Methane Production, Microbial Community, and Volatile Fatty Acids Profiling During Anaerobic Digestion Under Different Organic Loading
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Anaerobic Digestion Performance
2.3. Analytical Methods
2.4. Illumina MiSeq Sequencing
3. Results and Discussion
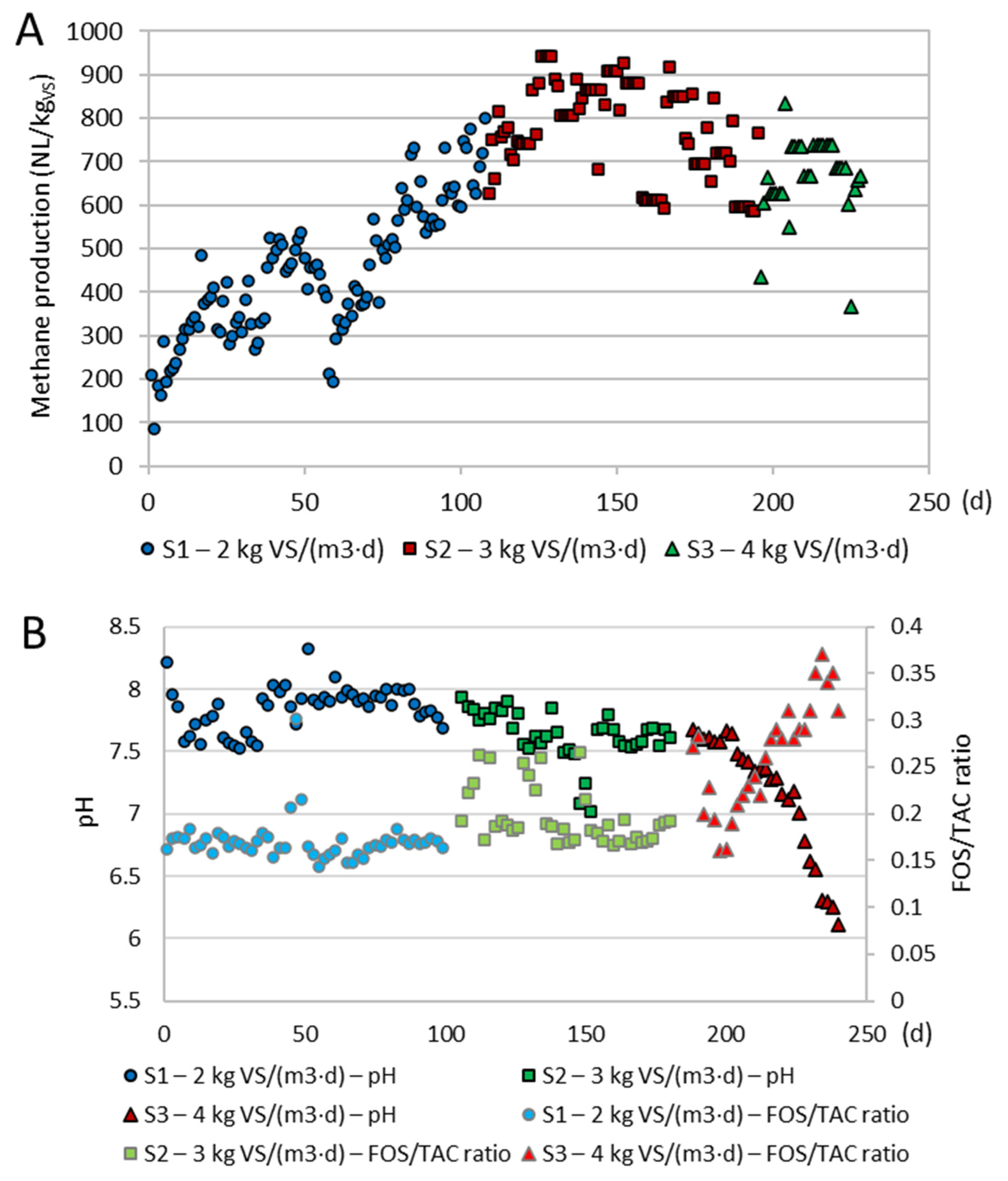
3.1. Long-Term Anaerobic Digestion
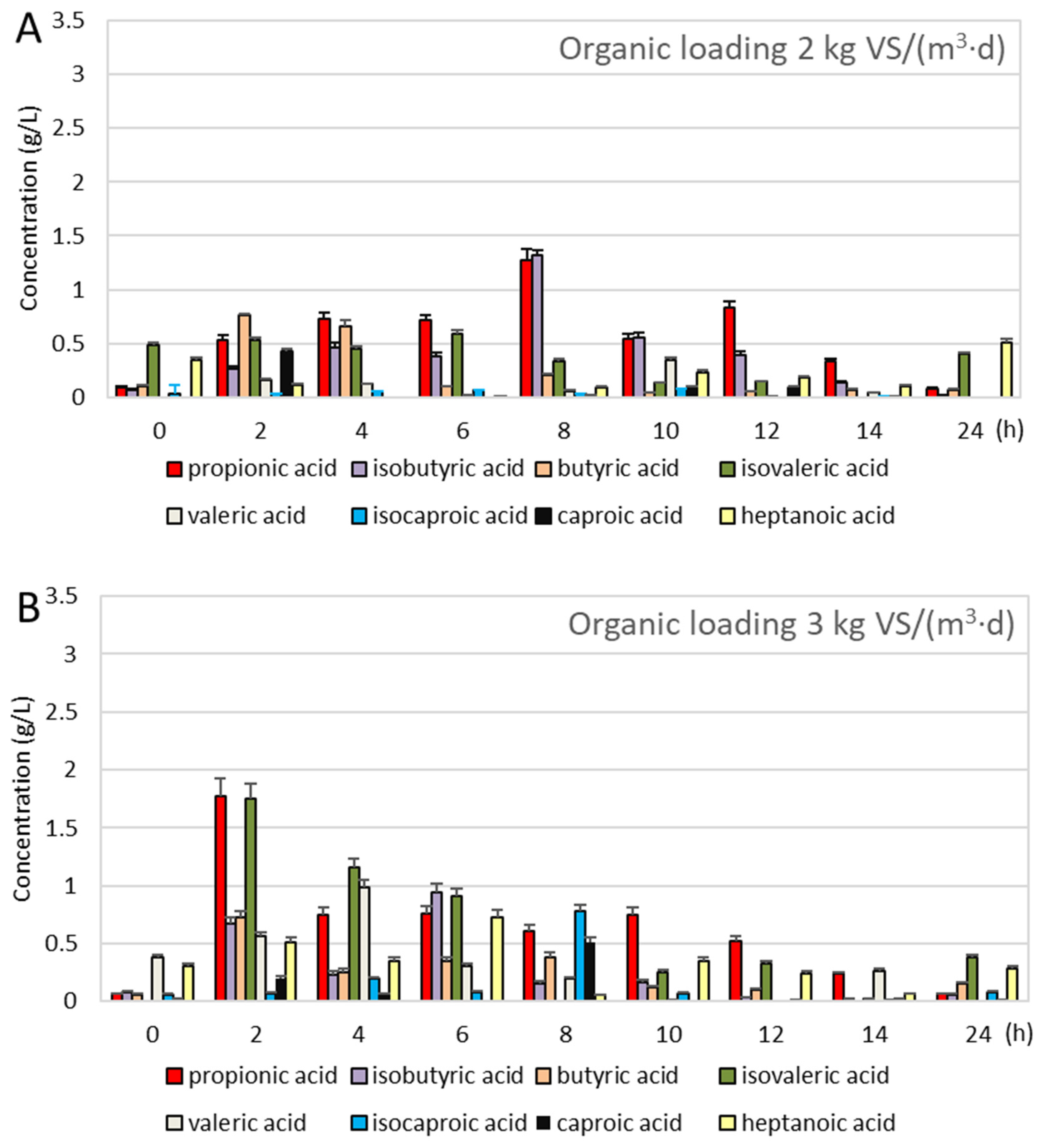
3.2. Anaerobic Digestion During the Operation Cycle
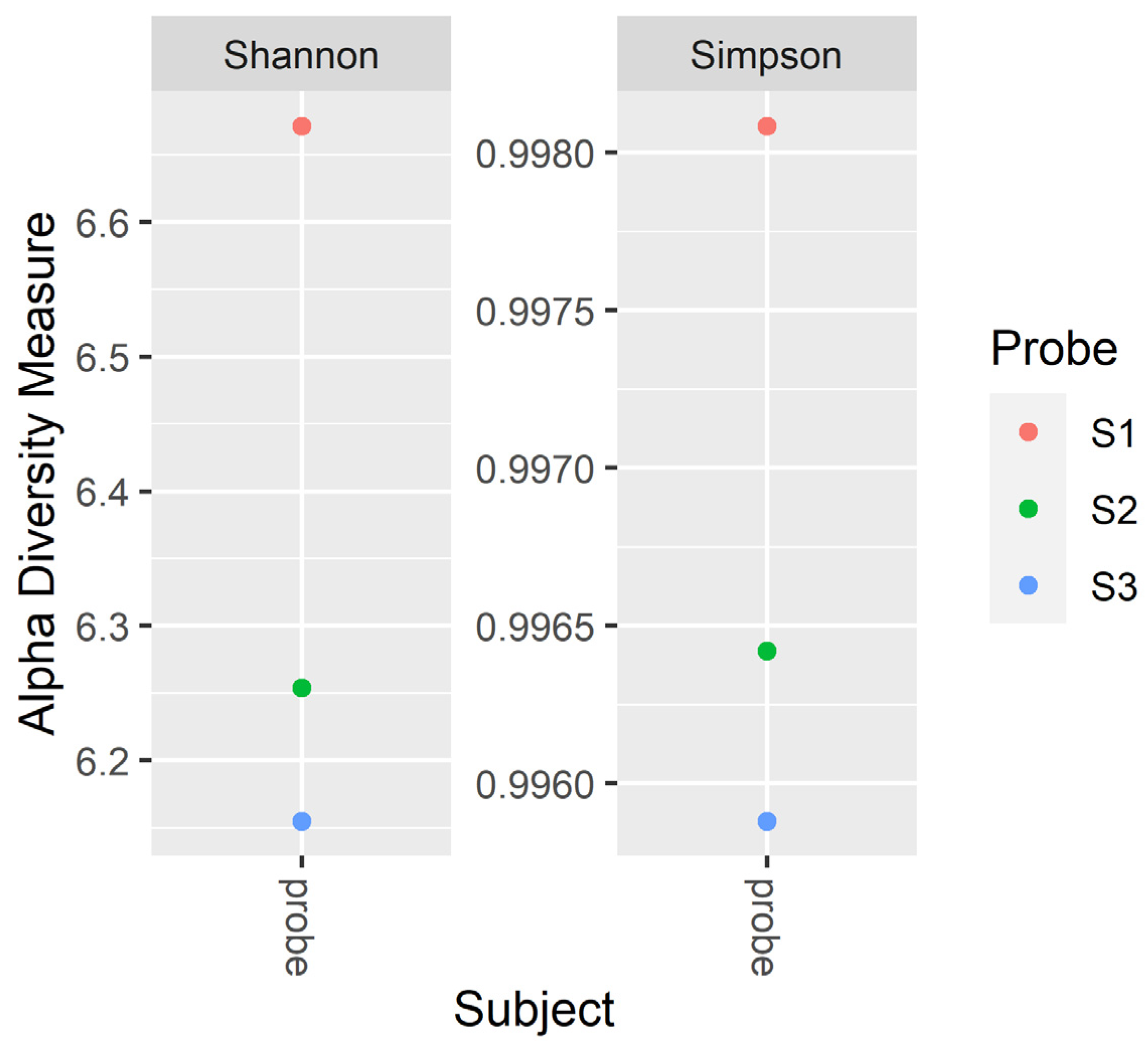
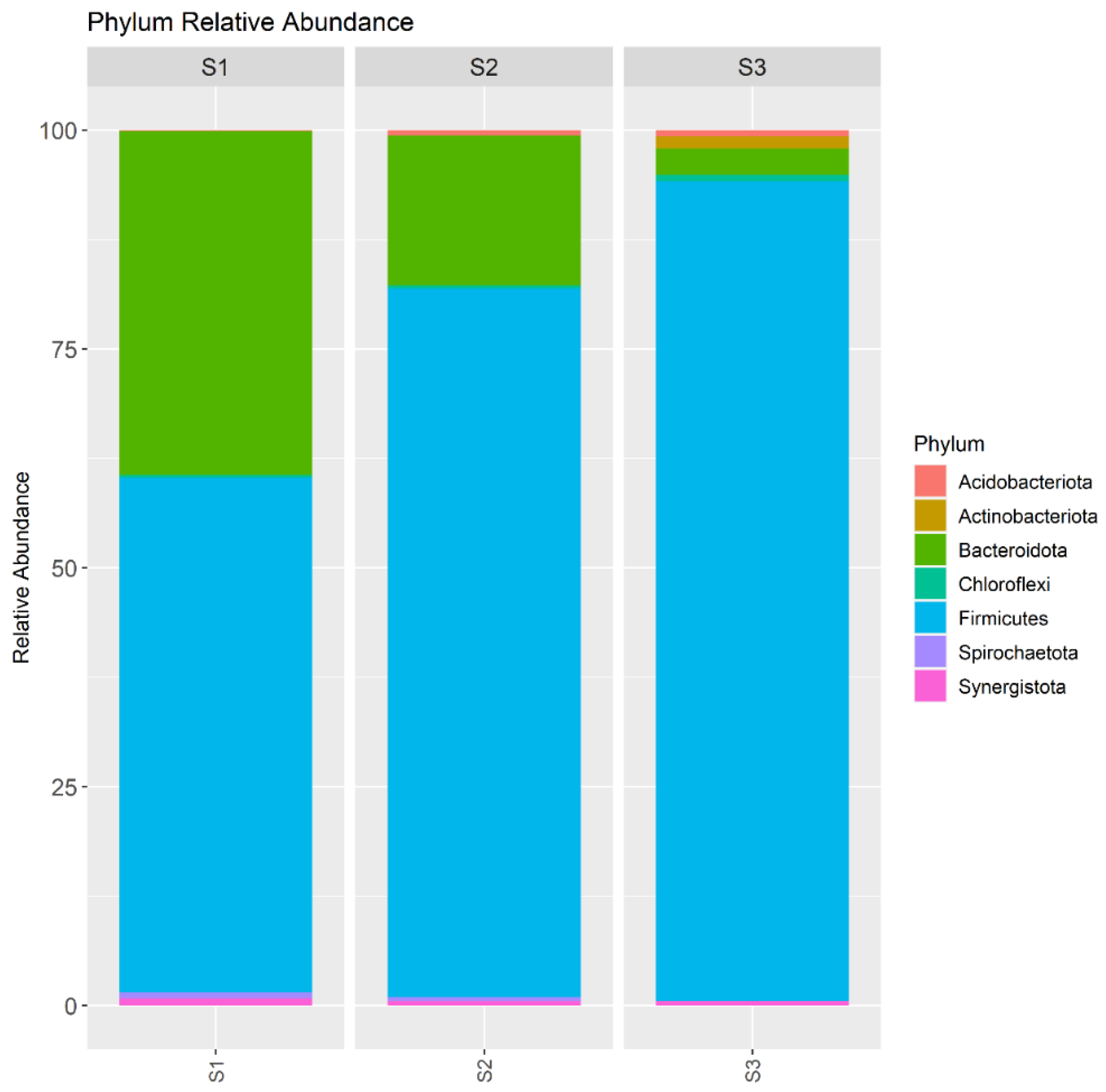
3.3. Microbial Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borkowska, H.; Molas, R. Two Extremely Different Crops, Salix and Sida, as Sources of Renewable Bioenergy. Biomass Bioenergy 2012, 36, 234–240. [Google Scholar] [CrossRef]
- Zielinski, M.; Rusanowska, P.; Krzywik, A.; Dudek, M.; Nowicka, A.; Dębowski, M. Application of Hydrodynamic Cavitation for Improving Methane Fermentation of Sida hermaphrodita Silage. Energies 2019, 12, 526. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of Microwave Thermohydrolysis and Liquid Hot Water Pretreatment of Energy Crop Sida hermaphrodita for Enhanced Methane Production. Biomass Bioenergy 2019, 128, 105324. [Google Scholar] [CrossRef]
- Marta, K.; Paulina, R.; Magda, D.; Anna, N.; Aleksandra, K.; Marcin, D.; Kazimierowicz, J.; Marcin, Z. Evaluation of Ultrasound Pretreatment for Enhanced Anaerobic Digestion of Sida hermaphrodita. Bioenerg. Res. 2020, 13, 824–832. [Google Scholar] [CrossRef]
- Barredo, M.S.; Evison, L.M. Effect of Propionate Toxicity on Methanogen-Enriched Sludge, Methanobrevibacter Smithii, and Methanospirillum Hungatii at Different PH Values. Appl. Environ. Microbiol. 1991, 57, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Thakur, N.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Saif, I.; Ali, G.; Zheng, Y.; Salama, E. Facilitated Lignocellulosic Biomass Digestibility in Anaerobic Digestion for Biomethane Production: Microbial Communities’ Structure and Interactions. J. Chem. Technol. Biotechnol. 2021, 96, 1798–1817. [Google Scholar] [CrossRef]
- Ünyay, H.; Yılmaz, F.; Başar, İ.A.; Altınay Perendeci, N.; Çoban, I.; Şahinkaya, E. Effects of Organic Loading Rate on Methane Production from Switchgrass in Batch and Semi-Continuous Stirred Tank Reactor System. Biomass Bioenergy 2022, 156, 106306. [Google Scholar] [CrossRef]
- Zieliński, M.; Rusanowska, P.; Zielińska, M.; Dudek, M.; Nowicka, A.; Purwin, C.; Fijałkowska, M.; Dębowski, M. Influence of Preparation of Sida hermaphrodita Silages on Its Conversion to Methane. Renew. Energy 2021, 163, 437–444. [Google Scholar] [CrossRef]
- Chamy, R.; Ramos, C. Factors in the determination of methanogenic potential of manure. Bioresour. Technol. 2011, 102, 7673–7677. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zielińska, M.; Zieliński, Z. Improvement of Biohydrogen Production Using a Reduced Pressure Fermentation. Bioprocess Biosyst. Eng. 2015, 38, 1925–1933. [Google Scholar] [CrossRef]
- Motulsky, H.J. Analyzing Data with GraphPad Prism; GraphPad Software Inc.: San Diego CA, USA, 1999; Available online: www.graphpad.com (accessed on 20 November 2024).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Rg Peplies, J.; Glö Ckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Mcmurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Nordberg, Å.; Jarvis, Å.; Stenberg, B.; Mathisen, B.; Svensson, B.H. Anaerobic Digestion of Alfalfa Silage with Recirculation of Process Liquid. Bioresour. Technol. 2007, 98, 104–111. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Huttunen, S.; Rintala, J.A. Laboratory Investigations on Co-Digestion of Energy Crops and Crop Residues with Cow Manure for Methane Production: Effect of Crop to Manure Ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Krishna, D.; Kalamdhad, A.S. Pre-Treatment and Anaerobic Digestion of Food Waste for High Rate Methane Production—A Review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Ren, N.; Liu, M.; Wang, A.; Ding, J.; Li, H. Organic Acids Conversion in Methanogenic-Phase Reactor of the Two-Phase Anaerobic Process. Huan Jing Ke Xue 2003, 24, 89–93. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of Volatile Fatty Acid Concentrations on Methane Yield and Methanogenic Bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Marchaim, U.; Krause, C. Propionic to Acetic Acid Ratios in Overloaded Anaerobic Digestion. Bioresour. Technol. 1993, 43, 195–203. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the Anaerobic Digestion of Food Waste for Biogas Production. Renew. Sustain. Energ. Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Otite, S.V.; Lag-Brotons, A.J.; Ezemonye, L.I.; Martin, A.D.; Pickup, R.W.; Semple, K.T. Volatile Fatty Acids Effective as Antibacterial Agents against Three Enteric Bacteria during Mesophilic Anaerobic Incubation. Molecules 2024, 29, 1908. [Google Scholar] [CrossRef] [PubMed]
- Wambugu, C.W.; Rene, E.R.; Van de Vossenberg, J.; Dupont, C.; van Hullebusch, E.D. Biochar from Various Lignocellulosic Biomass Wastes as an Additive in Biogas Production from Food Waste. In Waste Biorefinery: Integrating Biorefineries for Waste Valorisation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–217. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, H.; Wyckoff, K.N.; He, Q. Linkages of Firmicutes and Bacteroidetes Populations to Methanogenic Process Performance. J. Ind. Microbiol. Biotechnol. 2016, 43, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile Fatty Acids Production via Mixed Culture Fermentation: Revealing the Link between PH, Inoculum Type and Bacterial Composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef] [PubMed]
- Widyasti, E.; Shikata, A.; Hashim, R.; Sulaiman, O.; Sudesh, K.; Wahjono, E.; Kosugi, A. Biodegradation of Fibrillated Oil Palm Trunk Fiber by a Novel Thermophilic, Anaerobic, Xylanolytic Bacterium Caldicoprobacter sp. CL-2 Isolated from Compost. Enzym. Microb. Technol. 2018, 111, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.G.; Li, Q.; Wang, L.L.; Chen, Q.L.; Hu, H.W.; Cheng, D.J.; He, J.Z. Semi-Solid State Promotes the Methane Production during Anaerobic Co-Digestion of Chicken Manure with Corn Straw Comparison to Wet and High-Solid State. J. Environ. Manag. 2022, 316, 115264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Zhan, X.; Gardiner, G.E. Exploring the Roles of and Interactions among Microbes in Dry Co-Digestion of Food Waste and Pig Manure Using High-Throughput 16S RRNA Gene Amplicon Sequencing. Biotechnol. Biofuels 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.; Yulisa, A.; Hwang, S. Microbial Community Structure in Full Scale Anaerobic Mono-and Co-Digesters Treating Food Waste and Animal Waste. Bioresour. Technol. 2019, 282, 439–446. [Google Scholar] [CrossRef]
- Moestedt, J.; Westerholm, M.; Isaksson, S.; Schnürer, A. Inoculum Source Determines Acetate and Lactate Production during Anaerobic Digestion of Sewage Sludge and Food Waste. Bioengineering 2019, 7, 3. [Google Scholar] [CrossRef]
- Ghosh, P.; Kumar, M.; Kapoor, R.; Kumar, S.S.; Singh, L.; Vijay, V.; Vijay, V.K.; Kumar, V.; Thakur, I.S. Enhanced Biogas Production from Municipal Solid Waste via Co-Digestion with Sewage Sludge and Metabolic Pathway Analysis. Bioresour. Technol. 2020, 296, 122275. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cao, Q.; Sun, Y.; Huang, S.; Liu, X.; Li, D. 16S RRNA Genes- and Metagenome-Based Confirmation of Syntrophic Butyrate-Oxidizing Methanogenesis Enriched in High Butyrate Loading. Bioresour. Technol. 2022, 345, 126483. [Google Scholar] [CrossRef] [PubMed]


| Ratio of Silage/Cattle Manure/Water (Fresh Mass kg) (±2%) | Silage/Cattle Manure (Dry Mass kg % of Fresh Mass) | Silage/Cattle Manure (Dry Organic Mass % of Dry Mass) | Organic Load (kgVS/(m3·d)) | Hydraulic Retention Time (d) | |
|---|---|---|---|---|---|
| Serie 1 (S1) | 3.0/1.5/1.5 | 32% ± 1%/10% ± 1% | 90% ± 5%/85% ± 4% | 2 | 50.0 |
| Serie 2 (S2) | 4.5/2.25/2.25 | 3 | 37.5 | ||
| Serie 3 (S3) | 6.0/3.0/3.0 | 4 | 25.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanowska, P.; Zieliński, M.; Kisielewska, M.; Dudek, M.; Paukszto, Ł.; Dębowski, M. Methane Production, Microbial Community, and Volatile Fatty Acids Profiling During Anaerobic Digestion Under Different Organic Loading. Energies 2025, 18, 575. https://doi.org/10.3390/en18030575
Rusanowska P, Zieliński M, Kisielewska M, Dudek M, Paukszto Ł, Dębowski M. Methane Production, Microbial Community, and Volatile Fatty Acids Profiling During Anaerobic Digestion Under Different Organic Loading. Energies. 2025; 18(3):575. https://doi.org/10.3390/en18030575
Chicago/Turabian StyleRusanowska, Paulina, Marcin Zieliński, Marta Kisielewska, Magda Dudek, Łukasz Paukszto, and Marcin Dębowski. 2025. "Methane Production, Microbial Community, and Volatile Fatty Acids Profiling During Anaerobic Digestion Under Different Organic Loading" Energies 18, no. 3: 575. https://doi.org/10.3390/en18030575
APA StyleRusanowska, P., Zieliński, M., Kisielewska, M., Dudek, M., Paukszto, Ł., & Dębowski, M. (2025). Methane Production, Microbial Community, and Volatile Fatty Acids Profiling During Anaerobic Digestion Under Different Organic Loading. Energies, 18(3), 575. https://doi.org/10.3390/en18030575








