An Innovative Cryogenic Heat Exchanger Design for Sustainable Aviation
Abstract
1. Introduction
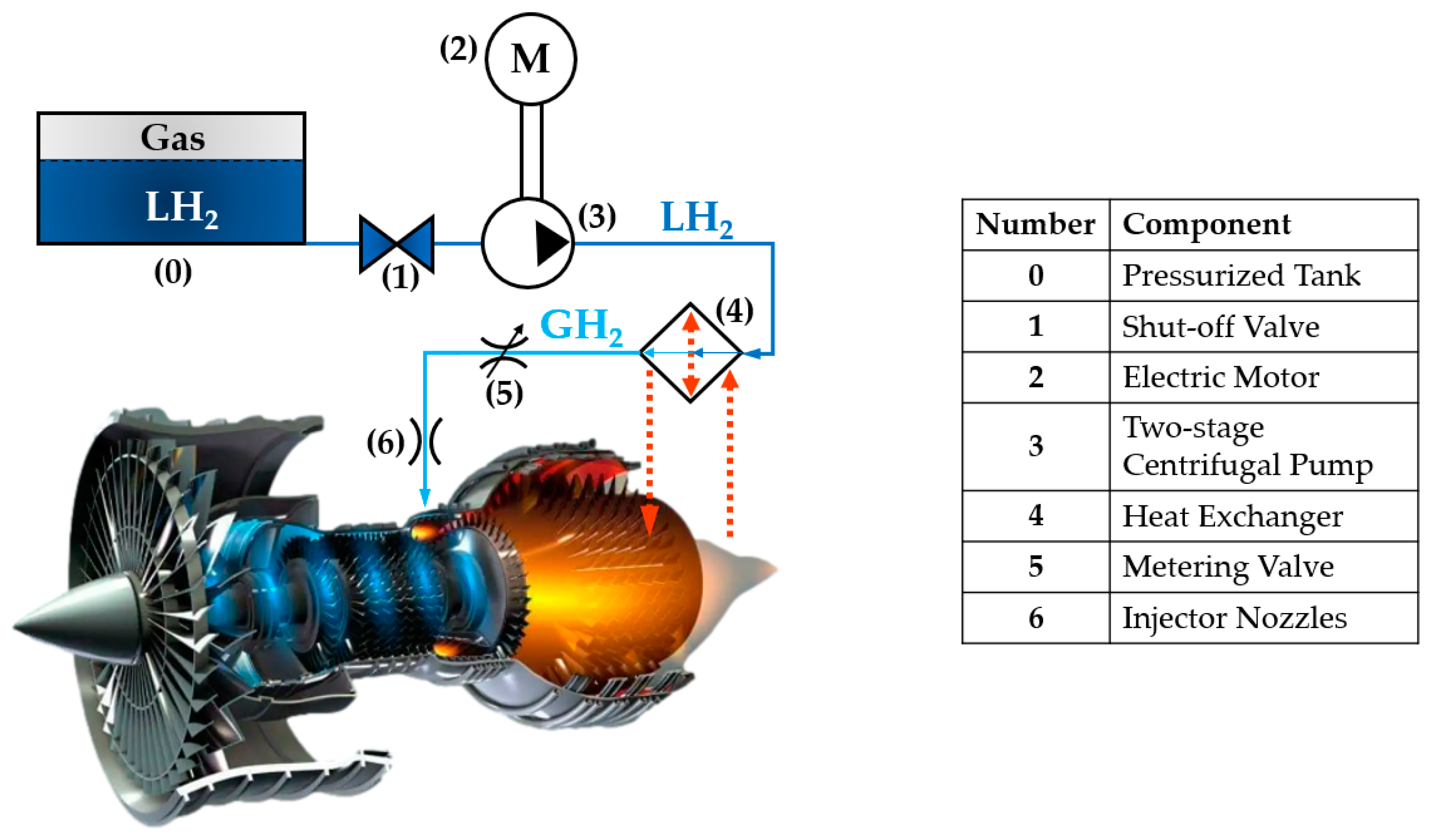
2. Heat Exchanger Layout
- ⮚
- N2 circuit: N2, a safe and inert gas with excellent thermophysical properties, is widely used in high-performance heat exchangers [42,43]. Specifically, N2 is particularly suitable for use with H2 under supercritical conditions, as its critical temperature is moderately higher than that of H2 and, for both fluids, slightly supercritical operative pressures are employed. This aspect makes N2 a suitable carrier fluid, favoured over commonly used alternatives like water, ethylene, and glycol, as their critical temperatures are excessively higher compared to that of H2, thus not allowing a correct heat exchange between the fluids in the exchanger. In this system, N2 operates within a closed-loop circuit as follows:
- It absorbs heat from the turbine’s exhaust gases (EGs), raising its temperature;
- This energy is then transferred to the H2 circuit to heat GH2 to high temperatures;
- After completing the heat exchange, N2 is recirculated and reheated by the EGs, enabling continuous operation.
- ⮚
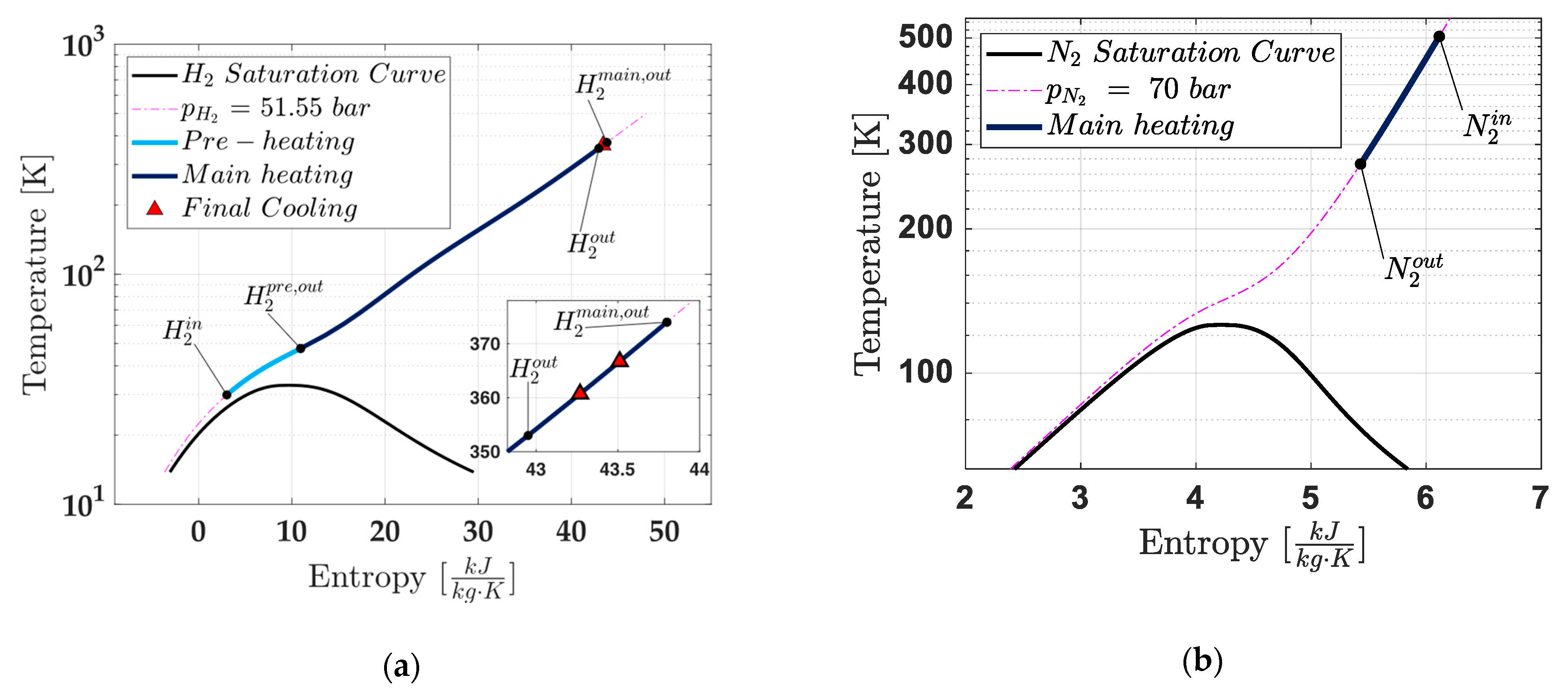
- H2 circuit: This circuit ensures the complete phase change of H2 from liquid form (LH2) to gaseous form (GH2) while achieving the desired thermodynamic conditions for metering. Key processes include the following:
- Pre–Heating: LH2 is initially vaporised using a portion of the heat from high-temperature GH2;
- Main Heating: The resulting GH2 is further heated through energy transfer from the N2 circuit;
- Final Cooling: A portion of the high-temperature GH2’s energy is used to vaporise incoming LH2. After this step, the GH2 exits the heat exchanger at the precise thermodynamic conditions required for metering.
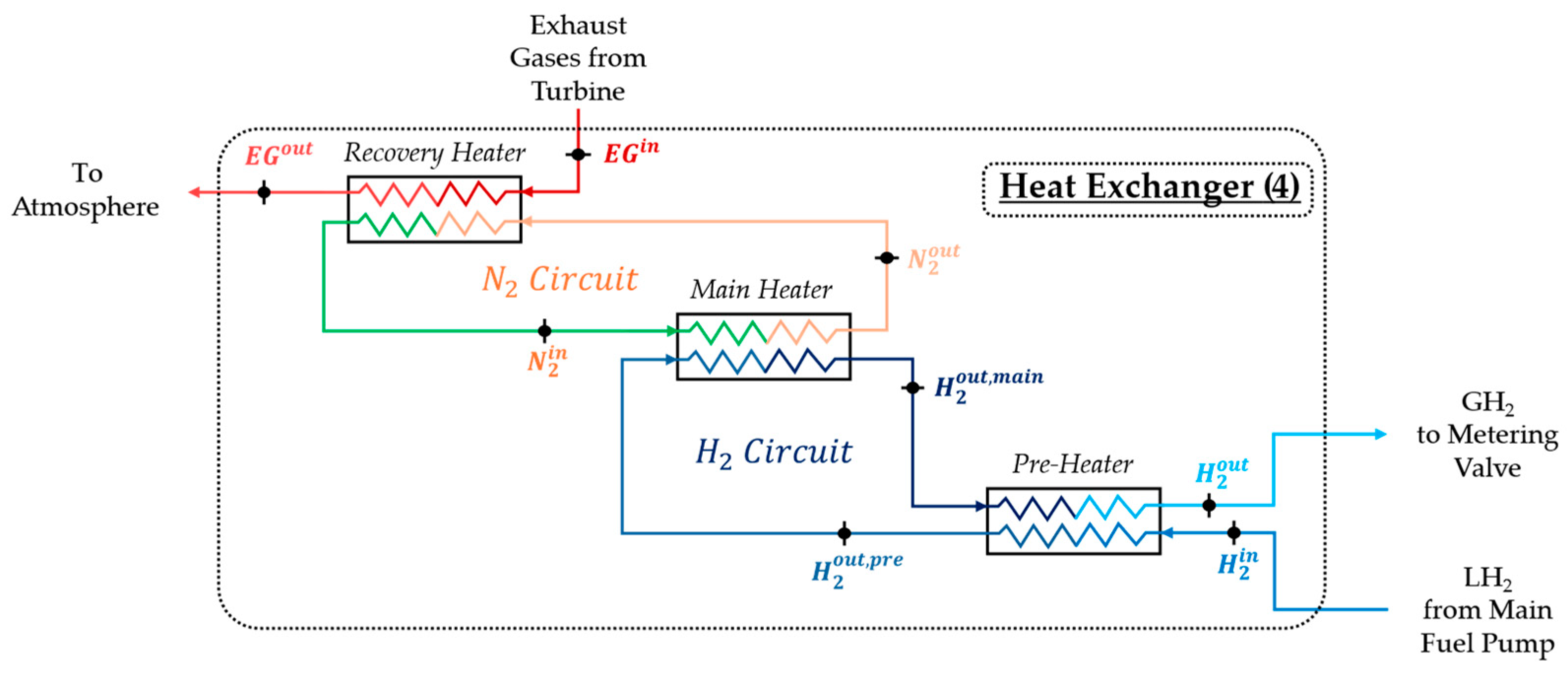
- Recovery Heater: The first subunit, the recovery heater, captures thermal energy from the EGs leaving the turbine (), which retain significant heat. The hot EGs transfer this heat to N2, increasing its temperature (). After the heat exchange, the EGs are discharged into the atmosphere (;
- Main Heater: The high-temperature N2 exiting the recovery heater () flows into the second subunit, called the main heater, where it transfers heat to the H2. This H2, already in gaseous form due to vaporisation by the third subunit (the pre-heater) in the H2 circuit (), receives additional heat from the N2, further increasing its temperature (). Since the N2 circuit is closed, the cooled nitrogen () exits the main heater and is recirculated back to the inlet of the recovery heater for reheating;
- Pre-Heater: LH2 from the main fuel pump enters the heat exchanger ) and flows into the third subunit, the pre-heater. Within this subunit, high-temperature GH2 from the main heater () transfers a part of heat to the incoming LH2, causing its vaporisation. After this process, the GH2 from the main heater exits the pre-heater at a slightly reduced temperature () and is ready to flow toward the metering system under the required thermodynamic conditions.
3. Heat Exchanger Numerical Model
3.1. Calculation of the Temperatures
- and are the enthalpies for N2 at the inlet of the main heater subunit and for H2 at inlet of the heat exchanger, respectively;
- and are the enthalpies for N2 at the outlet of the main heater subunit and for H2 at the outlet of the heat exchanger, respectively;
- is the temperature associated with .
3.2. Calculation of the Heat Transfer Coefficients
- The average densities of the hot and cold fluids (, ;
- The average thermal conductivities of the hot and cold fluids , ;
- The average viscosities of the hot and cold fluids , ;
- The average specific heats of the hot and cold fluids , .
- when
- when , the Gnielinski correlation can be used
- when , the Chilton–Coulburn correlation can be used.
3.3. Calculation of the Performance Parameters
4. Results
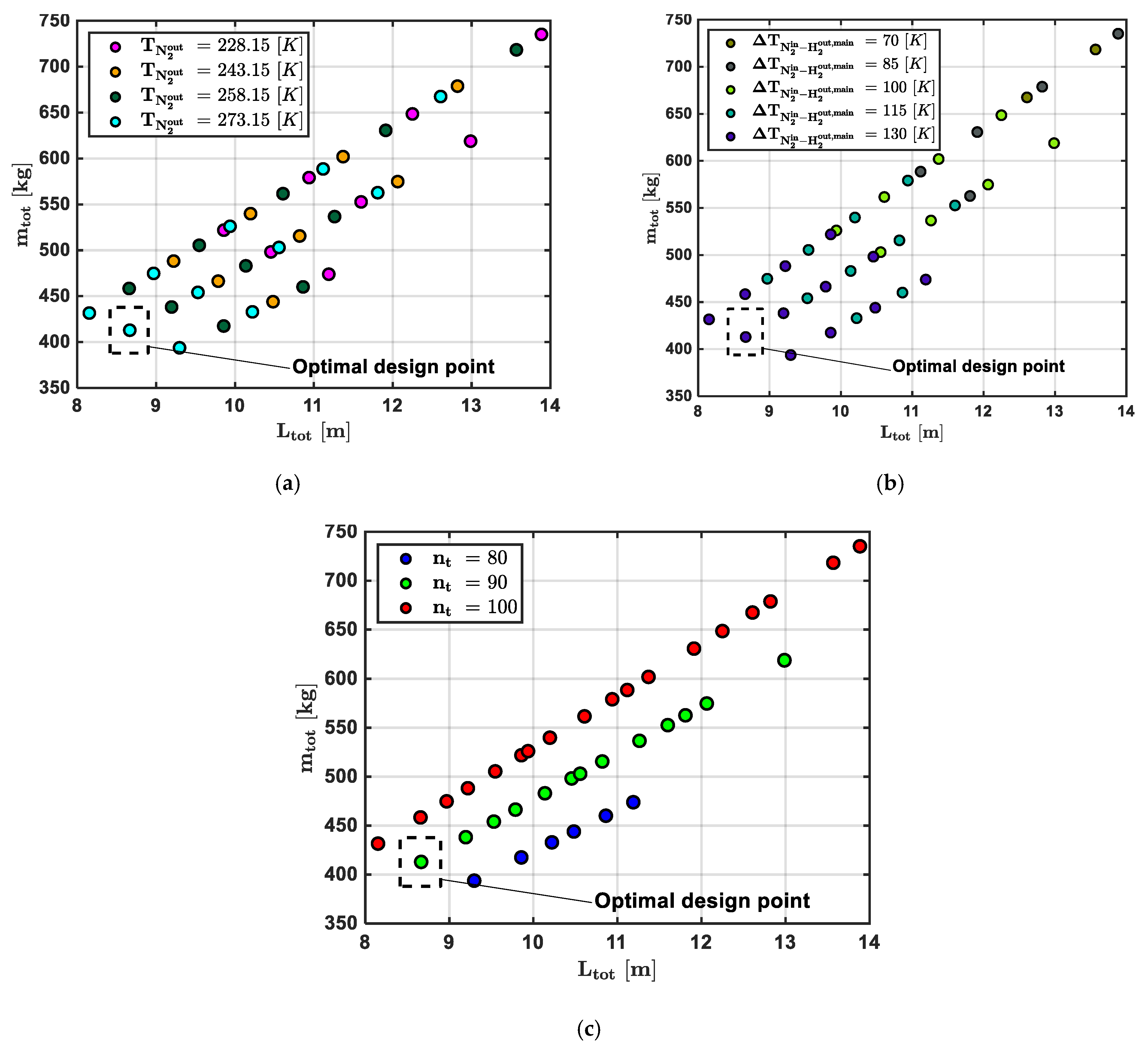
- The N2 inlet temperature to the main heater, , was set to 504 K. This value represents the temperature achieved by N2 after heat exchange with the EGs in the recovery heater;
- The pressure in the N2 circuit was set to 70 bar.
- The outlet N2 temperature from the main heater ().
- The temperature difference between the inlet N2 temperature to the main heater and the outlet H2 temperature from the main heater ().
- The number of pipes in both the pre-heater and main heater subunits ().
- The temperatures and enthalpies of H2 at the outlets of the pre-heater and main heater subunits.
- The required mass flow rate of N2 in the main heater subunit.
- The thermal power is absorbed by LH2 and released by GH2 in the pre-heater subunit.
- The thermal power is absorbed by GH2 and released by N2 in the main heater subunit.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Civil Aviation Organization Industry High Level Group. Aviation Benefits Report 2019. 2019. Available online: https://www.icao.int/sustainability/Documents/AVIATION-BENEFITS-2019-web.pdf (accessed on 30 July 2024).
- Lee, D.S.; Fahey, D.W.; Skowron, A.; Allen, M.R.; Burkhardt, U.; Chen, Q.; Doherty, S.J.; Freeman, S.; Forster, P.M.; Fuglestvedt, J.; et al. The contribution of global aviation to anthropogenic climate forcing for 2000 to 2018. Atmos. Environ. 2021, 244, 117834. [Google Scholar] [CrossRef] [PubMed]
- Undertaking, H.J. Hydrogen Powered Aviation: A Fact-Based Study of Hydrogen Technology, Economics, and Climate Impact by 2050. 2020. Available online: https://www.h2knowledgecentre.com/content/researchpaper1126 (accessed on 20 January 2025).
- Oesingmann, K.; Grimme, W.; Scheelhaase, J. Hydrogen in aviation: A simulation of demand, price dynamics, and CO2 emission reduction potentials. Int. J. Hydrogen Energy 2024, 64, 633–642. [Google Scholar] [CrossRef]
- Gössling, S.; Humpe, A. The global scale, distribution and growth of aviation: Implications for climate change. Glob. Environ. Change 2020, 65, 102194. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Fahey, D.W.; Forster, P.M.; Newton, P.J.; Wit, R.C.; Lim, L.L.; Owen, B.; Sausen, R. Aviation and global climate change in the 21st century. Atmos. Environ. 2009, 43, 3520–3537. [Google Scholar] [CrossRef] [PubMed]
- Valdes, R.M.A.; Burmaoglu, S.; Tucci, V.; da Costa Campos, L.M.B.; Mattera, L.; Comendador, V.F.G. Flight path 2050 and ACARE goals for maintaining and extending industrial leadership in aviation: A map of the aviation technology space. Sustainability 2019, 11, 2065. [Google Scholar] [CrossRef]
- Srinath, A.N.; Pena López, Á.; Miran Fashandi, S.A.; Lechat, S.; di Legge, G.; Nabavi, S.A.; Nikolaidis, T.; Jafari, S. Thermal management system architecture for hydrogen-powered propulsion technologies: Practices, thematic clusters, system architectures, future challenges, and opportunities. Energies 2022, 15, 304. [Google Scholar] [CrossRef]
- Kadyk, T.; Schenkendorf, R.; Hawner, S.; Yildiz, B.; Römer, U. Design of fuel cell systems for aviation: Representative mission profiles and sensitivity analyses. Front. Energy Res. 2019, 7, 440189. [Google Scholar] [CrossRef]
- Rutherford, D.; Zeinali, M. Efficiency Trends for New Commercial Jet Aircraft 1960–2008. 2009. Available online: https://theicct.org/wp-content/uploads/2021/06/ICCT_Aircraft_Efficiency_final.pdf (accessed on 20 January 2025).
- Thapa, N.; Ram, S.; Kumar, S.; Mehta, J. All electric aircraft: A reality on its way. Mater. Today Proc. 2021, 43, 175–182. [Google Scholar] [CrossRef]
- Meissner, R.; Sieb, P.; Wollenhaupt, E.; Haberkorn, S.; Wicke, K.; Wende, G. Towards climate-neutral aviation: Assessment of maintenance requirements for airborne hydrogen storage and distribution systems. Int. J. Hydrogen Energy 2023, 48, 29367–29390. [Google Scholar] [CrossRef]
- Abrantes, I.; Ferreira, A.F.; Silva, A.; Costa, M. Sustainable aviation fuels and imminent technologies-CO2 emissions evolution towards 2050. J. Clean. Prod. 2021, 313, 127937. [Google Scholar] [CrossRef]
- Adler, E.J.; Martins, J.R.R.A. Hydrogen-powered aircraft: Fundamental concepts, key technologies, and environmental impacts. Prog. Aerosp. Sci. 2023, 141, 100922. [Google Scholar] [CrossRef]
- Svensson, F.; Hasselrot, A.; Moldanova, J. Reduced environmental impact by lowered cruise altitude for liquid hydrogen-fuelled aircraft. Aerosp. Sci. Technol. 2004, 8, 307–320. [Google Scholar] [CrossRef]
- Timmons, D.; Terwel, R. Economics of aviation fuel decarbonization: A preliminary assessment. J. Clean. Prod. 2022, 369, 133097. [Google Scholar] [CrossRef]
- Yusaf, T.; Mahamude, A.S.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Dhahad, H.A.; Talib, A.R. Sustainable hydrogen energy in aviation—A narrative review. Int. J. Hydrogen Energy 2023, 52, 1026–1045. [Google Scholar] [CrossRef]
- Donateo, T.; Bonatesta, A.G.; Ficarella, A.; Lecce, L. Energy Consumption and Saved Emissions of a Hydrogen Power System for Ultralight Aviation: A Case Study. Energies 2024, 17, 3272. [Google Scholar] [CrossRef]
- Smith, T.D. Hydrogen-Powered Flight. 2005. Available online: https://ntrs.nasa.gov/citations/20050196728 (accessed on 20 January 2025).
- Rogers, H.L.; Lee, D.S.; Raper, D.W.; de F. Foster, P.M.; Wilson, C.W.; Newton, P.J. The impacts of aviation on the atmosphere. Aeronaut. J. 2002, 106, 521–546. [Google Scholar] [CrossRef]
- Haglind, F.; Hasselrot, A.; Singh, R. Potential of reducing the environmental impact of aviation by using hydrogen Part I: Background, prospects and challenges. Aeronaut. J. 2006, 110, 533–540. [Google Scholar] [CrossRef]
- Koroneos, C.J.; Moussiopoulos, N. Cryoplane–Hydrogen vs. kerosene as aircraft fuel. In Proceedings of the 7th International Conference on Environmental Science and Technology, Syros, Greece, 3–6 September 2001; pp. 484–493. [Google Scholar]
- Bock, L.; Burkhardt, U. Contrail cirrus radiative forcing for future air traffic. Atmos. Chem. Phys. 2019, 19, 8163–8174. [Google Scholar] [CrossRef]
- Winnefeld, C.; Kadyk, T.; Bensmann, B.; Krewer, U.; Hanke-Rauschenbach, R. Modelling and designing cryogenic hydrogen tanks for future aircraft applications. Energies 2018, 11, 105. [Google Scholar] [CrossRef]
- Huber, M.L.; Lemmon, E.W.; Bruno, T.J. Surrogate mixture models for the thermophysical properties of aviation fuel Jet-A. Energy Fuels 2010, 24, 3565–3571. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takahashi, K.; Ohmori, T. Thermal conductivity of polyurethane foam at liquid hydrogen temperature region. In Advances in Cryogenic Engineering: Part A; Springer: Boston, MA, USA, 1996; pp. 117–122. [Google Scholar]
- Buttner, W.J.; Ciotti, M.; Hartmann, K.; Schmidt, K.; Wright, H.; Weidner, E. Empirical profiling of cold hydrogen plumes formed from venting of LH2 storage vessels. Int. J. Hydrogen Energy 2021, 46, 32723–32734. [Google Scholar] [CrossRef]
- Coutinho, M.; Bento, D.; Souza, A.; Cruz, R.; Afonso, F.; Lau, F.; Suleman, A.; Barbosa, F.R.; Gandolfi, R.; Junior, W.A.; et al. A review on the recent developments in thermal management systems for hybrid-electric aircraft. Appl. Therm. Eng. 2023, 227, 120427. [Google Scholar] [CrossRef]
- Patrao, A.C.; Jonsson, I.; Xisto, C.; Lundbladh, A.; Lejon, M.; Grönstedt, T. The heat transfer potential of compressor vanes on a hydrogen fueled turbofan engine. Appl. Therm. Eng. 2024, 236, 121722. [Google Scholar] [CrossRef]
- Brewer, G.D. Hydrogen Aircraft Technology; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar] [CrossRef]
- ZEROe|Airbus. Available online: https://www.airbus.com/en/innovation/energy-transition/hydrogen/zeroe (accessed on 28 February 2025).
- Plummer, A.; Adeyemi, D.; Sell, N.; Sciatti, F.; Tamburrano, P.; Amirante, R. Fuel System Control for Hydrogen-Powered Aircraft. In Proceedings of the 2024 UKACC 14th International Conference on Control (CONTROL), Winchester, UK, 10–12 April 2024; pp. 201–202. [Google Scholar] [CrossRef]
- Sciatti, F.; Di Domenico, V.; Zagaria, L.; Adeyemi, D.; Tamburrano, P.; Plummer, A.R.; Sell, N.; Distaso, E.; Amirante, R. Preliminary Design and Modelling of a Hydrogen-Powered Aircraft Fuel System. In Fluid Power Systems Technology; American Society of Mechanical Engineers: New York, NY, USA, 2024; p. V001T01A028. [Google Scholar]
- Sciatti, F.; Tamburrano, P.; Distaso, E.; Amirante, R. Modelling of the Entire Aircraft Fuel System Through Simulink for Accurate Performance Evaluation. In Fluid Power Systems Technology; American Society of Mechanical Engineers: New York, NY, USA, 2023; p. V001T01A050. [Google Scholar]
- Sciatti, F.; Tamburrano, P.; Distaso, E.; Amirante, R. Digital Hydraulic Technology: Applications, Challenges, and Future Direction. J. Phys. Conf. Ser. 2023, 2648, 012053. [Google Scholar]
- Tamburrano, P.; Sciatti, F.; Distaso, E.; Amirante, R. Comprehensive Numerical Analysis of a Four-Way Two-Position (4/2) High-Frequency Switching Digital Hydraulic Valve Driven by a Ring Stack Actuator. Energies 2023, 16, 7355. [Google Scholar] [CrossRef]
- Tamburrano, P.; De Palma, P.; Plummer, A.R.; Distaso, E.; Sciatti, F.; Amirante, R. Simulation of a high frequency on/off valve actuated by a piezo-ring stack for digital hydraulics. E3S Web Conf. 2021, 312, 05008. [Google Scholar]
- Łapka, P.; Seredyński, M.; Ćwik, A. Preliminary study on supercritical hydrogen and bleed air heat exchanger for aircraft application. Proc. Inst. Mech. Eng. G J. Aerosp. Eng. 2018, 232, 2231–2243. [Google Scholar] [CrossRef]
- Vietze, M.; Evrim, C. Development of additively manufactured cryogenic heat exchangers for hydrogen-electric aircraft propulsion. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2024; p. 012007. [Google Scholar]
- Patrao, A.C.; Jonsson, I.; Xisto, C.; Lundbladh, A.; Grönstedt, T. Compact heat exchangers for hydrogen-fueled aero engine intercooling and recuperation. Appl. Therm. Eng. 2024, 243, 122538. [Google Scholar] [CrossRef]
- Di Domenico, V.; Tamburrano, P.; Sciatti, F.; Distaso, E.; Foglia, M.M.; Amirante, R. A Novel Hydrogen-Nitrogen Heat Exchanger For Aeronautical Applications. J. Phys. Conf. Ser. 2024, 2893, 012082. [Google Scholar]
- Olumayegun, O.; Wang, M.; Kelsall, G. Thermodynamic analysis and preliminary design of closed Brayton cycle using nitrogen as working fluid and coupled to small modular Sodium-cooled fast reactor (SM-SFR). Appl. Energy 2017, 191, 436–453. [Google Scholar] [CrossRef]
- Zhang, X.; Tiwari, R.; Shooshtari, A.H.; Ohadi, M.M. An additively manufactured metallic manifold-microchannel heat exchanger for high temperature applications. Appl. Therm. Eng. 2018, 143, 899–908. [Google Scholar] [CrossRef]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef]
- Riaz, A.; Qyyum, M.A.; Hussain, A.; Lee, M. Significance of ortho-para hydrogen conversion in the performance of hydrogen liquefaction process. Int. J. Hydrogen Energy 2023, 48, 26568–26582. [Google Scholar] [CrossRef]
- Teng, J.; Wang, K.; Zhu, S.; Bao, S.; Zhi, X.; Zhang, X.; Qiu, L. Comparative study on thermodynamic performance of hydrogen liquefaction processes with various ortho-para hydrogen conversion methods. Energy 2023, 271, 127016. [Google Scholar] [CrossRef]
- Yin, L.; Ju, Y. Review on the design and optimization of hydrogen liquefaction processes. Front. Energy 2020, 14, 530–544. [Google Scholar] [CrossRef]
- Çengel, Y.A. Introduction to Thermodynamics and Heat Transfer; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Taler, D.; Taler, J. Simple heat transfer correlations for turbulent tube flow. E3S Web Conf. 2017, 13, 02008. [Google Scholar] [CrossRef]
- Brewer, G.D. Hydrogen Aircraft Technology; Routledge: New York, NY, USA, 2017. [Google Scholar]
- Liu, J.; Zhao, M.; Rong, L. Overview of hydrogen-resistant alloys for high-pressure hydrogen environment: On the hydrogen energy structural materials. Clean Energy 2023, 7, 99–115. [Google Scholar] [CrossRef]
- Marchi, C.S.; Somerday, B.P. Comparison of Stainless Steels for High-Pressure Hydrogen Service. In Pressure Vessels and Piping Division (Publication) PVP; American Society of Mechanical Engineers: New York, NY, USA, 2014; Volume 6B. [Google Scholar] [CrossRef]
- Available online: https://engineering.esteco.com/modefrontier/ (accessed on 10 April 2024).





| Property [Unit] | Jet A-1 | LH2 | GH2 (350 [bar]) | GH2 (700 [bar]) |
|---|---|---|---|---|
| Specific energy [MJ/kg] | 43.2 | 120 | 120 | 120 |
| Energy density [MJ/L] | 34.9 | 8.5 | 2.9 | 4.8 |
| Storage temperature [K] | Ambient | 20 | Ambient | Ambient |
| Storage pressure [bar] | Ambient | ~2 | 350 | 700 |
| Storage density [kg/m3] | 804 | 71.4 | 23.3 | 39.2 |
| Cold Fluid (c) | Hot Fluid (h) | |
|---|---|---|
| Pre-heater | ||
| Main heater |
| Parameter | Symbol | Take-Off Value [Unit] | |
|---|---|---|---|
| Circuit | Mass flow rate | 0.411 [kg/s] | |
| LH2 inlet heat exchanger temperature (from main fuel pump) | 29.9 [K] | ||
| GH2 outlet heat exchanger temperature (to metering valve) | 353 [K] | ||
| Pressure | 51.55 [bar] |
| Parameter | Symbol | Value [Unit] | |
|---|---|---|---|
| Circuit | N2 inlet main heater temperature | 504 [K] | |
| Pressure | 70 [bar] |
| Propriety | Symbol | Value [Unit] | |
|---|---|---|---|
| Stainless Steel 316 | Density | 8060 [kg/m3] | |
| Tensile Strength, Yield | 200 [MPa] | ||
| Tensile Strength, Max | 550 [MPa] | ||
| Young Modulus | 193 [GPa] | ||
| Specific Heat Capacity | 500 [J/(kg∙K)] | ||
| Thermal Conductivity | 16 [W/(m∙K)] |
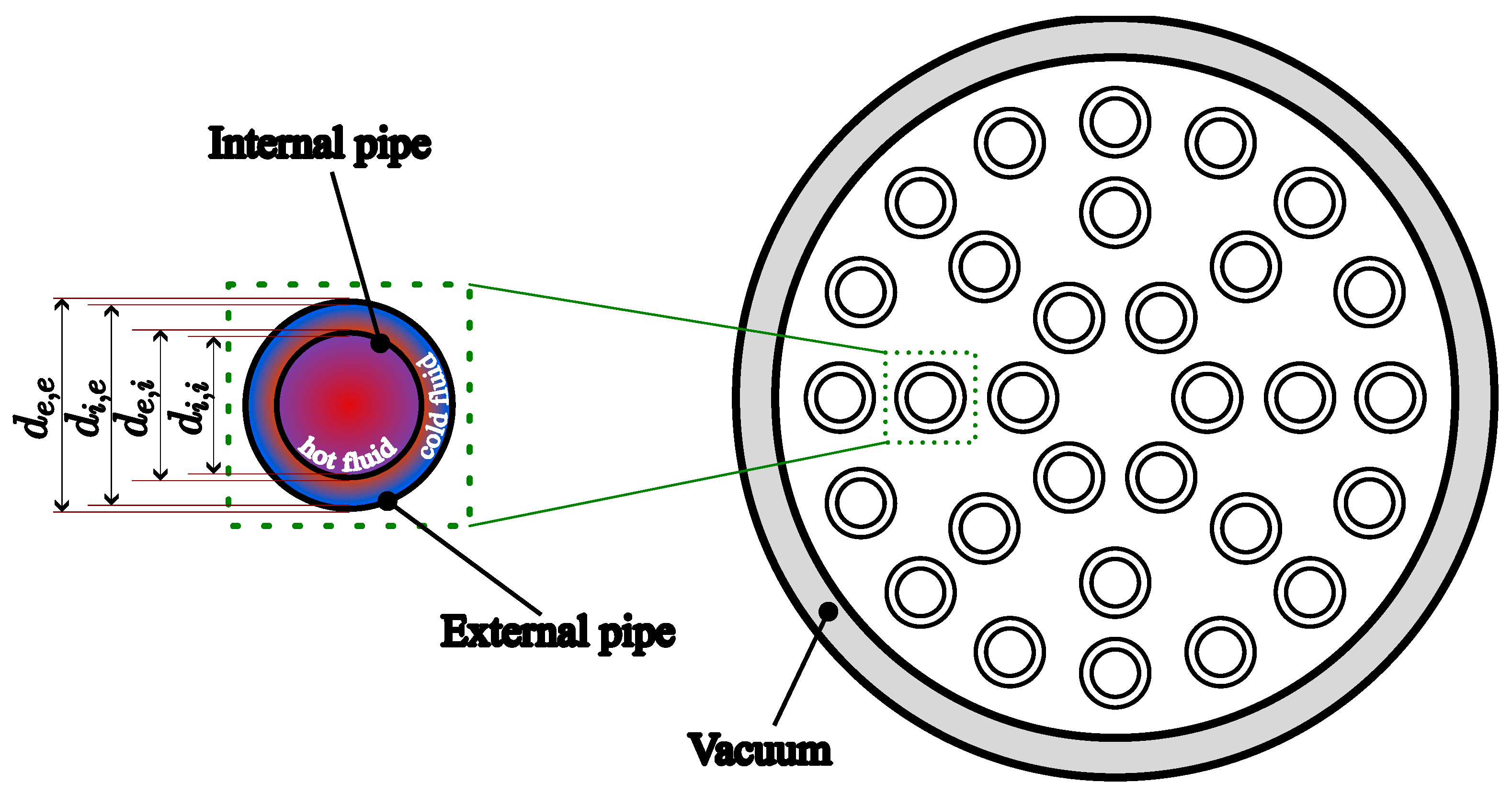
| Internal Tube | External Tube | ||||
|---|---|---|---|---|---|
| Parameter | Symbol | Value [Unit] | Symbol | Value [Unit] | |
| Geometry of Pipes (both Subunits) | Internal Diameter | 20 [mm] | 25 [mm] | ||
| External Diameter | 20.8 [mm] | 26 [mm] | |||
| Roughness | 0.2 [mm] | 0.2 [mm] | |||
| Safety factor for Mariotte’s law | 1.5 [-] | 1.5 [-] | |||
| Calculated Minimum Thickness | 0.22 [mm] | 0.48 [mm] | |||
| Actual Thickness | 0.8 [mm] | 1 [mm] | |||
| Parameter | Symbol | Range Values [Unit] | Step Size [Unit] | Number of Points | |
|---|---|---|---|---|---|
| Input Thermodynamic Variables | N2 outlet main heater temperature | (228.15–273.15) [K] | 15 [K] | 4 | |
| Temperature difference between N2 entering and H2 exiting the main heater | (70–130) [K] | 15 [K] | 5 | ||
| Input Geometric Variables | Number of pipes (both subunits) | (80–100) [-] | 10 [-] | 3 |
| Propriety | Symbol | Objective [Unit] | |
|---|---|---|---|
| Constraints | Pressure drops across pipes (both subunits) | [bar] |
| Parameter | Symbol | Design Point Value [Unit] | |
|---|---|---|---|
| Input Thermodynamic Variables | N2 outlet main heater temperature | 273.15 [K] | |
| Temperature difference between N2 entering and H2 exiting the main heater | 130 [K] | ||
| Input Geometric Variables | Number of pipes (both subunits) | 90 [-] |
| Parameter | Symbol | Value [Unit] | |
|---|---|---|---|
| H2 circuit | LH2 inlet enthalpy (from main fuel pump) | 148.7 [kJ/kg] | |
| GH2 outlet enthalpy (to metering valve) | 5264 [kJ/kg] | ||
| N2 circuit | N2 inlet enthalpy (to main heater) | 522.5 [kJ/kg] | |
| N2 outlet enthalpy (from main heater) | 265.8 [kJ/kg] |
| Parameter | Symbol | Value [Unit] | |
|---|---|---|---|
| H2 circuit | Vaporised H2 outlet temperature (from pre-heater) | 47.6 [K] | |
| Vaporised H2 outlet enthalpy (from pre-heater) | 457.14 [kJ/kg] | ||
| Thermal Power absorbed by LH2 (in the pre-heater) | 126.75 [kW] | ||
| GH2 outlet temperature (from main heater) | 374 [K] | ||
| GH2 outlet enthalpy (from main heater) | 5572.5 [kJ/kg] | ||
| Thermal Power absorbed by GH2 (in the main heater) | 2102.4 [kW] | ||
| Thermal Power released by GH2 (in the pre-heater) | −126.75 [kW] | ||
| N2 circuit | N2 mass flow rate (in the main heater) | 8.19 [kg/s] | |
| Thermal Power released by N2 (in the main heater) | −2102.4 [kW] |
| Parameter | Symbol | Value [Unit] | |
|---|---|---|---|
| Pre-heater | Length | 0.141 [m] | |
| Mass | 6.711 [kg] | ||
| Volume | 9.25 [m3] | ||
| Pressure drops hot fluid (GH2) | 5.74 [bar] | ||
| Pressure drops cold fluid (LH2) | 1.02 [bar] | ||
| Main heater | Length | 8.525 [m] | |
| Mass | 406.2 [kJ/kg] | ||
| Volume | 5.60 [m3] | ||
| Pressure drops hot fluid (N2) | 0.064 [bar] | ||
| Pressure drops cold fluid (GH2) | 0.059 [bar] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciatti, F.; Di Domenico, V.; Tamburrano, P.; Distaso, E.; Amirante, R. An Innovative Cryogenic Heat Exchanger Design for Sustainable Aviation. Energies 2025, 18, 1261. https://doi.org/10.3390/en18051261
Sciatti F, Di Domenico V, Tamburrano P, Distaso E, Amirante R. An Innovative Cryogenic Heat Exchanger Design for Sustainable Aviation. Energies. 2025; 18(5):1261. https://doi.org/10.3390/en18051261
Chicago/Turabian StyleSciatti, Francesco, Vincenzo Di Domenico, Paolo Tamburrano, Elia Distaso, and Riccardo Amirante. 2025. "An Innovative Cryogenic Heat Exchanger Design for Sustainable Aviation" Energies 18, no. 5: 1261. https://doi.org/10.3390/en18051261
APA StyleSciatti, F., Di Domenico, V., Tamburrano, P., Distaso, E., & Amirante, R. (2025). An Innovative Cryogenic Heat Exchanger Design for Sustainable Aviation. Energies, 18(5), 1261. https://doi.org/10.3390/en18051261