Thorium in Energy and Ecology: Prospects for Clean Fuel Sources and Protection of Water and Soil Systems from Radiation Risks
Abstract
1. Introduction
2. Global Distribution and Geochemistry of Thorium
3. Isotopic Composition and Ecological Significance of Thorium
- 238U series: 234Th (T½ = 24.1 days), 230Th (T½ ≈ 75,600 years);
- 232Th series: 232Th (T½ = 1.40 × 1010 years) and its daughter 228Th (T½ = 698.6 days);
- 235U series: 231Th (T½ = 25.5 h), 227Th (T½ = 18.7 days).
4. Thorium in Soils
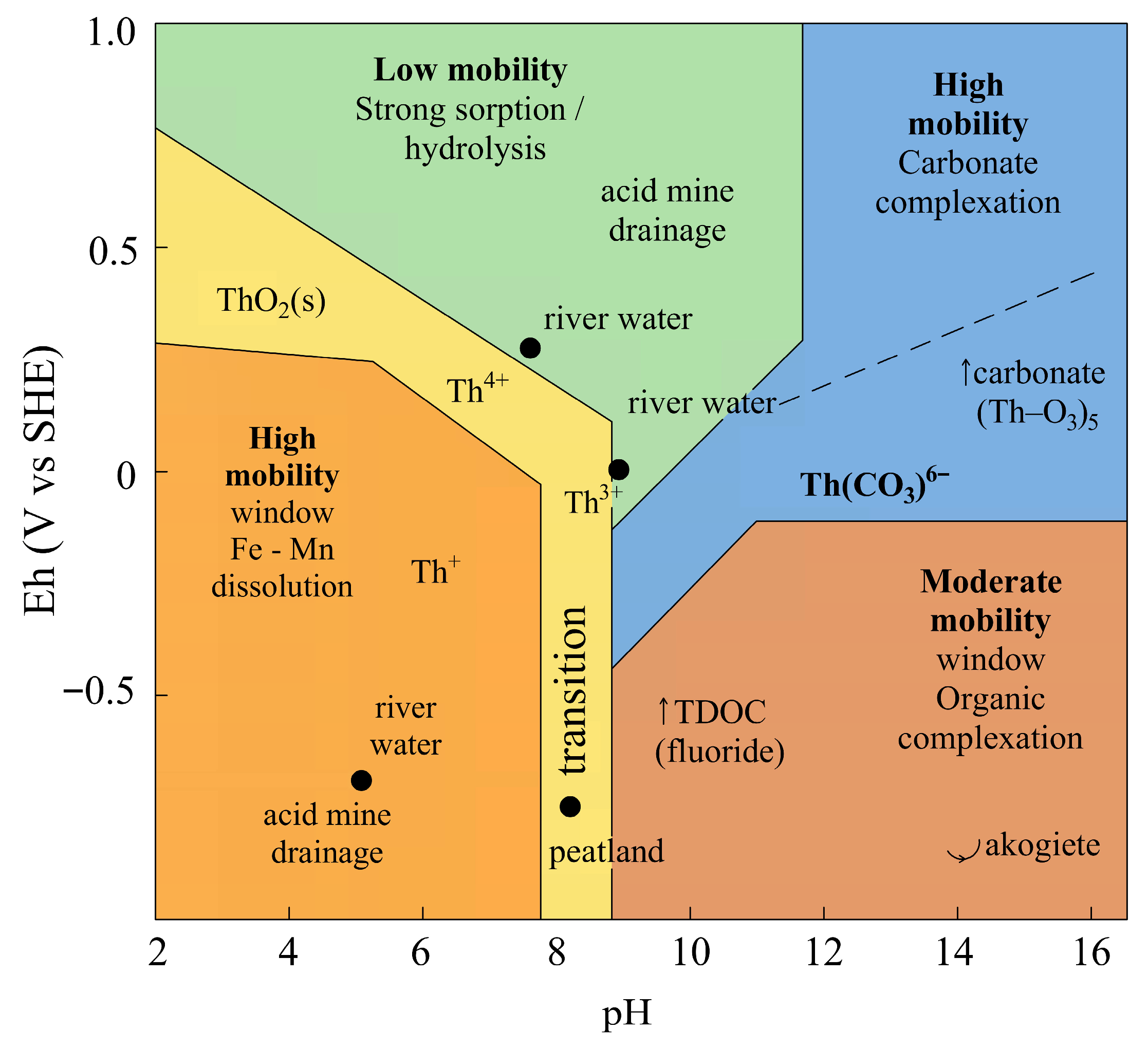
5. Ecology of Thorium in Aquatic Systems
6. Meta-Analytic Framework for Thorium Concentrations and Hydrogeochemical Mobility in Environmental Compartments
| Compartment/Setting | Units | Typical Range (P5–P95) | Indicative Median | Approx. IQR (P25–P75) | Notes/Dominant Controls |
|---|---|---|---|---|---|
| Soils, global background | mg kg−1 | 0.5–20 | 5–10 | 3–15 | Controlled by parent lithology and accessory Th minerals; higher in felsic terrains [14,67,68,69] |
| Soils, high-Th/mineralized (REE, phosphate, etc.) | mg kg−1 | 20–100+ | 40–70 | 30–80 | Monazite/xenotime-rich horizons, U–Th–REE mineralization, mine-affected or phosphatic soils [14,68,69,70] |
| Stream/river sediments, background | mg kg−1 | 3–40 | ~10 | ~5–20 | Enrichment in clays, Fe–Mn (oxyhydr)oxides and heavy minerals; lithology-controlled [14,41,72] |
| Stream/river sediments, mineralized | mg kg−1 | 20–250+ | 50–100 | 30–150 | Drainage of U–Th–REE deposits, monazite placers, mine tailings [14,41,72] |
| Suspended river particulates (SPM) | mg kg−1 | 5–40 (background) | 10–20 | 8–30 | Fine fraction; strong sorption to clays and Fe–Mn colloids; often enriched [14,41] |
| Fresh surface waters (rivers, lakes) | µg L−1 | 10−3–10−1 | ~10−2 | ~3 × 10−3–3 × 10−2 | Very low solubility; mainly colloidal Th; enhanced in DOC- and carbonate-rich systems [14,41] |
| Coastal/open seawater | µg L−1 | 0.1–1.0 | 0.3–0.5 | 0.2–0.7 | Particle–water exchange; resuspension and river inputs [14,73] |
| Impacted waters (mine drainage, geothermal, tailings seepage) | µg L−1–mg L−1 | 10−2 µg L−1–103 µg L−1 (site-specific) | highly variable | — | Strongly site-specific; up to mg L−1 near mining or mineralized groundwaters [14,41] |
6.1. Hydrogeochemical Context and Eh–pH Mobility Framework
6.2. Geochemical Significance
- -
- In oxidizing, near-neutral environments, Th is immobilized as ThO2(s) or strongly adsorbed phases.
- -
- Under reducing or acidic conditions, dissolution of Fe–Mn phases and proton-promoted desorption release Th into solution.
- -
- In alkaline, carbonate-rich waters, Th forms highly soluble anionic complexes.
- -
- In organic-rich reducing settings, Th occurs as stable organo-complexes of intermediate mobility.
7. Methods for Remediation of Thorium Contamination in Water and Soils
8. Conclusions
- Industrial thorium mining: Design selective and efficient solid-phase extraction methods for recovering thorium from minerals such as monazite while minimizing wastewater generation and ecological disturbance.
- Thorium biogeochemistry: Advance geochemical modeling and targeted experimental studies on the speciation, stability, and mobility of thorium in natural waters and during waste disposal or site remediation.
- Radiobiological effects: Deepen understanding of thorium’s cellular and systemic impacts in humans and experimental organisms to refine radiological safety standards and risk assessments.
- Decorporation strategies: Develop next-generation chelating agents and medical countermeasures to mitigate internal contamination in case of occupational or accidental exposure.
9. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSR | Molten salt reactor |
| USGS | United States Geological Survey |
| RES | Renewable energy sources |
| IUPAC | International Union of Pure and Applied Chemistry |
| US EPA | United States Environmental Protection Agency |
| COF | Covalent organic framework |
| EDTA | ethylenediaminetetraacetic acid |
References
- Haneklaus, N.; Qvist, S.; Gładysz, P.; Bartela, Ł. Why Coal-Fired Power Plants Should Get Nuclear-Ready. Energy 2023, 280, 128169. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Thorium Fuel Cycle—Potential Benefits Challenges (IAEA-TECDOC-1450); I.A.E.A.: Vienna, Austria, 2005; ISBN 92-0-103405-9. [Google Scholar]
- Schaffer, M.B. Abundant thorium as an alternative nuclear fuel: Important waste disposal and weapon proliferation advantages. Energy Policy 2013, 60, 4–12. [Google Scholar] [CrossRef]
- Insepov, Z.; Kalybay, A.; Alsar, Z.; Hassanein, A.; Sizyuk, Y.; Mansurov, Z.; Gajimuradova, A. Critical analysis of thorium reactor designs in nuclear-producing countries. Combust. Plasma Chem. 2025, 23, 9–23. [Google Scholar] [CrossRef]
- Insepov, Z. Nuclear-chemical characteristics of subcritical thorium reactors with an external neutron source: A review. Combust. Plasma Chem. 2024, 22, 297–308. [Google Scholar] [CrossRef]
- Kalybay, A.; Kurbanova, B.; Mansurov, Z.; Hassanein, A.; Alsar, Z.; Insepo, Z. Mathematical models of the thorium reactor core. Combust. Plasma Chem. 2024, 22, 279–295. [Google Scholar] [CrossRef]
- Vijayan, P.K.; Shivakumar, V.; Basu, S.; Sinha, R. Role of thorium in the Indian nuclear power programme. Prog. Nucl. Energy 2017, 101 Pt A, 43–52. [Google Scholar] [CrossRef]
- Xu, H. Thorium energy and molten salt reactor R&D in China. In Thorium Energy for the World, Proceedings of the ThEC13 Conference, CERN, Globe of Science and Innovation, Geneva, Switzerland, 27–31 October 2013; Springer: Cham, Switzerland, 2016; pp. 71–88. [Google Scholar] [CrossRef]
- Kumar, J.R.; De Melo, L.G.T.C.; Santos, R.M.; Yoon, H.-S. An overview of thorium as a prospective natural resource for future energy. Front. Energy Res. 2023, 11, 1132611. [Google Scholar] [CrossRef]
- Humphrey, U.E.; Khandaker, M.U. Viability of thorium-based nuclear fuel cycle for the next generation nuclear reactor: Issues and prospects. Renew. Sustain. Energy Rev. 2018, 97, 259–275. [Google Scholar] [CrossRef]
- Glahn, J. A Thorium Molten Salt Reactor that Refuels with Fertile Rather than Fissile Fuel. Modern Power Systems. Copenhagen Atomics. 2025. Available online: https://www.modernpowersystems.com/analysis/a-thorium-molten-salt-reactor-that-refuels-with-fertile-rather-than-fissile-fuel (accessed on 12 November 2025).
- World Nuclear News. Chinese Molten Salt Reactor Achieves Conversion of Thorium-Uranium Fuel; World Nuclear News: London, UK, 2025; Available online: https://www.world-nuclear-news.org/articles/chinese-msr-achieves-conversion-of-thorium-uranium-fuel (accessed on 12 November 2025).
- Santofimia, E.; González, F.J.; Rincón-Tomás, B.; López-Pamo, E.; Marino, E.; Reyes, J.; Bellido, E. The mobility of thorium, uranium and rare earth elements from Mid Ordovician black shales to acid waters and its removal by goethite and schwertmannite. Chemosphere 2022, 307 Pt 2, 135907. [Google Scholar] [CrossRef]
- Patel, K.S.; Sharma, S.; Maity, J.P.; Martín-Ramos, P.; Fiket, Ž.; Bhattacharya, P.; Zhu, Y. Occurrence of uranium, thorium and rare earth elements in the environment: A review. Front. Environ. Sci. 2023, 10, 1058053. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Hayat, M.A.; Iqbal, M. Assessment of thorium in the environment (A review). J. Chem. Soc. Pak. 2012, 34, 1012. Available online: https://jcsp.org.pk/ArticleUpload/4735-21611-1-CE.pdf (accessed on 12 November 2025).
- Rivotti, P.; Karatayev, M.; Sobral Mourao, Z.; Shah, N.; Clarke, M.L.; Konadu, D.D. Impact of future energy policy on water resources in Kazakhstan. Energy Strategy Rev. 2019, 24, 261–267. [Google Scholar] [CrossRef]
- Karatayev, M.; Clarke, M.L. A review of current energy systems and green energy potential in Kazakhstan. Renew. Sustain. Energy Rev. 2016, 55, 491–504. [Google Scholar] [CrossRef]
- Gad, S.C. Thorium and thorium dioxide. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 183–184. [Google Scholar] [CrossRef]
- Chroneos, A.; Goulatis, I.; Daskalopulu, A.; Tsoukalas, L.H. Thorium fuel revisited. Prog. Nucl. Energy 2023, 164, 104839. [Google Scholar] [CrossRef]
- Asylbaev, I.; Khabirov, I.K.; Gabbasova, I.M.; Rafikov, B.V.; Lukmanov, N.A. Geochemistry of thorium and uranium in soils of the Southern Urals. Eurasian Soil Sci. 2017, 50, 1406–1413. [Google Scholar] [CrossRef]
- Balachandran, G. Case study 1—Extraction of rare earths for advanced applications. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1291–1340. [Google Scholar] [CrossRef]
- Hazen, R.M.; Ewing, R.C.; Sverjensky, D.A. Evolution of uranium and thorium minerals. Am. Mineral. 2009, 94, 1293–1311. [Google Scholar] [CrossRef]
- Andersen, P.K.; Ghassemi, A.; Ghassemi, M. Nuclear waste. In Encyclopedia of Energy; Cleveland, C.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 449–463. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The principal rare earth elements deposits of the United States: A summary of domestic deposits and a global perspective. In Non-Renewable Resource Issues: Geoscientific and Societal Challenges; Springer: Dordrecht, The Netherlands, 2010; pp. 131–155. [Google Scholar] [CrossRef]
- Udayakumar, S.; Noor, A.F.M.; Hamid, S.A.R.S.A.; Putra, T.A.R.; Anderson, C.G. Chemical and mineralogical characterization of Malaysian monazite concentrate. Min. Metall. Explor. 2020, 37, 103–114. [Google Scholar] [CrossRef]
- Anderson, C. A Review of the Beneficiation of Bastnaesite and Monazite Rare Earth Bearing Minerals. 2024, Preprint. Available online: https://www.researchgate.net/profile/Corby-Anderson/publication/382338248_A_Review_of_the_Beneficiation_of_Bastnaesite_and_Monazite_Rare_Earth_Bearing_Minerals/links/669907a6cb7fbf12a45c6648/A-Review-of-the-Beneficiation-of-Bastnaesite-and-Monazite-Rare-Earth-Bearing-Minerals.pdf (accessed on 12 November 2025).
- Anitha, J.K.; Joseph, S.; Rejith, R.G.; Sundararajan, M. Monazite chemistry and its distribution along the coast of Neendakara–Kayamkulam belt, Kerala, India. SN Appl. Sci. 2020, 2, 812. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC technical report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Choppin, G.R.; Liljenzin, J.-O.; Rydberg, J. Radionuclides in nature. In Radiochemistry and Nuclear Chemistry, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2002; pp. 94–122. [Google Scholar] [CrossRef]
- Akbari, R.; Nasr, M.A.; D’Auria, F.; Cammi, A.; Maiorino, J.; Stefani, G. Analysis of thorium–transuranic fuel deployment in a LW-SMR: A solution toward sustainable fuel supply for the future plants. Nucl. Eng. Des. 2024, 421, 113090. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on the Biological Effects of Ionizing Radiations. Health Risks of Radon and Other Internally Deposited Alpha-Emitters: BEIR IV (Ch. 5: Thorium); National Academies Press: Washington, DC, USA, 1988. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218130/ (accessed on 12 November 2025).
- Walter, H.J.; van der Loeff, M.R.; Höltzen, H.; Bathmann, U. Reduced scavenging of 230Th in the Weddell Sea: Implications for paleoceanographic reconstructions in the South Atlantic. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2000, 47, 1369–1387. [Google Scholar] [CrossRef]
- Geibert, W.; Stimac, I.; van der Loeff, M.M.R.; Kuhn, G. Dating deep-sea sediments with 230Th excess using a constant rate of supply model. Paleoceanogr. Paleoclimatology 2019, 34, 1895–1912. [Google Scholar] [CrossRef]
- Ribeiro, F.C.A.; Silva, J.; Lima, E.; Sobrinho, N.D.A.; Perez, D.; Lauria, D. Natural radioactivity in soils of the state of Rio de Janeiro (Brazil): Radiological characterization and relationships to geological formation, soil types and soil properties. J. Environ. Radioact. 2018, 182, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, H.; Migdisov, A.A.; Williams-Jones, A.E.; van Hinsberg, V.J.; Xu, H.; Roback, R. The solubility of thorium in carbonate-bearing solutions at hydrothermal conditions. Geochim. Cosmochim. Acta 2022, 330, 80–92. [Google Scholar] [CrossRef]
- Guo, P.; Duan, T.; Song, X.; Xu, J.; Chen, H. Effects of soil pH and organic matter on distribution of thorium fractions in soil contaminated by rare-earth industries. Talanta 2008, 77, 624–627. [Google Scholar] [CrossRef]
- Ratnayake, S.; Lützenkirchen, J.; Schild, D.; Finck, N.; Eiche, E.; Gil-Díaz, T.; Weerasooriya, R.; Geckeis, H. Solid phase speciation and mobility of thorium in soil samples from a case study in Sri Lanka. Radiochim. Acta 2025, 113, 277–298. [Google Scholar] [CrossRef]
- Sartandel, S.; Jha, S.; Bara, S.; Tripathi, R.; Puranik, V. Spatial distribution of uranium and thorium in the surface soil around proposed uranium mining site at Lambapur and its vertical profile in the NagarjunaSagar Dam. J. Environ. Radioact. 2009, 100, 831–834. [Google Scholar] [CrossRef]
- Veerasamy, N.; Sahoo, S.K.; Inoue, K.; Arae, H.; Fukushi, M. Geochemical behavior of uranium and thorium in sand and sandy soil samples from a natural high background radiation area of the Odisha coast, India. Environ. Sci. Pollut. Res. 2020, 27, 31339–31349. [Google Scholar] [CrossRef]
- Grebenshchikova, V.; Gritsko, P.P.; Kuznetsov, P.V.; Doroshkov, A.A. Uranium and thorium in soil cover of the Irkutsk-Angarsk industrial zone (Baikal region). Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2017, 328, 93–104. [Google Scholar]
- Nascimento, R.; Nascimento, C.W.A.D.; da Silva, Y.J.A.B.; da Silva, R.J.A.B.; Collins, A.L. Thorium content in soil, water and sediment samples and fluvial sediment-associated transport in a catchment system with a semiarid-coastal interface, Brazil. Environ. Sci. Pollut. Res. 2019, 26, 33532–33540. [Google Scholar] [CrossRef]
- Chen, C.-L.; Wang, X.-K. Influence of pH, soil humic/fulvic acid, ionic strength and foreign ions on sorption of thorium(IV) onto γ-Al2O3. Appl. Geochem. 2007, 22, 436–445. [Google Scholar] [CrossRef]
- Hunter, K.A.; Hawke, D.J.; Choo, L.K. Equilibrium adsorption of thorium by metal oxides in marine electrolytes. Geochim. Cosmochim. Acta 1988, 52, 627–636. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, W. Review of allanite: Properties, occurrence and mineral processing technologies. Green Smart Min. Eng. 2024, 1, 40–52. [Google Scholar] [CrossRef]
- Vogel, C.; Hoffmann, M.C.; Taube, M.C.; Krüger, O.; Baran, R.; Adam, C. Uranium and thorium species in phosphate rock and sewage sludge ash based phosphorus fertilizers. J. Hazard. Mater. 2020, 382, 121100. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, S.; Cochran, J.K. Uranium–thorium series radionuclides in ocean profiles. Encycl. Ocean. Sci. 2016, 1, 377–391. [Google Scholar] [CrossRef]
- Rao, C.V.S.B.; Jayalakshmi, S.; Sivaraman, N.; Rao, P.R.V. Studies on extraction of actinides by unsymmetrical diamylbutyl phosphonate. Radiochim. Acta 2015, 103, 235–243. [Google Scholar] [CrossRef]
- Yusof, M.Y.M.; Idris, M.I.; Mohamed, F.; Nor, M.M. Adsorption of radioactive element by clay: A review. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Nuclear Science Technology and Engineering Conference 2019 (iNuSTEC2019), Bangi, Malaysia, 29–31 October 2019; IOP Publishing: Bristol, UK, 2020; Volume 785, p. 012020. [Google Scholar] [CrossRef]
- Wang, B.; Ye, S.; Zhang, S.; Fang, H.; Zhang, Y.; Xia, C.; Chen, W. Reactions of thorium oxide clusters with water: The effects of oxygen content. ChemPhysChem 2022, 23, e202200701. [Google Scholar] [CrossRef]
- Estevenon, P.; Causse, J.; Szenknect, S.; Welcomme, E.; Mesbah, A.; Moisy, P.; Poinssot, C.; Dacheux, N. In situ study of the synthesis of thorite (ThSiO4) under environmental representative conditions. Dalton Trans. 2020, 49, 11512–11521. [Google Scholar] [CrossRef]
- Kumar, P.; Dumpala, R.M.R.; Telmore, V.M.; Sadhu, B.; Sundararajan, M.; Yadav, A.K.; Bhattacharyya, D.; George, J.P. Thorium complexation with aliphatic and aromatic hydroxycarboxylates: A combined experimental and theoretical study. ACS Omega 2024, 9, 27289–27299. [Google Scholar] [CrossRef]
- Nisbet, H.; Migdisov, A.; Xu, H.; Guo, X.; van Hinsberg, V.; Williams-Jones, A.; Boukhalfa, H.; Roback, R. An experimental study of the solubility and speciation of thorium in chloride-bearing aqueous solutions at temperatures up to 250 °C. Geochim. Cosmochim. Acta 2018, 239, 363–373. [Google Scholar] [CrossRef]
- Kopylova, Y.; Guseva, N.; Shestakova, A.; Khvaschevskaya, A.; Arakchaa, K. Uranium and thorium behavior in groundwater of the natural spa area “Choygan mineral water” (East Tuva). In IOP Conference Series: Earth and Environmental Science, Proceedings of the XIX International Scientific Symposium in Honor of Academician M.A. Usov “Problems of Geology and Subsurface Development”, Tomsk, Russia, 6–10 April 2015; IOP Publishing: Bristol, UK, 2015; Volume 27, p. 012034. [Google Scholar] [CrossRef]
- Filistovič, V.; Maceika, E.; Tarasiuk, N.; Lukšienė, B.; Konstantinova, M.; Buivydas, Š.; Koviazina, E.; Puzas, A. Model of non-equilibrium multiphase contaminant transport in lake water–sediment system. Water Air Soil Pollut. 2015, 226, 202. [Google Scholar] [CrossRef]
- Schumann, R.R.; Zielinski, R.A.; Otton, J.K.; Pantea, M.P.; Orem, W.H. Uranium delivery and uptake in a montane wetland, north-central Colorado, USA. Appl. Geochem. 2017, 78, 363–379. [Google Scholar] [CrossRef]
- Duggal, V.; Rani, A.; Balaram, V. Assessment of age-dependent radiation dose due to intake of uranium and thorium in drinking water from Sikar district, Rajasthan, India. Radiat. Prot. Dosim. 2016, 171, ncw070. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.M.L.; Taddei, M.H.T.; Cheberle, L.T.V.; Silva, P.S.C.; Maihara, V.A. A comparative study using different resins to determine thorium isotopes. Braz. J. Radiat. Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Awudu, A.R.; Darko, E.O.; Schandorf, C.; Hayford, E.K.; Abekoe, M.K.; Ofori-Danson, P.K. Determination of activity concentration levels of 238U, 232Th, and 40K in drinking water in a gold mine in Ghana. Health Phys. 2010, 99 (Suppl. S2), S149–S153. [Google Scholar] [CrossRef] [PubMed]
- Sansone, U.; Kim, C.K.; Kis-Benedek, G.; Schorn, R.; Zeiller, E.; Qaribov, A.; Huseynov, V.; Chupov, A. Natural and anthropogenic radionuclides in the rivers of Azerbaijan. J. Radioanal. Nucl. Chem. 2008, 277, 357–364. [Google Scholar] [CrossRef]
- Newman, C.P.; Paschke, S.S.; Keith, G. Natural and anthropogenic geochemical tracers to investigate residence times and groundwater–surface-water interactions in an urban alluvial aquifer. Water 2021, 13, 871. [Google Scholar] [CrossRef]
- El-Arabi, A.E.-G.M.; Khalifa, I.H. Application of multivariate statistical analyses in the interpretation of geochemical behaviour of uranium in phosphatic rocks in the Red Sea, Nile Valley and Western Desert, Egypt. J. Environ. Radioact. 2002, 61, 169–190. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, B.J.; Feng, Z.G.; Wang, X.-L.; Chen, R.; Li, P.-S.; Huang, C. Geochemical behaviors of uranium and thorium during weathering and pedogenesis of carbonate rock: Constraint from their speciation. Environ. Sci. Pollut. Res. 2023, 30, 95348–95366. [Google Scholar] [CrossRef]
- Nisbet, H.; Migdisov, A.A.; Williams-Jones, A.E.; Xu, H.; van Hinsberg, V.J.; Roback, R. Challenging the thorium-immobility paradigm. Sci. Rep. 2019, 9, 17035. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy metals removal from water by efficient adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Adeoye, J.B.; Tan, Y.H.; Lau, S.Y.; Tan, Y.Y.; Chiong, T.; Mubarak, N.M.; Khalid, M. Advanced oxidation and biological integrated processes for pharmaceutical wastewater treatment: A review. J. Environ. Manag. 2024, 353, 120170. [Google Scholar] [CrossRef] [PubMed]
- Malikova, I.N.; Strakhovenko, V.D.; Ustinov, M.T. Uranium and thorium contents in soils and bottom sediments of Lake BolshoyeYarovoye, western Siberia. J. Environ. Radioact. 2020, 211, 106048. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Thorium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- De Vivo, B.; Cicchella, D.; Albanese, S.; Birke, M.; Demetriades, A.; De Vos, W.; Dinelli, E.; Lima, A.; O’Connor, P.J.; Salpeteur, I.; et al. Uranium, Thorium and Potassium Concentrations in Agricultural and Grazing Land Soils of Europe. Geophys. Res. Abstr. 2014, 16, EGU2014-7258. [Google Scholar]
- Escareño-Juarez, E.; Jiménez-Barredo, F.; Gascó-Leonarte, C.; Barrado-Olmedo, A.I.; Vega, M. Baseline Thorium Concentration and Isotope Ratios in Topsoil of Zacatecas State, Mexico. Chemosphere 2021, 268, 128915. [Google Scholar] [CrossRef]
- Azazi, A.; El-Araby, E.H.; El-Barbary, A.; Hassani, R.; El-Bialy, E.; Shabaan, D.H. Gamma Spectroscopy Analysis of Agricultural Soils in Jazan: Radiological Safety and Environmental Impact. J. Hazard. Mater. Adv. 2025, 18, 100729. [Google Scholar] [CrossRef]
- Ahmed, H.; Young, S.D.; Shaw, G. Factors Affecting Uranium and Thorium Fractionation and Profile Distribution in Contrasting Arable and Woodland Soils. J. Geochem. Explor. 2014, 145, 98–105. [Google Scholar] [CrossRef]
- Akakçe, N.; Öztürk Atay, N.; TüneyKizilkaya, I.; Uğur Görgün, A. Radioactivity and Heavy Metal Accumulation in Surface Sediment from the West Aegean Coast. Soil Sediment Contam. Int. J. 2023, 33, 560–577. [Google Scholar] [CrossRef]
- Akyil, S.; Yusof, A.M. The Distribution of Uranium and Thorium in Samples Taken from Different Polluted Marine Environment. J. Hazard. Mater. 2007, 144, 564–569. [Google Scholar] [CrossRef]
- OECD-NEA. Chemical Thermodynamics of Thorium; NEA No. 15; OECD Publishing: Paris, France, 2020. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- International Atomic Energy Agency (IAEA). Radiation Protection of the Public and the Environment; General Safety Guide No. GSG-8; IAEA: Vienna, Austria, 2018. [Google Scholar]
- Li, Z.; Chen, F.; Yuan, L.; Liu, Y.; Zhao, Y.; Chai, Z.; Shi, W. Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem. Eng. J. 2012, 210, 539–546. [Google Scholar] [CrossRef]
- Fallatah, O.; Qutub, M.T.; Alsulimani, E.F.; Alshehri, O.H.; Hafiz, L.M.; Altamrawi, A.A.; Khattab, M.R. Adsorption rate of uranium and thorium isotopes in soil and plants grown in a high background radiation area. Isot. Environ. Health Stud. 2024, 60, 417–427. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Cai, J. Sorption of Th(IV) from aqueous solution to GMZ bentonite: Effect of pH, ionic strength, fulvic acid and electrolyte ions. J. Radioanal. Nucl. Chem. 2012, 295, 947–955. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lai, C.-W.; Hsien, K.-J. Characterization and adsorption properties of diatomaceous earth modified by hydrofluoric acid etching. J. Colloid Interface Sci. 2006, 297, 749–754. [Google Scholar] [CrossRef]
- Yusan, S.; Gok, C.; Erenturk, S.; Aytas, S. Adsorptive removal of thorium(IV) using calcined and flux-calcined diatomite from Turkey: Evaluation of equilibrium, kinetic and thermodynamic data. Appl. Clay Sci. 2012, 67–68, 106–116. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Ismail, A.F.; Aziman, E.S. Ultra-effective modified clinoptilolite adsorbent for selective thorium removal from radioactive residue. Sci. Rep. 2023, 13, 9316. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Khalid, N.; Mirza, M.L. Kinetics, isotherm and thermodynamics for thorium ions adsorption from aqueous solutions by coal. Desalination Water Treat. 2017, 92, 291–300. [Google Scholar] [CrossRef]
- Zafar, S.; Khalid, N.; Daud, M.; Mirza, M.L. Kinetic studies of the adsorption of thorium ions onto rice husk from aqueous media: Linear and nonlinear approach. Nucleus 2015, 52, 14–19. [Google Scholar] [CrossRef]
- Khamseh, A.A.G.; Ghorbanian, S.A.; Amini, Y.; Shadman, M.M. Investigation of kinetic, isotherm and adsorption efficacy of thorium by orange peel immobilized on calcium alginate. Sci. Rep. 2023, 13, 8393. [Google Scholar] [CrossRef]
- Albayari, M.; Nordin, N.; Adnan, R.; Khalili, F.; Nazal, M. Assessing the sorption of uranium and thorium from simulated solutions using chemically treated biomass of Sargassum aquifolium macroalgae. Environ. Eng. Res. 2024, 29, 240004. [Google Scholar] [CrossRef]
- Tran, G.H.; Leu, H.-J.; Richards, D.; Lo, S.-S.; Tran, T.T. Biosynthesis and application of biological thin films for heavy metal ion biosorption from aqueous solution. J. Environ. Chem. Eng. 2025, 13, 115014. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Thorium biosorption by Aspergillus fumigatus, a filamentous fungal biomass. J. Hazard. Mater. 2009, 165, 670–676. [Google Scholar] [CrossRef]
- Zahakifar, F.; Khanramaki, F. Continuous removal of thorium from aqueous solution using functionalized graphene oxide: Study of adsorption kinetics in batch system and fixed bed column. Sci. Rep. 2024, 14, 14888. [Google Scholar] [CrossRef]
- Okyere, D.; Manso, R.H.; Tong, X.; Chen, J. Stability of polyethylene glycol-coated copper nanoparticles and their optical properties. Coatings 2022, 12, 776. [Google Scholar] [CrossRef]
- Noli, F.; Dafnomili, A.; Sarafidis, G.; Dendrinou-Samara, C.; Pliatsikas, N.; Kapnisti, M. Uranium and thorium water decontamination via novel coated Cu-based nanoparticles: The role of chemistry and environmental implications. Sci. Total Environ. 2022, 838, 156050. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bo, T.; Zhang, Y.; Wang, Y.; Li, X.; Zhang, S.; Liu, Y. Biomimetic phosphatization of nano zero-valent iron for thorium removal and waste remediation from rare earth leachates. Environ. Res. 2025, 285, 122390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, Y.; Weng, Z.; Wang, J.; Yan, Y.; Cheng, L.; Fan, Y.; Chen, L.; Zhang, H.; Chen, L.; et al. Thorium cluster synthesized by a solvent-free flux approach: The richest coordination diversity and application exploration. Inorg. Chem. 2024, 63, 14278–14283. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Mao, Y. Metal–organic framework-based materials for adsorption and detection of uranium(VI) from aqueous solution. ACS Omega 2022, 7, 14430–14456. [Google Scholar] [CrossRef]
- Liu, X.; Gao, F.; Jin, T.; Ma, K.; Shi, H.; Wang, M.; Gao, Y.; Xue, W.; Zhao, J.; Xiao, S.; et al. Efficient and selective capture of thorium ions by a covalent organic framework. Nat. Commun. 2023, 14, 5097. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, Z.; Zhang, W.; Gao, Q.; Dai, Y.; Xia, F.; Zhang, X. EDTA-based adsorbents for the removal of metal ions in wastewater. Coord. Chem. Rev. 2021, 434, 213809. [Google Scholar] [CrossRef]
- Bahmanova, F.N.; Hajiyeva, S.R.; Chyragov, F.M. Thorium(IV), sorbent, sorption, desorption, clay. Vestn. Astrakhan State Tech. Univ. 2021, 2021, 64–70. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Deng, B. Enhanced mercury ion adsorption by amine-modified activated carbon. J. Hazard. Mater. 2009, 166, 866–872. [Google Scholar] [CrossRef]
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K. Kinetic and isotherm analysis for selective thorium(IV) retrieval from aqueous environment using eco-friendly cellulose composite. Int. J. Environ. Sci. Technol. 2015, 12, 3095–3106. [Google Scholar] [CrossRef]
- Yiqing, Y.; Palaniandy, P.; Zaman, H.G.; Khan, A.H. Functionalized metal–organic frameworks for efficient uranium adsorption from seawater. J. Radioanal. Nucl. Chem. 2025, 334, 4457–4484. [Google Scholar] [CrossRef]
- Hassan, A.; Mollah, M.R.; Jayashree, R.; Jain, A.; Das, S.; Das, N. Ultrafast removal of thorium and uranium from radioactive waste and groundwater using highly efficient and radiation-resistant functionalized triptycene-based porous organic polymers TP-POP-SO3NH4. ACS Appl. Mater. Interfaces 2024, 16, 24547–24561. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Mohapatra, P.K.; Pathak, P.N.; Manchanda, V.K. Cation-exchange separation of uranium from thorium in nitric acid medium. J. Radioanal. Nucl. Chem. 2006, 268, 323–328. [Google Scholar] [CrossRef]
- Atta, A.M.; Akl, Z.F. Removal of thorium from water using modified magnetite nanoparticles capped with rosin amidoxime. Mater. Chem. Phys. 2015, 163, 253–261. [Google Scholar] [CrossRef]
- Muneer, I.; Javed, T.; Majeed, A.A.; Masood, H.T. A brief study of adsorption of Congo red dye over sawdust of Cedrus deodara. Desalination Water Treat. 2021, 235, 272–282. [Google Scholar] [CrossRef]
- Liaskos, C.; Tsioliaridou, A.; Pitsillides, A.; Akyildiz, I.F.; Kantartzis, N.V.; Lalas, A.X.; Dimitropoulos, X.; Ioannidis, S.; Kafesaki, M.; Soukoulis, C. Design and development of software defined metamaterials for nanonetworks. IEEE Circuits Syst. Mag. 2015, 15, 12–25. [Google Scholar] [CrossRef]
- World Nuclear Association. Thorium. Available online: https://world-nuclear.org/information-library/current-and-future-generation/thorium.aspx (accessed on 2 May 2024).
- Dixon, B.W.; Ganda, F.; Hoffman, E.; Hansen, J.K.; Schneider, E.; Shropshire, D.E.; Williams, K.A. Advanced Fuel Cycle Cost Basis Report, Module A2: Thorium (INL/EXT-17-43812). Idaho Falls, ID: Idaho National Laboratory. 2017. Available online: https://sai.inl.gov/content/uploads/29/2024/11/2017_advanced_fuel_cycle_cost_basis.pdf?__cf_chl_f_tk=yLDjOroVumIECu9vChl0hmCUbfbrD0RMt4XUaeNj1qk-1762158193-1.0.1.1-u9JyvVmtUf49joAvrctClV8tnywuHKEwq4Phcyj8stw (accessed on 12 November 2025).
- Nardi, A.; Idiart, A.; Trinchero, P.; de Vries, L.M.; Molinero, J. Interface COMSOL-PHREEQC (iCP), an efficient numerical framework for the solution of coupled multiphysics and geochemistry. Comput. Geosci. 2014, 69, 10–21. [Google Scholar] [CrossRef]
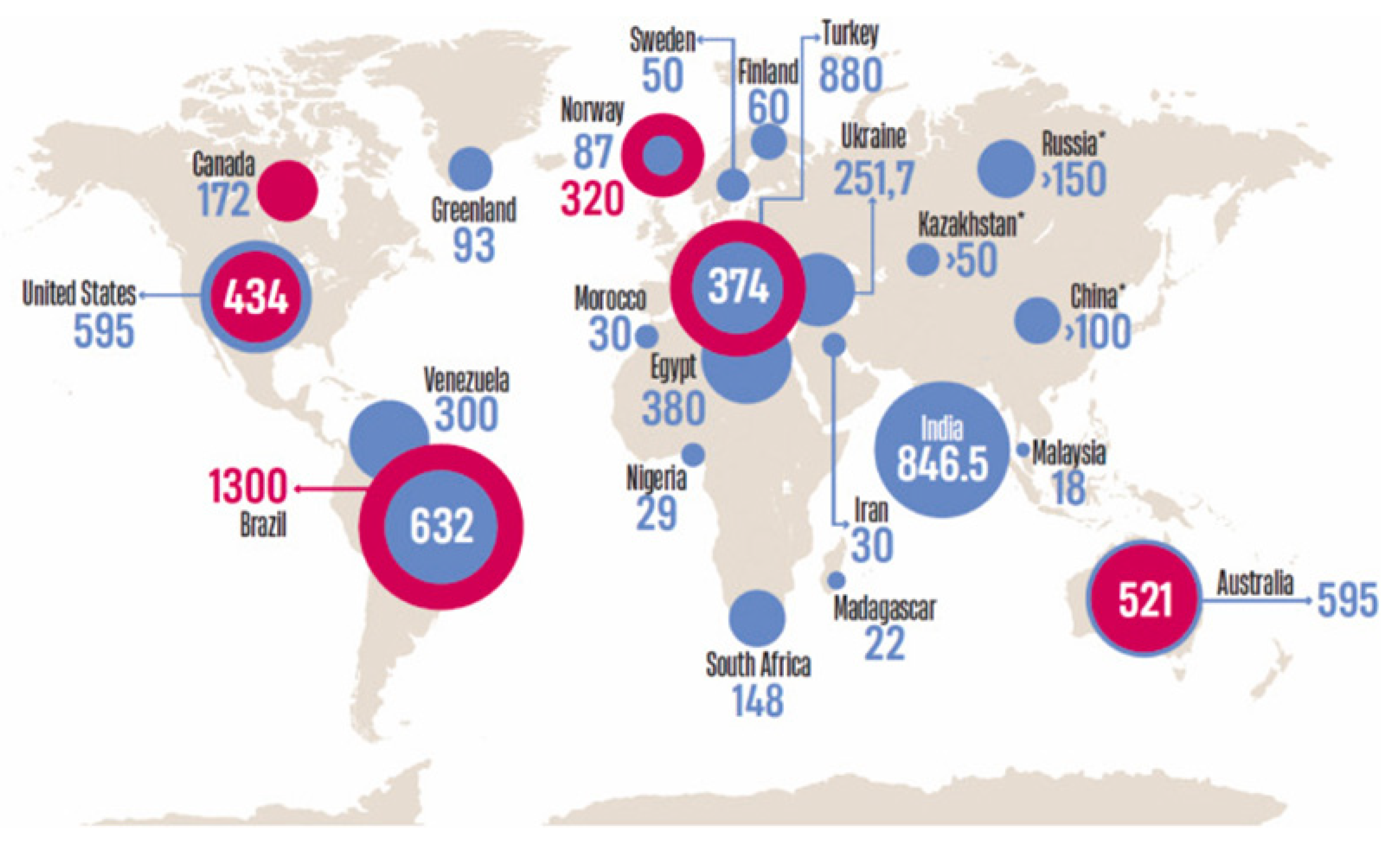
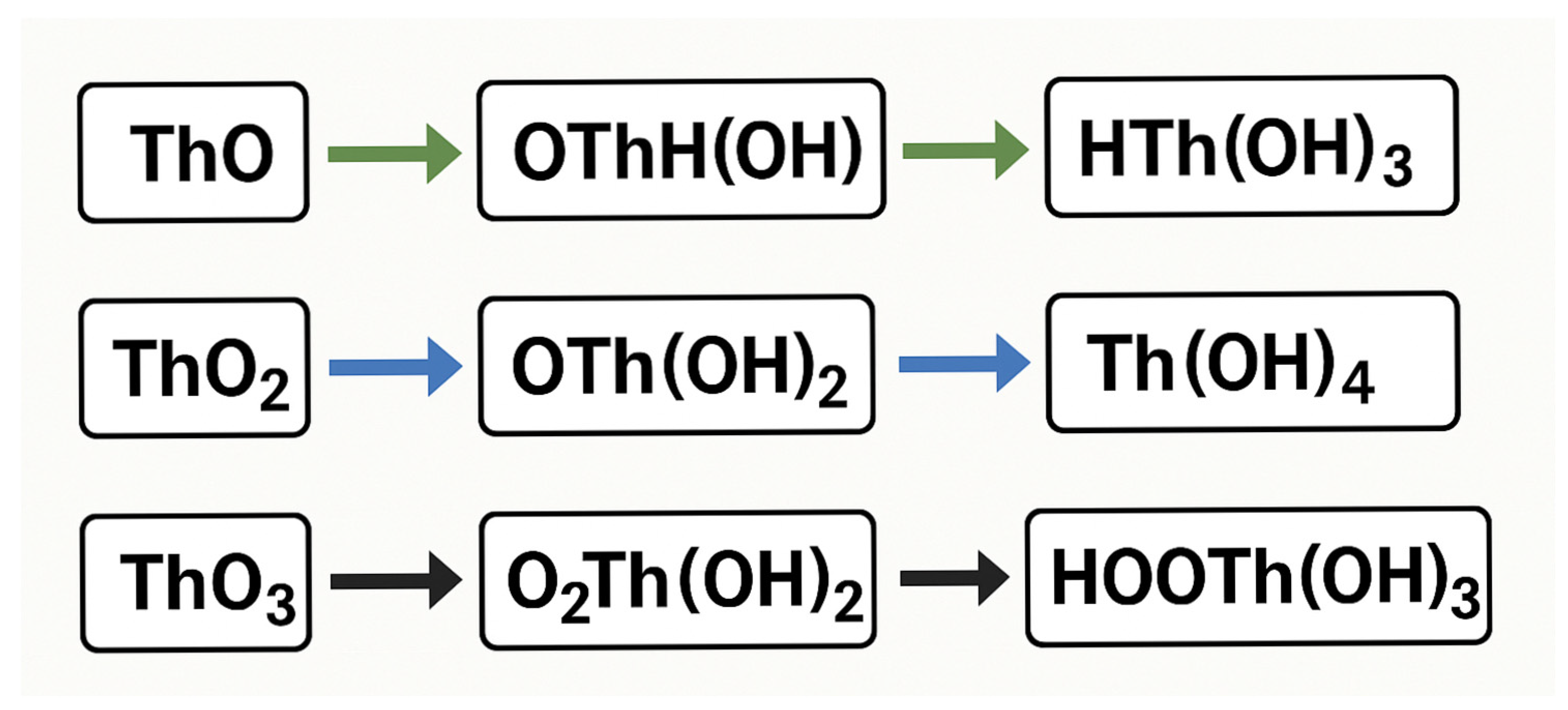
| Isotope | Half-Life | Decay Type | Ecological Significance |
|---|---|---|---|
| 232Th | 1.40 × 1010 years | α | The main natural isotope; determines long-term radiation background; potential source of 233U |
| 230Th | ~75,600 years | α | Member of the uranium series; accumulates in sediments; used in geochronology |
| 228Th | 698.6 days | α | Daughter product of 232Th; increases local radiation levels in water and soils |
| 234Th | 24.1 days | β− | Decay product of 238U; indicator of migration processes in the hydrosphere |
| 231Th | 25.5 h | β− | Member of the 235U series; relevant near uranium deposits |
| 227Th | 18.7 days | α | Short-lived but highly active; can contribute to contamination of surface waters |
| Factor | Conditions | Ecological Significance |
|---|---|---|
| Soil acidity (pH) | Low pH increases solubility and phytoavailability of thorium | Enhanced radiological risks, transition to mobile forms [36] |
| Organic matter | Humus and humic acids immobilize thorium; dissolved organic matter at high pH enhances migration | Formation of stable organo-mineral complexes or, conversely, promotion of transport [42] |
| Fe–Mn oxides | Act as active sorbents for Th(IV) | Reduced mobility, long-term fixation in soils [43] |
| Mineral composition | Isomorphic substitution in silicates, apatites, carbonates | Controls thorium distribution in geological environments [44] |
| Anthropogenic activity | Mining and ore processing, lignite combustion, phosphate fertilizers | Increased concentrations of Th, co-release of Ra and Po into soils [45] |
| Natural transport processes | Erosion and sedimentation in catchment basins | Redistribution of thorium from soils to rivers and oceans [14] |
| Form of Th in Water | Conditions of Formation | Ecological Behavior |
|---|---|---|
| Th(IV) aquo-complexes (soluble) | Acidic pH, presence of organic ligands, wastewater inputs | High mobility, risk of contamination of water bodies, bioavailability to aquatic organisms |
| Simple hydroxides (Th(OH)4) | Neutral media, hydrolysis of Th(IV) | Limited solubility, partial fixation in bottom sediments |
| Oxo- and peroxo-hydroxo complexes | Mixed redox conditions, presence of organic matter | Potential migration under changing conditions, possible bioaccumulation |
| Insoluble hydroxides | Alkaline media (pH > 7) | Precipitation and long-term fixation in sediments, reduced mobility |
| Colloidal forms | Suspended particles, minerals, organo-mineral associations | Transport with water flows, accumulation in silts and sediments, long-term ecological impact |
| Sorbent | Sorption Mechanism | Optimal pH | Capacity (mg/g) |
|---|---|---|---|
| Bentonite | Ion exchange [89] | 4.0–6.0 | 20–35 |
| Diatomite | Physical adsorption [90] | 5.0–6.0 | 15–25 |
| Biochar (from rice husk) | Chemical adsorption [94] | 4.5–6.5 | 45–65 |
| Algae (Sargassum spp.) | Carboxyl and sulfate groups [96] | 4.0–5.5 | 75–85 |
| Aspergillus niger mycelium | Ion exchange [98] | 3.0–4.5 | 40–50 |
| Ion exchange resins (Dowex 50WX8) | Ion exchange [102] | 2.0–3.5 | 100–130 |
| Graphene oxide | Surface adsorption [99] | 3.5–5.5 | 150–180 |
| Fe3O4@SiO2-NH2 | Chemical coordination [103] | 3.5–4.5 | 120–140 |
| Modified activated carbon (amino groups) | Chemisorption [104] | 4.0–5.5 | 60–80 |
| Chelating sorbent based on EDTA | Complexation [103] | 2.0–4.0 | 100–160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsar, Z.; Gajimuradova, A.; Mansurov, Z.; Gubaidullin, N.; Hassanein, A.; Insepov, Z. Thorium in Energy and Ecology: Prospects for Clean Fuel Sources and Protection of Water and Soil Systems from Radiation Risks. Energies 2025, 18, 6177. https://doi.org/10.3390/en18236177
Alsar Z, Gajimuradova A, Mansurov Z, Gubaidullin N, Hassanein A, Insepov Z. Thorium in Energy and Ecology: Prospects for Clean Fuel Sources and Protection of Water and Soil Systems from Radiation Risks. Energies. 2025; 18(23):6177. https://doi.org/10.3390/en18236177
Chicago/Turabian StyleAlsar, Zhanna, Aisarat Gajimuradova, Zulkhair Mansurov, Nurtai Gubaidullin, Ahmed Hassanein, and Zinetula Insepov. 2025. "Thorium in Energy and Ecology: Prospects for Clean Fuel Sources and Protection of Water and Soil Systems from Radiation Risks" Energies 18, no. 23: 6177. https://doi.org/10.3390/en18236177
APA StyleAlsar, Z., Gajimuradova, A., Mansurov, Z., Gubaidullin, N., Hassanein, A., & Insepov, Z. (2025). Thorium in Energy and Ecology: Prospects for Clean Fuel Sources and Protection of Water and Soil Systems from Radiation Risks. Energies, 18(23), 6177. https://doi.org/10.3390/en18236177







_Insepov.png)

