The Use of Coniferous Tree Cone Biomass as an Energy Source and a Reducing Agent in the Recycling of Metals from Oxide Secondary Raw Materials
Abstract
1. Introduction
2. Materials and Methods
- Characterization of the energetic characteristics, i.e., the determination of the heat of combustion of pine cones in calorimetric tests;
- Characterization of physical changes and their courses under high-temperature conditions, i.e., conducting thermogravimetric analysis (TGA) in both inert and oxidizing atmospheres;
- Verification of process suitability by performing reductive melting of metallurgical slags using pine cone biomass as a reductor.
2.1. Research Material
- Fe1.966O2.963—tetragonal maghemite;
- Cu2O—regular crystal-structured cuprite;
- Al2PbSi2O8 of monoclinic structure;
- PbO—lead (II) oxide orthorhombic structure (massicot).
2.2. Method
2.2.1. Heat of Combustion
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Reductive Melting Process
3. Results and Discussion
3.1. Calorimetry
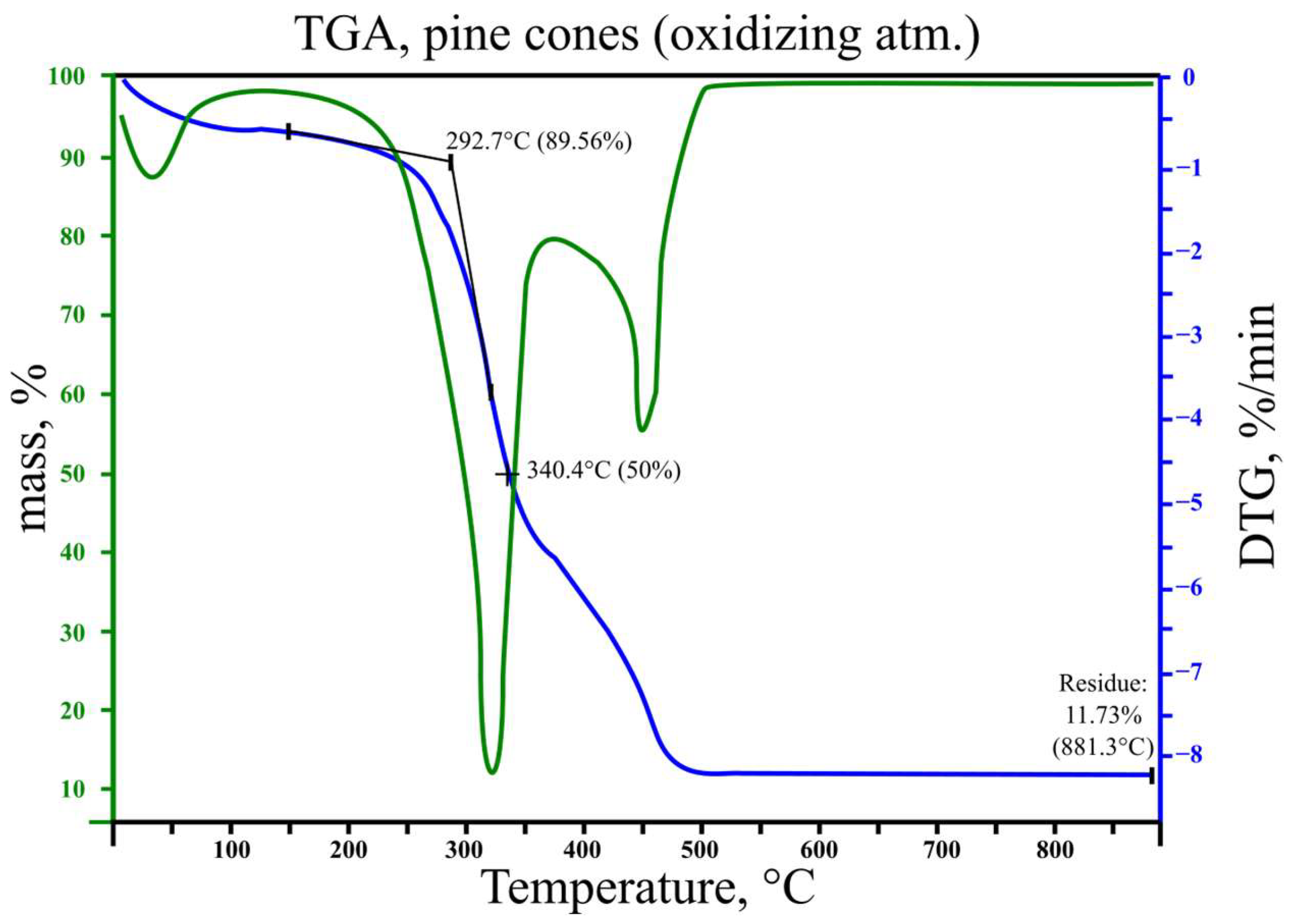
3.2. Results of Thermogravimetric Analysis
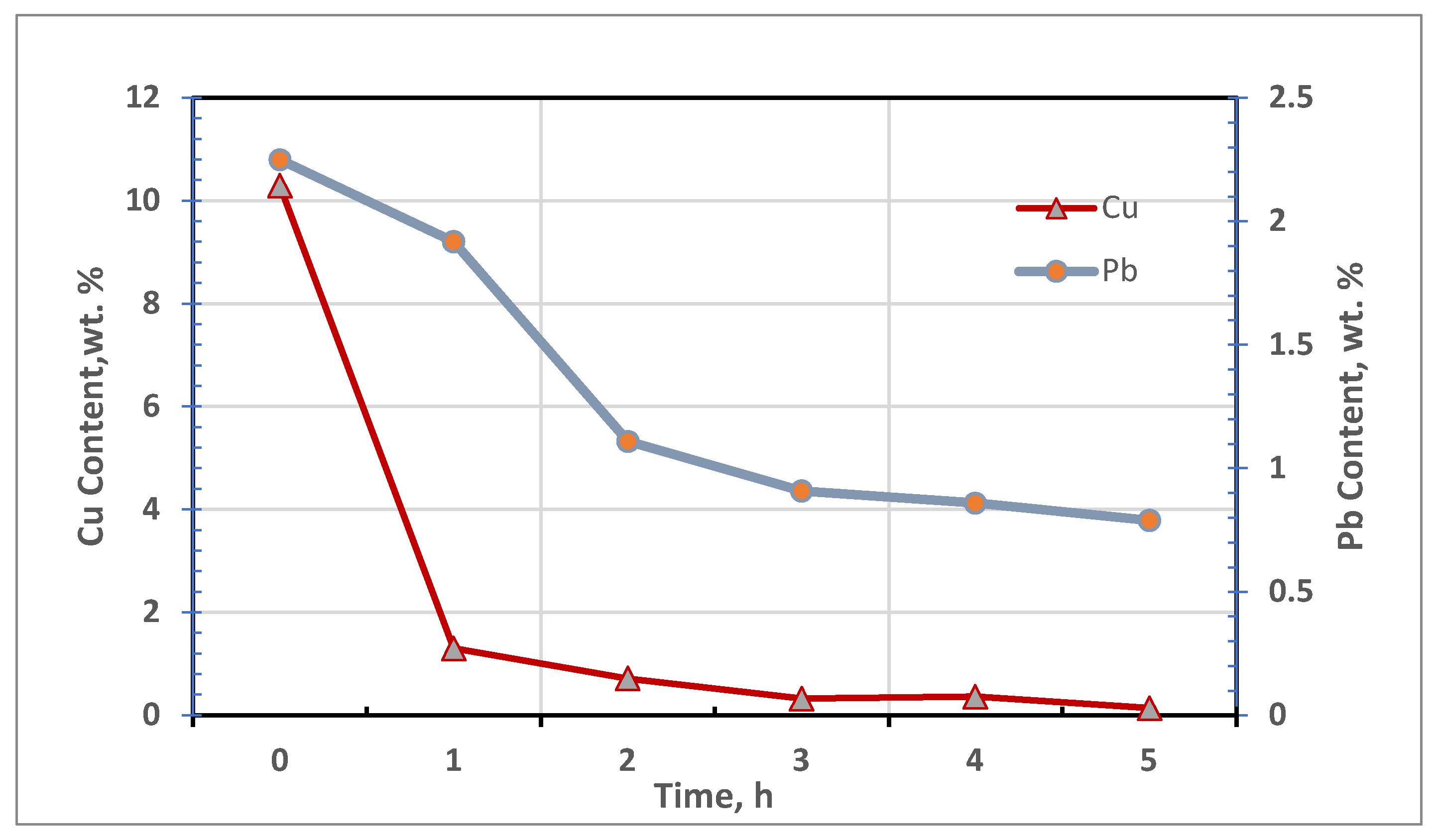
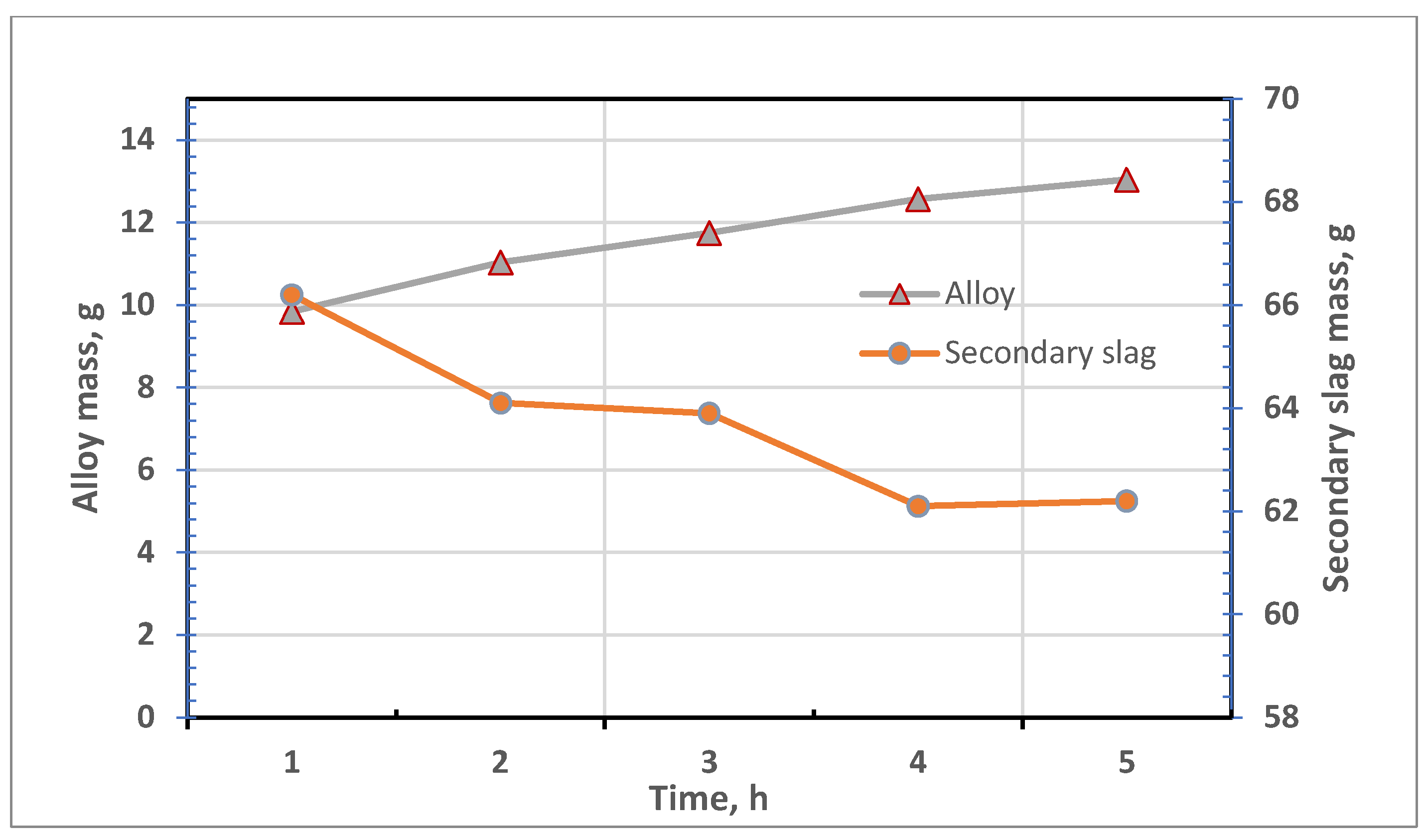
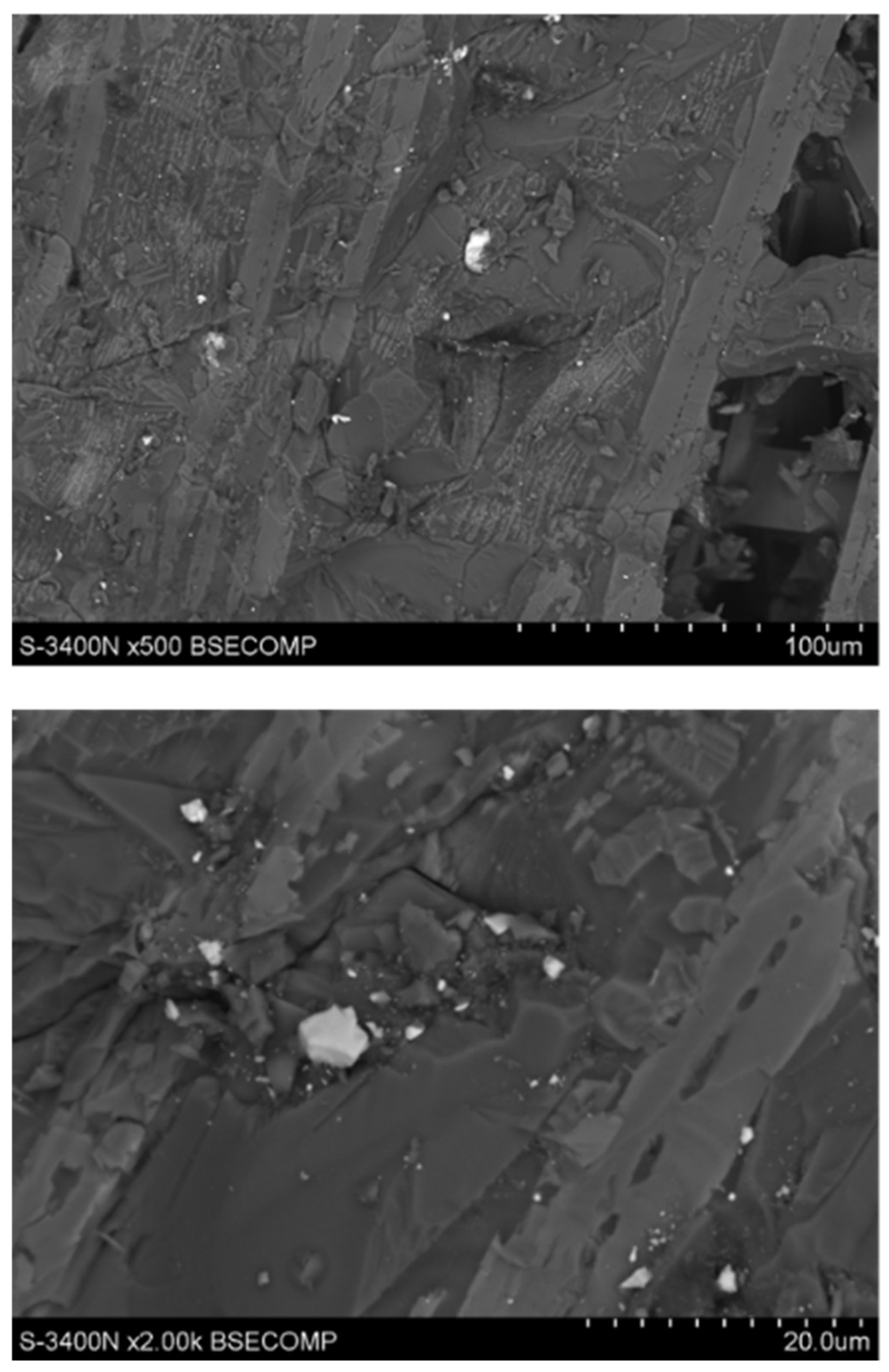
3.3. Results of Reductive Melting Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chudy, R.; Szuleck, K.; Siry, J.; Grala, R. Woody Biomass for Energy Production. Acad. Mag. Pol. Acad. Sci. 2021, 69, 62–65. [Google Scholar] [CrossRef]
- Aniszewska, M.; Gendek, A. Logistics of the supplies of selected forest tree species’cones. Part 1. Cone density and substitution coefficient. Ann. Wars Univ. Life Sci.-SGGW Agric. 2016, 67, 121–130. [Google Scholar]
- Aniszewska, M.; Gendek, A. Logistics of delivery of cones of selected species of foresttrees. Part 2: Cone transport. Ann. Wars Univ. Life Sci.-SGGW Agric. 2016, 68, 13–21. [Google Scholar]
- Kwiatkowski, J.; Sztejna, Z. Energy Efficiency of Conifer Cones and Seed Extraction Residue Biomass. Sustainability 2024, 16, 2693. [Google Scholar] [CrossRef]
- Malaťák, J.; Gendek, A.; Aniszewska, M.; Velebil, J. Emissions from combustion of renewable solid biofuels from coniferous tree cones. Fuel 2020, 276, 118001. [Google Scholar] [CrossRef]
- Chand, P.; Arya, D.; Singh, J. Pine Cone Biomass as a Potential Source of Bioenergy. Int. J. Adv. Eng. Manag. IJAEM 2024, 6, 776–780. [Google Scholar]
- Kumar, R.; Bhaduri, G.A. Biorefining of pine cone forest waste: Ultrasound assisted extraction followed with thermal degradation for a zero waste process. Ind. Crop. Prod. 2024, 224, 120278. [Google Scholar] [CrossRef]
- Boutaieb, M.; Guiza, M.; Román, S.; Nogales, S.; Ledesma, B.; Ouederni, A. Pine cone pyrolysis: Optimization of temperature for energy recovery. Environ. Prog. Sustain. Energy 2020, 39, 13272. [Google Scholar] [CrossRef]
- Li, K.; Tian, S.; Jiang, J.; Wang, J.; Chen, X.; Yan, F. Pine cone shell-based activated carbon used for CO2 adsorption. J. Mater. Chem. A 2016, 4, 5223–5234. [Google Scholar] [CrossRef]
- Gomez-Delgado, E.; Nunell, G.; Cukierman, A.L.; Bonelli, P. Tailoring activated carbons from pinus canariensis cones for post-combustion CO2 captureEnviron. Environ. Sci. Pollut. Res. 2020, 27, 13915–13929. [Google Scholar] [CrossRef]
- Nowicki, P.; Kuszyńska, I.; Przepiórski, J.; Pietrzak, R. The effect of chemical activation method on properties of activated carbons obtained from pine cones. Cent. Eur. J. Chem. 2013, 11, 78–85. [Google Scholar] [CrossRef]
- Muslim, A. Australian pine cones-based activated carbon for adsorption of copper in aqueous solution. J. Eng. Sci. Technol. 2017, 12, 280–295. [Google Scholar]
- Duman, G.; Onal, Y.; Okutucu, C.; Onenc, S.; Yanik, J. Production of activated carbon from pine cone and evaluation of its physical, chemical, and adsorption properties. Energy Fuels 2009, 23, 2197–2204. [Google Scholar] [CrossRef]
- Özhan, A.; Şahin, Ö.; Küçük, M.M.; Saka, C. Preparation and characterization of activated carbon from pine cone by microwave-induced ZnCl2 activation and its effects on the adsorption of methylene blue. Cellulose 2014, 21, 2457–2467. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Chen, H.; Chen, M.; Zhao, Y. Potential of bioethanol production from pine cones using enzymatic hydrolysis and fermentation. Bioresour. Technol. Rep. 2019, 6, 1622. [Google Scholar]
- Baatache, O.; Derbal, K.; Benalia, A.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Pine cone as bio-coagulant for heavy metal removal from industrial wastewater: Use of Box–Behnken design. Ind. Crops Prod. 2024, 210, 18185. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Aberkane, I.; Guizah, Q.E.; Khalfaoui, A.; Pizzi, A. Valorization of pine cones (Pinus nigras) for industrial wastewater treatment and crystal violet removal: A sustainable approach based on bio-coagulants and a bio-adsorbent. Water 2024, 16, 260. [Google Scholar] [CrossRef]
- Almendros, A.I.; Martín-Lara, M.A.; Ronda, A.; Perez, A.; Blázquez, G.; Calero, M. Physico-chemical characterization of pine cone shell and its use as biosorbent and fuel. Bioresour. Technol. 2015, 196, 406–412. [Google Scholar] [CrossRef]
- Trifol, J.; Quintero, D.C.M.; Moriana, R. Pine Cone Biorefinery: Integral Valorization of Residual Biomass into Lignocellulose Nanofibrils (LCNF)-Reinforced Composites for Packaging. ACS Sustain. Chem. Eng. 2021, 9, 2180–2190. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef]
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and Characterization of Cellulose Nanocrystals from Spruce Bark in a Biorefinery Perspective. Carbohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef]
- García-García, D.; Balart, R.; Lopez-Martinez, J.; Ek, M.; Moriana, R. Optimizing the Yield and Physico-Chemical Properties of Pine Cone Cellulose Nanocrystals by Different Hydrolysis Time. Cellulose 2018, 25, 2925–2938. [Google Scholar] [CrossRef]
- Yuan, P.; Shen, B.; Duan, D.; Adwek, G.; Mei, X.; Lu, F. Study on the formation of direct reduced iron by using biomass as reductants of carbon containing pellets in RHF process. Energy 2017, 141, 472–482. [Google Scholar] [CrossRef]
- Rath, S.S.; Rao, D.S.; Tripathy, A.; Biswal, S.K. Biomass briquette as an alternative reductant for low grade iron ore resources. Biomass Bioenergy 2018, 108, 447–454. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, G.; Cheng, Z. Thermal analysis and kinetic modeling of manganese oxide ore reduction using biomass straw as reductant. Hydrometallurgy 2010, 105, 96–102. [Google Scholar] [CrossRef]
- Ge, H.; Xie, F.; Wu, S.; Wang, W. Biomass as a clean reductant for recovery of zinc from zinc leaching residue. J. Clean. Prod. 2024, 434, 139926. [Google Scholar] [CrossRef]
- Nurjaman, F.; Hakim, H.Z.; Arham, L.O.; Septiansyah, B.; Handoko, A.S.; Bahfie, F.; Suharto, S.; Dahlan, Y.; Fatimah, T.S.; Sari, Y.; et al. Utilisation of biomass waste as a reductant in the smelting of saprolitic nickel ore using a DC-arc furnace. Miner. Process. Extr. Met. 2024, 133, 160–170. [Google Scholar] [CrossRef]
- Deng, J.; Ning, X.-A.; Shen, J.; Ou, W.; Chen, J.; Qiu, G.; Wang, Y.; He, Y. Biomass waste as a clean reductant for iron recovery of iron tailings by magnetization roasting. J. Environ. Manag. 2022, 317, 115435. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Mousa, E.; Sjöblom, K. Modeling and Optimization of Biochar Injection into Blast Furnace to Mitigate the Fossil CO2 Emission. Sustainability 2022, 14, 2393. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Jin, G.; Cao, Y.; Han, Y. Waste Corn Straw as a Green Reductant for Hematite Reduction Roasting: Phase Transformation, Microstructure Evolution and Process Mechanism. Minerals 2023, 13, 1541. [Google Scholar] [CrossRef]
- Das, D.; Anand, A.; Gautam, S. A Review of Biomass Utilization as a Reducing Agent in Iron Ore Reduction. Prog. Petrochem. Sci. 2023, 5, 498–502. [Google Scholar] [CrossRef]
- Das, D.; Gautam, S. Assessment of Utilization Potential of Biomass Volatiles and Biochar as a Reducing Agent for Reduction of Iron Ore Pellets. Available online: https://ssrn.com/abstract=3932836 (accessed on 21 November 2025). [CrossRef]
- Sachida, N.S.; Meikap, B.C.; Surendra, K.B. Magnetization roasting of waste iron ore beneficiation plant tailings using sawdust biomass; A novel approach to produce metallurgical grade pellets. J. Clean. Prod. 2022, 343, 130894. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K. Characteristics and Utilization of Copper Slag. Resour. Conserv. Recy. 2002, 39, 299–313. [Google Scholar] [CrossRef]
- Erdenebold, U.; Choi, M.H.; Wang, J.P. Recovery of pig iron from coper smelting slag by reduction smelting. Arch. Metall. Mater. 2018, 63, 1793–1798. [Google Scholar] [CrossRef]
- Heo, J.; Kim, B.; Park, J.H. Effect of CaO Addition on Iron Recovery from Copper Smelting Slags by Carbon. Metall. Mater. Trans. 2013, 44, 1352–1363. [Google Scholar] [CrossRef]
- Łabaj, J.; Blacha, L.; Jodkowski, M.; Smalcerz, A.; Fröhlichová, M.; Findorak, R. The use of waste, fine-grained carbonaceous material in the process of copper slag reduction. J. Clean. Prod. 2021, 288, 125640. [Google Scholar] [CrossRef]
- Qu, G.; Wei, Y.; Li, B.; Wang, H.; Yang, Y.; Mclean, A. Recovery of Copper Smelting Slag Using a Green Reductant. In 11th International Symposium on High-Temperature Metallurgical Processing; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2020; pp. 417–429. [Google Scholar] [CrossRef]
- Zuo, Z.; Yu, Q.; Wei, M.; Xie, H.; Duan, W.; Wang, K.; Qin, Q. Thermogravimetric study of the reduction of copper slag by biomass. J. Therm. Anal. Calorim. 2016, 126, 481–491. [Google Scholar] [CrossRef]
- Zuo, Z.; Yu, Q.; Xie, H.; Qin, Q.; Wei, M. Direct Reduction of Copper Slag Composite Pellets Within Lignite Using Biomass as Binder. In Energy Technology; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H. Cleaner recycling of iron from waste copper slag by using walnut shell char as green reductant. J. Clean. Prod. 2019, 217, 423–431. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Zhang, S.; Li, B.; Wang, H.; Yang, Y.; Barati, M. Reduction of copper smelting slag using waste cooking oil. J. Clean. Prod. 2019, 236, 117668. [Google Scholar] [CrossRef]
- Kortyka, L.; Labaj, J.; Ptak, S.; Smalcerz, A.; Blacha, L.; Mycka, L.; Matula, T.; Findorak, R. A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags. Sustainability 2024, 16, 9261. [Google Scholar] [CrossRef]
- Ptak, S.; Matula, T.; Smalcerz, A.; Labaj, J.; Blacha, L.; Findorak, R.; Vaskova, I. Determination of potential replacement of natural carbon raw materials with walnut waste in the reduction process of metal recovery. J. Achiev. Mater. Manuf. Eng. 2023, 121, 174–183. [Google Scholar] [CrossRef]
- Ptak, S.; Matuła, T.; Blacha, L.; Łabaj, J.; Smalcerz, A.; Półka, M. Reduction of metallurgical slags using sunflower pellets. Met. Mater. Trans. B 2024, 55, 1690–1699. [Google Scholar] [CrossRef]
- Matula, T.; Labaj, J.; Nowacki, K.; Blacha, L.; Kortyka, L.; Mycka, L.; Madej, P.; Jaworek, L.; Wojtal, T. Application of Spent Coffee Grounds (SCGs) as a fuel and alternative reducer of slags from the copper industry. Energies 2023, 16, 2415. [Google Scholar] [CrossRef]
- PN-EN ISO 1716:2018-08; (English version). Badania reakcji na ogień wyrobów—Określanie ciepła spalania brutto (wartości kalorycznej). Polish Committee for Standardization: Warsaw, Poland, 2018.
- Karchniwy, E.; Klimanek, A. Porównanie wybranych modeli spalania koksiku. In Inżynieria Środowiska w Energetyce i Motoryzacji; Politechnika Śląska: Gliwice, Poland, 2017. [Google Scholar]
- Kowalczyk, R.; Piwnicki, Ł. Pips of fruit as a valuable raw material of the food industry. Postęp. Technol. Przetwórstwa Spożywczego 2007, 2, 61–66. [Google Scholar]
- Rzeźnik, W.; Mielcarek, P.; Rzeźnik, I. Assessment of Energetic Potential of Cherry Stones in Poland. J. Res. Appl. Agric. Eng. 2016, 61, 84–87. [Google Scholar]
- Bryś, A.; Zielińska, J.; Głowacki, S.; Tulej, W.; Bryś, J. Analysis of Possibilities of Using Biomass from Cherry and Morello Cherry Stones for Energy Purposes. E3S Web Conf. 2020, 154, 01005. [Google Scholar] [CrossRef]
- Aniszewska, M.; Gendek, A. Comparison of heat of combustion and calorific value of the cones and wood of selected forest tree species. Leśne Prace Badawcze For. Res. Pap. 2014, 75, 231–236. [Google Scholar] [CrossRef]
- PN-EN ISO 11358-1:2022-09; (English version). Tworzywa sztuczne—Termograwimetria (TG) polimerów—Część 1: Zasady ogólne. Polish Committee for Standardization: Warsaw, Poland, 2022.
- ASTM E1131-20; Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM: West Conshohocken, PA, USA, 2025.
- PN-EN ISO 15309:2010; (Polish version). Charakteryzowanie odpadów i gleby—Oznaczanie składu pierwiastkowego za pomocą fluorescencji rentgenowskiej. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Butnaru, E.; Brebu, M. The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis. Energies 2022, 15, 3483. [Google Scholar] [CrossRef]
- Aniszewska, M.; Gendek, A.; Hýsek, Š.; Malaťák, J.; Velebil, J.; Tamelová, B. Changes in the Composition and Surface Properties of Torrefied Conifer Cones. Materials 2020, 13, 5660. [Google Scholar] [CrossRef]
- Butnaru, E.; Pamfil, D.; Stoleru, E.; Brebu, M. Characterization of bark, needles and cones from silver fir (Abies alba mill.) towards valorization of biomass forestry residues. Biomass Bioenergy 2022, 159, 106413. [Google Scholar] [CrossRef]
- Font, R.; Conesa, J.; Moltó, J.; Muñoz, M. Kinetics of pyrolysis and combustion of pine needles and cones. J. Anal. Appl. Pyrolysis 2009, 85, 276–286. [Google Scholar] [CrossRef]
- Kucharski, M. Effect of thermodynamic and physical properties of flash smelting slags on copper losses during slags cleaning in an electric furnace. Arch. Hut. 1987, 32, 307–323. [Google Scholar]
- Karwan, T.; Nowakowski, J.; Gostynski, Z.; Konefał, R. Structure of slags from fluidized-bed process. In Proceedings of the Conference Materials: Metalurgia Metali Nieżelaznych, Krakow, Poland, 17–19 November 2014. [Google Scholar]
- Sarfo, P.; Wyss, G.; Ma, G.; Das, A. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass. Miner. Eng. 2017, 107, 8–19. [Google Scholar] [CrossRef]
- HSC Chemistry Software 10: Thermodynamic Database and Applications. Outotec. Available online: https://www.metso.com/portfolio/hsc-chemistry/ (accessed on 21 November 2025).
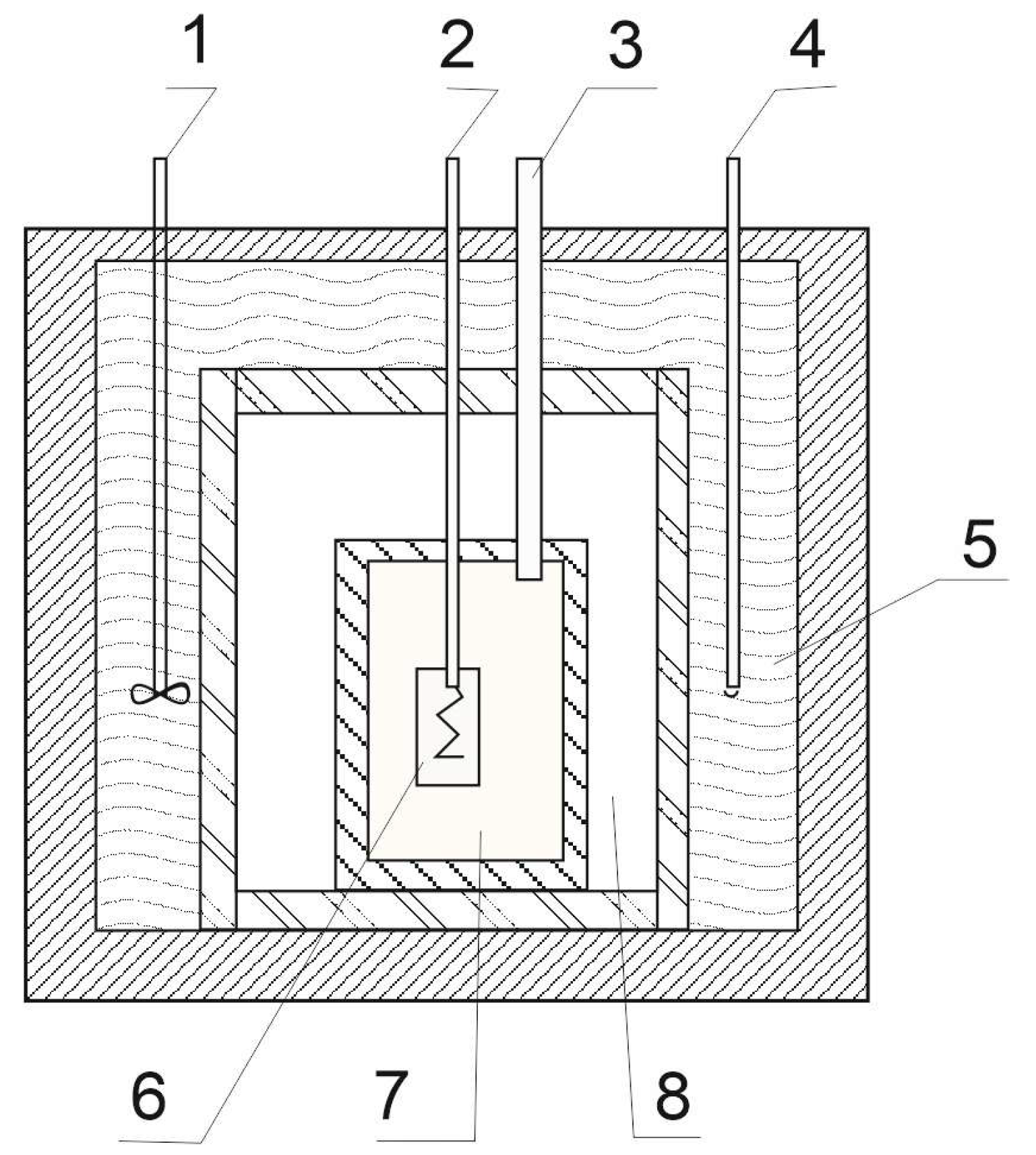


| Sample | Comments | ||
|---|---|---|---|
| Pine cones | 18,130 | 19,453 | Own research |
| 20,400 | |||
| 19,830 | |||
| Pine cones | - | 19,400 | [49] |
| Cherry stones | - | 19,540–22,020 | [50,51,52] |
| Coke breeze | - | 29,760–32,460 | [53] |
| Biomass Type | Content, % by Mass | Source | ||||
|---|---|---|---|---|---|---|
| C | H | S | O | N | ||
| Pine cones | 47.57 | 6.80 | 0.02 | 40.11 | 0.76 | Own research |
| Pine cones | 45–55 | 5.3–6.8 | <0.25 | 38–45 | 0.1–0.94 | [57,58,59,60] |
| Spruce cones | 46–54 | 5.1–5.7 | <0.03 | 37–41 | 0.37–0.8 | |
| Larch cones | 47–52 | 5.2–5.8 | <0.02 | 37–40 | 0.24–0.8 | |
| Fir cones | 54.26 | 6.3 | 0.48 | 35.89 | 0.37 | |
| Parameter | Inert Atmosphere | Oxidizing Atmosphere |
|---|---|---|
| Beginning of thermal decomposition, °C | 266 | 293 |
| 50% mass loss temperature, °C | 350 | 340 |
| 1st phase of pyrolysis/thermal decomposition, °C | 250–375 | 260–340 |
| Maximum DTG value in the 1st phase of pyrolysis/thermal decomposition (@ temperature), %/min @°C | 8.6 @355 | 8.3 @325 |
| 2nd phase of pyrolysis/thermal decomposition, °C | - | 440–475 |
| Maximum DTG value in the 2nd phase of pyrolysis/thermal decomposition (@ temperature), %/min @°C | - | 4.2 @455 |
| Ash content, %mass | 17.45 | 11.73 |
| No. | Reaction Number | , kJ/mol | ΔHT, kJ/mol |
|---|---|---|---|
| 1 | (R4) | −126.70 | −170.97 |
| 2 | (R5) | −140.89 | −141.32 |
| 3 | (R6) | −720.69 | −370.29 |
| 4 | (R7) | −72.41 | −97.16 |
| 5 | (R8) | −86.60 | −67.50 |
| 6 | (R9) | −503.52 | −75.05 |
| 7 | (R10) | −21.64 | −36.63 |
| 8 | (R11) | −64.21 | 52.34 |
| 9 | (R12) | −728.21 | 794.26 |
| Test no. | Reduction Time, h | Metal Mass, g | Slag Mass, g | Metal Content in Secondary Slag | ||
|---|---|---|---|---|---|---|
| Cu | Pb | Fe | ||||
| 1 | 1 | 9.84 | 66.2 | 1.30 | 1.92 | 12.50 |
| 2 | 2 | 11.04 | 64.1 | 0.72 | 1.11 | 12.07 |
| 3 | 3 | 11.75 | 63.9 | 0.33 | 0.91 | 13.36 |
| 4 | 4 | 12.57 | 62.1 | 0.37 | 0.86 | 12.19 |
| 5 | 5 | 13.05 | 62.2 | 0.15 | 0.79 | 11.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptak, S.; Łabaj, J.; Matuła, T.; Smalcerz, A.; Blacha, L.; Smagór, A.; Findorák, R. The Use of Coniferous Tree Cone Biomass as an Energy Source and a Reducing Agent in the Recycling of Metals from Oxide Secondary Raw Materials. Energies 2025, 18, 6183. https://doi.org/10.3390/en18236183
Ptak S, Łabaj J, Matuła T, Smalcerz A, Blacha L, Smagór A, Findorák R. The Use of Coniferous Tree Cone Biomass as an Energy Source and a Reducing Agent in the Recycling of Metals from Oxide Secondary Raw Materials. Energies. 2025; 18(23):6183. https://doi.org/10.3390/en18236183
Chicago/Turabian StylePtak, Szymon, Jerzy Łabaj, Tomasz Matuła, Albert Smalcerz, Leszek Blacha, Adrian Smagór, and Róbert Findorák. 2025. "The Use of Coniferous Tree Cone Biomass as an Energy Source and a Reducing Agent in the Recycling of Metals from Oxide Secondary Raw Materials" Energies 18, no. 23: 6183. https://doi.org/10.3390/en18236183
APA StylePtak, S., Łabaj, J., Matuła, T., Smalcerz, A., Blacha, L., Smagór, A., & Findorák, R. (2025). The Use of Coniferous Tree Cone Biomass as an Energy Source and a Reducing Agent in the Recycling of Metals from Oxide Secondary Raw Materials. Energies, 18(23), 6183. https://doi.org/10.3390/en18236183








