem. C 2019, 123, 1059–1068. [Google Scholar] [CrossRef]
- Ladhane, S.; Shah, S.; Shinde, P.; Punde, A.; Waghmare, A.; Hase, Y.; Bade, B.R.; Doiphode, V.; Rahane, S.N.; Kale, D.; et al. Enhanced Photoelectrochemical Activity Realized from WS2 Thin Films Prepared by RF-Magnetron Sputtering for Water Splitting. ChemElectroChem 2024, 11, e202400002. [Google Scholar] [CrossRef]
- Kozejova, M.; Bodnarova, R.; Latyshev, V.; Lisnichuk, M.; Girman, V.; You, H.; Komanicky, V. Structural dependence of hydrogen evolution reaction on transition metal catalysts sputtered at different temperatures in alkaline media. Int. J. Hydrog. Energy 2022, 47, 26987–26999. [Google Scholar] [CrossRef]
- Mathankumar, M.; Balasubramanian, S.; Hasin, P.; Lin, J.-Y. Pulsed laser deposition as an efficient tool to enhance the performance of electrocatalysis design, strategies and current perspectives. Int. J. Hydrog. Energy 2024, 60, 668–687. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Fominski, V.Y.; Romanov, R.I.; Volosova, M.A.; Shelyakov, A.V. Pulsed laser deposition of nanocomposite MoSex/Mo thin-film catalysts for hydrogen evolution reaction. Thin Solid Film. 2015, 592, 175–181. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Lv, Z.; Wang, M.; Dang, J. Recent strategies to improve the catalytic activity of pristine MOFs and their derived catalysts in electrochemical water splitting. Int. J. Hydrog. Energy 2023, 48, 30435–30463. [Google Scholar] [CrossRef]
- Mohsin, M.; Ishaq, T.; Bhatti, I.A.; Maryam; Jilani, A.; Melaibari, A.A.; Abu-Hamdeh, N.H. Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials 2023, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Ahmad, N.B.H.; Afroze, S.; Taweekun, J.; Sharifpur, M.; Azad, A.K. Hydrogen Production from Water Splitting through Photocatalytic Activity of Carbon-Based Materials. Chem. Eng. Technol. 2023, 46, 420–434. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U.; Inamuddin. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Karthik, G.; Rosaiah, P.; Albaqami, M.D.; Nunna, G.P.; Ko, T.J. Investigating the influence of oxygen-functionalized graphene nanosheets as an efficient multifunctional material for supercapacitor and electrocatalytic water splitting applications. Diam. Relat. Mater. 2024, 147, 111293. [Google Scholar] [CrossRef]
- Sadiq, I.; Ali, S.A.; Ahmad, T. Graphene-Based Derivatives Heterostructured Catalytic Systems for Sustainable Hydrogen Energy via Overall Water Splitting. Catalysts 2023, 13, 109. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, S.; Xia, Y.; Ma, S.; Zhou, Q.; Li, Y. The fundamental role and mechanism of reduced graphene oxide in rGO/Pt-TiO2 nanocomposite for high-performance photocatalytic water splitting. Appl. Catal. B Environ. 2017, 207, 335–346. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Bellamkonda, S.; Rao, G.R.; Shankar, M.V.; Bahnemann, D.W.; Neppolian, B. Construction of ternary hybrid layered reduced graphene oxide supported g-C3N4-TiO2 nanocomposite and its photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2018, 43, 3892–3904. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Li, Y.; Fan, X.; Zhang, G.; Zhang, F.; Peng, W. Increasing the heteroatoms doping percentages of graphene by porous engineering for enhanced electrocatalytic activities. J. Colloid Interface Sci. 2020, 577, 101–108. [Google Scholar] [CrossRef]
- Alotaibi, N.H.; Shah, J.H.; Nisa, M.U.; Mohammad, S.; Günhan Özcan, H.; Abid, A.G.; Allakhverdiev, S.I. Catalytic enhancement of graphene oxide by trace molybdenum oxide nanoparticles doping: Optimized electrocatalyst for green hydrogen production. Int. J. Hydrog. Energy 2024, 62, 488–497. [Google Scholar] [CrossRef]
- Louis, J.; Padmanabhan, N.T.; Jayaraj, M.K.; John, H. Exploring enhanced interfacial charge separation in ZnO/reduced graphene oxide hybrids on alkaline photoelectrochemical water splitting and photocatalytic pollutant degradation. Mater. Res. Bull. 2024, 169, 112542. [Google Scholar] [CrossRef]
- Younas, U.; Mobeen, F.; Saleem, A.; Ali, F.; Huwayz, M.A.; Ashraf, A.; Ahmad, A.; Alwadai, N.; Pervaiz, M.; Iqbal, M. Efficient hydrogen production via overall water splitting using CuO/ZnO decorated reduced graphene oxide as bifunctional electrocatalyst. Ceram. Int. 2024, 50, 30570–30578. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, R.; Dong, G.; Xiang, M.; Hui, J.; Ou, J.; Qin, H. Biochar Nanocomposite Derived from Watermelon Peels for Electrocatalytic Hydrogen Production. ACS Omega 2021, 6, 2066–2073. [Google Scholar] [CrossRef]
- Mian, M.M.; Alam, N.; Ahommed, M.S.; He, Z.; Ni, Y. Emerging applications of sludge biochar-based catalysts for environmental remediation and energy storage: A review. J. Clean. Prod. 2022, 360, 132131. [Google Scholar] [CrossRef]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, W.; Peng, H.; Pan, B.; Xing, B. Functional Biochar and Its Balanced Design. ACS Environ. Au 2022, 2, 115–127. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, M.; Li, Q.; Lu, S.; Zhou, D.; Feng, L.; Hou, Z.; Yu, J. Comparative analysis of the properties of biochars produced from different pecan feedstocks and pyrolysis temperatures. Ind. Crops Prod. 2023, 197, 116638. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Ho, S.-H.; Zhu, S.; Chang, J.-S. Recent advances in nanoscale-metal assisted biochar derived from waste biomass used for heavy metals removal. Bioresour. Technol. 2017, 246, 123–134. [Google Scholar] [CrossRef]
- Lou, T.; Yin, Y.; Wang, J. Recent advances in effect of biochar on fermentative hydrogen production: Performance and mechanisms. Int. J. Hydrog. Energy 2024, 57, 315–327. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, Q.; Gu, Y.; Nie, X.; Shan, R. Review of Advances in the Utilization of Biochar-Derived Catalysts for Biodiesel Production. ACS Omega 2023, 8, 8190–8200. [Google Scholar] [CrossRef]
- Amdeha, E. Biochar-based nanocomposites for industrial wastewater treatment via adsorption and photocatalytic degradation and the parameters affecting these processes. Biomass Convers. Biorefinery 2023, 14, 23293–23318. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Lee, J.S. Nanomaterials for photocatalytic hydrogen production: From theoretical perspectives. RSC Adv. 2017, 7, 34875–34885. [Google Scholar] [CrossRef]
- An, M.; Yang, Z.; Zhang, B.; Xue, B.; Xu, G.; Chen, W.; Wang, S. Construction of biochar-modified TiO2 anatase-rutile phase S-scheme heterojunction for enhanced performance of photocatalytic degradation and photocatalytic hydrogen evolution. J. Environ. Chem. Eng. 2023, 11, 110367. [Google Scholar] [CrossRef]
- Deng, C.; Peng, L.; Ling, X.; Wang, T.; Xu, R.; Zhu, Y.; Wang, C.; Qian, X.; Wang, L.; Wu, Y.; et al. Construction of S-scheme Zn0.2Cd0.8S/biochar aerogel architectures for boosting photocatalytic hydrogen production under sunlight irradiation. J. Clean. Prod. 2023, 414, 137616. [Google Scholar] [CrossRef]
- Deng, C.; Ling, X.; Peng, L.; Wang, T.; Xu, R.; Zhu, Y.; Zhang, W.; Sun, P.; Wu, Y.; Hu, H.; et al. Constructing nano CdS-decorated porous biomass-derived carbon for multi-channel synergetic photocatalytic hydrogen evolution under solar lighting. Appl. Surf. Sci. 2023, 623, 157065. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Gomari, K.A.; Hafeez, H.Y.; Mohammed, J.; Dankawu, U.M.; Ndikilar, C.E.; Suleiman, A.B. A recent development and future prospect of g–C3N4–based photocatalyst for stable hydrogen (H2) generation via photocatalytic water-splitting. Int. J. Hydrog. Energy 2024, 85, 598–624. [Google Scholar] [CrossRef]
- Kuila, S.K.; Guchhait, S.K.; Mandal, D.; Kumbhakar, P.; Chandra, A.; Tiwary, C.S.; Kundu, T.K. Dimensionality effects of g-C3N4 from wettability to solar light assisted self-cleaning and electrocatalytic oxygen evolution reaction. Chemosphere 2023, 333, 138951. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Z.; Wang, Y.; Ai, H.; Zhang, W.; Liu, X.; Han, X.; Zhao, J.; Zhang, H. Exploiting synergistic effects: Co3O4/g-C3N4 composite catalyst for enhanced oxygen evolution reaction. Int. J. Electrochem. Sci. 2023, 18, 100394. [Google Scholar] [CrossRef]
- Pan, J.; Wang, P.; Wang, P.; Yu, Q.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The photocatalytic overall water splitting hydrogen production of g-C3N4/CdS hollow core–shell heterojunction via the HER/OER matching of Pt/MnOx. Chem. Eng. J. 2021, 405, 126622. [Google Scholar] [CrossRef]
- BaQais, A.; Shariq, M.; Qamar, M.A.; Alhasmialameer, D.; Alharbi, A.F.; Althikrallah, H.A.; Alrahili, M.R.; Alrashdi, K.S. Synthesis and characterization of (Ni, Mn)-ZnO/g-C3N4 nanocomposite for efficient electrochemical water splitting: The role of electrocatalyst for OER. Diam. Relat. Mater. 2024, 147, 111343. [Google Scholar] [CrossRef]
- Paul, A.M.; Sajeev, A.; Nivetha, R.; Gothandapani, K.; Bhardwaj, P.; Raghavan, V.; Jacob, G.; Sellapan, R.; Jeong, S.K.; Grace, A.N. Cuprous oxide (Cu2O)/graphitic carbon nitride (g-C3N4) nanocomposites for electrocatalytic hydrogen evolution reaction. Diam. Relat. Mater. 2020, 107, 107899. [Google Scholar] [CrossRef]
- Sowmya, S.; Vijaikanth, V. g-C3N4/Chlorocobaloxime Nanocomposites as Multifunctional Electrocatalysts for Water Splitting and Energy Storage. ACS Omega 2023, 8, 32940–32954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).