Absorption and Desorption Heat of Carbon Dioxide Capture Based on 2-Amino-2-Methyl-1-Propanol
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Apparatus
2.3. Experimental Conditions and Procedures
2.4. Experimental Principles and Methods
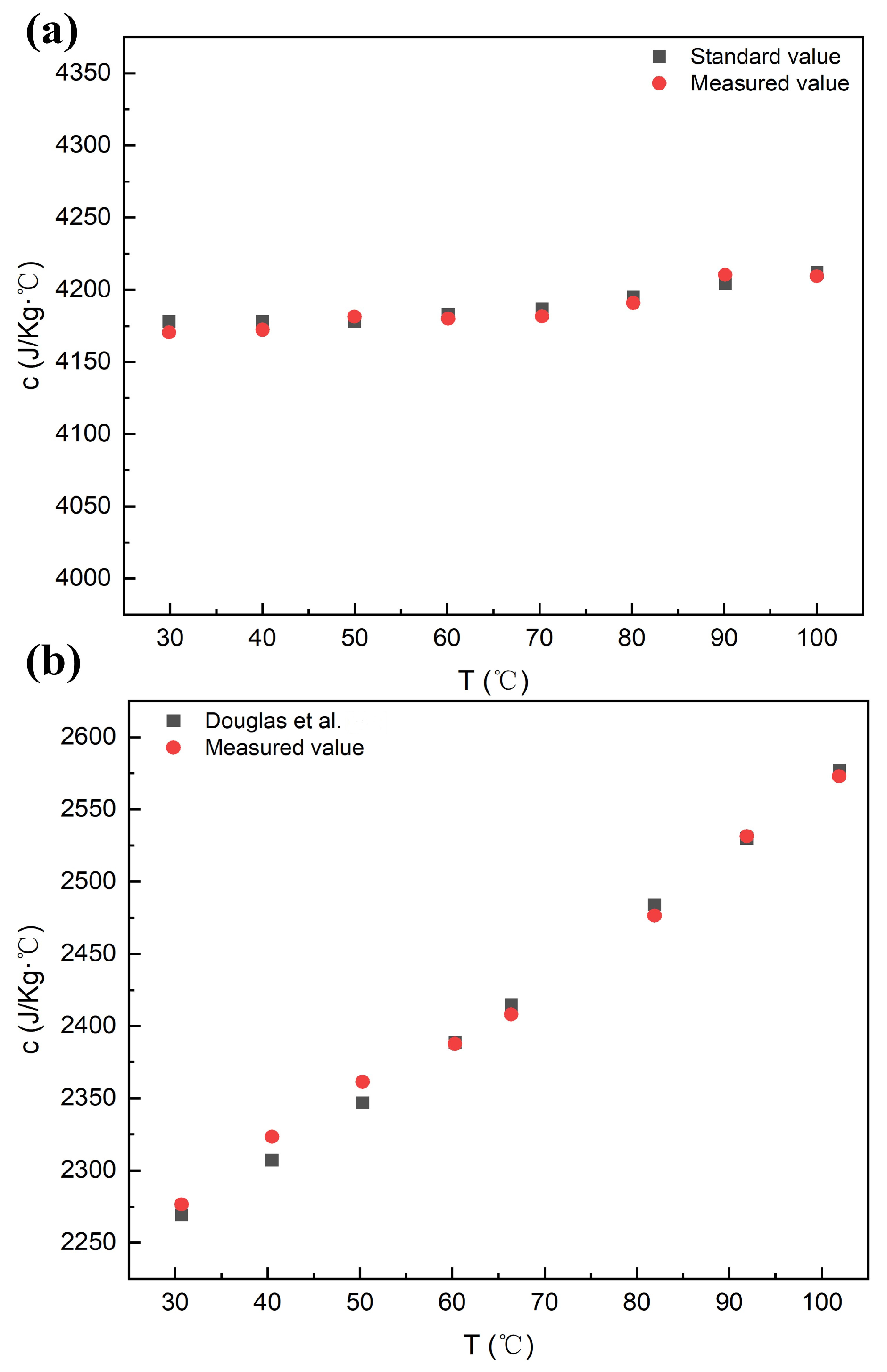
2.5. Reliability Verification of the Experimental System
3. Results and Discussion
3.1. Single-Component Amine
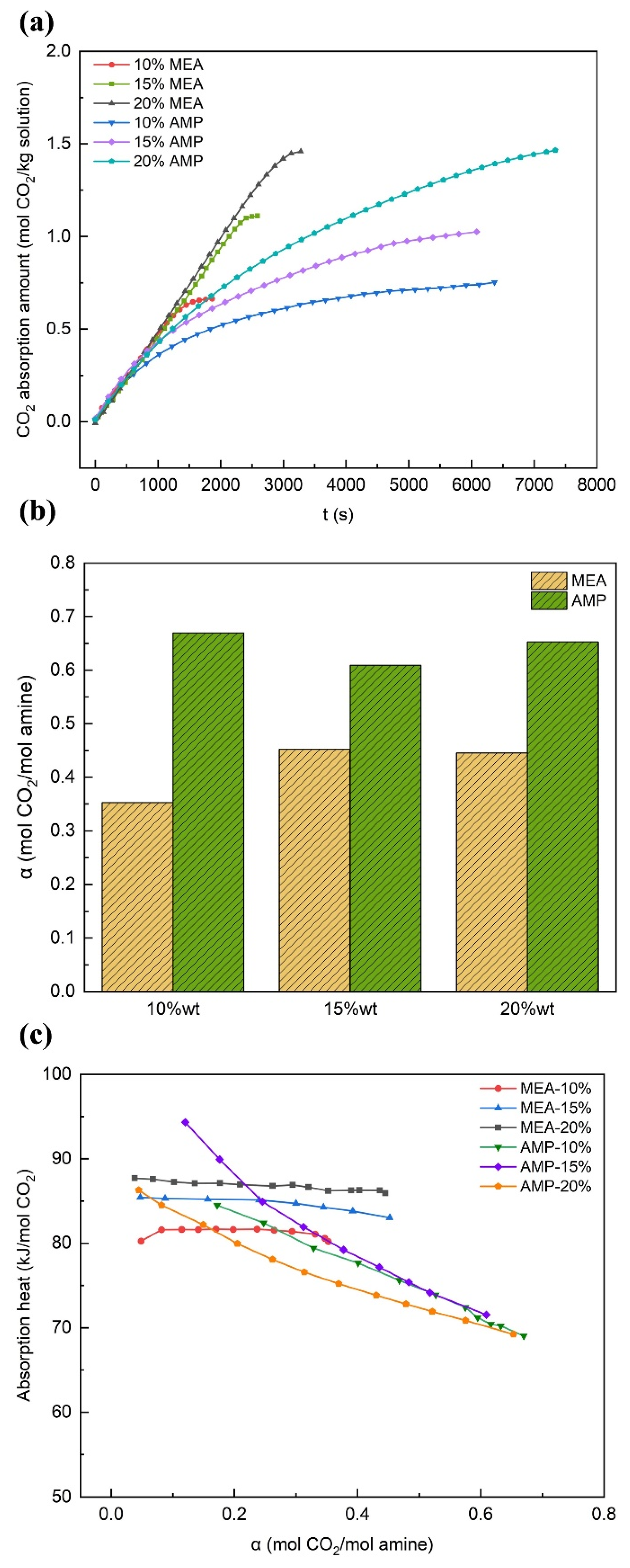
3.1.1. The Effect of Concentration
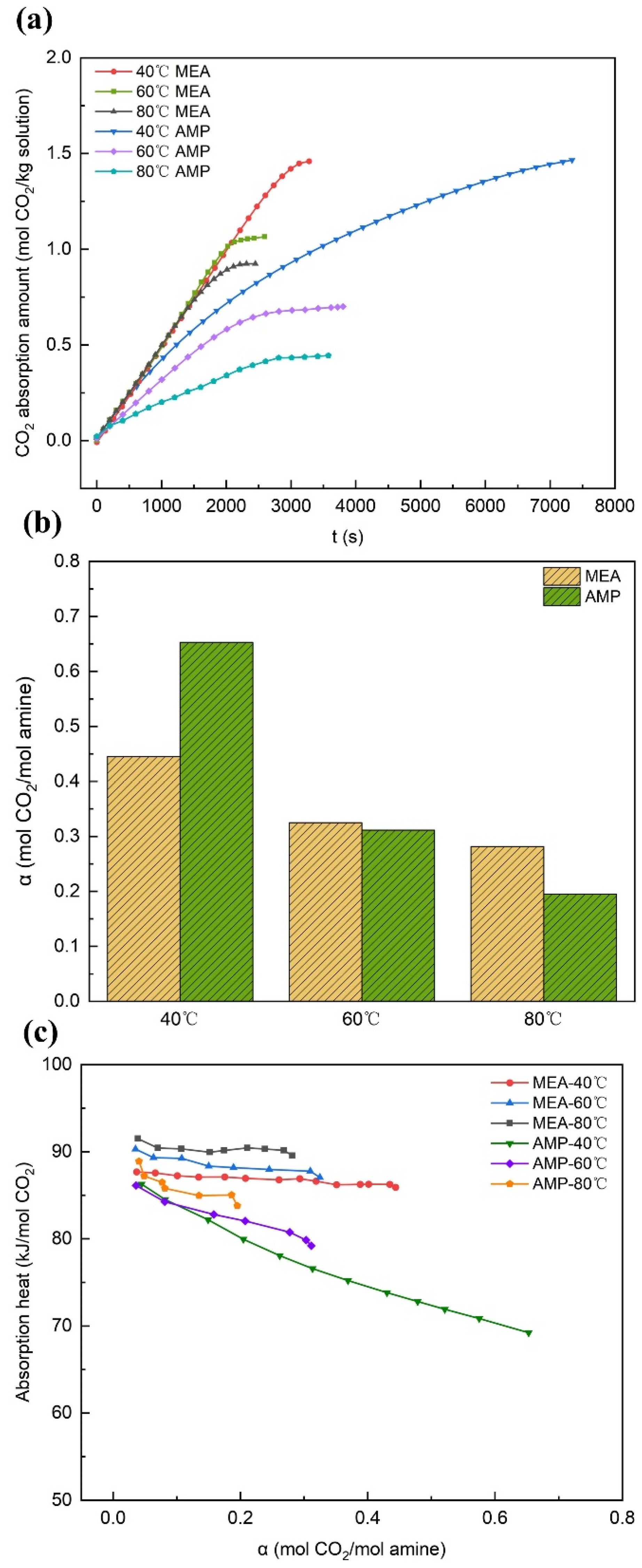
3.1.2. The Effect of Temperature
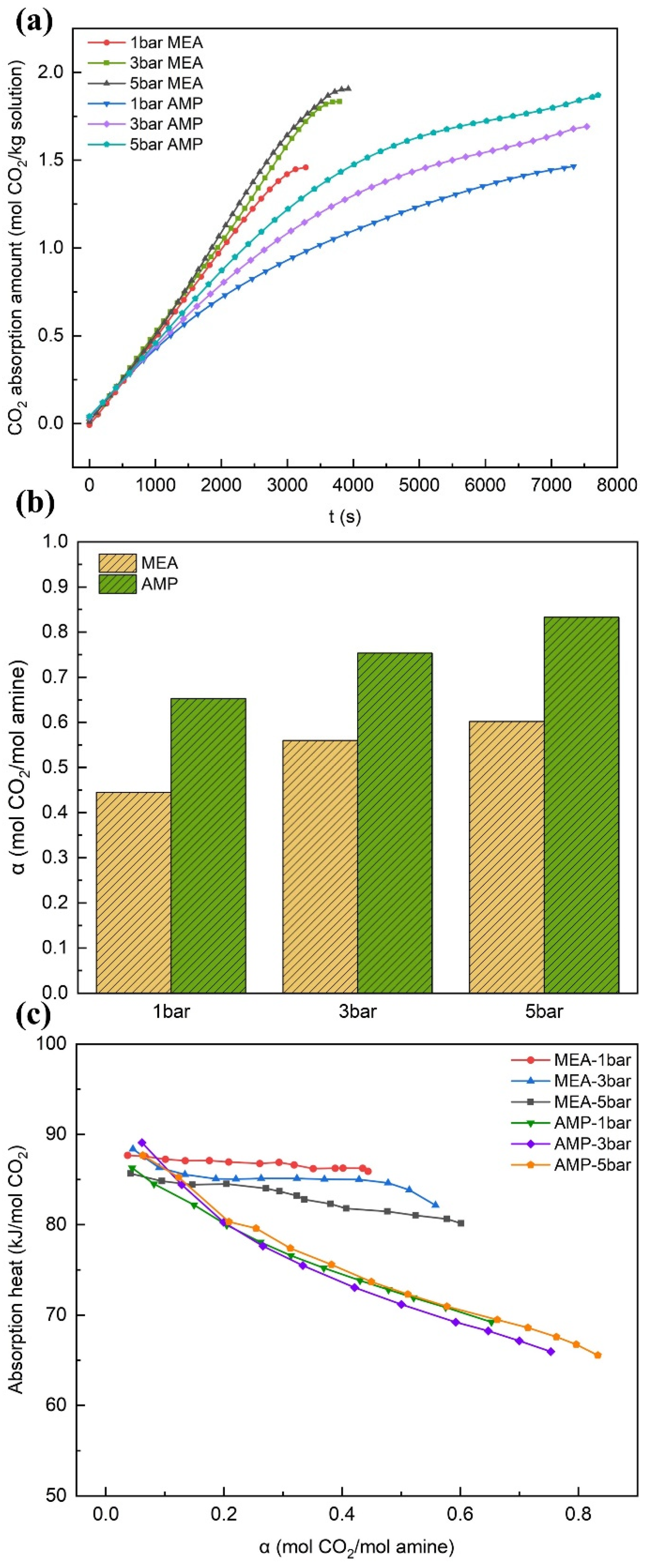
3.1.3. The Effect of Pressure
3.2. Binary Blended Amines with AMP as the Main Absorbent
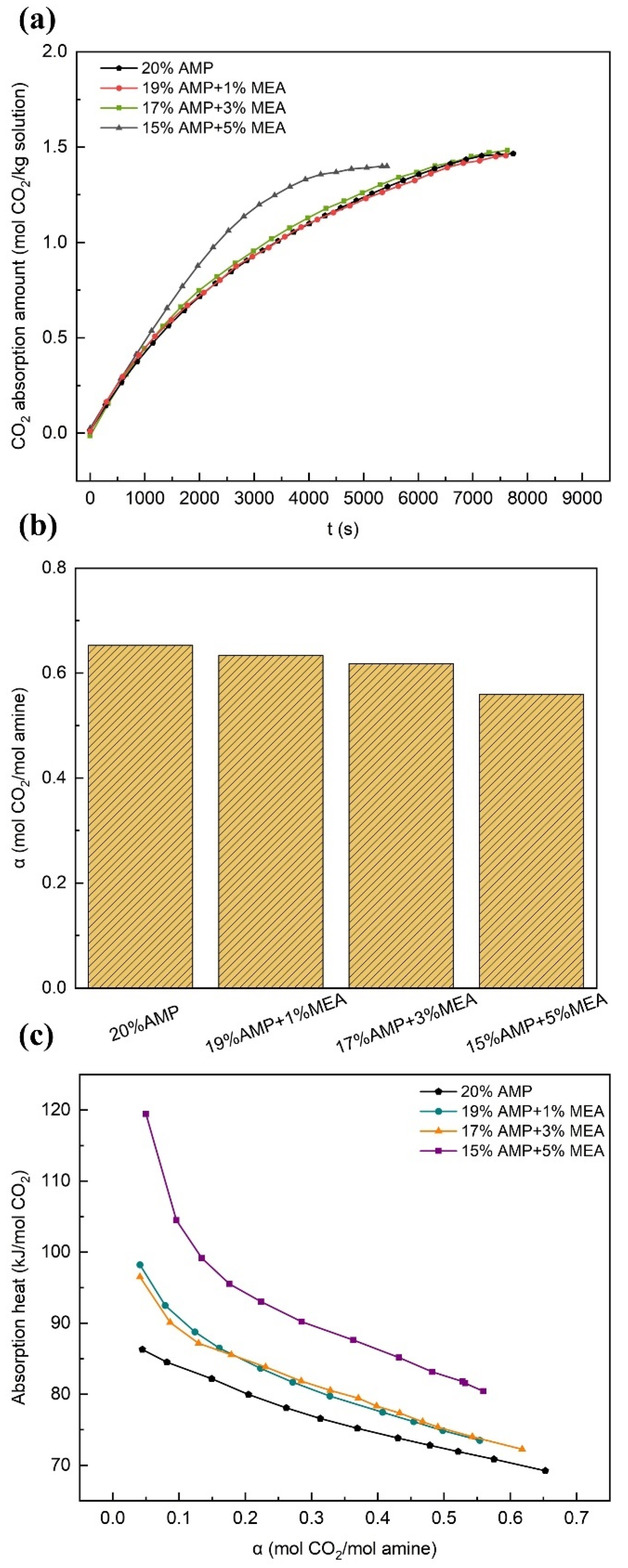
3.2.1. Effects of MEA
3.2.2. Effects of DGA
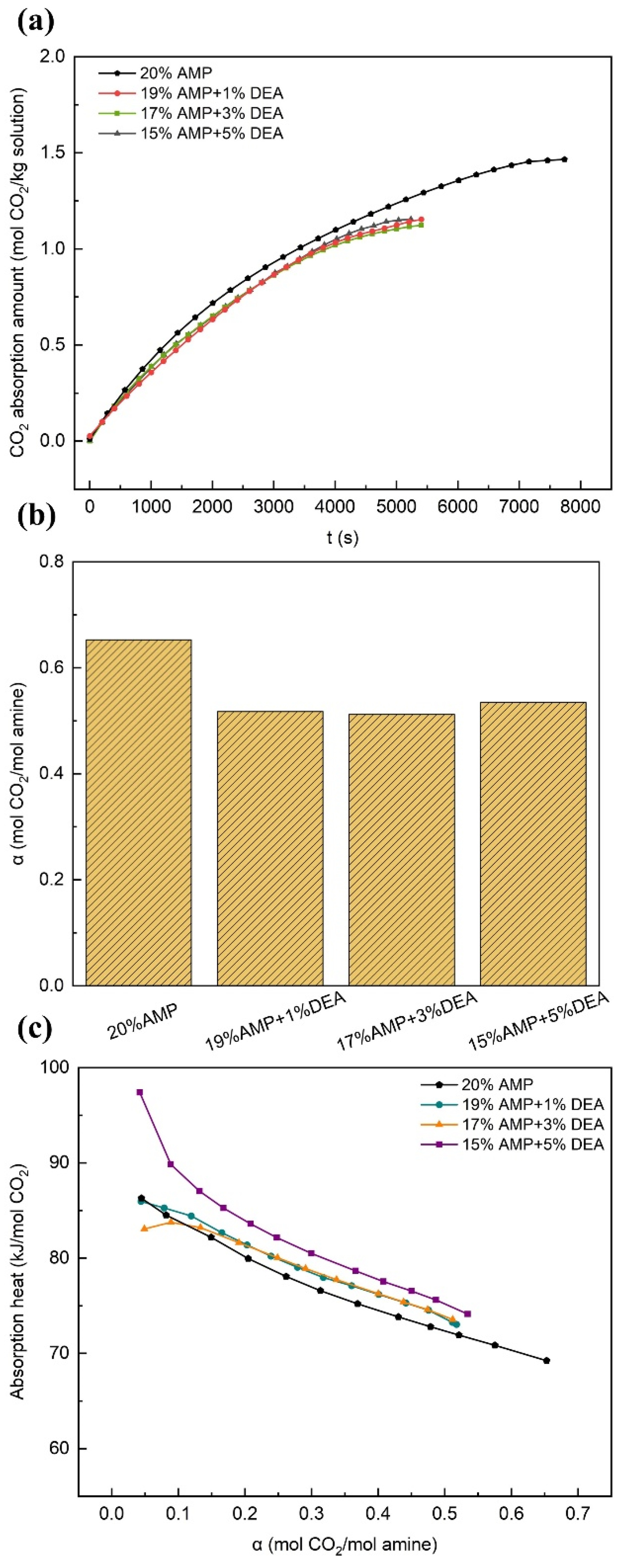
3.2.3. Effects of DEA
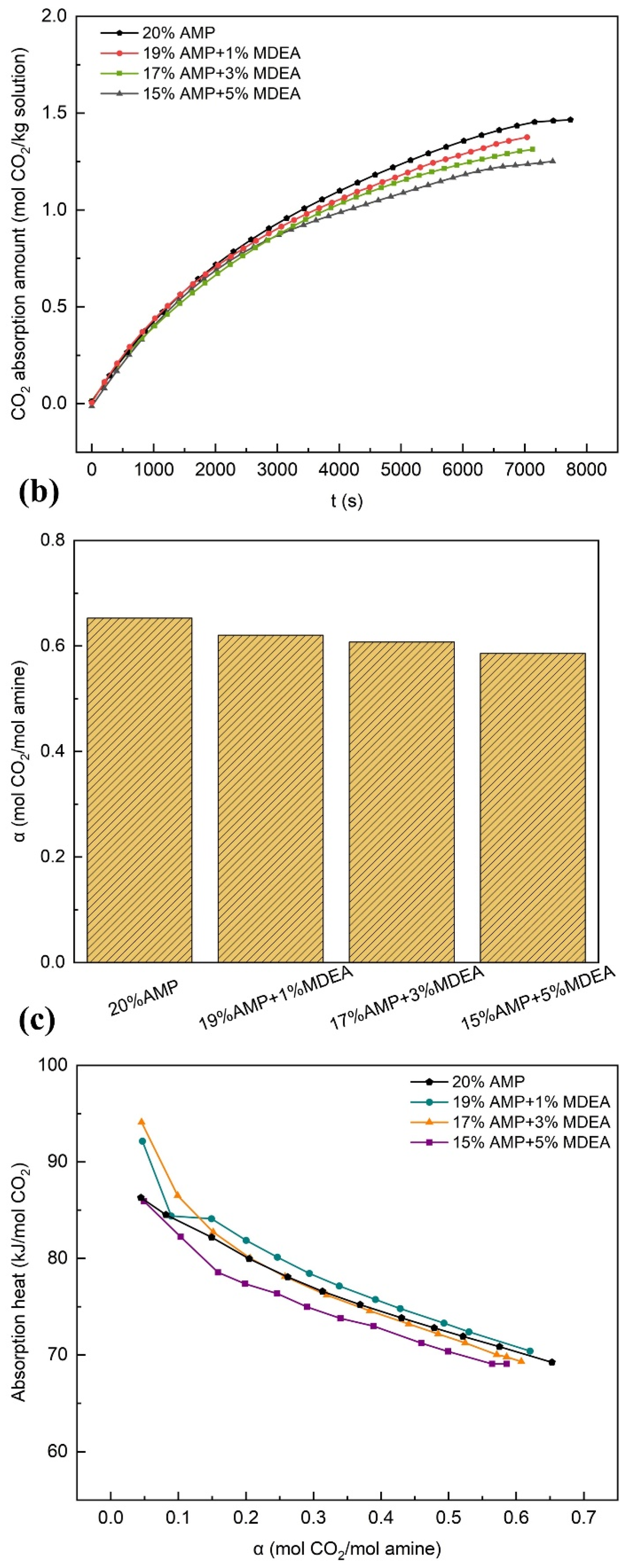
3.2.4. Effects of MDEA
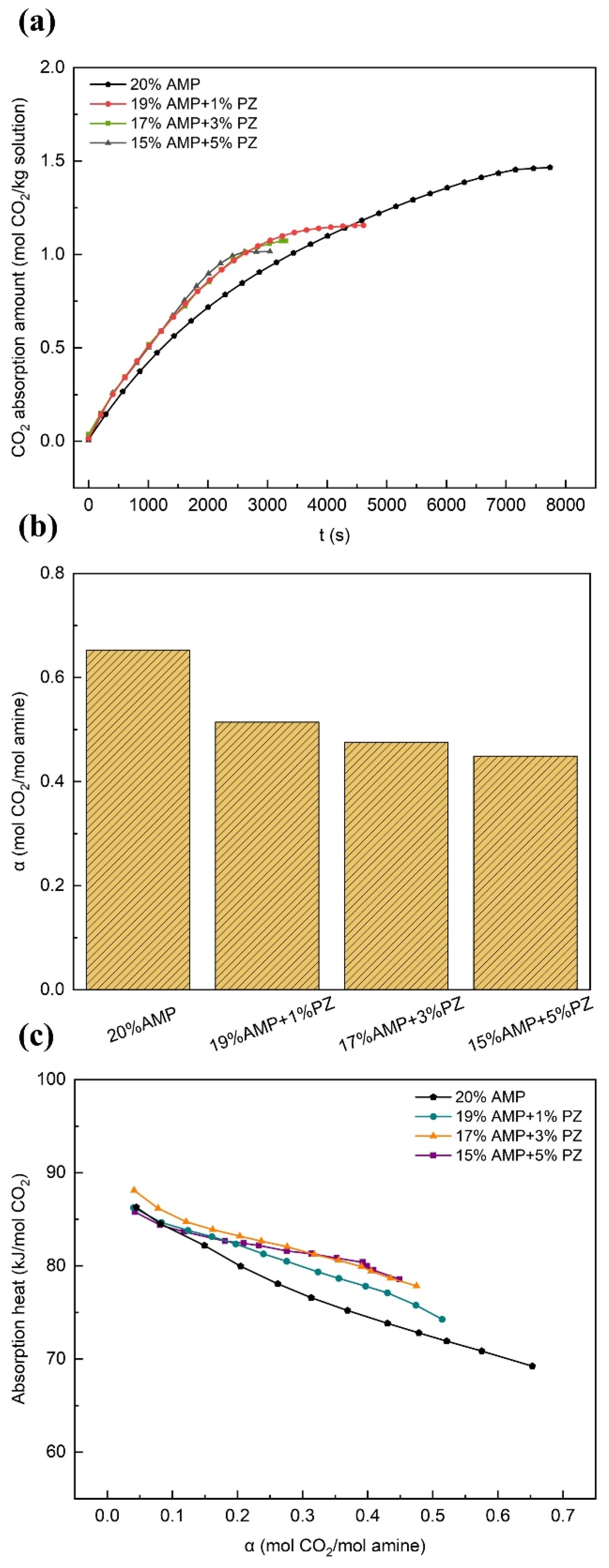
3.2.5. Effects of PZ
4. Conclusions
- (1)
- In the case of MEA, an upward trend was observed in the CO2 absorption capacity as the solution concentration increased. Specifically, the difference in terms of absorption rate and maximum carbon loading between the 15 wt% and 20 wt% solutions could be neglected. With a rise in solution concentration, the reaction heat grew higher, while the removal rate of the rich solution declined. When the absorption temperature was increased, the absorption capacity of the solution was reduced, accompanied by an increase in regeneration energy consumption. However, a slight improvement in the removal rate was noted. By contrast, raising the absorption pressure could augment the absorption capacity, reduce the absorption heat, and increase the removal rate of the rich solution, yet it exerted a little influence on the desorption heat.
- (2)
- In the case of AMP, with the increase in the solution concentration, a fast rise in the CO2 absorption capacity and absorption rate was observed. By contrast, the variation tendencies of the maximum carbon loading, absorption heat, and the removal rate of the rich solution were less conspicuous. Meanwhile, the desorption heat demonstrated a slight increment. The increase in the absorption temperature exerted negative influences on both the absorption and desorption performances. When the absorption pressure was increased, it served to boost the absorption capacity and the removal rate of the rich solution, yet it exerted a relatively minor impact on the heat of the reaction. In comparison with MEA, AMP demonstrated a slower overall absorption rate; however, it showed stronger carbon loading capability and cyclic capacity, a lower reaction heat, more favorable regeneration energy consumption levels, and a more remarkable effect in terms of rich solution removal.
- (3)
- The experimental results demonstrated that the overall performance of the solution was significantly enhanced or promoted by even minute quantities of additives. In comparison with the single-component AMP solution, the AMP-MEA solutions showed higher absorption rates, while the total absorption amounts remained nearly unchanged; however, this was accompanied by higher reaction heats. The absorption performances of the AMP-DGA solutions and AMP-DEA solutions declined, yet certain ratios manifested enhanced desorption performances. As for the AMP-MDEA solutions, both their absorption capacities and rates decreased, but the desorption performances of all three ratios were shown to increase remarkably. The AMP-PZ solutions exhibited high absorption rates and faster kinetics, yet their absorption capacities were significantly decreased, along with an increase in the heat of the reactions.
- (4)
- Due to the interactions among diverse components in the binary blended solvents, typically only one or two specific properties of the absorbent were enhanced, but overall performance improvements were not achieved. Through a comprehensive analysis of the experimental data, the 15 wt% AMP + 5 wt% MDEA solution demonstrated a distinct advantage in terms of the heat of reaction. Future research is recommended to develop multi-component blends based on this formulation and pilot-scale CO2 capture experiments to optimize the design parameters and operating conditions for industrial processes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Shen, X.; Jiao, X.; Xie, F.; Hua, J.; Lin, H.; Yan, F.; Wu, H.; Zhang, Z. Novel Tri-Solvent Amines Absorption for Flue Gas CO2 Capture: Efficient Absorption and Regeneration with Low Energy Consumption. Chem. Eng. J. 2024, 493, 152699. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A Systematic Review on CO2 Capture with Ionic Liquids: Current Status and Future Prospects. Renew. Sust. Energ. Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Arshad, N.; Alhajaj, A. Process Synthesis for Amine-Based CO2 Capture from Combined Cycle Gas Turbine Power Plant. Energy 2023, 274, 127391. [Google Scholar] [CrossRef]
- Baudoux, A.; Demeyer, F.; Paepe, W.D. Advanced Configurations of Amine Based Post-Combustion Carbon Capture Process Applied to Combined Cycle Gas Turbine. Energy Convers. Manag. 2024, 22, 100537. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Son, J.; Bara, J.E.; O’Harra, K.E.; Engelhard, M.H.; Heldebrant, D.J.; Rousseau, R.; Yu, X.-Y. The Interfacial Compatibility between a Potential CO2 Separation Membrane and Capture Solvents. Carbon Capture Sci. Technol. 2022, 2, 100037. [Google Scholar] [CrossRef]
- Guo, R.-T.; Li, G.-Y.; Liu, Y.; Pan, W. Recent Progress on CO2 Capture Based on Sterically Hindered Amines: A Review. Energy Fuels 2023, 37, 15429–15452. [Google Scholar] [CrossRef]
- Kimura, N.; Omata, K.; Kiga, T.; Takano, S.; Shikisma, S. Characteristics of Pulverized Coal Combustion in O2/CO2 Mixtures for CO2 Recovery. Energy Convers. Manag. 1995, 36, 805–808. [Google Scholar] [CrossRef]
- Wolsky, A.M.; Daniels, E.J.; Jody, B.J. CO2 Capture from The Flue Gas of Conventional Fossil-Fuel-Fired Power Plants. Environ. Prog. 1994, 13, 214–219. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Environmental Impacts of Absorption-Based CO2 Capture Unit for Post-Combustion Treatment of Flue Gas from Coal-Fired Power Plant. Int. J. Greenh. Gas Control 2007, 1, 318–342. [Google Scholar] [CrossRef]
- MacInnes, J.M.; Ayash, A.A.; Dowson, G.R.M. CO2 Absorption Using Diethanolamine-Water Solutions in a Rotating Spiral Contactor. Chem. Eng. J. 2017, 307, 1084–1091. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kim, J.-K. Operational Optimization for Part-Load Performance of Amine-Based Post-Combustion CO2 Capture Processes. Energy 2018, 146, 57–66. [Google Scholar] [CrossRef]
- Bui, T.Q.; Khokarale, S.G.; Shukla, S.K.; Mikkola, J.-P. Switchable Aqueous Pentaethylenehexamine System for CO2 Capture: An Alternative Technology with Industrial Potential. ACS Sustain. Chem. Eng. 2018, 6, 10395–10407. [Google Scholar] [CrossRef]
- Varghese, A.M.; Karanikolos, G.N. CO2 Capture Adsorbents Functionalized by Amine—Bearing Polymers: A Review. Int. J. Greenh. Gas Control 2020, 96, 103005. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon Dioxide Capture Using Liquid Absorption Methods: A Review. Environ. Chem. Lett. 2020, 19, 77–109. [Google Scholar] [CrossRef]
- Rochelle, G.; Chen, E.; Freeman, S.; Van Wagener, D.; Xu, Q.; Voice, A. Aqueous Piperazine as the New Standard for CO2 Capture Technology. Chem. Eng. J. 2011, 171, 725–733. [Google Scholar] [CrossRef]
- Meng, F.; Ju, T.; Han, S.; Lin, L.; Li, J.; Chen, K.; Jiang, J. Study on Biogas Upgrading Characteristics and Reaction Mechanism of Low Energy Consumption 2-Amino-2-Methylpropanol (AMP)/Piperazine (PZ)/H2O Mixed Amines. Sep. Purif. Technol. 2023, 310, 123195. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Liu, T.; Li, C.; Xiao, M.; Ling, H.; Hu, X.; Zhang, X.; Tang, F.; Luo, H. Thermodynamic Studies for Improving the Prediction of CO2 Equilibrium Solubility in Aqueous 2-Dimethylamino-2-Methyl-1-Propanol. Sep. Purif. Technol. 2022, 295, 121292. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Ramli, R.M.; Lock, S.S.M.; Farooqi, A.S.; Shahid, M.Z.; Wajahat ul Hasnain, S.M.; Hira, N.; Abdullah, B. Removal of Carbon Dioxide and Hydrogen Sulfide from Natural Gas Using a Hybrid Solvent of Monoethanolamine and N-Methyl 2-Pyrrolidone. ACS Omega 2024, 9, 25704–25714. [Google Scholar] [CrossRef]
- Chakma, A. CO2 Capture Processes Opportunities for Improved Energy Efficiencies. J. Energy Convers. Manag. 1997, 38, S51–S56. [Google Scholar] [CrossRef]
- Ma’mun, S.; Nilsen, R.; Svendsen, H.F.; Juliussen, O. Solubility of Carbon Dioxide in 30 mass % Monoethanolamine and 50 mass % Methyldiethanolamine Solutions. J. Chem. Eng. Data 2005, 50, 630–634. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Mahajani, V.V. Kinetics of the Reaction of CO2 with Aqueous Formulated Solution Containing Monoethanolamine, N-Methyl-2-pyrrolidone, and Diethylene Glycol. Ind. Eng. Chem. Res. 2005, 44, 1868–1873. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Verheyen, T.V.; Adeloju, S.B.; Meuleman, E.; Feron, P. Towards Commercial Scale Postcombustion Capture of CO2 with Monoethanolamine Solvent: Key Considerations for Solvent Management and Environmental Impacts. Environ. Sci. Technol. 2012, 46, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Ayittey, F.K.; Saptoro, A.; Kumar, P.; Wong, M.K. Energy-Saving Process Configurations for Monoethanolamine-Based CO2 Capture System. Asia-Pac. J. Chem. Eng. 2020, 16, e2576. [Google Scholar] [CrossRef]
- He, X.; He, H.; Barzagli, F.; Amer, M.W.; Li, C.E.; Zhang, R. Analysis of the Energy Consumption in Solvent Regeneration Processes Using Binary Amine Blends for CO2 Capture. Energy 2023, 270, 126903. [Google Scholar] [CrossRef]
- Kontos, G.; Leontiadis, K.; Tsivintzelis, I. CO2 Solubility in Aqueous Solutions of Blended Amines: Experimental Data for Mixtures with MDEA, AMP and MPA and Modeling with the Modified Kent-Eisenberg Model. Fluid Phase Equilibria 2023, 570, 113800. [Google Scholar] [CrossRef]
- Veawab, A.; Tontiwachwuthikul, P.; Bhole, S.D. Studies of Corrosion and Corrosion Control in CO2-2-Amino-2-Methyl-1-Propanol (AMP) Environment. Ind. Eng. Chem. Res. 1997, 36, 264–269. [Google Scholar] [CrossRef]
- Choi, B.-K.; Kim, S.-M.; Lee, J.-S.; Park, Y.C.; Chun, D.-H.; Shin, H.-Y.; Sung, H.-J.; Min, B.-M.; Moon, J.-H. Effect of Blending Ratio and Temperature on CO2 Solubility in Blended Aqueous Solution of Monoethanolamine and 2-Amino-2-Methyl-Propanol: Experimental and Modeling Study Using the Electrolyte Nonrandom Two-Liquid Model. ACS Omega 2020, 5, 28738–28748. [Google Scholar] [CrossRef] [PubMed]
- Bougie, F.; Iliuta, M.C. Sterically Hindered Amine-Based Absorbents for The Removal of CO2 from Gas Streams. J. Chem. Eng. Data 2012, 57, 635–669. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; An, S.; Wang, L. Kinetics of CO2 Absorption in Concentrated K2CO3/Pz Mixture Using a Wetted-Wall Column. Energy Fuels 2016, 30, 7496–7502. [Google Scholar] [CrossRef]
- An, S.; Huang, X.; Li, N.; Li, Q.; Qi, T.; Wang, R.; Wang, L. Preventing Crystallization at High Concentrated 2-Amino-2-Methyl-1-Propanol for Energy-Saving CO2 Capture. Sep. Purif. Technol. 2024, 330, 125484. [Google Scholar] [CrossRef]
- Paul, S.; Ghoshal, A.K.; Mandal, B. Kinetics of Absorption of Carbon Dioxide into Aqueous Solution of 2-(1-Piperazinyl)-Ethylamine. Chem. Eng. Sci. 2009, 64, 313–321. [Google Scholar] [CrossRef]
- Chakravarty, T.; Phukan, U.K.; Weilund, R.H. Reaction of Acid Gases with Mixtures of Amines. Chem. Eng. Prog. 1985, 81, 4. [Google Scholar]
- Fu, W.; Yu, K.; Song, H.; Zhang, K.; Yang, W. Design Strategy of Polyamine for CO2 Absorption Guided by a Novel Descriptor of Hydrogen Bond Strength. Fuel 2024, 359, 130393. [Google Scholar] [CrossRef]
- Nwaoha, C.; Saiwan, C.; Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Rongwong, W.; Al-Marri, M.J.; Benamor, A. Carbon Dioxide (CO2) Capture Performance of Aqueous Tri-Solvent Blends Containing 2-Amino-2-Methyl-1-Propanol (Amp) and Methyldiethanolamine (Mdea) Promoted by Diethylenetriamine (Deta). Int. J. Greenh. Gas Control 2016, 53, 292–304. [Google Scholar] [CrossRef]
- Meng, F.; Han, S.; Meng, Y.; Ju, T.; Lin, L.; Jiang, J. A Comparative Study of the Effects of Aqueous Mixed Amines on Biogas Upgrading Based on 13c Nuclear Magnetic Resonance (Nmr) Analysis. J. Clean. Prod. 2022, 369, 133288. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous Amine Solution Characterization for Post-Combustion CO2 Capture Process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Zhang, R.; Li, T.; Zhang, Y.; Ha, J.; Xiao, Y.; Li, C.; Zhang, X.; Luo, H. Cuo Modified Kit-6 as a High-Efficiency Catalyst for Energy-Efficient Amine Solvent Regeneration. Sep. Purif. Technol. 2022, 300, 121702. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, L.; He, S. Analysis of the Mechanism of Energy Consumption for CO2 Capture in a Power System. Energy 2023, 262, 125103. [Google Scholar] [CrossRef]
- Muchan, P.; Saiwan, C.; Narku-Tetteh, J.; Idem, R.; Supap, T.; Tontiwachwuthikul, P. Screening Tests of Aqueous Alkanolamine Solutions Based on Primary, Secondary, and Tertiary Structure for Blended Aqueous Amine Solution Selection in Post Combustion CO2 Capture. Chem. Eng. J. 2017, 170, 574–582. [Google Scholar] [CrossRef]
- Nwaoha, C.; Idem, R.; Supap, T.; Saiwan, C.; Tontiwachwuthikul, P.; Rongwong, W.; Al-Marri, M.J.; Benamor, A. Heat Duty, Heat of Absorption, Sensible Heat and Heat of Vaporization of 2–Amino–2–Methyl–1–Propanol (AMP), Piperazine (PZ) and Monoethanolamine (Mea) Tri–Solvent Blend for Carbon Dioxide (CO2) Capture. Chem. Eng. Sci. 2017, 170, 26–35. [Google Scholar] [CrossRef]
- Sherwood, A.E.; Prausnitz, J.M. The Heat of Solution of Gases at High Pressure. AIChE J. 2004, 8, 519–521. [Google Scholar] [CrossRef]
- Zambonin, P.G.; Jordan, J. Gas enthalpimetry. Anal. Chem. 1969, 41, 437–442. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D.; Mather, A.E. The Solubility of H2S and CO2 in Aqueous Monoethanolamine Solutions. Can. J. Chem. Eng. 1974, 52, 803. [Google Scholar] [CrossRef]
- Jou, F.-Y.; Otto, F.D.; Mather, A.E. Vapor-Liquid Equilibrium of Carbon Dioxide in Aqueous Mixture of Monoethanolamine and Methyldi-ethanolamine. Ind. Eng. Chem. Res. 1994, 33, 2002. [Google Scholar] [CrossRef]
- Kim, I.; Hoff, K.A.; Hessen, E.T.; Haug-Warberg, T.; Svendsen, H.F. Enthalpy of Absorption of CO2 with Alkanolamine Solutions Predicted from Reaction Equilibrium Constants. Chem. Eng. Sci. 2009, 64, 2027–2038. [Google Scholar] [CrossRef]
- Merkley, K.E.; Christensen, J.J.; Izatt, R.M. Enthalpies of Absorption of Carbon Dioxide in Aqueous Methyldietiianolamine Solutions. Thermochim. Acta 1987, 121, 437–446. [Google Scholar] [CrossRef]
- Oscarson, J.L.; Van Dam, R.H.; Christensen, J.J. Enthalpies of Absorption of Carbon Dioxide in Aqueous Diethanolamine Solutions. Thermochim. Acta 1989, 146, 107–111. [Google Scholar] [CrossRef]
- Kim, I.; Svendsen, H.F. Comparative Study of the Heats of Absorption of Post-Combustion CO2 Absorbents. Int. J. Greenh. Gas Control 2011, 5, 390–395. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Svendsen, H.F.; Idrees, M.U.; Kim, I.; Chen, C. Heat of Absorption of CO2 in Aqueous Ammonia, Piperazine Solutions and Their Mixtures. Int. J. Greenh. Gas Control 2012, 9, 148–159. [Google Scholar] [CrossRef]
- Dallos, A.T.; Altsach, L.; Kotsis, L. Enthalpies of Absorption and Solubility of Carbon Dioxide in Aqueous Polyamine Solutions. J. Therm. Anal. Calorim. 2001, 65, 419–423. [Google Scholar] [CrossRef]
- Zheng, Y.; El Ahmar, E.; Simond, M.; Ballerat-Busserolles, K.; Zhang, P. CO2 Heat of Absorption in Aqueous Solutions of Mdea and Mdea/Piperazine. J. Chem. Eng. Data 2020, 65, 3784–3793. [Google Scholar] [CrossRef]
- Lu, S.; Yang, F.; Zhang, J.; Wang, N.; Liu, L.; Kang, G.; Zhao, D.; Yu, X.; Li, Q. Experimental Analysis of Reaction Heat of CO2 Absorption of Phase Change Absorber Aep-Dpa at Low Partial Pressure. Energies 2023, 16, 1867. [Google Scholar] [CrossRef]
- Douglas, T.B.; George, T.; Furukawa, R.E.; Mccoskey Ball, A.F. Calorimetric properties of normal heptane from 0-degrees to 520-degrees-k. J. Res. Natl. Bur. Stand. 1954, 53, 139. [Google Scholar] [CrossRef]
- Mathonat, C.; Majer, V.; Mather, A.E.; Grolier, J.P.E. Use of Flow Calorimetry for Determining Enthalpies of Absorption and The Solubility of CO2 in Aqueous Monoethanolamine Solutions. Ind. Eng. Chem. Res. 1998, 37, 4136–4141. [Google Scholar] [CrossRef]
- Kim, I.; Svendsen, H.F. Heat of Absorption of Carbon Dioxide (CO2) in Monoethanolamine (MEA) and 2-(Aminoethyl)ethanolamine (AEEA) Solutions. Ind. Eng. Chem. Res. 2007, 46, 5803–5809. [Google Scholar] [CrossRef]
- Arcis, H.; Ballerat-Busserolles, K.; Rodier, L.; Coxam, J.-Y. Enthalpy of Solution of Carbon Dioxide in Aqueous Solutions of Monoethanolamine at Temperatures of 322.5 K and 372.9 K and Pressures up to 5 MPa. J. Chem. Eng. Data 2011, 56, 3351–3362. [Google Scholar] [CrossRef]
- Kim, Y.E.; Lim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of Carbon Dioxide Absorption in Aqueous MEA, DEA, TEA, and AMP Solutions. Bull. Korean Chem. Soc. 2013, 34, 783–787. [Google Scholar] [CrossRef]
- Mathonat, C.; Majer, V.; Mather, A.E.; Grolier, J.P.E. Enthalpies of Absorption and Solubility of CO2 In Aqueous Solutions of Methyldiethanolamine. Fluid Phase Equilibria 1997, 140, 171–182. [Google Scholar] [CrossRef]
- McCann, N.; Maeder, M.; Hasse, H. Prediction of the Overall Enthalpy of CO2 Absorption in Aqueous Amine Systems from Experimentally Determined Reaction Enthalpies. Energy Procedia 2011, 4, 1542–1549. [Google Scholar] [CrossRef]
- Kundu, M.; Bandyopadhyay, S.S. Solubility of CO2 in Water + Diethanolamine + 2-Amino-2-methyl-1-propanol. J. Chem. Eng. Data 2006, 51, 398–405. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Continuous Cycles of CO2 Absorption and Amine Regeneration with Aqueous Alkanolamines: A Comparison of the Efficiency Between Pure and Blended DEA, MDEA And AMP Solutions by 13C NMR Spectroscopy. Energy Environ. Sci. 2010, 3, 772–779. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Thermodynamics of Piperazine/Methyldiethanolamine/Water/Carbon Dioxide. Ind. Eng. Chem. Res. 2002, 41, 604–612. [Google Scholar] [CrossRef]
- Xie, Q.; Aroonwilas, A.; Veawab, A. Measurement of Heat of CO2 Absorption into 2-Amino-2-Methyl-1-Propanol (Amp)/Piperazine (Pz) Blends Using Differential Reaction Calorimeter. Energy Procedia 2013, 37, 826–833. [Google Scholar] [CrossRef]


| Chemical Reagent | Molecular Formula | Molar Mass (g/mol) | CAS. No. | Chemical Structure |
|---|---|---|---|---|
| N-Heptane | CH3(CH2)5CH3 | 114.23 | 142-82-5 |  |
| MEA | H2NCH2CH2OH | 61.08 | 141-43-5 |  |
| AMP | (CH3)2C(NH2)CH2OH | 89.14 | 124-68-5 |  |
| DGA | H2NCH2CH2OCH2CH2OH | 105.14 | 929-06-6 |  |
| DEA | HN(H2CH2OH)2 | 105.14 | 111-42-2 |  |
| MDEA | NCH3N(CH2CH2OH)2 | 119.16 | 105-59-9 |  |
| PZ | NHC2H4NHC2H4 | 82.10 | 110-85-0 |  |
| Amine System | Concentration | Absorption Temperature (°C) | Absorption Pressure (bar (G)) | Desorption Temperature (°C) | Desorption Pressure |
|---|---|---|---|---|---|
| MEA | 10 wt%, 15 wt%, 20 wt% | 40, 60, 80 | 1, 3, 5 | 105 | Follows the saturated vapor pressure of the water solution |
| AMP | 10 wt%, 15 wt%, 20 wt% | 40, 60, 80 | 1, 3, 5 | ||
| AMP + MEA | 19 wt% + 1 wt%, 17 wt% + 3 wt%, 15 wt% + 5 wt% | 40 | 1 | ||
| AMP + DGA | 19 wt% + 1 wt% *, 17 wt% + 3 wt%, 15 wt% + 5 wt% | 40 | 1 | ||
| AMP + DEA | 19 wt% + 1 wt%, 17 wt% + 3 wt%, 15 wt% + 5 wt% | 40 | 1 | ||
| AMP + MDEA | 19 wt% + 1 wt%, 17 wt% + 3 wt%, 15 wt% + 5 wt% * | 40 | 1 | ||
| AMP + PZ | 19 wt% + 1 wt%, 17 wt% + 3 wt%, 15 wt% + 5 wt% | 40 | 1 |
| Sources | Equipment | T (°C) | P (bar) | (bar) | Absorption Heat (kJ/mol CO2) |
|---|---|---|---|---|---|
| Mathonat et al. [55] | C-80 | 40~120 | 20~100 | 81~102 | |
| Kim et al. [56] | CPA 122 | 40~120 | 1~3 | 84~110 | |
| El Hadri et al. [37] | URC | 40 | 0.15 | 85.13 | |
| Arcis et al. [57] | C-80 | 50~100 | 5~50 | 83~96 | |
| This study | Independent development | 40 | 1 | 86.44 |
| Amine System | Concentration (wt%) | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|---|
| MEA | 10 | 0.24 | 60.13 | 95.10 |
| MEA | 15 | 0.24 | 52.47 | 97.89 |
| MEA | 20 | 0.12 | 27.40 | 101.05 |
| AMP | 10 | 0.46 | 68.71 | 81.92 |
| AMP | 15 | 0.41 | 67.72 | 82.18 |
| AMP | 20 | 0.46 | 69.58 | 85.16 |
| Amine System | Absorption Temperature (°C) | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|---|
| MEA | 40 | 0.12 | 27.4 | 101.05 |
| MEA | 60 | 0.09 | 27.5 | 108.01 |
| MEA | 80 | 0.09 | 32.5 | 116.76 |
| AMP | 40 | 0.46 | 69.58 | 85.16 |
| AMP | 60 | 0.17 | 55 | 86.8 |
| AMP | 80 | 0.10 | 49.14 | 88.87 |
| Amine System | Absorption Pressure (bar) | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|---|
| MEA | 1 | 0.12 | 27.4 | 101.05 |
| MEA | 3 | 0.28 | 50.49 | 102.84 |
| MEA | 5 | 0.44 | 76.08 | 101.81 |
| AMP | 1 | 0.46 | 69.58 | 85.16 |
| AMP | 3 | 0.66 | 87.27 | 86.3 |
| AMP | 5 | 0.72 | 86.08 | 85.71 |
| Amine System | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|
| 20 wt% AMP | 0.46 | 69.58 | 85.16 |
| 19 wt% AMP + 1 wt% MEA | 0.48 | 75.05 | 88.17 |
| 17 wt% AMP + 3 wt% MEA | 0.43 | 69.94 | 91.12 |
| 15 wt% AMP + 5 wt% MEA | 0.39 | 68.84 | 97.68 |
| Amine System | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|
| 20 wt% AMP | 0.46 | 69.58 | 85.16 |
| 19 wt% AMP + 1 wt% DGA | 0.41 | 72.19 | 78.54 |
| 17 wt% AMP + 3 wt% DGA | 0.37 | 71.72 | 84.21 |
| 15 wt% AMP + 5 wt% DGA | 0.30 | 65.52 | 89.12 |
| Amine System | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|
| 20 wt% AMP | 0.46 | 69.58 | 85.16 |
| 19 wt% AMP + 1 wt% DEA | 0.35 | 67.42 | 86.63 |
| 17 wt% AMP + 3 wt% DEA | 0.40 | 78.57 | 87.32 |
| 15 wt% AMP + 5 wt% DEA | 0.42 | 79.15 | 93.711 |
| Amine System | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|
| 20 wt% AMP | 0.46 | 69.58 | 85.16 |
| 19 wt% AMP + 1 wt% MDEA | 0.48 | 77.69 | 84.35 |
| 17 wt% AMP + 3 wt% MDEA | 0.48 | 78.55 | 81.45 |
| 15 wt% AMP + 5 wt% MDEA | 0.46 | 76.61 | 76.24 |
| Amine System | Cyclic Capacity (mol CO2/mol Amine) | Removal Rate (%) | Desorption Heat (kJ/mol CO2) |
|---|---|---|---|
| 20 wt% AMP | 0.46 | 69.58 | 85.16 |
| 19 wt% AMP + 1 wt% PZ | 0.39 | 76.76 | 87.68 |
| 17 wt% AMP + 3 wt% PZ | 0.32 | 66.4 | 89.35 |
| 15 wt% AMP + 5 wt% PZ | 0.23 | 52.35 | 88.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wang, X.; Li, Y.; Li, Q.; Liu, H.; Wang, H. Absorption and Desorption Heat of Carbon Dioxide Capture Based on 2-Amino-2-Methyl-1-Propanol. Energies 2025, 18, 1075. https://doi.org/10.3390/en18051075
Guo J, Wang X, Li Y, Li Q, Liu H, Wang H. Absorption and Desorption Heat of Carbon Dioxide Capture Based on 2-Amino-2-Methyl-1-Propanol. Energies. 2025; 18(5):1075. https://doi.org/10.3390/en18051075
Chicago/Turabian StyleGuo, Jia, Xin Wang, Yi Li, Qingfang Li, Haili Liu, and Hui Wang. 2025. "Absorption and Desorption Heat of Carbon Dioxide Capture Based on 2-Amino-2-Methyl-1-Propanol" Energies 18, no. 5: 1075. https://doi.org/10.3390/en18051075
APA StyleGuo, J., Wang, X., Li, Y., Li, Q., Liu, H., & Wang, H. (2025). Absorption and Desorption Heat of Carbon Dioxide Capture Based on 2-Amino-2-Methyl-1-Propanol. Energies, 18(5), 1075. https://doi.org/10.3390/en18051075






