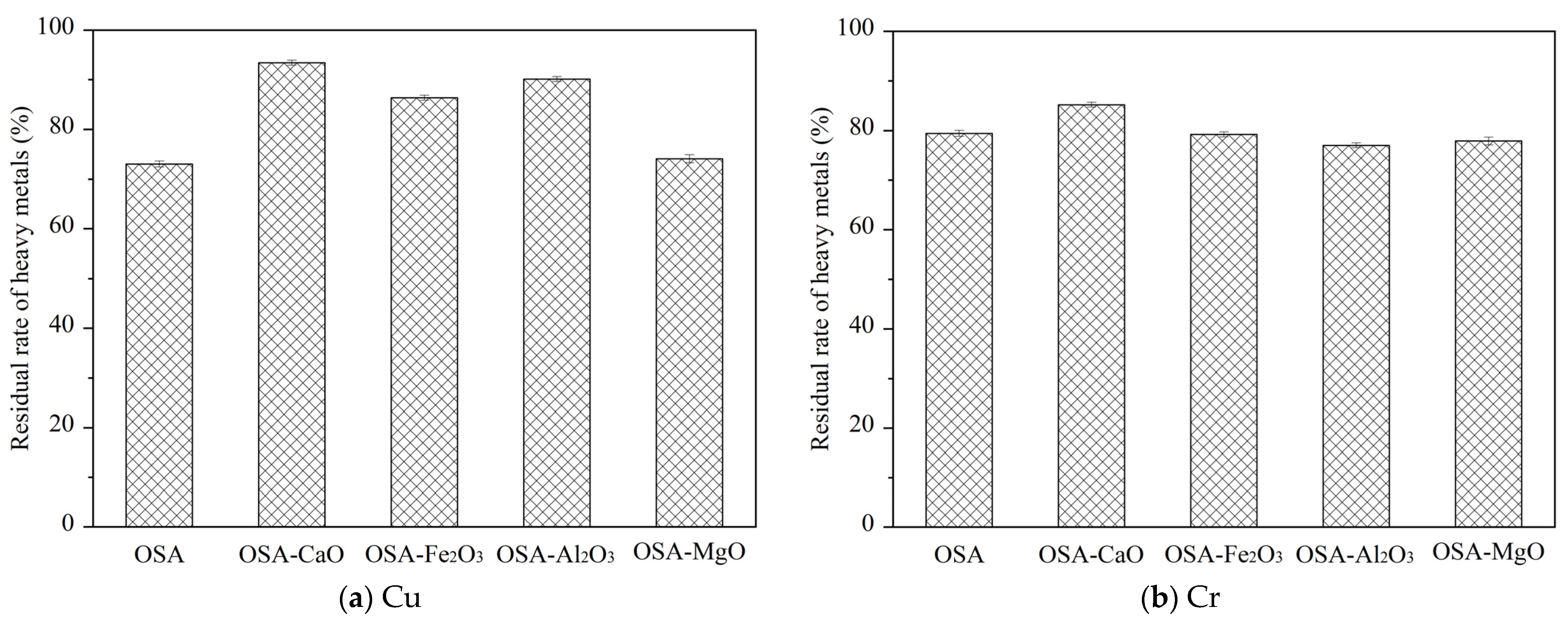
3.1. Effect of Mineral Compounds on the Total Amount of Heavy Metals
The concentrations of heavy metals in different samples are shown in
Table 2. In order to more accurately represent the residue of heavy metals after oily sludge incineration, the concept of residual rate R is introduced, as shown in Formula (1), which represents the percentage share of the absolute content of heavy metals in oily sludge transferred to the incineration bottom ash. The results are shown in
Figure 1.
where
C1 is the concentration of heavy metals in oily sludge (mg/kg).
C2 is the concentration of heavy metals in incineration bottom ash (mg/kg).
M1 is the weight of oily sludge (g).
M2 is the weight of incineration bottom ash (g).
It can be seen that the concentration of Cu in OSA increased significantly, indicating that CaO, Fe2O3, Al2O3, and MgO all promote the retention of Cu in the incineration bottom ash. Compared with OSA, the residual rate of Cu in OSA-Fe2O3 increased from 73.02% to 86.41%. This is because when the incineration temperature is low, Cu mainly exists in the form of solid CuO. As the temperature rises, Cu reacts with Fe2O3 to generate CuO-Fe2O3, indicating that Fe2O3 promotes the residue of Cu in the incineration bottom ash. At the same time, some scholars found that CuO-Fe2O3 is relatively stable, and only when the temperature is higher than 1300 °C can it be decomposed. Therefore, Cu can stably exist in the incineration bottom ash at 800 °C. The residual rate of Cu in OSA-Al2O3 is increased to 90.12%. This is attributed to the reaction between CuO in oily sludge and Al2O3, forming aluminate CuO-Al2O3 at high temperature. CuO-Al2O3 has a high melting and boiling point and is not easy to volatilize, making it easier for Cu to remain in the bottom ash. Additionally, Al2O3 can form a eutectic mixture with adsorbed Cu during melting, encapsulating the heavy metals and preventing their escape. Therefore, Al2O3 can effectively reduce the volatilization of Cu in flue gas. In addition, it can be found that the promotion effect of different mineral compounds on Cu residue in the bottom ash is in the order of CaO > Al2O3 > Fe2O3 > MgO.
The residual rate of Cr in OSA reached 79.44%, which is due to the low volatility and high boiling point of Cr, so it is more likely to remain in the bottom ash. At the same time, it was found that the residual rate of Cr in OSA-CaO reached 85.21%, while the residual rate of Cr in OSA-Fe2O3, OSA-Al2O3, and OSA-MgO had little change. The order of promoting effects of different mineral compounds on Cr residue in bottom ash was CaO > Fe2O3 > MgO > Al2O3.
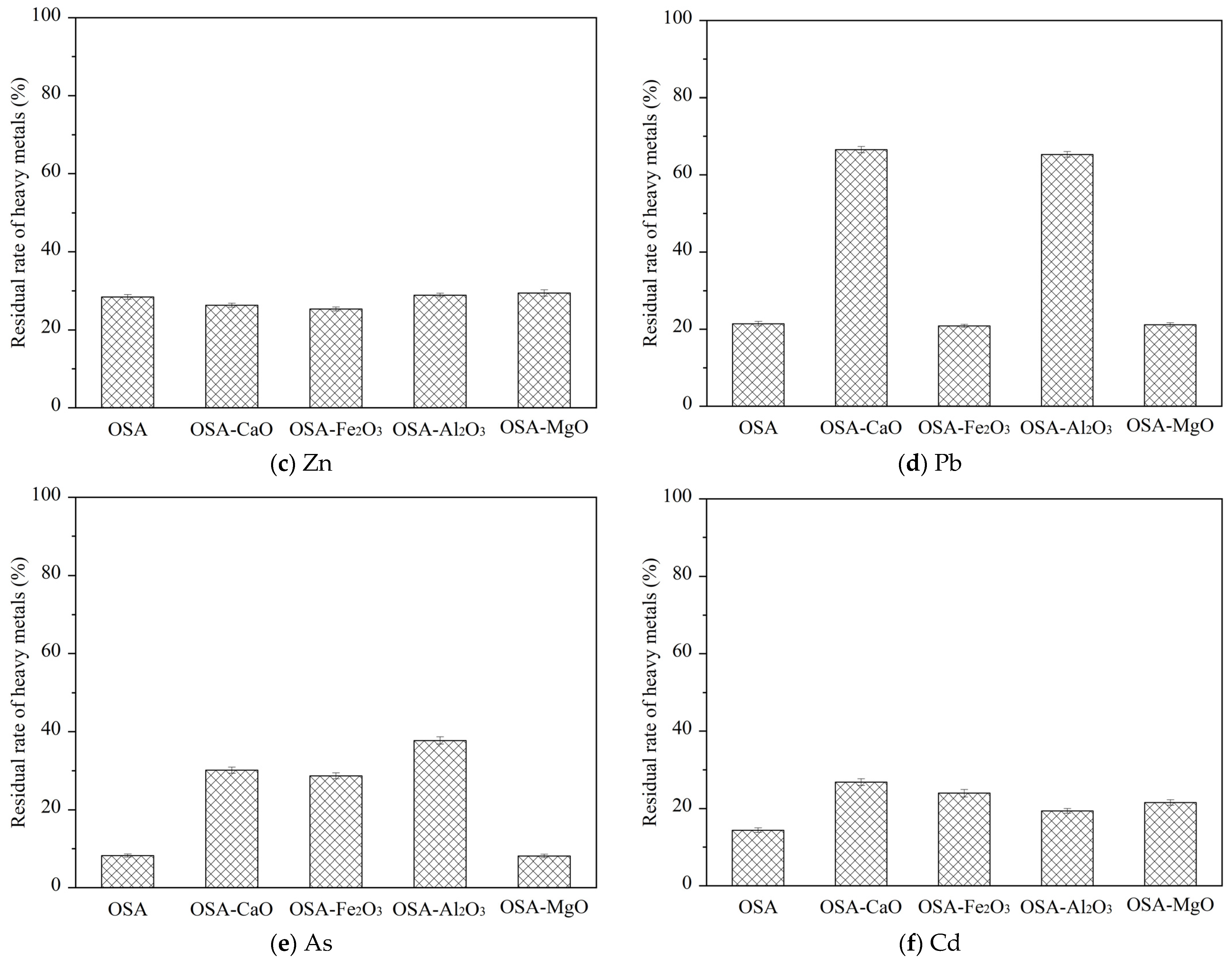
The concentration of Zn in OSA significantly decreased, and the residue rate is only 28.41%. It can be seen that most of Zn is volatilized into the flue gas, because the boiling point of oxides and sulfides of Zn is low, which is a volatile element. The residual rate of Zn in OSA-Al2O3 was 28.89%, which is higher. This is because ZnO reacts with SiO2 and Al2O3 in oily sludge during incineration to generate stable Zn2SiO4 and ZnAl2O4, thus inhibiting the evaporation of Zn. The order of the promoting effect of different mineral compounds on Zn residue in the bottom ash is MgO > Al2O3 > CaO > Fe2O3.
The concentration of Pb in OSA is low, which was because Pb is a volatile element, which was more likely to volatilize into the flue gas during incineration. The residual rate of Pb in OSA-Al
2O
3 was 65.27%, which was significantly higher than that in OSA. This is because, after adding Al
2O
3 during the incineration of oily sludge, Pb and Si react with Al
2O
3 to generate PbO-Al
2O
3-2SiO
2, which promotes the enrichment of Pb into bottom ash [
16]. It can be seen that the order of the effect of different mineral compounds on promoting Pb residue in the bottom ash is CaO > Al
2O
3 > MgO > Fe
2O
3.
The residual rate of As in OSA was 8.23%, indicating that most of As has volatilized into the flue gas. The residue rates of As in OSA-Fe2O3 and OSA-Al2O3 were 28.67% and 37.77%, respectively, which were higher than those in OSA. This is because As mainly exists in the form of gaseous As2O3 during incineration. However, when Fe2O3 or Al2O3 was added to oily sludge, gaseous As2O3 reacted with inorganic compounds to generate arsenate, which has a good ability to capture As and improves the residual rate of As in incineration bottom ash. The order of promoting effects of different mineral compounds on As residue in bottom ash is Al2O3 > CaO > Fe2O3 > MgO.
The residual rate of Cd in OSA was 21.61%. The boiling point of Cd, its oxides, and sulfides are low, belonging to high volatile elements. The residual rate of Cd in OSA-CaO increased significantly, reaching 50.02%, indicating that CaO has little adsorption effect on Cd. The order of the promoting effect of different mineral compounds on Cd residue in the bottom ash is CaO > Al2O3 > MgO > Fe2O3.
In general, the adsorption capacities of different mineral compounds for heavy metals vary significantly. Among them, CaO has a significant adsorption effect on Cu, Cr, Pb, As, Cd, while Fe2O3 has a great adsorption effect on Cu, As, and Cd but a weak adsorption effect on Cr, Zn, and Pb, while Al2O3 has a great adsorption effect on other heavy metals except Cr, especially on Pb, As, and other volatile heavy metals. MgO has a significant adsorption effect on Cd, while its adsorption capacity for other heavy metals is relatively moderate.
3.2. Effect of Mineral Compounds on the Leaching Characteristics of Heavy Metals
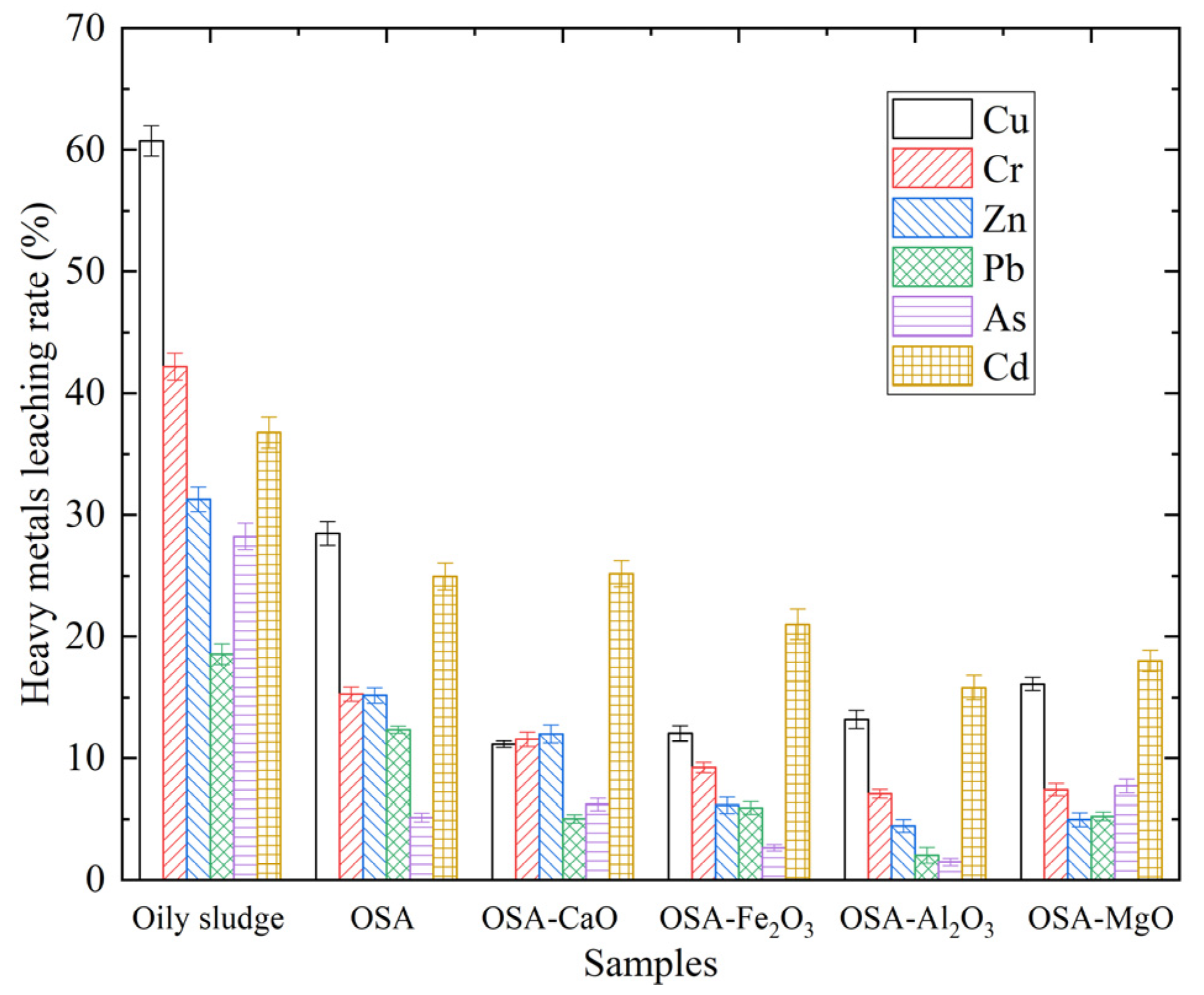
The leaching characteristics of heavy metals in the obtained incineration bottom ash were analyzed, with the leaching concentrations presented in
Table 3. To more accurately represent the leaching of heavy metals in the sample, the leaching rate
L of heavy metals is introduced, as shown in Formula (2), which is defined as the percentage of the leaching concentration and the total concentration of heavy metals, and the change trend of the leaching rate of different heavy metals with the incineration temperature is shown in
Figure 2.
where
LC is the leaching concentration of heavy metals in samples (mg/L).
TC is the total concentration of heavy metals in samples (mg/L).
Compared with oily sludge, the leaching rate of Cu in OSA reduced from 60.73% to 28.47%, indicating that the incineration treatment can reduce the leaching toxicity of Cu and lessen the harm to the environment. Further research showed that the inhibition order of different mineral compounds on the leaching toxicity of Cu in incineration bottom ash was CaO > Fe2O3 > Al2O3 > MgO.
The leaching rate of Cr in OSA was reduced from 42.19% to 15.29%, indicating that incineration treatment can also reduce the leaching toxicity of Cr. It can be seen that the inhibition order of different mineral compounds on the leaching toxicity of Cr in incineration bottom ash was Al2O3 > MgO > Fe2O3 > CaO.
The leaching concentration of Zn in OSA was also reduced from 111.11 mg/L to 35.19 mg/L, and the leaching rate was reduced from 31.27% to 15.18%. The inhibition order of different mineral compounds on the leaching toxicity of Zn in incineration bottom ash was Al2O3 > MgO > Fe2O3 > CaO.
After incineration, the leaching concentration of Pb in OSA also decreased from 10.93 mg/L to 3.66 mg/L, and the leaching rate decreased from 18.53% to 12.34%. The inhibition order of different mineral compounds on the leaching toxicity of Pb in incineration bottom ash was Al2O3 > CaO > MgO > Fe2O3.
Compared with OS, the leaching concentration of As in OSA decreased from 11.29 mg/L to 0.53 mg/L, and the leaching rate decreased from 28.21% to 5.14%, indicating that incineration treatment can significantly reduce the leaching toxicity of As. The inhibition order of different mineral compounds on the leaching toxicity of As in incineration bottom ash was Al2O3 > Fe2O3 > CaO > MgO.
The leaching concentration of Cd in OSA decreased from 41.99 mg/L to 22.03 mg/L, indicating that incineration can reduce the leaching toxicity of Cr. At the same time, it was found that the inhibition order of different mineral compounds on the leaching toxicity of Cd in incineration bottom ash was Al2O3 > MgO > Fe2O3 > CaO.
In general, the leaching concentration of heavy metals decreased after incineration. Specifically, the leaching rate of Cu, Cr, Zn, Pb, As, and Cd in OSA were reduced by 32%, 27%, 16%, 6%, 23%, and 12%, respectively. Obviously, incineration treatment can significantly reduce the leaching toxicity of Cu and thus mitigate its environmental impact. The effect of mineral compounds on the toxic leaching of heavy metals is influenced by multiple factors, but it is primarily determined by the stability of the products formed through chemical reactions involving heavy metals during the incineration process. If the stability of these products is poor, they are easy to be leached out; otherwise, they can be stably retained in the incineration bottom ash.
It can be seen from
Table 3 and
Figure 2 that CaO exhibited significant inhibition of the leaching of Cu, Cr, Zn, and Pb but had a weaker inhibition effect on the leaching of Cd and As. Fe
2O
3 can inhibit the leaching of Cu, Cr, Zn, Pb, Cd, and As, especially the leaching inhibition effect of Cu and Zn. Al₂O₃ also demonstrated leaching inhibition for Cu, Cr, Zn, Pb, Cd, and As, with the most pronounced effects on Cu and Zn. MgO showed good inhibition of the leaching of Cu, Cr, Zn, Pb, and Cd but had a relatively weaker performance on As.
3.3. Effect of Mineral Compounds on Morphological Distribution of Heavy Metals
The speciation fractions of heavy metals include exchangeable fraction (F1), reducible fraction (F2), oxidizable fraction (F3), and residual fraction (F4), each exhibiting distinct characteristics.
Exchangeable Fraction (F1): This fraction is adsorbed on solid surfaces or exists in carbonate forms. It is highly mobile, toxic, and poses significant risks to the ecological environment.
Reducible Fraction (F2): This fraction is associated with iron and manganese oxides. It is less toxic than the exchangeable fraction but still poses notable risks.
Oxidizable Fraction (F3): This fraction involves metal ions combined with organic matter and sulfides. It exhibits potential biological toxicity and bioavailability.
Residual Fraction (F4): This fraction is stable under natural conditions and can remain in sediments for extended periods. It is chemically inert and has minimal biological toxicity or bioavailability.
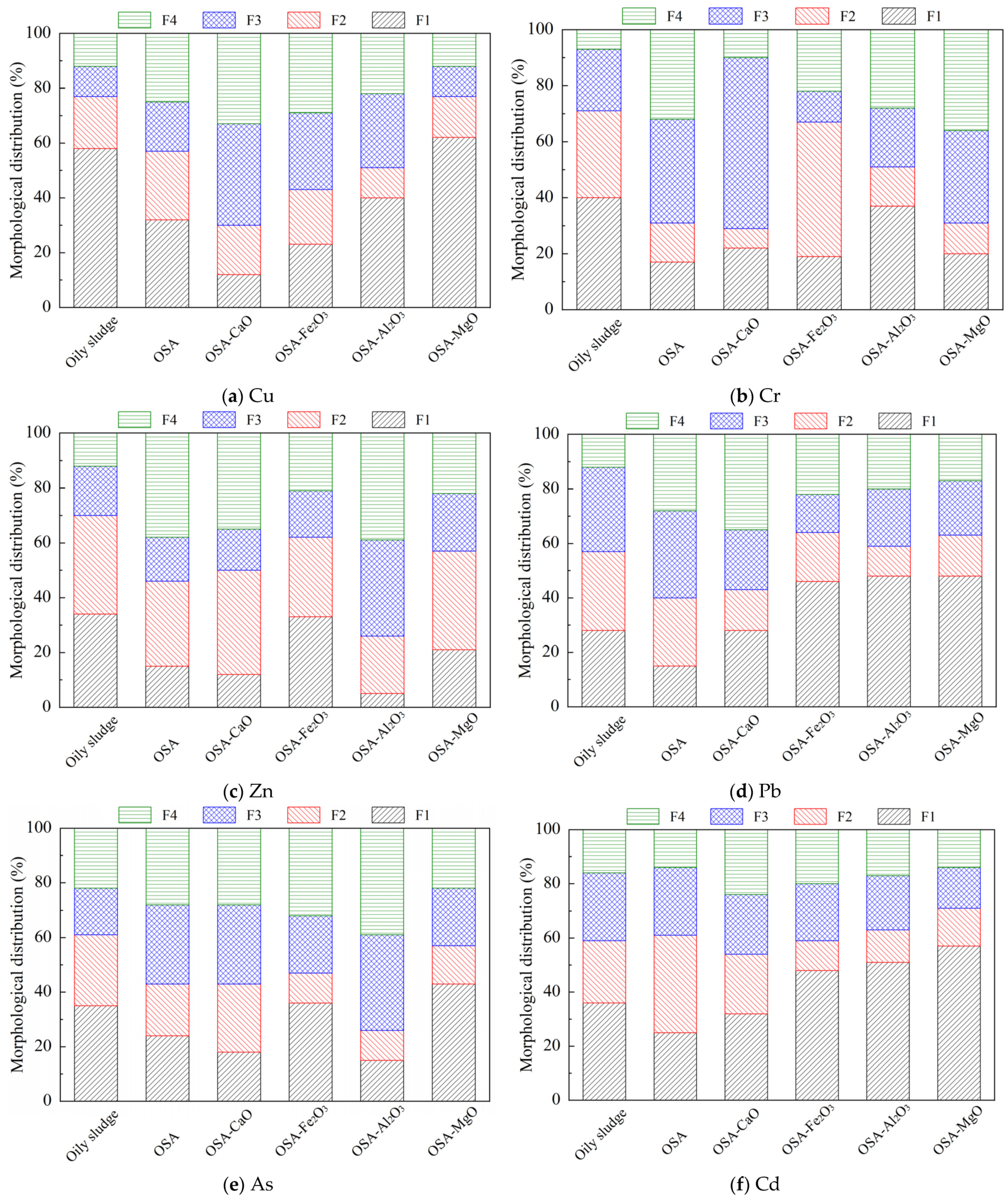
The morphological distribution of heavy metals in different incineration bottom ashes was analyzed, and the concentrations of different heavy metals in incineration bottom ashes were obtained, as shown in
Table S3. In order to verify the validity of the data, the recovery rate
Rec is introduced. The sum of the four speciation fractions was compared with the total concentration of heavy metals in oily sludge or incineration bottom ash, and the recovery rate of each heavy metal was calculated, as shown in Formula (3).
where
F1 is concentration of heavy metals in exchangeable form (mg/L).
F2 is the concentration of heavy metals in reducible form (mg/L).
F3 is the concentration of heavy metals in oxidizable form (mg/L).
F4 is the concentration of heavy metals in residual form (mg/L).
It can be seen from
Table S3 that the recovery rate of heavy metals was between 94.21% and 98.54%, indicating that the data of heavy metals obtained by BCR extraction method are effective and reliable. At the same time, the proportion of different forms of heavy metals was further analyzed, and the trends of the morphological distribution of heavy metals in different incineration bottom ashes were obtained, as shown in
Figure 3.
As illustrated in
Figure 3, Cu in both oily sludge and OSA mainly exists in the form of exchangeable form, accounting for 58% and 32%, respectively. This form has strong mobility and poses significant environmental risks. The addition of different mineral compounds influences the morphological distribution of Cu in various ways. In OSA-CaO, the oxidizable state and residual state of Cu accounted for a relatively high proportion, of which the oxidizable form accounted for 37% and the residual form accounted for 33%, indicating that CaO has an obvious solidification effect on Cu, which can reduce the mobility of Cu, and promoted the stable existence of Cu in the incineration bottom ash. The four forms of Cu in the OSA-Fe
2O
3 accounted for roughly the same proportion. The highest proportion of Cu in OSA-Al
2O
3 was in exchangeable form (40%), and the lowest proportion was in reducible form (11%). The exchangeable Cu accounted for the highest proportion in OSA-MgO (62%), which was higher than that of oily sludge and OSA. Overall, CaO and Fe
2O
3 had a very obvious effect on reducing the risk of Cu, while Al
2O
3 and MgO exhibited a poor effect.
Cr mainly exists in the form of exchangeable and reducible form in oily sludge, in which the exchangeable forms accounted for 40%, and the reducible form accounted for 31%. After incineration, it was found that Cr in OSA had changed from unstable form to stable form, of which the proportion of oxidizable Cr was 37% and the proportion of residual Cr was 32%. Different mineral compounds exhibited different effects on the morphological distribution of Cr. The oxidizable Cr accounted for the highest proportion of 61% in OSA-CaO, indicating that adding CaO reduced the mobility of Cr. Cr in OSA-Fe2O3 mainly existed in reducible form, accounting for 48%. The proportion of oxidizable Cr was relatively low, only 11%. Cr in OSA-Al2O3 mainly existed in the exchangeable form, accounting for 37%, while the reducible from accounted for only 14%. Cr mainly existed in residue form in OSA-MgO, accounting for 36%. The proportion of reducible form was relatively low, only 11%. It can be seen that mineral compounds used in this work showed a poor effect on reducing the risk of Cr in the incineration bottom ash.
Zn mainly existed in exchangeable and reducible form, accounting for 34% and 36%, respectively. Zn changed from unstable form to stable form after incineration, in which the residual Zn accounted for 38% in OSA. It was also found that different mineral compounds had different effects on the morphological distribution of Zn. The reducible and residual Zn accounted for 38% and 35% in OSA-CaO, respectively. It showed that CaO has a certain solidification effect on Zn and promoted the stable existence of Zn in OSA-CaO. The exchangeable Zn in OSA-Fe2O3 accounted for the highest proportion of 33%. While the oxidizable Zn accounted for a relatively low proportion of 17%, indicating that Fe2O3 has a poor curing effect on Zn. In OSA-Al2O3, the proportion of residual Zn was the highest, 39%, and the proportion of exchangeable was relatively low, only 5%, indicating that Al2O3 played an obvious role in Zn solidification. The reducible Zn accounts for the highest proportion of 36% in OSA-MgO. The proportion of the other three forms was relatively uniform, about 20%. In general, CaO and Al2O3 have a significant effect on reducing the risk of Zn, while Fe2O3 and MgO increased the risk of Zn in the bottom ash compared with OSA.
The proportions of exchangeable Pb, reducible Pb, and oxidable Pb in oily sludge were 28%, 29%, and 31%, respectively. Through incineration, Pb has obviously changed from unstable form to stable form, in which the proportion of oxidizable Pb was the highest, reaching 32%. At the same time, different mineral compounds exhibited different effects on the morphological distribution of Pb. The residual Pb accounted for the highest proportion of 35% in OSA-CaO. It showed that CaO promoted the stable existence of Pb during incineration. The exchangeable Pb in OSA-Fe2O3 accounted for the highest proportion, reaching 46%, while the oxidized Pb accounted for the lowest proportion of 14% in OSA-Fe2O3. In OSA-Al2O3, the exchangeable Pb accounted for the highest proportion, up to 48%, and the reducible Pb accounted for the lowest proportion, only 11%. The highest and lowest proportion of Pb were exchangeable and reducible in OSA-MgO, accounting for 48% and 15%, respectively. It can be seen that the effect of mineral compounds was poor on reducing the risk of Pb in incineration bottom ash.
The exchangeable As in OS accounts for the highest proportion, up to 35%, posing a great threat to the environment. Through incineration, the fixed form of As increased significantly, of which the proportion of oxidizable As was the highest, reaching 29%. The proportion of residual As was also high in OSA, reaching 28%, indicating that incineration improved the stability of As. At the same time, different mineral compounds showed different effects on the morphological distribution of As. In OSA-CaO, the proportion of As in oxidizable form and residue form was also high, 29% and 28%, respectively. It showed that CaO promoted the stable existence of As. The exchangeable form accounted for the highest proportion in OSA-Fe2O3, reaching 36%, while the reducible As accounted for 11%. The residual As in OSA-Al2O3 accounted for the highest proportion, up to 39%, while the reducible As accounted for 11%. The highest and lowest proportion of As were residual and reducible in OSA-MgO, accounting for 48% and 15%, respectively. It can be seen that CaO and Al2O3 have a significant effect on decreasing the risk of As, while Fe2O3 and MgO showed a poor effect.
The exchangeable Cd decreased from 36% in oily sludge to 25% in OSA after incineration, while the proportion of oxidizable Cd increased to 36% in OSA. At the same time, different mineral compounds showed different effects on the morphological distribution of Cd. The exchangeable Cd is relatively high in OSA-CaO, reaching 32%. It showed that CaO has little effect on the stabilization of Cd. The exchangeable Cd also accounted for a relatively high proportion, reaching 51%, while the reducible Cd accounted for the lowest proportion of 12% in OSA-Al2O3. The exchangeable Cd in OSA-MgO was as high as 57%, while the other three forms accounted for about 14%~15%. The proportion of exchangeable Cd in OSA-Fe2O3 was also high, reaching 48%. The proportion of reducible state was the lowest of 11%. It can be seen that mineral compounds exhibited a poor effect on reducing the risk of Cd during incineration.
Overall, incineration can effectively facilitate the transformation of heavy metals from unstable forms to more stable forms. The selected mineral compounds exhibited distinct effects on the morphological transformation of heavy metals during the incineration process. Specifically, CaO promotes the formation of oxidizable Cr and residual Cu. Fe2O3 enhances the formation of residual As and residual Cd. Al2O3 aids in the formation of oxidizable Zn and residual As. MgO supports the formation of residual Cr. The addition of these mineral compounds enhances the stability of certain heavy metals in incineration bottom ash, thereby reducing potential threats to the natural environment.
Indeed, besides focusing on the harmless disposal of solid products that has been studied in this work, the control of harmful gases during oily sludge incineration is also a crucial step. The effective management of gaseous emissions not only helps to mitigate environmental impacts but also ensures compliance with stringent regulatory standards, thereby safeguarding public health and ecological integrity.