3.1. Stability Analysis of the Air Operating Conditions
For an initial analysis of the oxy-fuel burner, the CO concentration in the flue gas is used as an indicator to characterize the stability of different air operating conditions. In
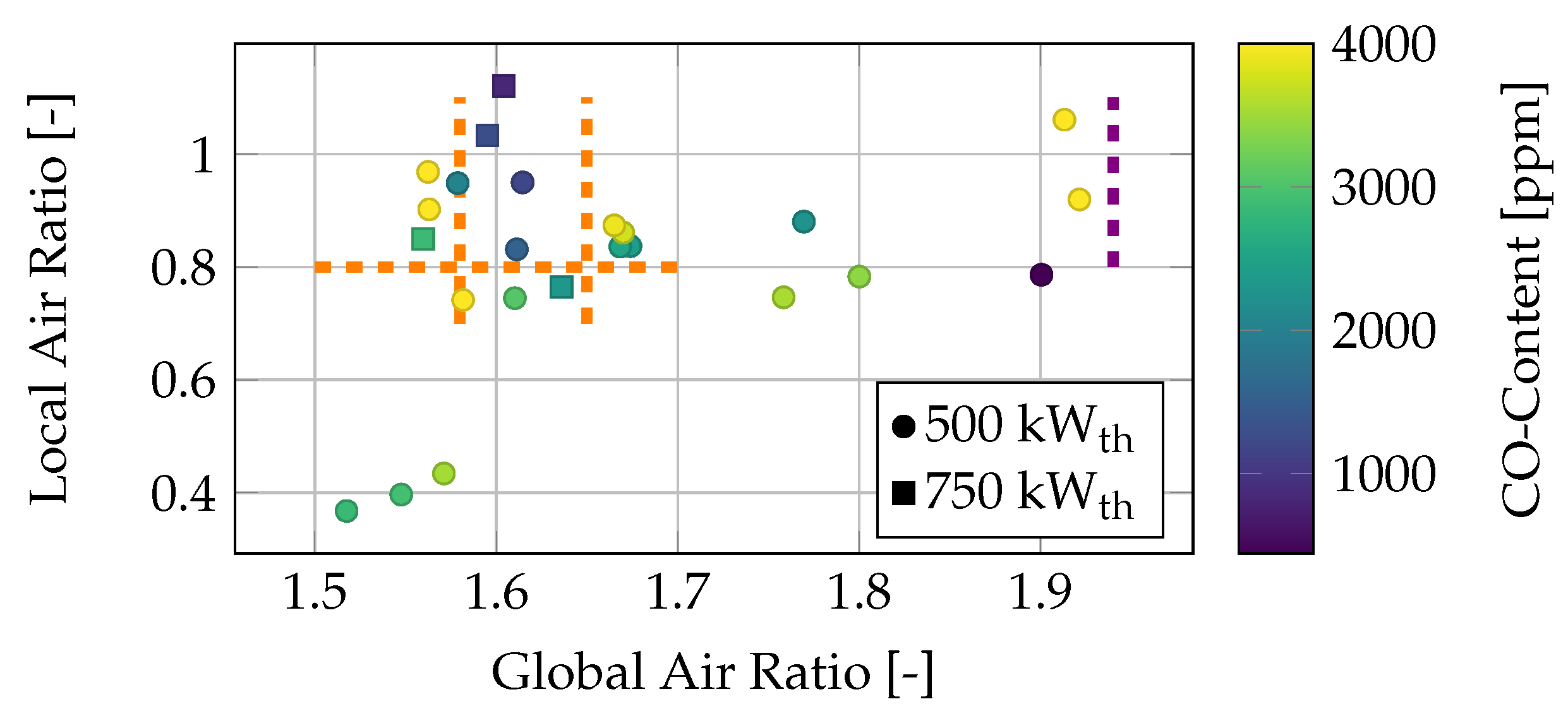
Figure 4, the CO concentrations in various operating conditions (refer to
Table 5) are plotted as a function of the local and global air ratios. The data are mostly gathered during two weeks of operation.
The CO content in the flue gas ranges from 500 ppm to 6000 ppm, with all operating conditions showing CO concentrations greater than 4000 ppm, as marked in yellow. Generally, the CO emissions are much higher than those typically observed in commercial boilers. This is attributed to the large particle size of the walnut shells, combined with the small combustion chamber dimensions for biomass combustion, which increases the likelihood of flame quenching due to the longer flame length [
35]. Operating conditions with a local air ratio higher than 1 and a global air ratio around
tend to have relatively low CO concentrations in the flue gas. This could be due to the significant amount of oxygen in the primary combustion zone. In contrast, it is anticipated that, for higher global air ratios, the increased tertiary stream may quench the flame before complete combustion occurs, leading to higher CO emissions. In the upper left portion of
Figure 4, where the local air ratio is around
and the global air ratio is
, a decrease in CO emissions is observed, followed by a subsequent increase. This area, marked by three dashed orange lines, represents the most stable operational range for the use of pulverized walnut shells as fuel. All tested operating conditions in this region exhibit CO emissions below 1500 ppm. Both the 500 kW
th and 750 kW
th operating conditions display the same trend. On the right side of
Figure 4, the lean blowout limit is marked with a violet line. The most stable operating conditions are found within a global air ratio range of 1.57–1.65 and a local air ratio higher than
. However, especially to reduce NOx emissions, a local air ratio lower than one should be used. It is assumed that a high tertiary air flow, and thus a high global air ratio, promotes rapid mixing and faster combustion in the fringe areas of the combustion chamber before the flame is thermally quenched by the walls. For thermal powers below 500 kW
th, a classical air staging approach is not feasible due to insufficient swirl momentum, which is discussed in more detail later. To achieve a higher swirl momentum in low-power operation, a local air ratio greater than one is necessary.
To better understand the individual phenomena of the flame, the CO content in the flue gas is plotted as a function of the global air ratio in
Figure 5. It is observed that CO emissions reach a minimum at a global air ratio of
. From furnace camera observations, it can be seen that, with a decreasing global air ratio, the flame touches the walls, suggesting that flame quenching occurs upon contact with the walls. For higher global air ratios, it is assumed that the flame is quenched by the high flow velocity of the tertiary stream. The CO emissions from the flame exhibit significant fluctuations, with high overall emissions under unstable operating conditions. In contrast, when the system operates under stable conditions at a global air ratio of
, both the fluctuations and the overall CO emission are reduced.
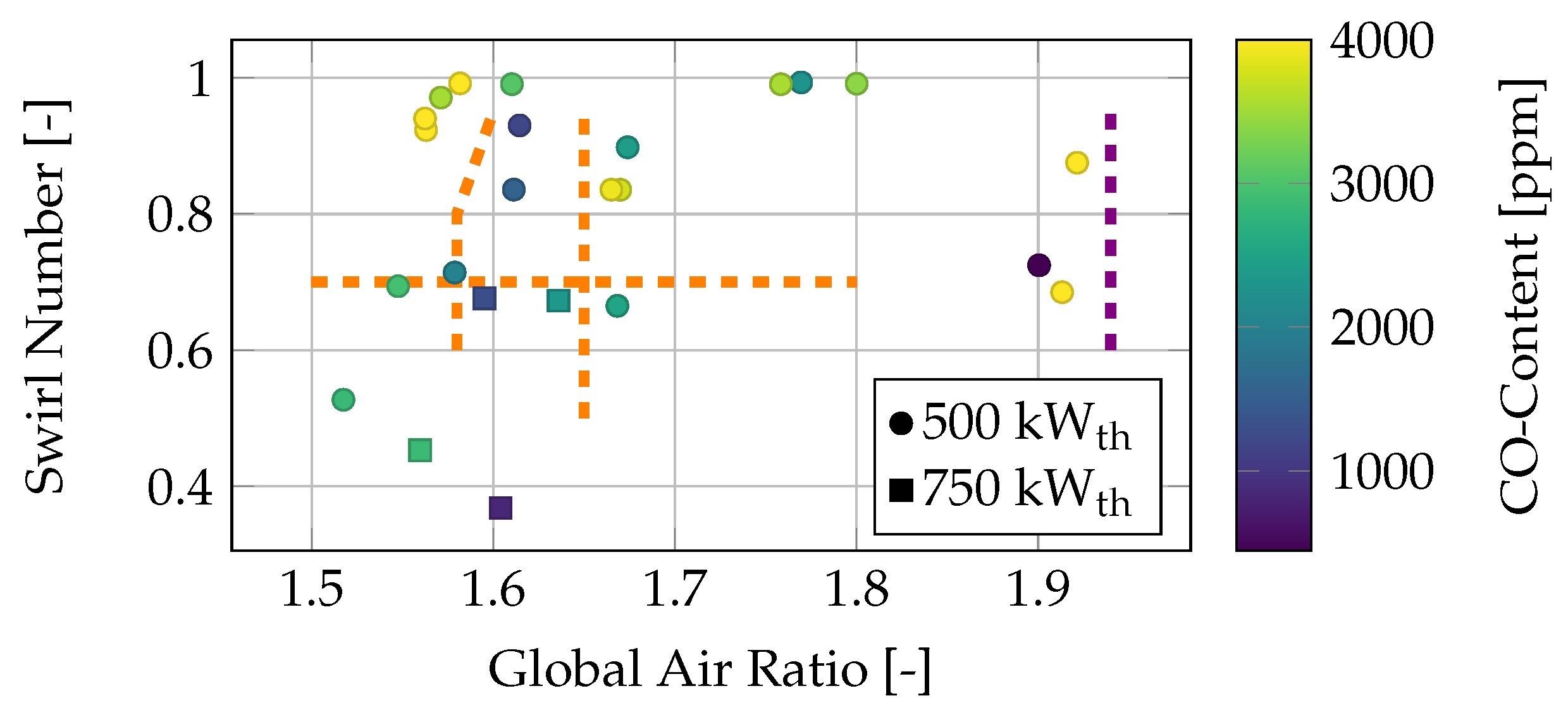
The second characteristic, the swirl number, is shown in
Figure 6.
The CO emissions of different air operating conditions are visualized in relation to the swirl number and global air ratio.
Figure 6 displays the same operating conditions as
Figure 4. Again, an area with decreased CO emissions is observed between a global air ratio of
and
, with all three limits marked by dashed orange lines in the diagram. The lean blowout limit is also shown in violet. Due to the high fuel momentum, which is greater than other types of fuel injection, a higher swirl momentum is required to form a proper recirculation zone in the center of the flame. This recirculation zone plays a key role in flame stabilization by providing a feedback loop of hot, burned gases to the diffusor. The recirculation ensures early ignition and contributes to the reduction of unburned components, such as CO. When comparing the 750 kW
th operating conditions (marked with a square) with the 500 kW
th conditions, it is evident that, for the higher power, a lower swirl is sufficient to achieve low CO concentrations in the flue gas and thus ensure stable flame operation. This difference is attributed to the air-to-fuel momentum ratio, which is discussed later.
All operating conditions with a local air ratio greater than
also show low CO concentrations in the flue gas, even at low swirl numbers. This correlation can be better understood by examining the axial and swirl velocities of the secondary stream in more detail, as shown in
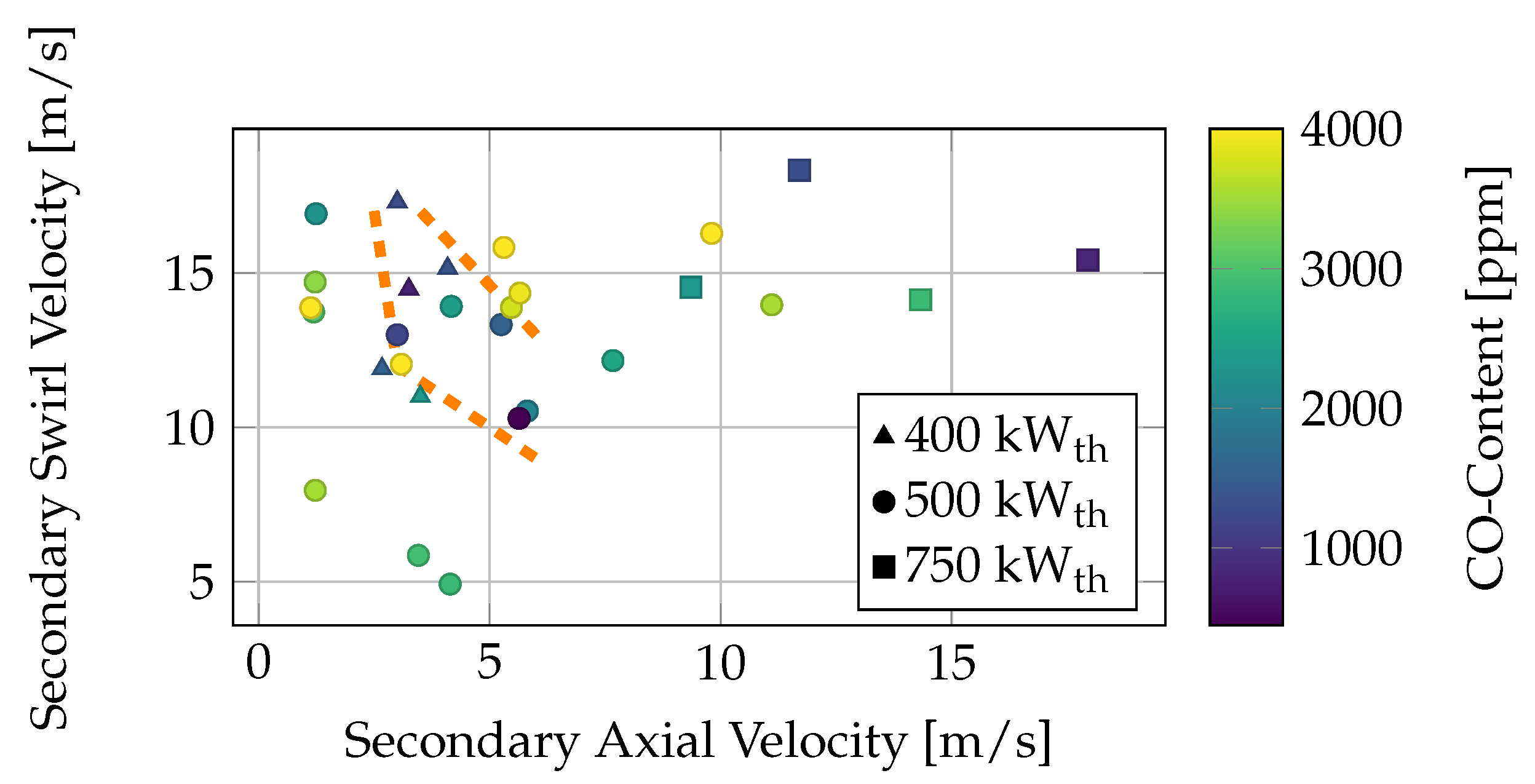
Figure 7.
The CO concentration of the different operating conditions is visualized as a function of the inlet velocity of the secondary axial and swirl streams. It is observed that the most stable operating conditions for the lower thermal power of 500 kW
th (marked with a dot) lie within an axial velocity range of 3–8 m/s and a swirl inlet velocity range of 10–14 m/s. These operational values are lower than the designed inlet velocity of 18 m/s (Richter et al. [
32]). For higher thermal power, the inlet velocity increases, and the CO content in the flue gas decreases. One operating condition must be excluded, namely, the yellow operating condition, which has a swirl velocity of 14 m/s and an axial inlet velocity of 44 m/s. Despite having the same inlet velocities as the other stable operating conditions, it has a lower global air ratio, leading to higher CO emissions (see
Figure 4). Increasing both the axial and swirl velocities results in higher CO emissions for the 500 kW
th operating conditions. The transition from stable operating conditions to lean blowout occurs very quickly, causing the operating conditions to be clustered closely together. For lower swirl velocities, the flame becomes unstable due to a high fuel momentum flux, leading to a dominant axial moment in the combustion chamber (orange line). This is also noticeable in the operating conditions for the lower thermal power of 400 kW
th, which exhibit stable behavior only when the local air ratio exceeds one. These conditions fall within the same area as the stable 500 kW
th operating conditions.
For higher power, the most stable operating conditions shift to higher inlet velocities of both the axial and swirl streams. This shift is due to the higher total volume flow of the oxidizer with increased power. Additionally, with a higher fuel-to-air momentum ratio, the burner exhibits more stable operating conditions, even at lower swirl numbers. This phenomenon is described by the fuel-to-air momentum ratio in Equation (
4) (Chen et al. [
36]):
with
This equation describes the ratio between the momentum that shapes the recirculation zone and the momentum that counteracts it. Notably, the fuel mass flow increases with the thermal power, but the air mass flow also increases correspondingly. Since the transport gas volume flow remains constant, the fuel inlet velocity does not change significantly, resulting in a decrease in the fuel-to-air momentum flux ratio. When calculating the momentum ratio for three stable operating conditions at different thermal powers, the ratio is found to vary between
for the highest power and
for the lowest. In the literature, two types of recirculating flames are described: strongly recirculating flames, which occur at low fuel momentum ratios, and fuel-penetrating recirculation flames, where the fuel jet penetrates the recirculation zone (Marinov et al. [
37]). Chen et al. [
36] discusses the differences between fuel jet-dominated flames and strongly recirculating flames, demonstrating that a momentum ratio greater than
results in a fuel-penetrating recirculation zone. This can also be observed in the analysis of the flame shape in the following section. Despite the influence of fuel momentum, the swirl plays a significant role in shaping the flame and determining its stability.
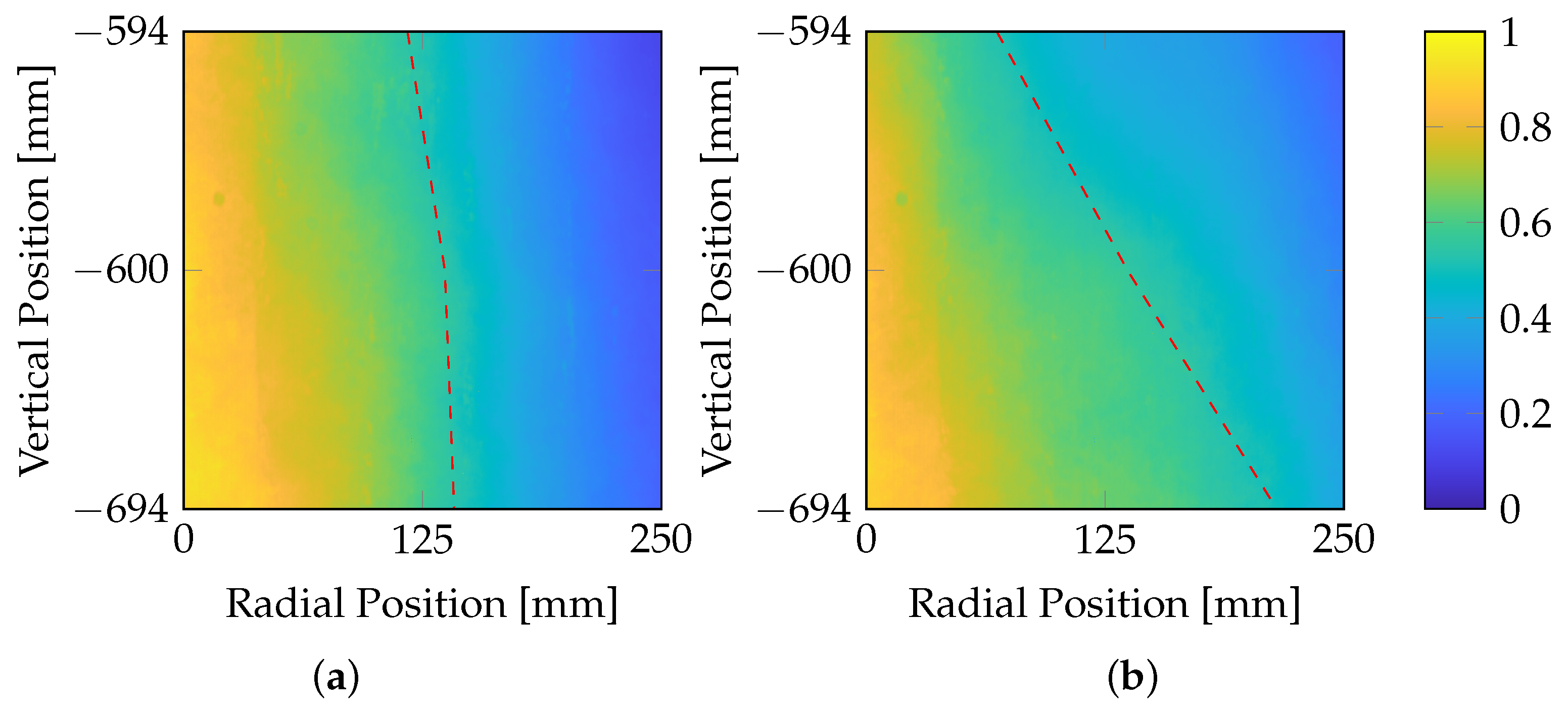
To obtain a better insight, two different swirl numbers and thus swirl inlet velocities are shown in
Figure 8.
An analysis of different operating conditions reveals that the swirl number has the most pronounced influence on the flame shape at port heights three and four. It is evident that the flame with a higher swirl (a) exhibits a larger diameter in the upper part of the flame than the flame with a lower swirl (b). However, as the distance from the burner increases, the flame with the lower swirl (b) shows greater radial spread. This suggests that, with an increased swirl, the flame exhibits better spread and enhanced mixing in the middle part of the flame, compared to a lower swirl. From the stability analysis, it can be inferred that a sufficient amount of tertiary air is essential to prevent flame quenching at the combustion chamber walls. The high fuel momentum of the solid fuel combined with the transport gas leads to a fuel-dominated swirl flame, characterized by a more jet-like shape. A high swirl momentum is crucial for ensuring the recirculation of flue gas, thereby maintaining stable combustion.
3.2. Comparison of Oxy-Fuel and Air Operating Conditions
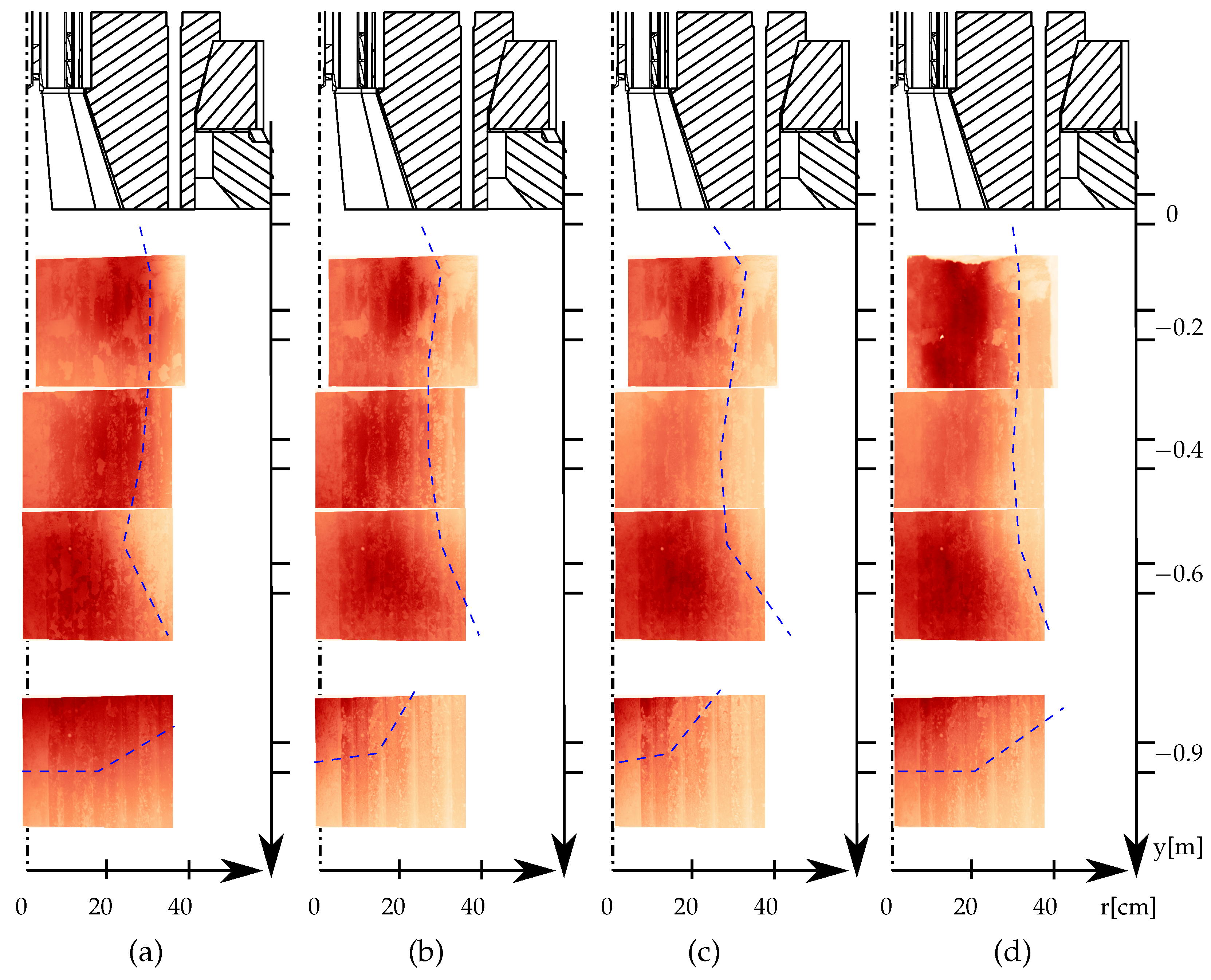
Figure 9 shows flame images of the air (a) and the corresponding oxy (b)–(d) operating conditions. On the top, the burner diffusor is sketched. The first image starts at the
y-position
mm. Each image shows a field of view of
mm of the combustion chamber.
Each image is normalized by its maximum intensity value, allowing for an easier analysis of the position of the highest flame intensity in each image. However, a direct quantitative comparison between different images is not feasible. By identifying the region of highest radiation intensity, the position of heat release can be estimated. The pattern observed in the background of the images is caused by reflections from the wall. For all four operating conditions, the highest intensity is found in the first two radial positions, which correspond to the diffusor’s radial extent (with a radius of 219 mm). This suggests that primary combustion occurs in the shear layer between the burner-attached secondary air and the centrally injected fuel. Images taken at port three indicate a widening flame behavior, with the flame spreading radially over a larger area. At a distance of m from the burner plane, the highest intensity is predominantly located in the upper region, indicating that this image captures the end of the flame.
When comparing the air and oxy-fuel operating conditions, it is observed that the oxy-fuel flames (OXY-27
p and OXY-30
p) exhibit less radial spread than the air case at the first two heights. Additionally, the oxy-fuel flames appear shorter and more compact. This is particularly evident in the image at a height of
m, where the inclination angle of the blue line in image three is lower. This difference is assumed to be due to the increased oxygen content in the oxy-fuel operating conditions, which accelerates ignition and results in a shorter flame. As the oxygen content and thermal power increase, the flame shape becomes more similar to the air case, as seen in the radial position, shape, and length of the flame. This was also demonstrated by Schneider et al. [
12] for a similar combustion chamber operating at 40 kW
th and led to a more similar flow field for the oxy-fuel operation with 33% and air combustion. The shift of the heat release zone closer to the burner plane is further corroborated by the temperature profile, which aligns with findings of Hjärtstam et al. [
16] and Smart et al. [
15].
Figure 10 illustrates the temperature and its standard deviation at three different heights in the combustion chamber. All four operating conditions display similar trends. The highest temperatures are observed at the second position, located
mm from the burner plane. This is consistent with the flame’s radial widening observed at
mm, which leads to the transport of hot gases near the wall. At the first position, temperatures are similar across all operating conditions, as noted by Toporov et al. [
7]. However, with an increasing distance from the burner, the differences between the air and oxy-fuel conditions become more pronounced. These differences can be attributed to the higher thermal load under the oxy-fuel operating conditions, as well as the increased reactivity and higher adiabatic flame temperature due to the elevated oxygen content.
Changes in the intensity distribution and position of the flame not only affect the temperature distribution within the combustion chamber but also influence the radiation emissions of the flame. Richter et al. [
32] conducted radiative heat flux measurements in the same combustion chamber using natural gas, Rhenish lignite, and walnut shells as fuels. These measurements show a behavior similar to that observed in the flame images: the radiative heat flux increases from port three to port four and then decreases again for all operating conditions. Like the temperature profiles, the oxy-fuel operating conditions exhibit higher radiative heat flux values than air combustion at a constant thermal power. At a distance of
mm from the burner plane, the radiative heat flux and temperature are comparable across both air and oxy-fuel conditions. However, as the distance from the burner increases, the differences between the air and oxy-fuel conditions become more pronounced. Overall, the results show that the application of oxy-fuel biomass combustion with flue gas recirculation is possible with the constant flow field approach used in this publication.