Optimization of Biomass to Bio-Syntetic Natural Gas Production: Modeling and Assessment of the AIRE Project Plant Concept
Abstract
1. Introduction
2. Material and Methods
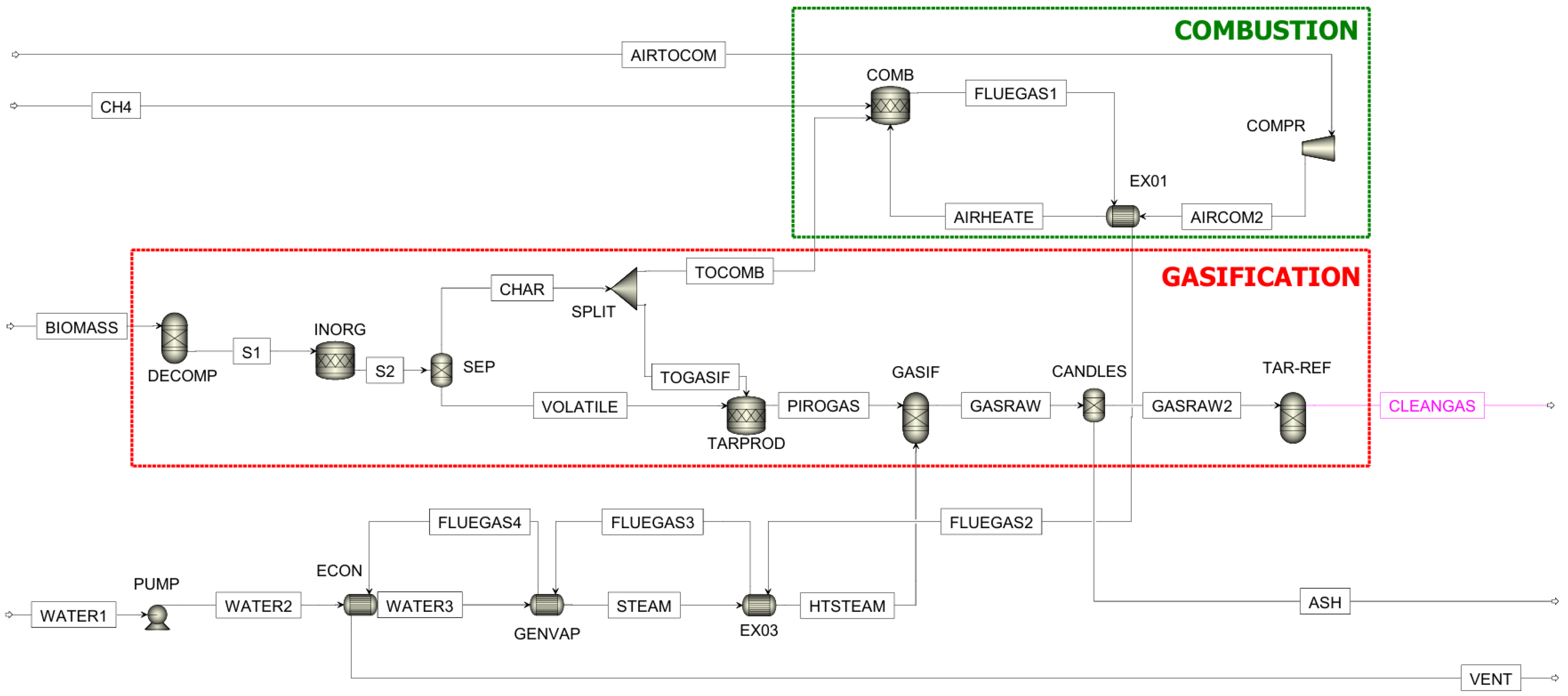
2.1. Gasification Process Simulation
Gasification Reactions
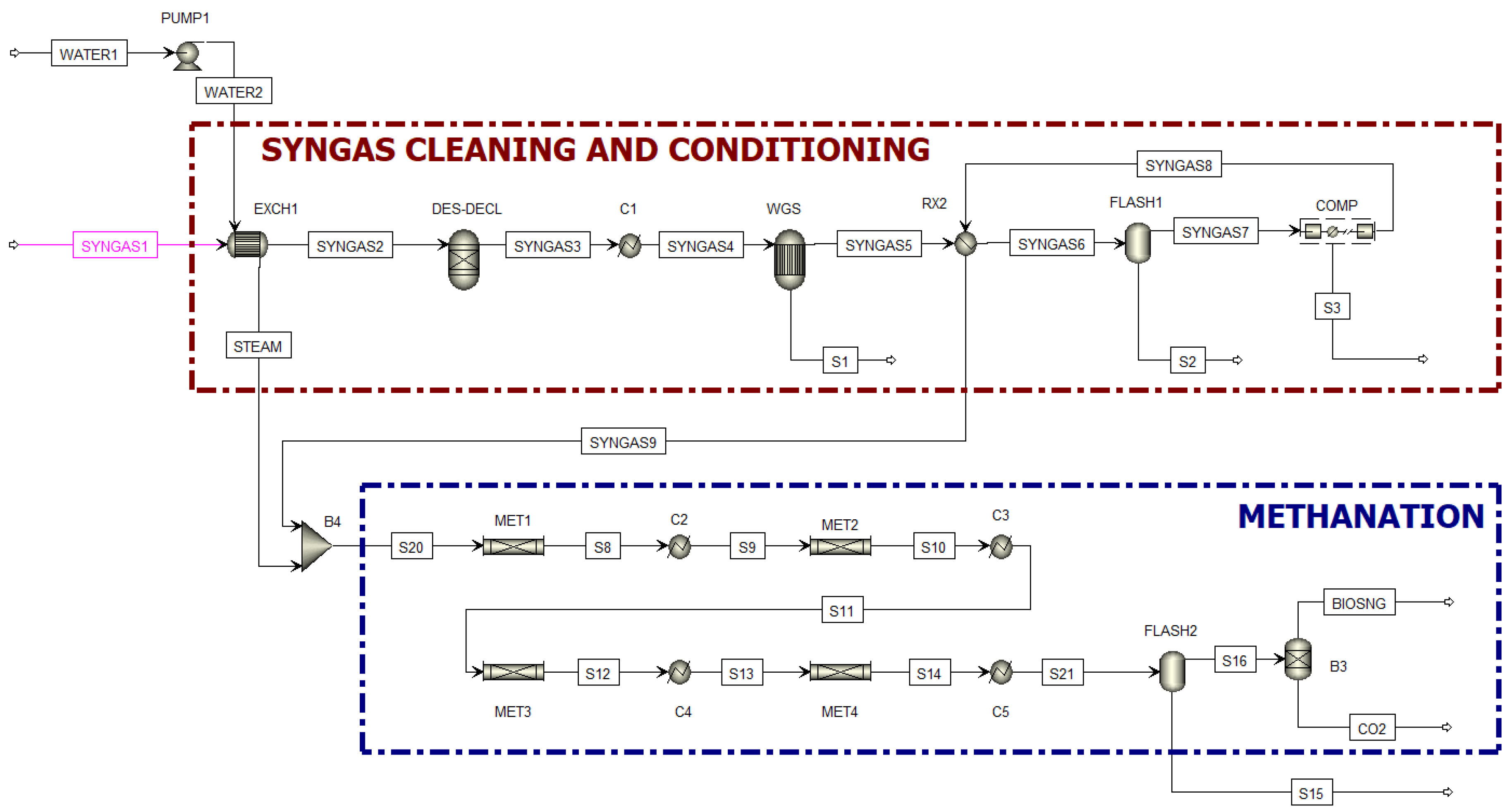
2.2. Methanation Process Simulation
Methanation Reaction
2.3. Parameters for the Performance Evaluation
2.4. Optimization Strategies
- Investigate the thermal feasibility by analyzing the operative temperatures to avoid thermal crossovers and ensure adequate driving force for heat exchange.
- Investigate the energy feasibility by analyzing the energy demand of each thermal operation.
3. Results
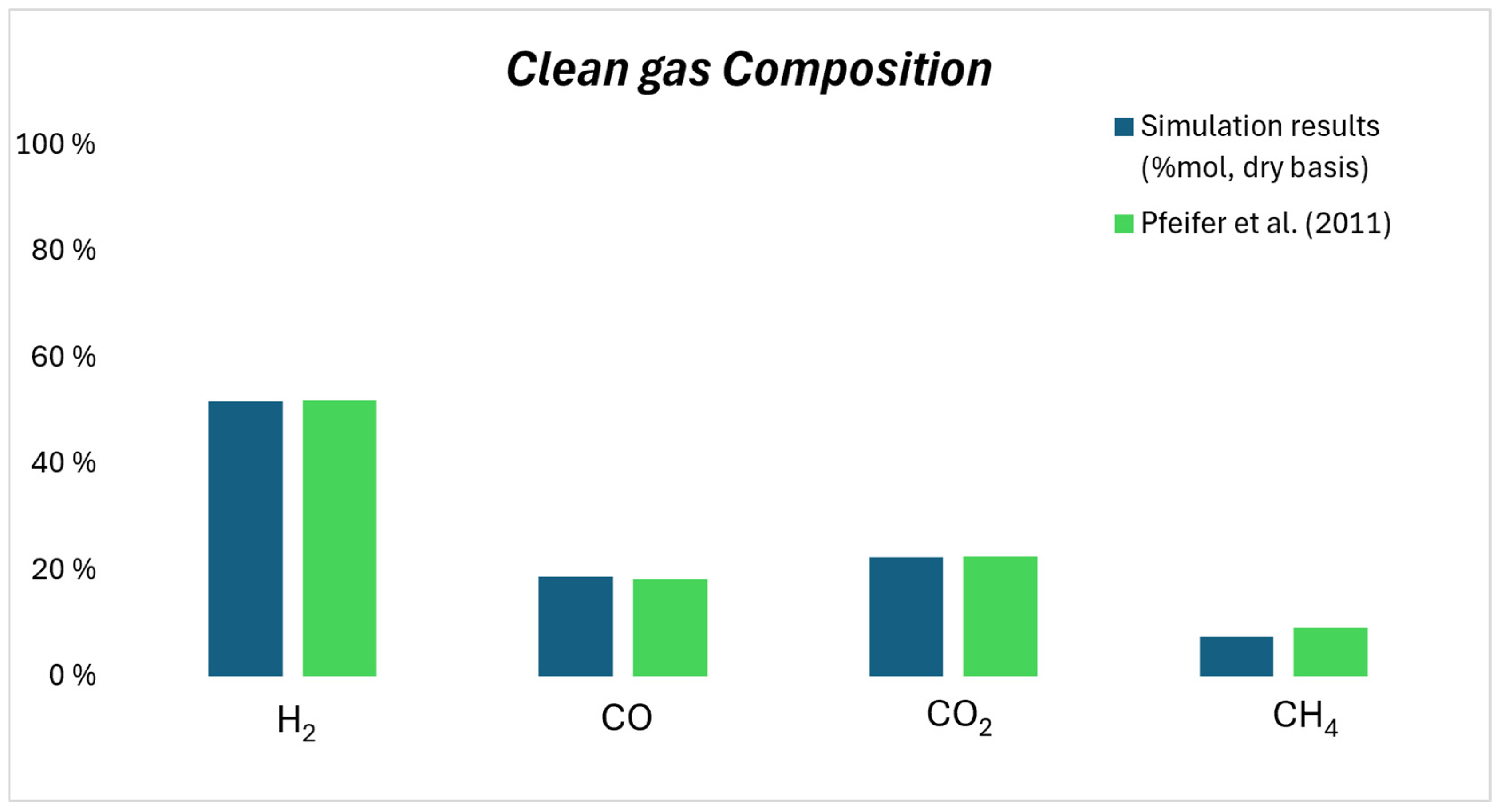
3.1. Gasification Results
3.2. Methanation Results
3.3. Optimization Results
- ➢
- Thermal recovery: Heat is recovered between methanation reactors, thereby reducing thermal losses.
- ➢
- Efficient sensible heat utilization: A portion of the sensible heat from the syngas exiting the gasification block is used to superheat the steam for gasification.
- ➢
- Flue gas optimization: The sensible heat of the flue gas exiting the burner is used to preheat the air entering the combustor, potentially reducing or even eliminating the need for auxiliary fuel.
- DFB gasification with direct methanation of the DFB product gas (case I).
- Methanation starting from DFB gasification supported by external hydrogen in the product gas (cases II and III).
- DFB gasification with in-situ CO2 removal (SER process) and direct methanation (case IV).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIRE | Advanced integration for renewable energies |
| Bio-SNG | Bio-synthetic natural gas |
| CC | Carbon conversion |
| CFB | Circulating fluidized bed |
| CGE | Cold gas efficiency |
| DFB | Dual fluidized bed |
| Fi | Flow rate (kg/h) of species i |
| HHV | High heating value |
| ICE | Internal combustion engine |
| IEA | International energy agency |
| PR-BM | Peng-Robinson with Boston–Mathias alpha |
| RES | Renewable energy source |
| SER | Sorption-enhanced reforming |
| S/B | Steam-to-biomass |
| WC | Water conversion |
| WGS | Water–gas shift |
| WMO | Word Meteorological Organization |
References
- World Meteorological Organization (WMO). State of the Global Climate 2023; WMO: Geneva, Switzerland, 2024. [Google Scholar]
- Gu, D.; Andreev, K.; Dupre, M.E. Major Trends in Population Growth Around the World. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Prospects 2024: Summary of Results; United Nations: New York, NY, USA, 2024. [Google Scholar]
- Harjanne, A.; Korhonen, J.M. Abandoning the concept of renewable energy. Energy Policy 2019, 127, 330–340. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.-U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Dees, M.; Elbersen, B.; Fitzgerald, J.; Vis, M.; Ramirez-Almeyda, J.; Glavonjic, B.; Staritsky, I.; Verkerk, H.; Monti, A.; Datta, P.; et al. S2Biom Project Grant Agreement n°608622 D1.1 Roadmap for Regional End-Users on How to Collect, Process, Store and Maintain Biomass Supply Data Delivery of Sustainable Supply of Non-Food Biomass to Support a “Resource-Efficient” Bioeconomy in Europe; Institute of Forest Sciences, University of Freiburg: Freiburg, Germany, 2017. [Google Scholar]
- Li, S.; Xu, S.; Liu, S.; Yang, C.; Lu, Q. Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Gallifuoco, A.; Papa, A.A.; Spera, A.; Taglieri, L.; Di Carlo, A. Dynamics of liquid-phase platform chemicals during the hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. Rep. 2022, 19, 101177. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, S.K. A review on recent gasification methods for biomethane gas production. Int. J. Energy Eng. 2016, 6, 32–43. [Google Scholar] [CrossRef]
- Papa, A.A.; Bartolucci, L.; Cordiner, S.; Di Carlo, A.; Mele, P.; Mulone, V.; Vitale, A. The effect of pyrolysis temperature on the optimal conversion of residual biomass to clean syngas through fast-pyrolysis/steam gasification integration. Int. J. Hydrogen Energy 2024, 95, 1316–1327. [Google Scholar] [CrossRef]
- Palone, O.; Ramezani, R.; Navarro, C.; Di Felice, L.; Borello, D.; Grasa, G.; Gallucci, F. On the reduction of NiFe/Al2O3 oxygen carrier in high-pressure chemical looping applications. Int. J. Hydrogen Energy 2024, 49, 1304–1317. [Google Scholar] [CrossRef]
- Rey, J.R.C.; Longo, A.; Rijo, B.; Pedrero, C.M.; Tarelho, L.A.C.; Brito, P.S.D.; Nobre, C. A review of cleaning technologies for biomass-derived syngas. Fuel 2024, 377, 132776. [Google Scholar] [CrossRef]
- Di Carlo, A.; Savuto, E.; Foscolo, P.U.; Papa, A.A.; Tacconi, A.; Del Zotto, L.; Aydin, B.; Bocci, E. Preliminary Results of Biomass Gasification Obtained at Pilot Scale with an Innovative 100 kWth Dual Bubbling Fluidized Bed Gasifier. Energies 2022, 15, 4369. [Google Scholar] [CrossRef]
- Papa, A.A.; Tacconi, A.; Savuto, E.; Ciro, E.; Hatunoglu, A.; Foscolo, P.U.; Del Zotto, L.; Aydin, B.; Bocci, E.; Di Carlo, A. Performance evaluation of an innovative 100 kWth dual bubbling fluidized bed gasifier through two years of experimental tests: Results of the BLAZE project. Int. J. Hydrogen Energy 2023, 48, 27170–27181. [Google Scholar] [CrossRef]
- Marchetti, L.; Guastaferro, M.; Annunzi, F.; Tognotti, L.; Nicolella, C.; Vaccari, M. Two-stage thermal pyrolysis of plastic solid waste: Set-up and operative conditions investigation for gaseous fuel production. Waste Manag. 2024, 179, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Enebe, N.L.; Chigor, C.B.; Obileke, K.; Lawal, M.S.; Enebe, M.C. Biogas and Syngas Production from Sewage Sludge: A Sustainable Source of Energy Generation. Methane 2023, 2, 192–217. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip Rev. Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Materazzi, M.; Taylor, R.; Cozens, P.; Manson-Whitton, C. Production of BioSNG from waste derived syngas: Pilot plant operation and preliminary assessment. Waste Manag. 2018, 79, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.T. Bio-synthetic natural gas as fuel in steel industry reheating furnaces—A case study of economic performance and effects on global CO2 emissions. Energy 2013, 57, 699–708. [Google Scholar] [CrossRef]
- Thunman, H.; Seemann, M.; Vilches, T.B.; Maric, J.; Pallares, D.; Ström, H.; Berndes, G.; Knutsson, P.; Larsson, A.; Breitholtz, C.; et al. Advanced biofuel production via gasification—Lessons learned from 200 man-years of research activity with Chalmers’ research gasifier and the GoBiGas demonstration plant. Energy Sci. Eng. 2018, 6, 6–34. [Google Scholar] [CrossRef]
- Bartik, A.; Benedikt, F.; Fuchs, J.; Hofbauer, H.; Müller, S. Experimental investigation of hydrogen-intensified synthetic natural gas production via biomass gasification: A technical comparison of different production pathways. Biomass Convers Biorefin. 2024, 14, 23091–23110. [Google Scholar] [CrossRef]
- Ciccone, B.; Murena, F.; Ruoppolo, G.; Urciuolo, M.; Brachi, P. Methanation of syngas from biomass gasification: Small-scale plant design in Aspen Plus. Appl. Therm. Eng. 2024, 246, 122901. [Google Scholar] [CrossRef]
- Wan, H.; Feng, F.; Yan, B.; Liu, J.; Chen, G.; Yao, J. Methanation of syngas from biomass gasification in a dual fluidized bed: An Aspen plus modeling. Energy Convers Manag. 2024, 318, 118902. [Google Scholar] [CrossRef]
- Luca del Zotto, Progetto AIRE, le Potenzialità Delle Biomasse Residuali. 2021. Available online: https://www.rinnovabili.it/energia/biomassa/progetto-aire-potenzialita-biomasse-residuali/ (accessed on 15 January 2025).
- Kuczynski, S.; Łaciak, M.; Olijnyk, A.; Szurlej, A.; Włodek, T. Thermodynamic and technical issues of hydrogen and methane-hydrogen mixtures pipeline transmission. Energies 2019, 12, 569. [Google Scholar] [CrossRef]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill Education: Berkshire, UK, 2008; Available online: https://www.accessengineeringlibrary.com/content/book/9780071422949 (accessed on 18 December 2024).
- Alptekin, F.M.; Celiktas, M.S. Review on Catalytic Biomass Gasification for Hydrogen Production as a Sustainable Energy Form and Social, Technological, Economic, Environmental, and Political Analysis of Catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Xu, G.; Suda, T.; Matsuzawa, Y.; Tani, H.; Fujimori, T. Some process fundamentals of biomass gasification in dual fluidized bed. Fuel 2007, 86, 244–255. [Google Scholar] [CrossRef]
- Li, Y.H.; Chen, Z.; Watkinson, P.; Bi, X.; Grace, J.; Lim, C.J.; Ellis, N. A novel dual-bed for steam gasification of biomass. Biomass Convers. Biorefin. 2018, 8, 357–367. [Google Scholar] [CrossRef]
- Colelli, L.; Verdone, N.; Bassano, C.; Segneri, V.; Vilardi, G. Optimization of Power to Gas system with cooled reactor for CO2 methanation: Start-up and shut-down tests with Ru-based and Ni-based kinetics. Energy 2024, 312, 133554. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Introduction in Process Simulation. Comput. Aided Chem. Eng. 2014, 35, 35–71. [Google Scholar] [CrossRef]
- Solís, A.; Rocha, S.; König, M.; Adam, R.; Garcés, H.O.; Candia, O.; Muñoz, R.; Azócar, L. Preliminary assessment of hazelnut shell biomass as a raw material for pellet production. Fuel 2023, 333, 126517. [Google Scholar] [CrossRef]
- ASTM D5373-93 (1997); Standard Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 1993.
- ASTM D4239-18e1; Standard Test Method for Sulfur in the Analysis Sample of Coal and Coke Using High-Temperature Tube Furnace Combustion. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2361-95; Standard Test Method for Chlorine in Coal. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM: West Conshohocken, PA, USA, 2015.
- Boie, W. Fuel Technology calculations. Energietechnik 1952, 3, 309–316. [Google Scholar]
- Fercher, E.; Hofbauer, H.; Fleck, T.; Rauch, R.; Veronik, G. Two Years Experience with the Ficfb-Gasification Process. In Biomass for Energy and Industry, Proceedings of the 10th European Conference and Technology Exhibition Biomass for Energy and Industry, Würzburg, Germany, 8–11 June 1998; Commission of the European Communities: Brussels, Belgium, 1998. [Google Scholar]
- Puig-Gamero, M.; Pio, D.T.; Tarelho, L.A.C.; Sánchez, P.; Sanchez-Silva, L. Simulation of biomass gasification in bubbling fluidized bed reactor using aspen plus®. Energy Convers Manag 2021, 235, 113981. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Monarca, D. Development of a Chemical Quasi-Equilibrium Model of Biomass Waste Gasification in a Fluidized-Bed Reactor by Using Aspen Plus. Energies 2020, 13, 53. [Google Scholar] [CrossRef]
- El-Rub, Z.A.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar] [CrossRef]
- Rapagnà, S.; Jand, N.; Kiennemann, A.; Foscolo, P.U. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Han, J.; Liang, Y.; Hu, J.; Qin, L.; Street, J.; Lu, Y.; Yu, F. Modeling downdraft biomass gasification process by restricting chemical reaction equilibrium with Aspen Plus. Energy Convers. Manag. 2017, 153, 641–648. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D. The effect of air preheating in a biomass CFB gasifier using ASPEN Plus simulation. Biomass Bioenergy 2009, 33, 1158–1167. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sustain. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. In Energy Procedia; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2022–2027. [Google Scholar] [CrossRef]
- Vitale, A.; Papa, A.A.; Iannello, S.; Ciro, E.; Hatunoglu, A.; Corradetti, V.; Rovelli, N.; Foscolo, P.U.; Di Carlo, A. Devolatilization of Polypropylene Particles in Fluidized Bed. Energies 2023, 16, 6324. [Google Scholar] [CrossRef]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Catalysts for dual fluidised bed biomass gasification-an experimental study at the pilot plant scale. Biomass Convers. Biorefin. 2011, 1, 63–74. [Google Scholar] [CrossRef]
- Taylor, D.G.; Taylor, D.; Raju, A. Renewable Syngas Methanation; California Energy Commission: Sacramento, CA, USA, 2024.
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]


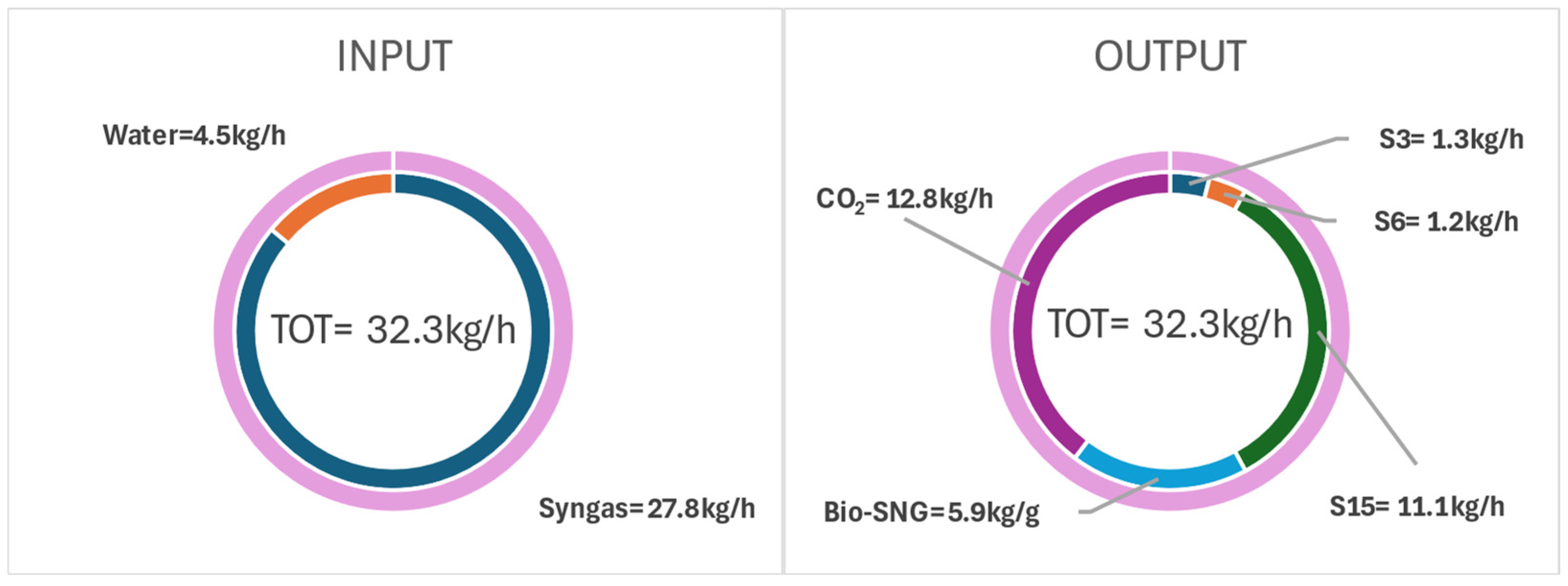
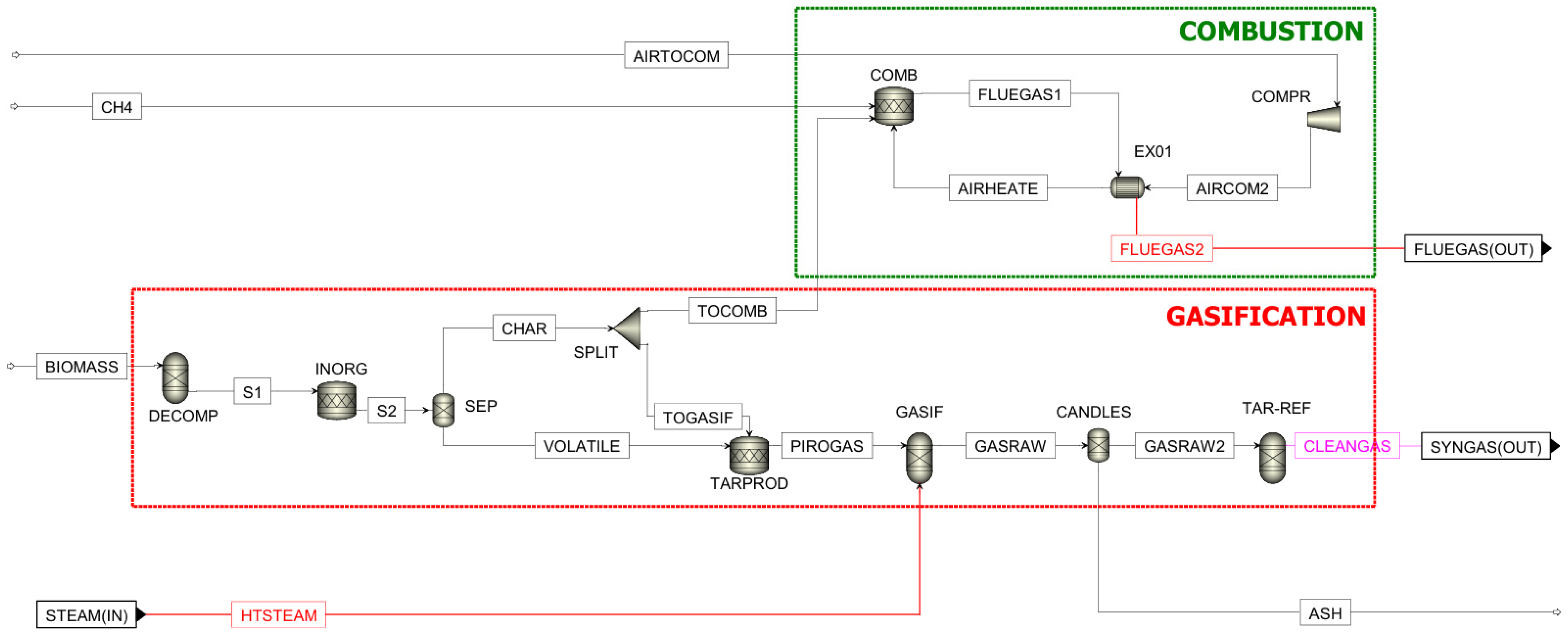
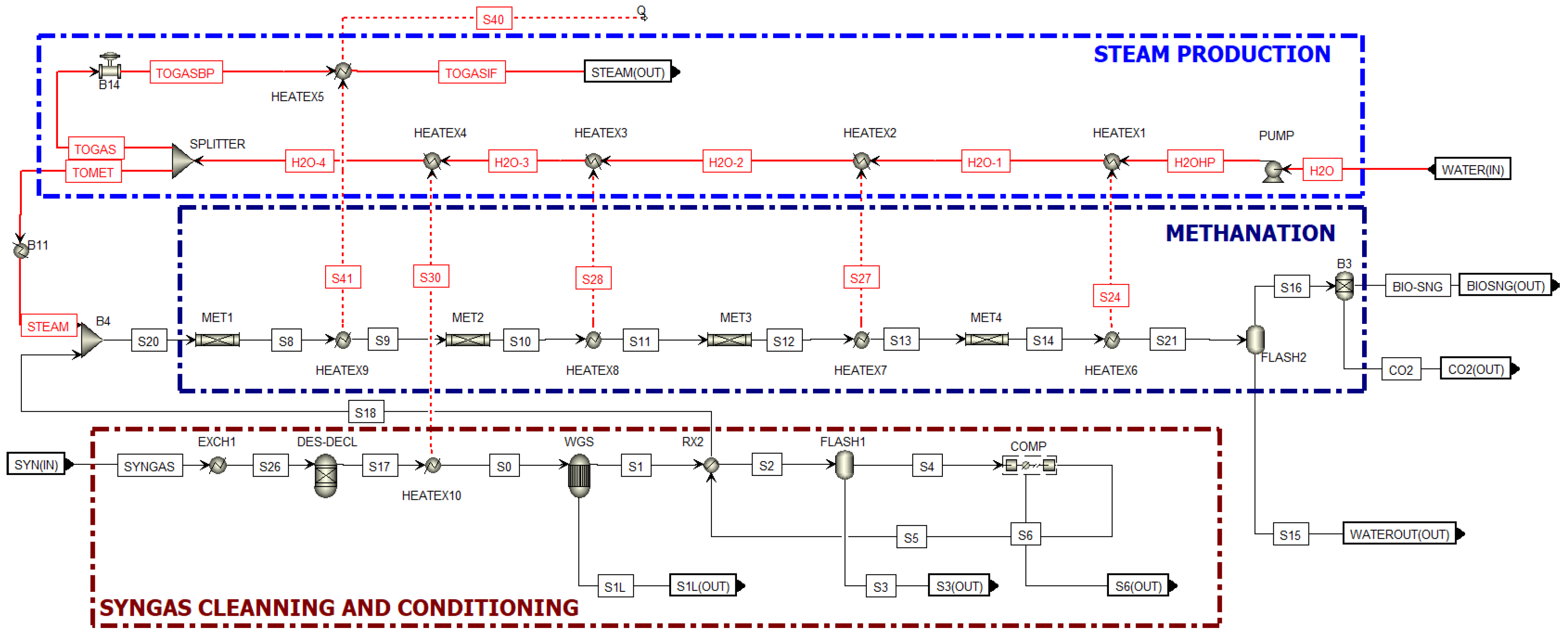
| Ultimate Analysis | Proximate Analysis | ||
|---|---|---|---|
| Ash (wt.%dry) | 1.20 | Moisture (wt.%) | 7.90 |
| Carbon (wt.%dry) | 50.96 | Fixed Carbon (wt.%dry) | 23.3 |
| Hydrogen (wt.%dry) | 5.72 | Volatile Matter (wt.%dry) | 75.5 |
| Nitrogen (wt.%dry) | 0.42 | Ash (wt.%dry) | 1.20 |
| Chlorine (wt.%dry) | 0.02 | ||
| Sulfur (wt.%dry) | 0.03 | HHV (MJ/kg) | 19.27 |
| Oxygen (wt.%dry) | 41.65 | ||
| ASPEN Plus® Unit | Block ID | Description |
|---|---|---|
| RYIELD | DECOMP | Converts the non-conventional stream “BIOMASS” into conventional components, from which weight fraction is calculated according to biomass characterization. |
| RSTOIC | INORG | Converts the inorganic components of the biomass (N2, S, Cl) into syngas contaminants (NH3, H2S, HCL). |
| TARPROD | Simulates tar content produced during the process by establishing the conversion of C and H2 obtained in the previous step. | |
| COMB | Converts char and CH4 to produce energy for gasification. | |
| SEP | SEP | Separates char from volatile compounds. |
| CANDLES | Separates ashes from the raw gas. | |
| RGIBBS | GASIF | Produces gaseous fuel. |
| TAR-REF | Produces H2 from tar. | |
| FSPLIT | SPLIT | Divides the char stream, sending some to the combustion chamber and some to the TARPROD reactor. |
| COMPR | COMPAIR | Isentropic compressor that exerts slight overpressure. |
| HEATX | EX01 | Recovers the sensible heat in the flue gas leaving the combustor by bringing the air to combustion temperature. |
| ASPEN Plus® Unit | Block ID | Description |
|---|---|---|
| RGIBBS | DES-DECL | Removes H2S and HCl. |
| REQUIL | WGS | Adiabatic reactor where the WGS reaction takes place. |
| RPLUG | MET1, MET2, MET3, MET4 | Adiabatic reactors where the Bio-SNG is produced. |
| PUMP | PUMP1 | Water is pressurized from 1 to 20 atm. |
| HEATX | EXCH1, C1, RX2, C2, C3, C4, C5 | Heat exchanger. |
| FLASH | FLASH1–FLASH2 | Flash separator used to separate water from the gas. |
| SEP | B3 | Separator for CO2 removal from Bio-SNG. |
| MCOMPR | COMP | Gas undergoes methanation and is compressed from 1 to 20 atm. |
| MIXER | B4 | Gas and steam are mixed before entering the methanators. |
| H2 (wt.%) | H2O (wt.%) | O2 (wt.%) | C (wt.%) | N2 (wt.%) | S (wt.%) | Cl (wt.%) | Ash (wt.%) |
|---|---|---|---|---|---|---|---|
| 5.27 | 7.90 | 38.36 | 46.93 | 0.39 | 0.03 | 0.02 | 1.11 |
| T (°C) | 612 |
| H2 (mol.%, dry basis) | 51.6% |
| CO (mol.%, dry basis) | 18.7% |
| CO2 (mol.%, dry basis) | 22.3% |
| CH4 (mol.%, dry basis) | 7.4% |
| HHV (MJ/kg) | 12.25 |
| Stream | Temperature (°C) | Energy Content (kW) | Sensible Heat (kW) | |
|---|---|---|---|---|
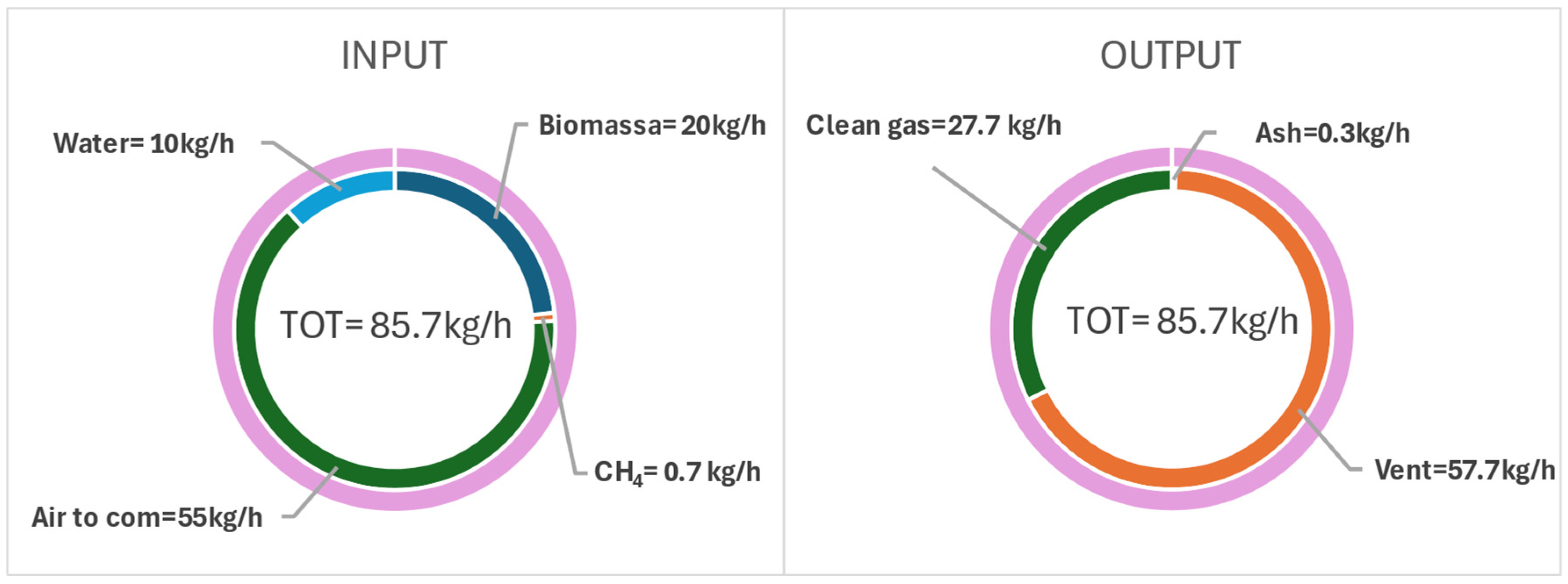
| INPUT | Biomass | 25 | 100.03 | - |
| Methane | 25 | 10.80 | - | |
| OUTPUT | Vent | 88.06 | 1.56 | |
| Clean gas | 612 | 93.75 | 12.78 | |
| Ash | 850 | 1.85 | 0.05 |
| Block Name | Energy Duty (kW) |
|---|---|
| DECOMP | 38.14 |
| TARPROD | 0.05 |
| GASIF | −20.7 |
| COMB | −18.41 |
| INORG | −0.10 |
| H2 (%mol) | CO (%mol) | CO2 (%mol) | CH4 (%mol) | H2O (%mol) | N2 (%mol) | HHV (MJ/kg) |
|---|---|---|---|---|---|---|
| 10.4 | 0.4 | 4.2 | 83.8 | 0.7 | 0.6 | 48.9 |
| Stream | Temperature (°C) | Energy Content (kW) | Sensible Heat (kW) | |
|---|---|---|---|---|
| INPUT | Clean gas | 612 | 94.56 | 12.78 |
| OUTPUT | Bio-SNG | 40.0 | 79.63 | 0.01 |
| Block Name | Energy Duty (kW) |
|---|---|
| C1 (heater) | −2.36 |
| FLASH1 | −1.73 |
| C2 (heater) | −5.77 |
| C3 (heater) | −0.82 |
| C4 (heater) | −3.44 |
| C5 (heater) | −3.49 |
| FLASH2 (Flash) | −9.01 |
| B3 (Sep) | −0.03 |
| Stream | Water2 | Water3 | Steam | HTSTEAM |
|---|---|---|---|---|
| From | Pump | ECONOM | GENVAP | EX03 |
| To | ECONOM | GENVAP | EX03 | GASIF |
| Temperature (°C) | 20 | 112 | 112 | 450 |
| Heat duty (kW) | 1.16 | 6.4 | 1.88 |
| Stream | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S21 |
|---|---|---|---|---|---|---|---|---|
| From | MET1 | C2 | MET2 | C3 | MET3 | C4 | MET4 | C5 |
| To | C2 | MET2 | C3 | MET3 | C4 | MET4 | C5 | FLASH2 |
| Temperature (°C) | 630.7 | 290 | 342 | 290 | 504.2 | 290 | 385.5 | 150.5 |
| Stream | Temperature (°C) | Energy Content (kW) | Sensible Heat (kW) | |
|---|---|---|---|---|
| INPUT | Biomass | 25 | 100.03 | - |
| OUTPUT | Flue gas | 105 | - | 1.27 |
| Clean gas | 615.1 | 94.56 | 12.78 | |
| Ash | 850 | 1.85 | 0.05 | |
| Bio-SNG | 40 | 79.62 | 0.01 |
| Block Name | Heat Duty (kW) |
|---|---|
| DECOMP | 38.19 |
| TARPROD | 0.05 |
| GASIF | −21.56 |
| COMB | −17.89 |
| INORG | −0.10 |
| B13 (heater) | −2.14 |
| B12 (heater) | −3.56 |
| FLASH1 | −1.73 |
| FLASH2 (Flash) | −9.49 |
| B3 (Sep) | −0.03 |
| B11 (heater) | 0.50 |
| CGEGasification | 94% |
| CGEMethanation | 84% |
| CGEOverall | 79% |
| Syngas yield wet | 1.78 | Nm3Syngas/kgBio |
| Syngas yield dry | 1.43 | Nm3Syngas/kgBio |
| Bio-SNG yield | 0.29 | Nm3Bio-SNG_dry/Nm3CleanGas_dry |
| Overall Bio-SNG yield | 0.41 | Nm3Bio-SNG_dry/kgBio |
| Bio-SNG methane content | 83.9 | vol.% |
| (Equation (18)) | 84.2 | - |
| (Equation (19)) | 79.6 | - |
| Bartik et al. [22] | Wan et al. [24] | |||||
|---|---|---|---|---|---|---|
| Aspen Plus Modeling | Experimental Investigation | ASPEN Plus Modeling | ||||
| (Case I) | (Case II) | (Case III) | (Case IV) | |||
| CGE (Equation (15)) | 79% | 58% | 58–59% | 58–59% | 66% | 61% |
| CH4 (vol.%) | 85.5% | 42.0% | 62.40% | 58.80% | 70.00% | 96.11% |
| CO (vol.%) | 0.3% | 0% | 0.3% | 0.3% | 0.3% | 0.00% |
| CO2 (vol.%) | 4.1% | 46.0% | 11.0% | 6.0% | 17.7% | 3.47% |
| H2 (vol.%) | 10.2% | 12.0% | 26.30% | 34.50% | 12.00% | 0.42% |
| Stechiometrich Number: SN (*) | 0.91 | 1.04 | ||||
| Methanation Temperature | 290 °C | 360 °C | 358 °C | 364 °C | 342 °C | 300 °C |
| Methanation Pressure | 10 bar | 1 bar | 1 bar | 1 bar | 1 bar | 30 bar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bisceglie, E.; Papa, A.A.; Vitale, A.; Pasqual Laverdura, U.; Di Carlo, A.; Bocci, E. Optimization of Biomass to Bio-Syntetic Natural Gas Production: Modeling and Assessment of the AIRE Project Plant Concept. Energies 2025, 18, 753. https://doi.org/10.3390/en18030753
Di Bisceglie E, Papa AA, Vitale A, Pasqual Laverdura U, Di Carlo A, Bocci E. Optimization of Biomass to Bio-Syntetic Natural Gas Production: Modeling and Assessment of the AIRE Project Plant Concept. Energies. 2025; 18(3):753. https://doi.org/10.3390/en18030753
Chicago/Turabian StyleDi Bisceglie, Emanuele, Alessandro Antonio Papa, Armando Vitale, Umberto Pasqual Laverdura, Andrea Di Carlo, and Enrico Bocci. 2025. "Optimization of Biomass to Bio-Syntetic Natural Gas Production: Modeling and Assessment of the AIRE Project Plant Concept" Energies 18, no. 3: 753. https://doi.org/10.3390/en18030753
APA StyleDi Bisceglie, E., Papa, A. A., Vitale, A., Pasqual Laverdura, U., Di Carlo, A., & Bocci, E. (2025). Optimization of Biomass to Bio-Syntetic Natural Gas Production: Modeling and Assessment of the AIRE Project Plant Concept. Energies, 18(3), 753. https://doi.org/10.3390/en18030753












