A Review of Operational Conditions of the Agroforestry Residues Biomethanization for Bioenergy Production Through Solid-State Anaerobic Digestion (SS-AD)
Abstract
1. Introduction
2. Principle of Solid-State Anaerobic Digestion (SS-AD)
2.1. Solid-State Anaerobic Digestion Steps
2.2. Major Inputs and Outputs Determining the Process Efficiency of SS-AD
3. Solid-State Anaerobic Digestion of Forestry and Agricultural Biomass
4. Advantages Associated with Anaerobic Co-Digestion of Agroforestry Residues
4.1. Case of Binary Co-Digestion of Lignocellulosic Biomass and Bovine Manure
4.2. Case of Ternary Co-Digestion
5. Viable Strategies to Enhance Biomethanization
5.1. At the Matter Level: The Pretreatment Step
5.2. At the Level of Reactor Design
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3c-DES | Three-constituent deep eutectic solvent |
| AD | Anaerobic digestion |
| ASWs | Agricultural solid wastes |
| BMP | Biochemical methane potential |
| C | Carbon |
| CH4 | Methane |
| ChCl | Choline chloride |
| ChM | Chicken manure |
| CM | Cattle manure |
| Co | Cobalt |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| COD | Chemical oxygen demand |
| CS | Corn stover |
| D | Discontinuous |
| DM | Dairy manure |
| EFBOP | Empty fruit bunches of oil palm |
| Fe | Iron |
| GHC | Greenhouse gas |
| H2 | Dihydrogen |
| H2S | Hydrogen sulfide |
| H2SO4 | Sulfuric acid |
| LS-AD | Liquid-state anaerobic digestion |
| MS | Moisture content |
| N | Nitrogen |
| NaOH | Sodium hydroxide |
| NH3 | Ammonia |
| NH4+ | Ammonium |
| NH4+–N | Ammonium–nitrogen |
| Ni | Nickel |
| NIRS | Near-infrared spectroscopy |
| ORL | Organic loading rate |
| PW | Poplar waste |
| RG | Roadside grass |
| RS | Rice straw |
| S/I | Substrate/inoculum |
| SC | Semi-continuous |
| SRT | Solid retention time |
| SS-AD | Solid-state anaerobic digestion |
| TM | Tomato residues |
| TS | Total solid content |
| VFA | Volatile fatty acid |
| VS | Volatile solid content |
References
- Hong, C.; Burney, J.A.; Pongratz, J.; Nabel, J.E.M.S.; Mueller, N.D.; Jackson, R.B.; Davis, S.J. Global and Regional Drivers of Land-Use Emissions in 1961–2017. Nature 2021, 589, 554–561. [Google Scholar] [CrossRef]
- Sharma, H.B.; Vanapalli, K.R.; Samal, B.; Cheela, V.R.S.; Dubey, B.K.; Bhattacharya, J. Circular Economy Approach in Solid Waste Management System to Achieve UN-SDGs: Solutions for Post-COVID Recovery. Sci. Total Environ. 2021, 800, 149605. [Google Scholar] [CrossRef] [PubMed]
- Venkatramanan, V.; Shah, S.; Rai, A.K.; Prasad, R. Nexus Between Crop Residue Burning, Bioeconomy and Sustainable Development Goals Over North-Western India. Front. Energy Res. 2021, 8, 614212. [Google Scholar] [CrossRef]
- Subbarao, P.M.V.; D’ Silva, T.C.; Adlak, K.; Kumar, S.; Chandra, R.; Vijay, V.K. Anaerobic Digestion as a Sustainable Technology for Efficiently Utilizing Biomass in the Context of Carbon Neutrality and Circular Economy. Environ. Res. 2023, 234, 116286. [Google Scholar] [CrossRef]
- Rekleitis, G.; Haralambous, K.-J.; Loizidou, M.; Aravossis, K. Utilization of Agricultural and Livestock Waste in Anaerobic Digestion (A.D): Applying the Biorefinery Concept in a Circular Economy. Energies 2020, 13, 4428. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic Digestion Process: Technological Aspects and Recent Developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of Agricultural Residues: Potential, Limitations and Possible Solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Fiallos, C.G.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic Digestate Management, Environmental Impacts, and Techno-Economic Challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef]
- Uddin, M.M.; Wright, M.M. Anaerobic Digestion Fundamentals, Challenges, and Technological Advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Naji, A.; Dujany, A.; Guerin Rechdaoui, S.; Rocher, V.; Pauss, A.; Ribeiro, T. Optimization of Liquid-State Anaerobic Digestion by Defining the Optimal Composition of a Complex Mixture of Substrates Using a Simplex Centroid Design. Water 2024, 16, 1953. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.; Wu, G.; Zhan, X. Impact of Total Solids Content on Anaerobic Co-Digestion of Pig Manure and Food Waste: Insights into Shifting of the Methanogenic Pathway. Waste Manag. 2020, 114, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, C.; Zhang, Y.; Li, Y.; Wang, Y.; Li, G.; Luo, W. Anaerobic Digestion of Agricultural Wastes from Liquid to Solid State: Performance and Environ-Economic Comparison. Bioresour. Technol. 2021, 332, 125080. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, J.; Zhao, Q.; Li, L.; Wang, K.; Wei, L. Effects of Organic Loading Rates on High-Solids Anaerobic Digestion of Food Waste in Horizontal Flow Reactor: Methane Production, Stability and Mechanism. Chemosphere 2022, 293, 133650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Zhan, X.; Gardiner, G.E. Inhibition of Volatile Fatty Acids on Methane Production Kinetics during Dry Co-Digestion of Food Waste and Pig Manure. Waste Manag. 2018, 79, 302–311. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Wang, Z.-W. Mathematical Modeling of Solid-State Anaerobic Digestion. Prog. Energy Combust. Sci. 2015, 51, 49–66. [Google Scholar] [CrossRef]
- Di Capua, F.; Spasiano, D.; Giordano, A.; Adani, F.; Fratino, U.; Pirozzi, F.; Esposito, G. High-Solid Anaerobic Digestion of Sewage Sludge: Challenges and Opportunities. Appl. Energy 2020, 278, 115608. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of Bacteria and Archaea Participating in the Bioconversion of Organic Waste for Methane Production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Frear, C.; Wang, Z.; Yu, L.; Zhao, Q.; Li, X.; Chen, S. A Simple Methodology for Rate-Limiting Step Determination for Anaerobic Digestion of Complex Substrates and Effect of Microbial Community Ratio. Bioresour. Technol. 2013, 134, 391–395. [Google Scholar] [CrossRef]
- Lim, J.W.; Park, T.; Tong, Y.W.; Yu, Z. Chapter One—The Microbiome Driving Anaerobic Digestion and Microbial Analysis. In Advances in Bioenergy; Li, Y., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 1–61. [Google Scholar]
- Ozsefil, I.C.; Miraloglu, I.H.; Ozbayram, E.G.; Ince, B.; Ince, O. Bioaugmentation of Anaerobic Digesters with the Enriched Lignin-Degrading Microbial Consortia through a Metagenomic Approach. Chemosphere 2024, 355, 141831. [Google Scholar] [CrossRef]
- Singh, A.; Müller, B.; Schnürer, A. Profiling Temporal Dynamics of Acetogenic Communities in Anaerobic Digesters Using Next-Generation Sequencing and T-RFLP. Sci. Rep. 2021, 11, 13298. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciezkowska, M.; Krawczyk, P.S.; Sobczak, A.; Drewniak, L.; Dziembowski, A.; Lipinski, L. Comparative Analysis of Deep Sequenced Methanogenic Communities: Identification of Microorganisms Responsible for Methane Production. Microb. Cell. Fact. 2018, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Ajayi-Banji, A.; Rahman, S. A Review of Process Parameters Influence in Solid-State Anaerobic Digestion: Focus on Performance Stability Thresholds. Renew. Sustain. Energy Rev. 2022, 167, 112756. [Google Scholar] [CrossRef]
- Hamilton, D.W. Solid State Anaerobic Digestion. Okla. Coop. Ext. Serv. 2017, BAE-1764, 2. [Google Scholar]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Biological Treatment of Organic Materials for Energy and Nutrients Production—Anaerobic Digestion and Composting. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 121–181. ISBN 978-0-12-817710-5. [Google Scholar]
- Su, L.; Sun, X.; Liu, C.; Ji, R.; Zhen, G.; Chen, M.; Zhang, L. Thermophilic Solid-State Anaerobic Digestion of Corn Straw, Cattle Manure, and Vegetable Waste: Effect of Temperature, Total Solid Content, and C/N Ratio. Archaea 2020, 2020, 8841490. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between Carbon/Nitrogen Ratio, Synergy and Microbial Characteristics of Long-Term Semi-Continuous Anaerobic Co-Digestion of Food Waste, Cattle Manure and Corn Straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef] [PubMed]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Croce, S.; Wei, Q.; D’Imporzano, G.; Dong, R.; Adani, F. Anaerobic Digestion of Straw and Corn Stover: The Effect of Biological Process Optimization and Pretreatment on Total Bio-Methane Yield and Energy Performance. Biotechnol. Adv. 2016, 34, 1289–1304. [Google Scholar] [CrossRef]
- Baek, G.; Kim, D.; Kim, J.; Kim, H.; Lee, C. Treatment of Cattle Manure by Anaerobic Co-Digestion with Food Waste and Pig Manure: Methane Yield and Synergistic Effect. Int. J. Environ. Res. Public Health 2020, 17, 4737. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; Pezzolla, D.; Tacconi, C.; Gigliotti, G. Anaerobic Co-Digestion of a Lignocellulosic Residue with Different Organic Wastes: Relationship between Biomethane Yield, Soluble Organic Matter and Process Stability. Biomass Bioenergy 2021, 153, 106209. [Google Scholar] [CrossRef]
- Hupfauf, S.; Winkler, A.; Wagner, A.O.; Podmirseg, S.M.; Insam, H. Biomethanation at 45 °C Offers High Process Efficiency and Supports Hygienisation. Bioresour. Technol. 2020, 300, 122671. [Google Scholar] [CrossRef]
- Li, H.; Jin, C.; Zhang, Z.; O’Hara, I.; Mundree, S. Environmental and Economic Life Cycle Assessment of Energy Recovery from Sewage Sludge through Different Anaerobic Digestion Pathways. Energy 2017, 126, 649–657. [Google Scholar] [CrossRef]
- Li, Y.-F.; Nelson, M.C.; Chen, P.-H.; Graf, J.; Li, Y.; Yu, Z. Comparison of the Microbial Communities in Solid-State Anaerobic Digestion (SS-AD) Reactors Operated at Mesophilic and Thermophilic Temperatures. Appl. Microbiol. Biotechnol. 2015, 99, 969–980. [Google Scholar] [CrossRef]
- Albalate-Ramírez, A.; Alcalá-Rodríguez, M.M.; Miramontes-Martínez, L.R.; Padilla-Rivera, A.; Estrada-Baltazar, A.; López-Hernández, B.N.; Rivas-García, P. Energy Production from Cattle Manure within a Life Cycle Assessment Framework: Statistical Optimization of Co-Digestion, Pretreatment, and Thermal Conditions. Sustainability 2022, 14, 16945. [Google Scholar] [CrossRef]
- Rajagopal, R.; Bellavance, D.; Rahaman, M.S. Psychrophilic Anaerobic Digestion of Semi-Dry Mixed Municipal Food Waste: For North American Context. Process Saf. Environ. Prot. 2017, 105, 101–108. [Google Scholar] [CrossRef]
- Kinnunen, M.; Hilderbrandt, D.; Grimberg, S.; Rogers, S.; Mondal, S. Comparative Study of Methanogens in One- and Two-Stage Anaerobic Digester Treating Food Waste. Renew. Agric. Food Syst. 2015, 30, 515–523. [Google Scholar] [CrossRef]
- Gensollen, G.; Pourcher, A.-M.; Duedal, A.-L.; Picard, S.; Le Roux, S.; Peu, P. Impact of pH in the First-Stage of a Two-Stage Anaerobic Digestion on Metabolic Pathways and Methane Production. Bioresour. Technol. Rep. 2022, 20, 101256. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karim, T.; Onik, M.H.; Kumar, D.; Rahman, M.A.; Yousuf, A.; Uddin, M.R. Impact of Temperature, Inoculum Flow Pattern, Inoculum Type, and Their Ratio on Dry Anaerobic Digestion for Biogas Production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef]
- Khaled, M.; Kamal, G.; Ahmed Porosh, K.; Mashfy, M.M.; Rahman, H. Experimental Approach of Producing Biogas from Fallen Leaves with Co-Digestion. BIO Web Conf. 2023, 62, 03003. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2019, 25, 1–17. [Google Scholar] [CrossRef]
- Giménez-Lorang, A.; Vázquez-Padín, J.R.; Dorado-Barragán, C.; Sánchez-Santos, G.; Vila-Armadas, S.; Flotats-Ripoll, X. Treatment of the Supernatant of Anaerobically Digested Organic Fraction of Municipal Solid Waste in a Demo-Scale Mesophilic External Anaerobic Membrane Bioreactor. Front. Bioeng. Biotechnol. 2021, 9, 642747. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, P.; Wang, Y.; Wu, P.; Li, Y.; Ma, L. Enhancing Methane Production in Anaerobic Co-Digestion of Sewage Sludge and Food Waste by Regulating Organic Loading Rate. Bioresour. Technol. 2022, 363, 127988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiao, P.; Zhang, M.; Wu, P.; Zhang, Y.; Wang, Y.; Xu, K.; Yu, J.; Ma, L. Impacts of Organic Loading Rate and Hydraulic Retention Time on Organics Degradation, Interspecies Interactions and Functional Traits in Thermophilic Anaerobic Co-Digestion of Food Waste and Sewage Sludge. Bioresour. Technol. 2023, 370, 128578. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef]
- Kong, X.; Yu, S.; Xu, S.; Fang, W.; Liu, J.; Li, H. Effect of Fe0 Addition on Volatile Fatty Acids Evolution on Anaerobic Digestion at High Organic Loading Rates. Waste Manag. 2018, 71, 719–727. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C. Synergistic Effect of Activated Carbon and Encapsulated Trace Element Additive on Methane Production from Anaerobic Digestion of Food Wastes—Enhanced Operation Stability and Balanced Trace Nutrition. Bioresour. Technol. 2019, 278, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, J.; Kong, X.; Yuan, J.; Liu, J.; Zhang, C. A Comprehensive Review of the Impact of Trace Elements on Anaerobic Digestion for Organic Solid Wastes. Process Saf. Environ. Prot. 2024, 192, 1172–1189. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, Y.; Ye, X.; Du, J.; Kong, X.; Guo, D.; Xiao, Q. Enhanced Anaerobic Biogas Production from Wheat Straw by Herbal-Extraction Process Residues Supplementation. Front. Bioeng. Biotechnol. 2021, 9, 623594. [Google Scholar] [CrossRef]
- Yasim, N.S.E.M.; Buyong, F. Comparative of Experimental and Theoretical Biochemical Methane Potential Generated by Municipal Solid Waste. Environ. Adv. 2023, 11, 100345. [Google Scholar] [CrossRef]
- Da Silva, C.; Astals, S.; Peces, M.; Campos, J.L.; Guerrero, L. Biochemical Methane Potential (BMP) Tests: Reducing Test Time by Early Parameter Estimation. Waste Manag. 2018, 71, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Shitophyta, L.M.; Putri, S.R.; Salsabiella, Z.A.; Budiarti, G.I.; Rauf, F.; Khan, A. Theoretical Biochemical Methane Potential Generated by the Anaerobic Digestion of Mustard Green Residues in Different Dilution Volumes. Pol. J. Environ. Stud. 2023, 32, 4799–4804. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, C.; Wang, N.; Shi, J.; Zhang, B.; Liu, C.; Sun, Y. Rapid Biochemical Methane Potential Evaluation of Anaerobic Co-Digestion Feedstocks Based on Near Infrared Spectroscopy and Chemometrics. Energies 2021, 14, 1460. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane Gas Separation Technologies for Biogas Upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Li, Y.; Alaimo, C.P.; Kim, M.; Kado, N.Y.; Peppers, J.; Xue, J.; Wan, C.; Green, P.G.; Zhang, R.; Jenkins, B.M.; et al. Composition and Toxicity of Biogas Produced from Different Feedstocks in California. Environ. Sci. Technol. 2019, 53, 11569–11579. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Removal of Sulfur Contaminants from Biogas to Enable Direct Catalytic Methanation. Biomass Conv. Bioref. 2021, 11, 1823–1834. [Google Scholar] [CrossRef]
- Sanaye, S.; Yazdani, M. Energy, Exergy, Economic and Environmental Analysis of a Running Integrated Anaerobic Digester-Combined Heat and Power System in a Municipal Wastewater Treatment Plant. Energy Rep. 2022, 8, 9724–9741. [Google Scholar] [CrossRef]
- Das, J.; Ravishankar, H.; Lens, P.N.L. Biological Biogas Purification: Recent Developments, Challenges and Future Prospects. J. Environ. Manag. 2022, 304, 114198. [Google Scholar] [CrossRef]
- Sihlangu, E.; Luseba, D.; Regnier, T.; Magama, P.; Chiyanzu, I.; Nephawe, K.A. Investigating Methane, Carbon Dioxide, Ammonia, and Hydrogen Sulphide Content in Agricultural Waste during Biogas Production. Sustainability 2024, 16, 5145. [Google Scholar] [CrossRef]
- Ardolino, F.; Cardamone, G.F.; Parrillo, F.; Arena, U. Biogas-to-Biomethane Upgrading: A Comparative Review and Assessment in a Life Cycle Perspective. Renew. Sustain. Energy Rev. 2021, 139, 110588. [Google Scholar] [CrossRef]
- Mahmoodi-Eshkaftaki, M.; Houshyar, E. Biogas Recirculation Technology: Effect on Biogas Purification, Slurry Characteristics, Microbial Activity and Energy Consumption. Environ. Technol. Innov. 2020, 19, 100867. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and Strategies for Solid-State Anaerobic Digestion of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, Z. Solid-State Anaerobic Digestion for Waste Management and Biogas Production. In Solid State Fermentation: Research and Industrial Applications; Steudler, S., Werner, A., Cheng, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 147–168. ISBN 978-3-030-23675-5. [Google Scholar]
- Seruga, P.; Krzywonos, M.; Paluszak, Z.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł.; Pińkowska, H. Pathogen Reduction Potential in Anaerobic Digestion of Organic Fraction of Municipal Solid Waste and Food Waste. Molecules 2020, 25, 275. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Šlepetys, J.; Cesevičienė, J. Towards a Full Circular Economy in Biogas Plants: Sustainable Management of Digestate for Growing Biomass Feedstocks and Use as Biofertilizer. Energies 2021, 14, 4272. [Google Scholar] [CrossRef]
- Brown, D.; Shi, J.; Li, Y. Comparison of Solid-State to Liquid Anaerobic Digestion of Lignocellulosic Feedstocks for Biogas Production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Motte, J.-C.; Escudié, R.; Bernet, N.; Delgenes, J.-P.; Steyer, J.-P.; Dumas, C. Dynamic Effect of Total Solid Content, Low Substrate/Inoculum Ratio and Particle Size on Solid-State Anaerobic Digestion. Bioresour. Technol. 2013, 144, 141–148. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Zamani, A.; Amiri, H.; Horváth, I.S. Enhanced Solid-State Biogas Production from Lignocellulosic Biomass by Organosolv Pretreatment. BioMed Res. Int. 2014, 2014, 350414. [Google Scholar] [CrossRef]
- López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; López-González, J.A.; Verstichel, S.; Debeer, L.; Wierinck, I.; Moreno, J. Biodelignification of Agricultural and Forest Wastes: Effect on Anaerobic Digestion. Biomass Bioenergy 2013, 58, 343–349. [Google Scholar] [CrossRef]
- Ge, X.; Matsumoto, T.; Keith, L.; Li, Y. Fungal Pretreatment of Albizia Chips for Enhanced Biogas Production by Solid-State Anaerobic Digestion. Energy Fuels 2015, 29, 200–204. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Horváth, I.S. Improvement of Solid-State Biogas Production from Wood by Concentrated Phosphoric Acid Pretreatment. BioResources 2016, 11, 3230–3243. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S.; Kafle, G.K. Importance of “Weak-Base” Poplar Wastes to Process Performance and Methane Yield in Solid-State Anaerobic Digestion. J. Environ. Manag. 2017, 193, 423–429. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, C.; Wang, Y.; Li, Y.; Han, T.; Gong, X.; Shan, M.; Li, G.; Luo, W. Enhancing Biogas Production from Livestock Manure in Solid-State Anaerobic Digestion by Sorghum-Vinegar Residues. Environ. Technol. Innov. 2022, 26, 102276. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Li, Y. Solid-State Anaerobic Digestion of Fungal Pretreated Miscanthus Sinensis Harvested in Two Different Seasons. Bioresour. Technol. 2015, 185, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg-Eliasson, K.; Nadeau, E.; Levén, L.; Schnürer, A. Production Efficiency of Swedish Farm-Scale Biogas Plants. Biomass Bioenergy 2017, 97, 27–37. [Google Scholar] [CrossRef]
- Dastyar, W.; Mohammad Mirsoleimani Azizi, S.; Dhadwal, M.; Ranjan Dhar, B. High-Solids Anaerobic Digestion of Organic Fraction of Municipal Solid Waste: Effects of Feedstock to Inoculum Ratio and Percolate Recirculation Time. Bioresour. Technol. 2021, 337, 125335. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, S.; Jo, H.; Jeong, J.; Mulbry, W.; Rhaman, S.; Ahn, H. Solid-State Anaerobic Digestion of Dairy Manure from a Sawdust-Bedded Pack Barn: Moisture Responses. Energies 2018, 11, 484. [Google Scholar] [CrossRef]
- Jo, H.; Lee, S.; Lee, J.; Kim, E.; Ahn, H. Evaluation of Mixing Effects on Solid-state Anaerobic Digestion Performance of Dairy Manure and Sawdust Bedding Mixtures. Korean J. Soil. Sci. Fertil. 2016, 49, 227–234. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Ha, D.-M.; Na, Y.; Kim, D.-H. Fermentation Characteristics of Bedded Pack Barn Dairy Cattle Manure on Methane Yield, Carbon, and Nitrogen Content in Solid-State Anaerobic Digestion. PeerJ 2022, 10, e14134. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jo, H.-S.; Lee, M.-G.; Yabe, M.; Ahn, H. Effect of Substrate to Inoculum Ratio on Methane Production and Organic Matter Removal during Solid State Anaerobic Digestion of Beef Manure and Sawdust Mixture. J. Fac. Agric. Kyushu Univ. 2017, 62, 197–203. [Google Scholar] [CrossRef]
- Walter, A.; Franke-Whittle, I.H.; Wagner, A.O.; Insam, H. Methane Yields and Methanogenic Community Changes during Co-Fermentation of Cattle Slurry with Empty Fruit Bunches of Oil Palm. Bioresour. Technol. 2015, 175, 619–623. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.A.; Rahman, S.; Sunoj, S.; Igathinathane, C. Impact of Corn Stover Particle Size and C/N Ratio on Reactor Performance in Solid-State Anaerobic Co-Digestion with Dairy Manure. J. Air Waste Manag. Assoc. 2020, 70, 436–454. [Google Scholar] [CrossRef] [PubMed]
- André, L.; Zdanevitch, I.; Pineau, C.; Lencauchez, J.; Damiano, A.; Pauss, A.; Ribeiro, T. Dry Anaerobic Co-Digestion of Roadside Grass and Cattle Manure at a 60 L Batch Pilot Scale. Bioresour. Technol. 2019, 289, 121737. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, J.; Xu, H.; Zhang, N.; Chen, Y.; Yang, J.; Wang, K.; Jiang, J. A Coupling Strategy Combined with Acid-Hydrothermal and Novel DES Pretreatment: Enhancing Biomethane Yield under Solid-State Anaerobic Digestion and Efficiently Producing Xylo-Oligosaccharides and Recovered Lignin from Poplar Waste. Int. J. Biol. Macromol. 2024, 274, 133443. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Enhancing the Production of Biogas through Anaerobic Co-Digestion of Agricultural Waste and Chemical Pretreatments. Chemosphere 2020, 255, 126805. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid State Anaerobic Co-Digestion of Tomato Residues with Dairy Manure and Corn Stover for Biogas Production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Xu, F.; Li, Y.; Li, D.; Wang, G.; Li, S.; Zhang, H.; Wu, Y.; Shah, A.; et al. Reactor Performance and Economic Evaluation of Anaerobic Co-Digestion of Dairy Manure with Corn Stover and Tomato Residues under Liquid, Hemi-Solid, and Solid State Conditions. Bioresour. Technol. 2018, 270, 103–112. [Google Scholar] [CrossRef]
- Mothe, S.; Bella, K.; Sukesh, M.J.; Gopal, B.; Rao, P.V.; Sridhar, P. Anaerobic Co-Digestion of Rice Straw with Ternary Mixtures for Enhanced Methane Production. J. Environ. Manag. 2024, 340, 117960. [Google Scholar]
- Qu, J.; Sun, Y.; Awasthi, M.K.; Liu, Y.; Xu, X.; Meng, X.; Zhang, H. Effect of Different Aerobic Hydrolysis Time on the Anaerobic Digestion Characteristics and Energy Consumption Analysis. Bioresour. Technol. 2021, 320, 124332. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Xu, X.; Li, P.; Li, N.; Zhang, H.; Sun, Y. Effect of Aerobic Hydrolysis on Anaerobic Fermentation Characteristics of Various Parts of Corn Stover and the Scum Layer. Energies 2019, 12, 381. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, T.; Chang, J.; Tang, Q.; Luo, T.; Cui, Z. Effect of Substrate to Inoculum Ratio on Biogas Production and Microbial Community During Hemi-Solid-State Batch Anaerobic Co-Digestion of Rape Straw and Dairy Manure. Appl. Biochem. Biotechnol. 2019, 189, 884–902. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Use of Inoculum, Water and Percolate as Strategy to Avoid Inhibition on Dry-Batch Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Waste Biomass Valor. 2022, 13, 227–239. [Google Scholar] [CrossRef]
- Coutu, A.; André, L.; Guérin, S.; Rocher, V.; Pauss, A.; Ribeiro, T. Transport and Retention Modeling of the Liquid Phase through a Stratified Porous Leach-Bed. Application for Solid-State Anaerobic Co-Digestion of Cattle Manure and Roadside Grass. Bioresour. Technol. Rep. 2022, 18, 101114. [Google Scholar] [CrossRef]
- Coutu, A.; Mottelet, S.; Guérin, S.; Rocher, V.; Pauss, A.; Ribeiro, T. Methane Yield Optimization Using Mix Response Design and Bootstrapping: Application to Solid-State Anaerobic Co-Digestion Process of Cattle Manure and Damp Grass. Bioresour. Technol. Rep. 2022, 17, 100883. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Jia, S.; Chen, J.; Qi, C.; Han, Y.; Shan, M.; Li, G.; Li, Y. Comparison of Batch and Fed-Batch Solid-State Anaerobic Digestion of on-Farm Organic Residues: Reactor Performance and Economic Evaluation. Environ. Technol. Innov. 2021, 24, 101977. [Google Scholar] [CrossRef]
- Hernandez-Shek, M.A.; Peultier, P.; Pauss, A.; Ribeiro, T. Rheological Evolution of Straw-Cattle Manure (SCM) Treated by Dry Anaerobic Digestion in Batch and in Continuous Pilot Reactors. Waste Manag. 2022, 144, 411–420. [Google Scholar] [CrossRef]
- Li, P.; Ning, Z.; Li, Z.; Feng, J.; Meng, H.; Ye, B. Rheological Properties and Microbial Community Structure in Continuous Dry Co-Digestion of Corn Straw and Cow Manure. J. Environ. Chem. Eng. 2023, 11, 110294. [Google Scholar] [CrossRef]

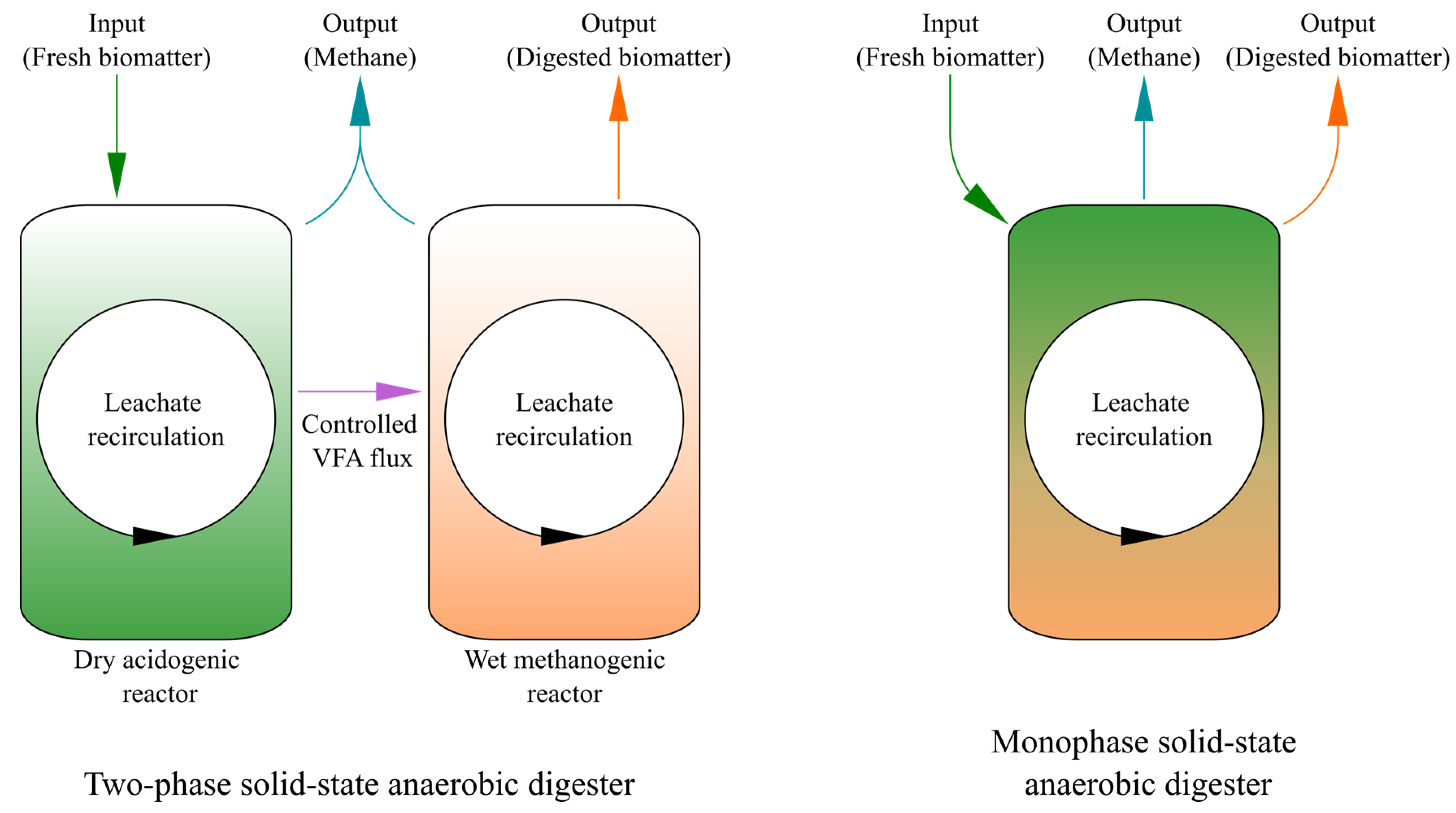
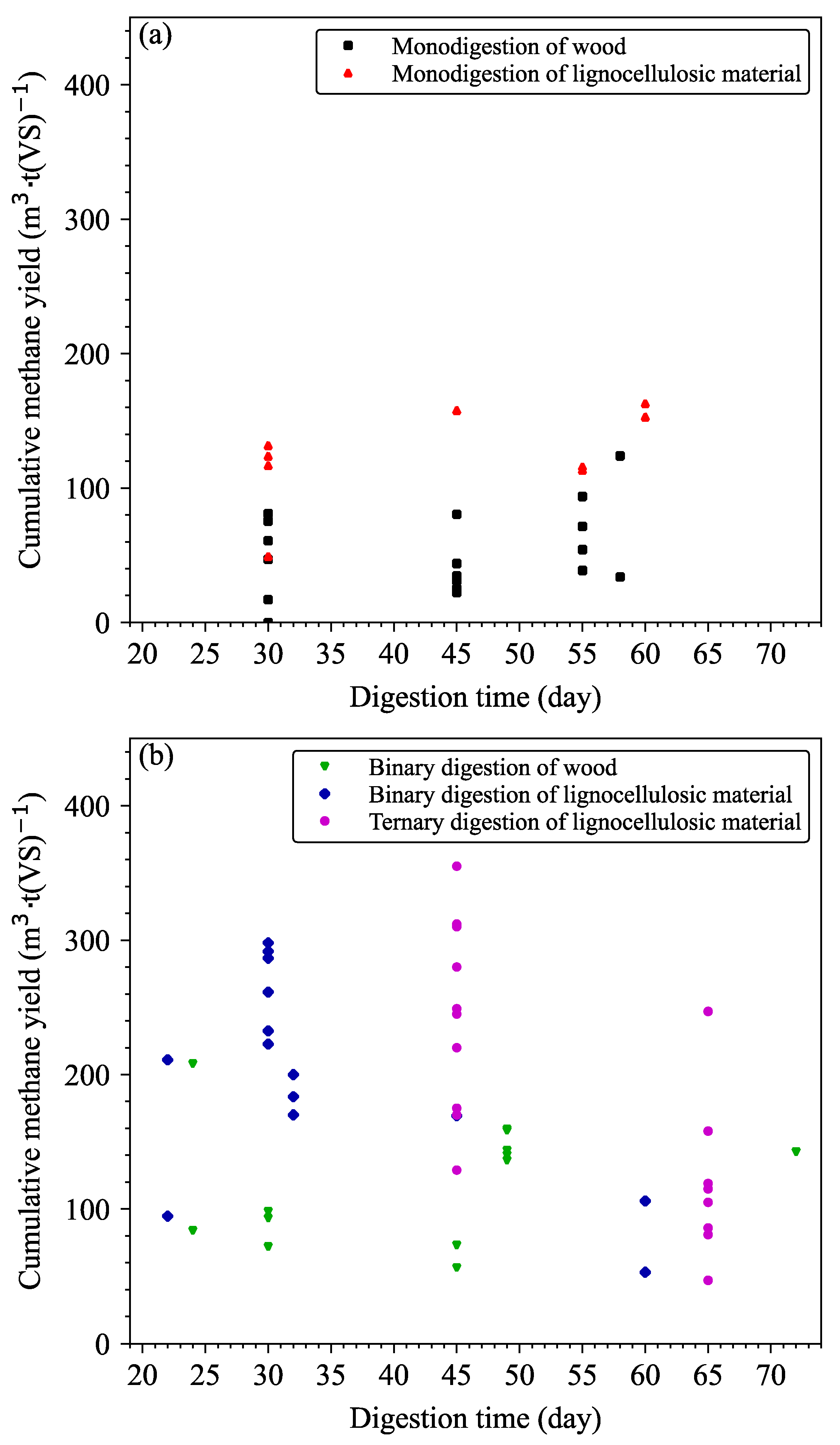
| Wood Biomass | Source of Added Inoculum | Time and Temperature of Digestion | Cumulative Methane Production (Nm3·t(VS)−1) | Complementary Information | Ref |
|---|---|---|---|---|---|
| Maple wood | Effluent from mesophillic LS-AD | 30 days at 37 °C | 46.9 * | - | [68] |
| Pine wood | Effluent from mesophillic LS-AD | 30 days at 37 °C | 17.0 * | - | [68] |
| Leaves | Effluent from mesophillic LS-AD | 30 days at 37 °C | 75.3 * | - | [68] |
| Corn stover | Effluent from mesophillic LS-AD | 30 days at 37 °C | 131.8 * | - | [68] |
| Wheat straw | Effluent from mesophillic LS-AD | 30 days at 37 °C | 123.9 * | - | [68] |
| Switchgrass | Effluent from mesophillic LS-AD | 30 days at 37 °C | 116.9 * | - | [68] |
| Yard wastes | Effluent from mesophillic LS-AD | 30 days at 37 °C | 49.3 * | - | [68] |
| Wheat straw | From mesophilic SS-AD pilot | 273 days at 35 °C | 108.8 | A three-level Box–Behnken plan was applied to the TScontent, particle size, and substrate/inoculum (S/I) ratio | [69] |
| Untreated rice straw | Effluent of a 7000 m3 mesophilic AD | 55 days at 39 °C | 115.9 ± 12.8 * | - | [70] |
| Untreated elmwood | Effluent of a 7000 m3 mesophilic AD | 55 days at 39 °C | 54.2 ± 3.5 * | - | [70] |
| Untreated pinewood | Effluent of a 7000 m3 mesophilic AD | 55 days at 39 °C | 38.7 ± 4.1 * | - | [70] |
| Organosolv-treated rice straw | Effluent of a 7000 m3 mesophilic AD | 55 days at 39 °C | 113.4 ± 1.6 * | Pretreatment with an ethanol/sulfuric acid at 180 °C and 1 h | [70] |
| Organosolv-treated elmwood | Effluent of a 7000 m3 mesophilic AD | 55 days at 39 °C | 93.7 ± 0.9 * | Pretreatment with an ethanol/sulfuric acid at 180 °C and 1 h | [70] |
| Organosolv-treated pinewood | Effluent of a 7000 m3 mesophilic AD | 55 days at 39 °C | 71.4 ± 3.7 * | Pretreatment with an ethanol/sulfuric acid at 180 °C and 1 h | [70] |
| Untreated wood fiber | Derived from a properly operating AD | 30 days at 52 °C | No methane production observed | - | [71] |
| Fungus-treated wood fiber | Derived from a properly operating AD | 30 days at 52 °C | 75.6 ** | Pretreated with P. flavido-alba fungus for 21 days at 30 °C | [71] |
| Untreated Albizia chips | From mesophilic LS-AD | 58 days at 37 °C | 33.9 * | - | [72] |
| Fungal-treated Albizia chips | From mesophilic LS-AD | 58 days at 37 °C | 123.9 * | Pretreated with C. subvermispora fungus for 48 days at 28 °C | [72] |
| Untreated pine wood | Effluent of a 7000 m3 mesophilic AD | 45 days at 37 °C | 34.7 | Ratio feed/inoculum 3/1 | [73] |
| Untreated poplar wood | Effluent of a 7000 m3 mesophilic AD | 45 days at 37 °C | 22.3 | Ratio feed/inoculum 3/1 | [73] |
| Untreated berry wood | Effluent of a 7000 m3 mesophilic AD | 45 days at 37 °C | 80.4 | Ratio feed/inoculum 3/1 | [73] |
| Treated pine wood | Effluent of a 7000 m3 mesophilic AD | 45 days at 37 °C | 25.0 | Pretreatment with COSLIF, ratio feed/inoculum 3/1 | [73] |
| Treated poplar wood | Effluent of a 7000 m3 mesophilic AD | 45 days at 37 °C | 31.1 | Pretreatment with COSLIF, ratio feed/inoculum 3/1 | [73] |
| Treated berry wood | Effluent of a 7000 m3 mesophilic AD | 45 days at 37 °C | 43.9 | Pretreatment with COSLIF, ratio feed/inoculum 3/1 | [73] |
| Untreated poplar waste | From biogas plant | 30 days at 35 °C | 60.8 * | - | [74] |
| Treated poplar waste | From biogas plant | 30 days at 35 °C | 81.1 * | Treated with 3% NaOH by weight | [74] |
| Sorghum vinegar residues | From industrial-scale mesophilic AD | 45 days at 35 °C | 157.9 * | - | [75] |
| Spring-harvested Miscanthus | Effluent from operating mesophilic AD | 60 days at 37 °C | 175 * | Ratio feed/inoculum 2/1 and TS of 20% | [76] |
| Spring-harvested Miscanthus | Effluent from operating mesophilic AD | 60 days at 37 °C | 163 * | Ratio feed/inoculum 4/1 and TS of 20% | [76] |
| Fall-harvested Miscanthus | Effluent from operating mesophilic AD | 60 days at 37 °C | 172 * | Ratio feed/inoculum 2/1 and TS of 20% | [76] |
| Fall-harvested Miscanthus | Effluent from operating mesophilic AD | 60 days at 37 °C | 153 * | Ratio feed/inoculum 4/1 and TS of 20% | [76] |
| Wood Biomass | Co-Digester | Lignocellulosic Biomass/Co-digester Ratio | Source of Added Inoculum | Time and Temperature of Digestion | Cumulative Methane Production (Nm3·t(VS)−1) | Complementary Information | Ref |
|---|---|---|---|---|---|---|---|
| PWa | CM | PW/CoM 2/1 | From biogas plant | 30 days at 35 °C | 93.2 * | PW treated with 3% wt. NaOH | [74] |
| PWa | CM | PW/CoM 1/1 | From biogas plant | 30 days at 35 °C | 98.2 * | PW treated with 3% wt. NaOH | [74] |
| PWa | CM | PW/CoM 1/2 | From biogas plant | 30 days at 35 °C | 71.9 * | PW treated with 3% wt. NaOH | [74] |
| Sorghum vinegar residues | CM | Mixed at a volatile solid (VS) ratio of 1/1 | From industrial-scale mesophilic AD | 45 days at 35 °C | 169.4 * | - | [75] |
| Pine tree sawdust | DM | Not given, sawdust bedding from dairy farm | Not given | 85 days at 37 °C | 64 | Moisture content of 70% | [79] |
| Pine tree sawdust | DM | Not given, sawdust bedding from dairy farm | Not given | 85 days at 37 °C | 73 | Moisture content of 76% | [79] |
| Pine tree sawdust | DM | Not given, sawdust bedding from dairy farm | Not given | 85 days at 37 °C | 90 | Moisture content of 83% | [79] |
| Sawdust | DM | Not given, sawdust bedding from dairy farm | Not given | 45 days at 37 °C | 73.1 | Unmixed | [80] |
| Sawdust | DM | Not given, sawdust bedding from dairy farm | Not given | 45 days at 37 °C | 56.3 | Mixed every 3 days | [80] |
| Sawdust | DM | Not given, sawdust bedding from dairy farm | From batch-type mesophilic anaerobic digester | 72 days at 39 °C | 142.5 | - | [81] |
| Sawdust | CM | Not given, sawdust bedding from beef farm | Not given | 49 days at 37 °C | 136.2 * | No inoculum | [82] |
| Sawdust | CM | Not given, sawdust bedding from beef farm | Not given | 49 days at 37 °C | 143.6 * | S/I 1/1 | [82] |
| Sawdust | CM | Not given, sawdust bedding from beef farm | Not given | 49 days at 37 °C | 140.3 * | S/I 1/2 | [82] |
| Sawdust | CM | Not given, sawdust bedding from beef farm | Not given | 49 days at 37 °C | 159.4 * | S/I 1/4 | [82] |
| Sawdust | CM | Not given, sawdust bedding from beef farm | Not given | 49 days at 37 °C | 158.4 * | S/I 1/50 | [82] |
| EFBOP | CM | EFBOP/CM 2/1 | Dairy farm | 22 days at 37 °C | 94.7 * | - | [83] |
| EFBOP | CM | EFBOP/CM 2/1 | Dairy farm | 22 days at 37 °C | 211.0 * | - | [83] |
| CS | DM | DM/CS/inoculum 34.6/32.2/33.2 | Mesophilic digester | 60 days at 37 °C | 53 * | Corn stover with 0.18–0.42 mm particle size | [84] |
| CS | DM | DM/CS/inoculum 34.6/32.2/33.2 | Mesophilic digester | 60 days at 37 °C | 106 * | Corn stover with 0.42–0.84 mm particle size | [84] |
| RG | CM | RG/CM 50/50 | Experimental farm | 32 days at 37 °C | 200 | Filling in layers, SS-AD in 60 L reactors | [85] |
| RG | CM | RG/CM 40/60 | Experimental farm | 32 days at 37 °C | 186 | Filling in layers, SS-AD in 60 L reactors | [85] |
| RG | CM | RG/CM 25/75 | Experimental farm | 32 days at 37 °C | 170 | Filling in layers, SS-AD in 60 L reactors | [85] |
| Untreated PW | CM | PW/CM 10/4.3 | Not given | 24 days at 37 °C | 83.9 | - | [86] |
| Pretreated PW | CM | PW/CM 10/4.3 | Not given | 24 days at 37 °C | 208 | Pretreated by a combination of acetic acid-hydrothermal and deep-eutectic solvents | [86] |
| ASWs | CM | ASWs/CM 20/80 | Not given | 30 days at 35 °C | 222.7 | S/I = 0.5 gVS/gVS | [87] |
| ASWs | CM | ASWs/CM 30/70 | Not given | 30 days at 35 °C | 261.4 | S/I = 0.5 gVS/gVS | [87] |
| ASWs | CM | ASWs/CM 40/60 | Not given | 30 days at 35 °C | 232.5 | S/I = 0.5 gVS/gVS | [87] |
| ASWs | CM | ASWs/CM 50/50 | Not given | 30 days at 35 °C | 286.6 | S/I = 0.5 gVS/gVS | [87] |
| ASWs | CM | ASWs/CM 60/40 | Not given | 30 days at 35 °C | 297.9 | S/I = 0.5 gVS/gVS | [87] |
| ASWs | CM | ASWs/CM 80/20 | Not given | 30 days at 35 °C | 291.6 | S/I = 0.5 gVS/gVS | [87] |
| Residues | Ratios | Source of Added Inoculum | Time and Temperature of Digestion (°C and Days) | Cumulative Methane Production (m3·t(VS)−1) | Complementary Information | Ref |
|---|---|---|---|---|---|---|
| DM/CS/TR | 33/13/54 | From a mesophilic LS-AD | 45 days at 35 °C | 175 | - | [88] |
| DM/CS/TR | 33/27/40 | From a mesophilic LS-AD | 45 days at 35 °C | 280 | - | [88] |
| DM/CS/TR | 33/40/27 | From a mesophilic LS-AD | 45 days at 35 °C | 312 | - | [88] |
| DM/CS/TR | 33/54/13 | From a mesophilic LS-AD | 45 days at 35 °C | 249 | - | [88] |
| DM/CS/TR | 13/33/54 | From a mesophilic LS-AD | 45 days at 35 °C | 245 | - | [88] |
| DM/CS/TR | 27/33/40 | From a mesophilic LS-AD | 45 days at 35 °C | 310 | - | [88] |
| DM/CS/TR | 40/33/27 | From a mesophilic LS-AD | 45 days at 35 °C | 355 | - | [88] |
| DM/CS/TR | 54/33/13 | From a mesophilic LS-AD | 45 days at 35 °C | 416 | - | [88] |
| DM/CS/TR | 24/16/60 | Digested sludge from a mesophilic LS-AD | 45 days at 35 °C | 129 | - | [89] |
| DM/CS/TR | 36/24/40 | Digested sludge from a mesophilic LS-AD | 45 days at 35 °C | 170 | - | [89] |
| DM/CS/TR | 48/32/20 | Digested sludge from a mesophilic LS-AD | 45 days at 35 °C | 220 | - | [89] |
| CM/RS/SS | - | Digestate from LS-AD plant | 65 days at 37 °C | 115 | TS = 15% | [90] |
| CM/RS/SS | - | Digestate from LS-AD plant | 65 days at 37 °C | 105 | TS = 20% | [90] |
| CM/RS/SS | - | Digestate from LS-AD plant | 65 days at 37 °C | 81 | TS = 25% | [90] |
| CM/RS/SS | - | Digestate from LS-AD plant | 65 days at 37 °C | 86 | TS = 30% | [90] |
| CM/RS/ChM | - | Digestate from LS-AD plant | 65 days at 37 °C | 158 | TS = 15% | [90] |
| CM/RS/ChM | - | Digestate from LS-AD plant | 65 days at 37 °C | 247 | TS = 20% | [90] |
| CM/RS/ChM | - | Digestate from LS-AD plant | 65 days at 37 °C | 119 | TS = 25% | [90] |
| CM/RS/ChM | - | Digestate from LS-AD plant | 65 days at 37 °C | 47 | TS = 30% | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaouefi, Z.; Lecoublet, M.; Taktek, S.; Lafontaine, S.; LeBihan, Y.; Braghiroli, F.L.; Horchani, H.; Koubaa, A. A Review of Operational Conditions of the Agroforestry Residues Biomethanization for Bioenergy Production Through Solid-State Anaerobic Digestion (SS-AD). Energies 2025, 18, 1397. https://doi.org/10.3390/en18061397
Dhaouefi Z, Lecoublet M, Taktek S, Lafontaine S, LeBihan Y, Braghiroli FL, Horchani H, Koubaa A. A Review of Operational Conditions of the Agroforestry Residues Biomethanization for Bioenergy Production Through Solid-State Anaerobic Digestion (SS-AD). Energies. 2025; 18(6):1397. https://doi.org/10.3390/en18061397
Chicago/Turabian StyleDhaouefi, Zaineb, Morgan Lecoublet, Salma Taktek, Simon Lafontaine, Yann LeBihan, Flavia Lega Braghiroli, Habib Horchani, and Ahmed Koubaa. 2025. "A Review of Operational Conditions of the Agroforestry Residues Biomethanization for Bioenergy Production Through Solid-State Anaerobic Digestion (SS-AD)" Energies 18, no. 6: 1397. https://doi.org/10.3390/en18061397
APA StyleDhaouefi, Z., Lecoublet, M., Taktek, S., Lafontaine, S., LeBihan, Y., Braghiroli, F. L., Horchani, H., & Koubaa, A. (2025). A Review of Operational Conditions of the Agroforestry Residues Biomethanization for Bioenergy Production Through Solid-State Anaerobic Digestion (SS-AD). Energies, 18(6), 1397. https://doi.org/10.3390/en18061397












