Phosphoric Acid Addition: Insight into the Mechanism Governing Biochar Structural Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Materials
2.2. Biochar Preparation
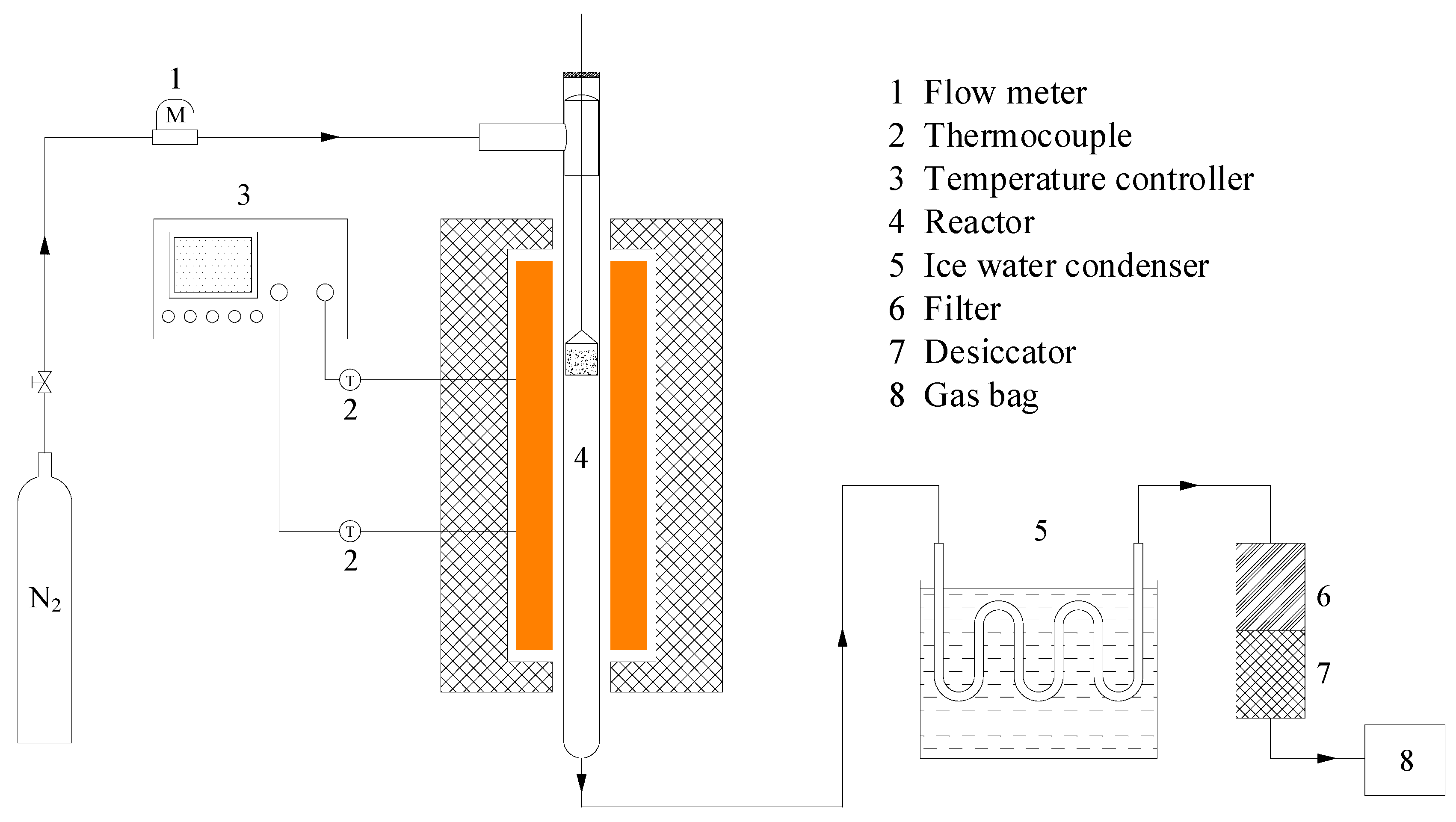
2.3. Characterization of Biochar
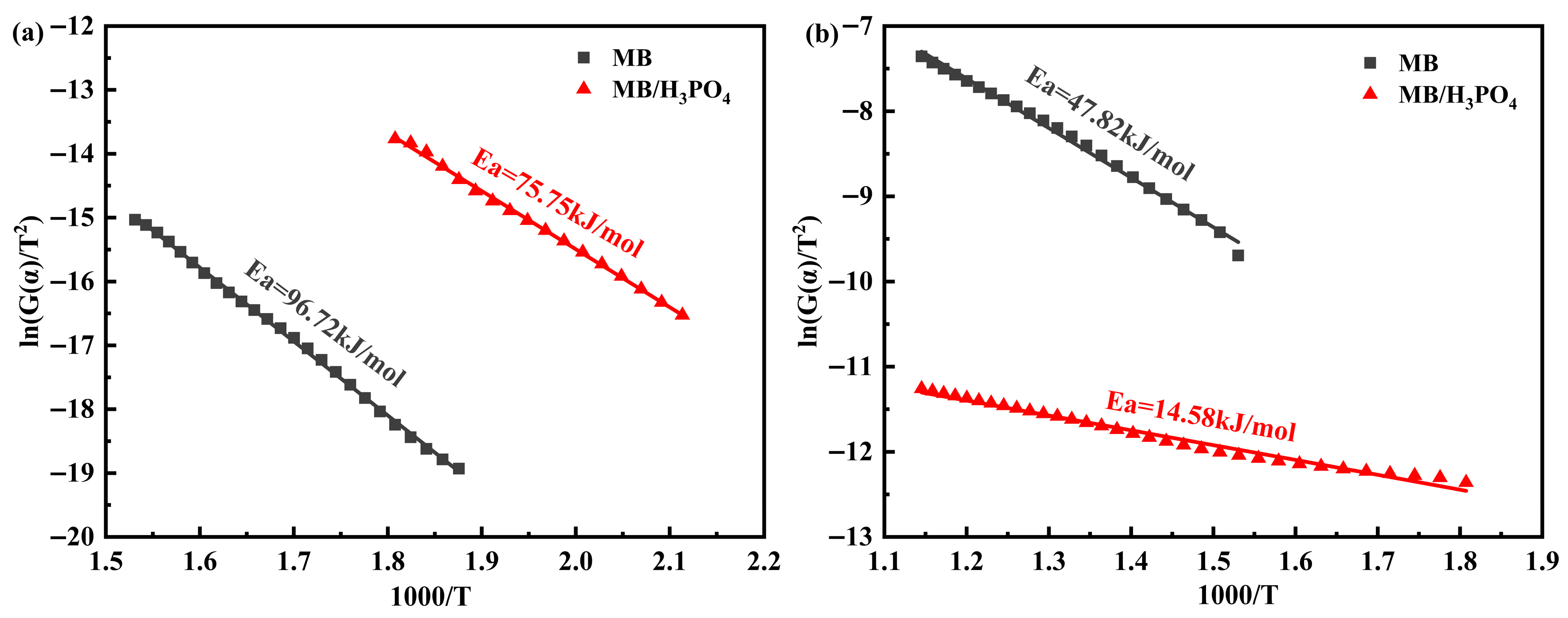
2.4. Pyrolysis Kinetics Analysis Method
2.5. D-PCIS Analysis Method
3. Results and Discussion
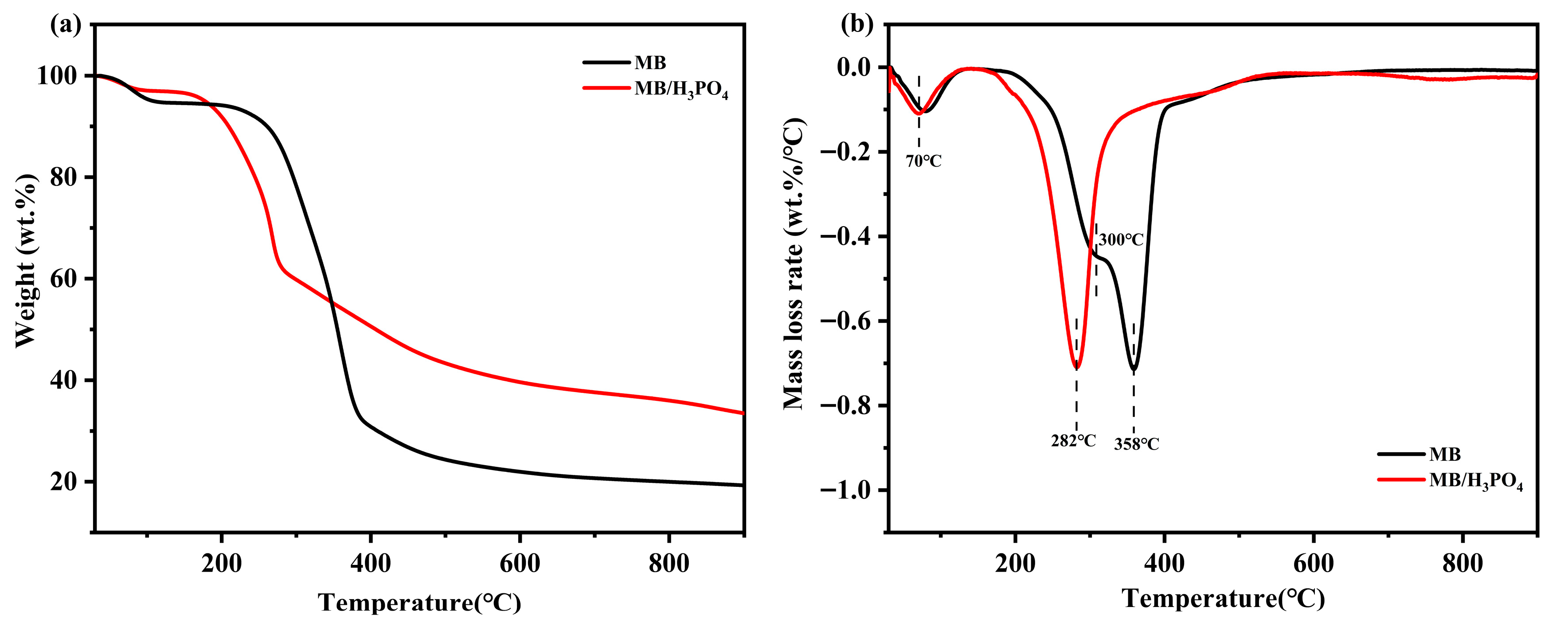
3.1. Pyrolysis Properties Determined by Thermogravimetric Analyses
3.2. Biochar’s Carbon Skeleton Analysis
3.3. Evolution of Biochar’s Functional Group
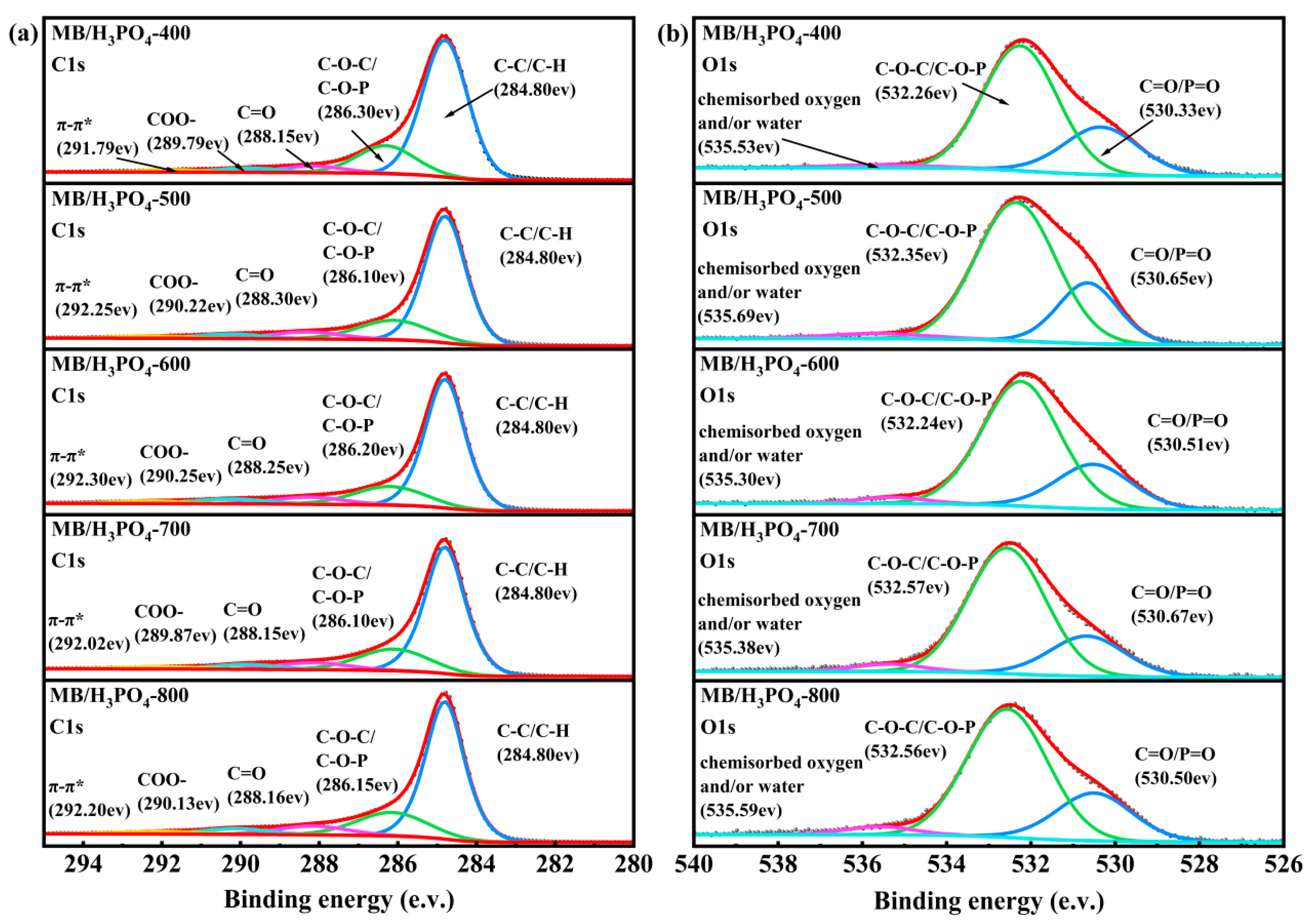
3.3.1. XPS Analysis
3.3.2. FTIR Analysis
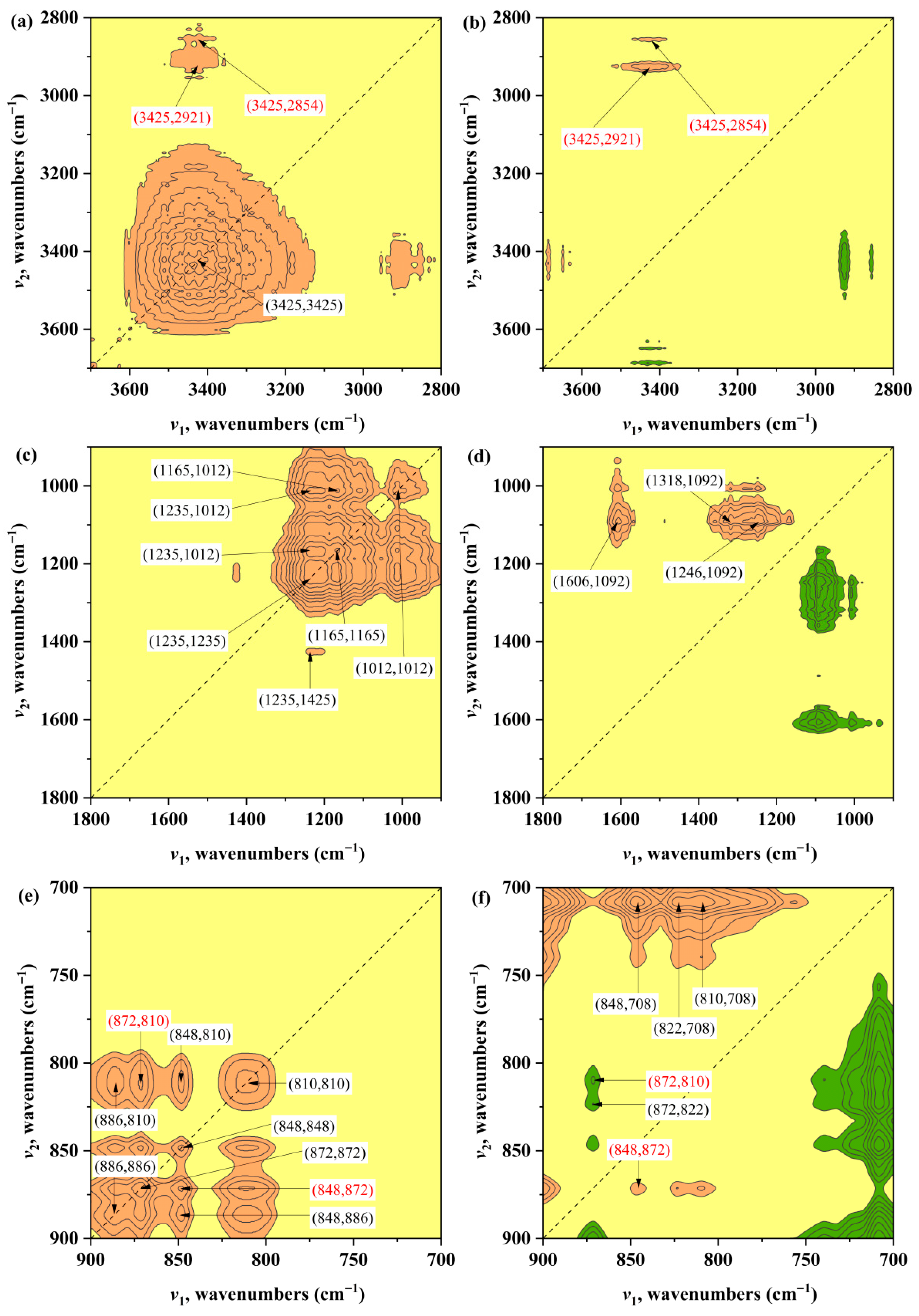
3.3.3. D-PCIS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, C.; Lv, Z.; Li, Z.; Sun, K. Green finance, renewable energy and carbon neutrality in OECD countries. Renew. Energy 2023, 211, 279–284. [Google Scholar] [CrossRef]
- Xia, S.W.; Yang, H.P.; Lei, S.S.; Lu, W.; Cai, N.; Xiao, H.Y.; Chen, Y.Q.; Chen, H.P. Iron salt catalytic pyrolysis of biomass: Influence of iron salt type. Energy 2023, 262, 125415. [Google Scholar] [CrossRef]
- Xia, S.W.; Yang, H.P.; Lu, W.; Cai, N.; Xiao, H.Y.; Chen, X.; Chen, Y.Q.; Wang, X.H.; Wang, S.R.; Wu, P.; et al. Fe-Co based synergistic catalytic graphitization of biomass: Influence of the catalyst type and the pyrolytic temperature. Energy 2022, 239, 122262. [Google Scholar] [CrossRef]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The key role of biochar in amending acidic soil: Reducing soil acidity and improving soil acid buffering capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Das, N.; Pandey, P. Biochar-driven rhizoremediation of soil contaminated with organic pollutants: Engineered solutions, microbiome enrichment, and bioeconomic benefits for ecosystem restoration. Biochar 2025, 7, 101. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Z.; Lu, Y.; Shan, S.; Zhuang, H.; Gong, C.; Cui, X.; Zhang, F.; Li, P. Facilitating mitigation of agricultural non-point source pollution and improving soil nutrient conditions: The role of low temperature co-pyrolysis biochar in nitrogen and phosphorus distribution. Bioresour. Technol. 2024, 394, 130179. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, H.; Bartocci, P.; Fantozzi, F.; Mašek, O.; Agblevor, F.A.; Wei, Z.; Yang, H.; Chen, H.; Lu, X.; et al. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 2021, 12, 1698. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. Production of syngas via gasification using optimum blends of biomass. J. Clean. Prod. 2020, 242, 118499. [Google Scholar] [CrossRef]
- Leng, L.J.; Xu, S.Y.; Liu, R.F.; Yu, T.; Zhuo, X.M.; Leng, S.Q.; Xiong, Q.; Huang, H.J. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Chen, H.Y.; Shan, R.; Zhao, F.X.; Gu, J.; Zhang, Y.Y.; Yuan, H.R.; Chen, Y. A review on the NOx precursors release during biomass pyrolysis. Chem. Eng. J. 2023, 451, 138979. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ. Technol. Rev. 2019, 8, 47–64. [Google Scholar] [CrossRef]
- Rizwan, M.; Eisa, M.H.; Zada, A.; Azizi, S.; Alkaoud, A.M.; Albishi, M.S.; Chen, H. Cutting edge advancements in biochar applications: Thermochemical, fermentative, photocatalytic and electrocatalytic hydrogen production and storage. Fuel 2025, 402, 136016. [Google Scholar] [CrossRef]
- Rana, P.; Soni, V.; Sharma, S.; Poonia, K.; Patial, S.; Singh, P.; Selvasembian, R.; Chaudhary, V.; Hussain, C.M.; Raizada, P. Harnessing nitrogen doped magnetic biochar for efficient antibiotic adsorption and degradation. J. Ind. Eng. Chem. 2025, 148, 174–195. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Q.; Wang, C.; Wu, Y.; Liu, L.; Shen, G.; Chen, Q. Single-atom Fe-decorated N-doped porous carbon from waste biomass as a high-performance air-cathode for wastewater treatment in microbial fuel cells. Water Res. 2025, 280, 123518. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Wu, C.L.; Zhang, G.J.; Liu, J.; Li, Y.L.; Li, G.Q. Synthesis and characterization of magnetic K2CO3-activated carbon produced from bamboo shoot for the adsorption of Rhodamine b and CO2 capture. Fuel 2023, 332, 126107. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, G.G.; Zhang, Q.; Yang, Y.T.; Zhou, J.C.; Cai, J.J. Hierarchical porous carbons from biowaste: Hydrothermal carbonization and high-performance for Rhodamine B adsorptive removal. J. Mol. Liq. 2021, 330, 115580. [Google Scholar] [CrossRef]
- He, Y.Y.; Ni, L.M.; Gao, Q.; Ren, H.; Su, M.F.; Hou, Y.M.; Liu, Z.J. Activated Carbon with Ultrahigh Specific Surface Derived from Bamboo Shoot Shell through K2FeO4 Oxidative Pyrolysis for Adsorption of Methylene Blue. Molecules 2023, 28, 3410. [Google Scholar] [CrossRef]
- Fakhar, A.; Canatoy, R.C.; Galgo, S.J.C.; Rafique, M.; Sarfraz, R. Advancements in modified biochar production techniques and soil application: A critical review. Fuel 2025, 400, 135745. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, L.; Liu, H.; de Jesus Puy Alquiza, M.; Li, Y. Biochar application to soils can regulate soil phosphorus availability: A review. Biochar 2025, 7, 13. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.-B.; Yang, X.-C.; Dong, C.-Q.; Zhu, X.-F. Catalytic fast pyrolysis of biomass impregnated with K3PO4 to produce phenolic compounds: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2013, 104, 139–145. [Google Scholar] [CrossRef]
- Zhurinsh, A.; Dobele, G.; Pomilovskis, R.; Volperts, A.; Jurkjane, V. Evolution of 1,6-anhydrosugars in the pyrolysis of biomass with phosphoric acid and P-containing activated carbon. Catal. Today 2021, 367, 51–57. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; He, Y.; An, X.; Fan, D.; Wu, Z. Microwave-assisted catalytic pyrolysis of apple wood to produce biochar: Co-pyrolysis behavior, pyrolysis kinetics analysis and evaluation of microbial carriers. Bioresour. Technol. 2021, 320, 124345. [Google Scholar] [CrossRef]
- Yang, L.; Tan, W.-F.; Mumford, K.; Ding, L.; Lv, J.-W.; Zhang, X.-W.; Wang, H.-Q. Effects of phosphorus-rich sawdust biochar sorption on heavy metals. Sep. Sci. Technol. 2018, 53, 2704–2716. [Google Scholar] [CrossRef]
- Xiang, W.; Gan, L.H.; Wang, Y.; Yang, N.W.; Wang, W.J.; Li, L.J.; Liu, Z.J.; Feng, Y.P.; Chen, D.F.; Wang, R.J. Enhanced adsorption performance of phosphoric acid activated biochar from in-situ pre-carbonized bamboo shoot shells. Ind. Crops Prod. 2025, 226, 120719. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, W.; Zhou, X.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Coeffect of pyrolysis temperature and potassium phosphate impregnation on characteristics, stability, and adsorption mechanism of phosphorus-enriched biochar. Bioresour. Technol. 2022, 344, 126273. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.P.; Xu, H.C.; Xu, X.Y.; Qiu, H.; Cao, X.D.; Zhao, L. Development of phosphorus composite biochar for simultaneous enhanced carbon sink and heavy metal immobilization in soil. Sci. Total Environ. 2022, 831, 154845. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Tang, Z.; Yao, D.; Yang, H.; Shao, J.; Chen, H. Thermal behavior, kinetics and gas evolution characteristics for the co-pyrolysis of real-world plastic and tyre wastes. J. Clean. Prod. 2020, 260, 121102. [Google Scholar] [CrossRef]
- Liu, H.; Chen, B.; Wang, C. Pyrolysis kinetics study of biomass waste using Shuffled Complex Evolution algorithm. Fuel Process. Technol. 2020, 208, 106509. [Google Scholar] [CrossRef]
- Noda, I. Generalized 2-Dimensional Correlation Method Applicable to Infrared, Raman, and Other Types of Spectroscopy. Appl. Spectrosc. 1993, 47, 1329–1336. [Google Scholar] [CrossRef]
- Lasch, P.; Noda, I. Two-Dimensional Correlation Spectroscopy (2D-COS) for Analysis of Spatially Resolved Vibrational Spectra. Appl. Spectrosc. 2019, 73, 359–379. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P.-y. Applications of Two-dimensional Correlation Infrared Spectroscopy in the Characterization of Polymers. Acta Polym. Sin. 2022, 53, 522–538. [Google Scholar] [CrossRef]
- Li, J.; Dou, B.; Zhang, H.; Zhang, H.; Chen, H.; Xu, Y.; Wu, C. Pyrolysis characteristics and non-isothermal kinetics of waste wood biomass. Energy 2021, 226, 120358. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wang, C.; Zhu, X. Catalytic effects of ammonium dihydrogen phosphate on the pyrolysis of lignocellulosic biomass: Selective production of furfural and levoglucosenone. Fuel Process. Technol. 2020, 209, 106525. [Google Scholar] [CrossRef]
- Yi, H.; Yang, Z.; Tang, X.; Zhao, S.; Gao, F.; Wang, J.; Huang, Y.; Yang, K.; Shi, Y.; Xie, X. Variations of apparent activation energy based on thermodynamics analysis of zeolitic imidazolate frameworks including pyrolysis and combustion. Energy 2018, 151, 782–798. [Google Scholar] [CrossRef]
- Jia, Y.; Lu, Y.; Zhang, G.; Liang, Y.; Zhang, F. Facile synthesis of an eco-friendly nitrogen-phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J. Mater. Chem. A 2017, 5, 9970–9981. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman-Spectroscopy of Carbon Materials—Structural Basis of Observed Spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Sheng, C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, W.; Masek, O.; Chen, X.; Gu, B.; Sharma, B.K.; Cao, X. Roles of Phosphoric Acid in Biochar Formation: Synchronously Improving Carbon Retention and Sorption Capacity. J. Environ. Qual. 2017, 46, 393–401. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wisniewski, M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Y.; Zhang, H.; Wang, W.; Zhu, J.; Chen, Y.; Zhang, S.; Chen, H. Effect mechanism of phosphorous-containing additives on carbon structure evolution and biochar stability enhancement. Biochar 2024, 6, 39. [Google Scholar] [CrossRef]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Valero-Romero, M.J.; Garcia-Mateos, F.J.; Rodriguez-Mirasol, J.; Cordero, T. Role of surface phosphorus complexes on the oxidation of porous carbons. Fuel Process. Technol. 2017, 157, 116–126. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Liu, B.; Chen, Y.; Xiao, J.; Dong, Z.; Gong, M.; Chen, H. Hemicellulose pyrolysis mechanism based on functional group evolutions by two-dimensional perturbation correlation infrared spectroscopy. Fuel 2020, 267, 117302. [Google Scholar] [CrossRef]
- Harvey, O.R.; Herbert, B.E.; Kuo, L.-J.; Louchouarn, P. Generalized Two-Dimensional Perturbation Correlation Infrared Spectroscopy Reveals Mechanisms for the Development of Surface Charge and Recalcitrance in Plant-Derived Biochars. Environ. Sci. Technol. 2012, 46, 10641–10650. [Google Scholar] [CrossRef]
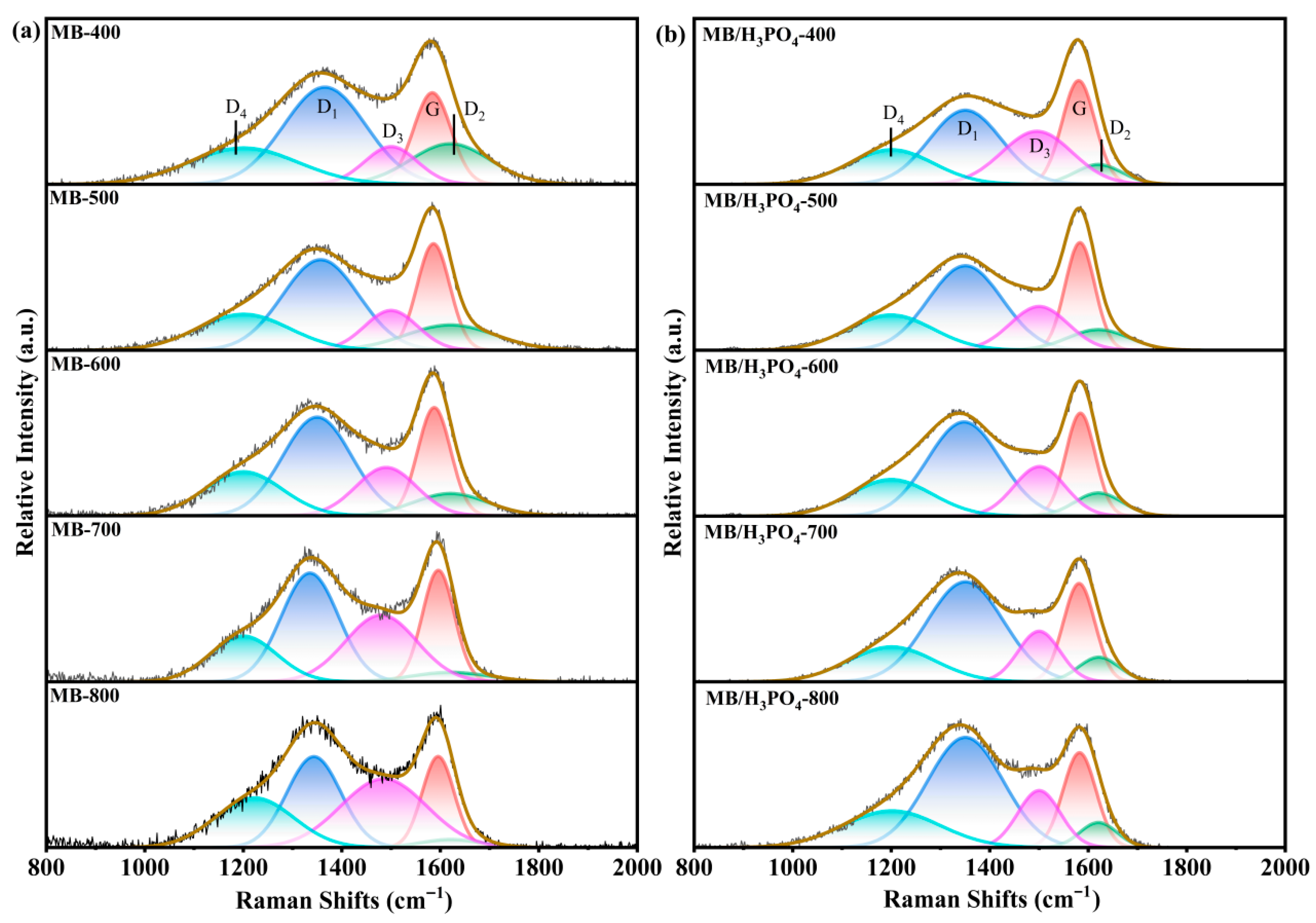
| Samples | FWHG(D1)/cm−1 | FWHG(G)/cm−1 | IG/ITotal | ID1/IG |
|---|---|---|---|---|
| MB-400 | 196.75 | 89.23 | 16.59 | 2.34 |
| MB-500 | 182.90 | 78.64 | 19.32 | 1.98 |
| MB-600 | 164.60 | 78.55 | 19.40 | 1.91 |
| MB-700 | 136.17 | 76.42 | 19.45 | 1.74 |
| MB-800 | 129.54 | 76.36 | 16.08 | 1.69 |
| MB/H3PO4-400 | 172.15 | 78.89 | 20.94 | 1.56 |
| MB/H3PO4-500 | 175.98 | 70.85 | 19.68 | 1.95 |
| MB/H3PO4-600 | 174.01 | 70.38 | 18.33 | 2.26 |
| MB/H3PO4-700 | 182.80 | 75.49 | 18.39 | 2.46 |
| MB/H3PO4-800 | 180.30 | 78.62 | 17.00 | 2.66 |
| Samples | C1s | O1s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C-C/ C-H | C-O-C/ C-O-P | C=O | COO- | π-π* Satellite | Total of Oxygenated Carbon | C=O/ P=O | C-O-C/ C-O-P | Chemisorbed Oxygen and/or Water | |
| MB/H3PO4-400 | 71.52 | 18.77 | 4.86 | 3.13 | 1.71 | 26.76 | 27.49 | 70.18 | 2.33 |
| MB/H3PO4-500 | 69.53 | 18.26 | 6.48 | 3.63 | 2.10 | 28.37 | 24.29 | 72.25 | 3.46 |
| MB/H3PO4-600 | 69.21 | 17.39 | 6.60 | 4.55 | 2.24 | 28.54 | 24.99 | 71.76 | 3.25 |
| MB/H3PO4-700 | 67.55 | 19.66 | 6.42 | 4.05 | 2.31 | 30.13 | 23.17 | 72.91 | 3.92 |
| MB/H3PO4-800 | 65.97 | 19.70 | 7.39 | 4.37 | 2.56 | 31.46 | 25.17 | 70.93 | 3.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xia, S.; Ren, X.; Dong, Y. Phosphoric Acid Addition: Insight into the Mechanism Governing Biochar Structural Evolution. Energies 2025, 18, 6165. https://doi.org/10.3390/en18236165
Wang Z, Xia S, Ren X, Dong Y. Phosphoric Acid Addition: Insight into the Mechanism Governing Biochar Structural Evolution. Energies. 2025; 18(23):6165. https://doi.org/10.3390/en18236165
Chicago/Turabian StyleWang, Zhongwei, Sunwen Xia, Xiaohan Ren, and Yong Dong. 2025. "Phosphoric Acid Addition: Insight into the Mechanism Governing Biochar Structural Evolution" Energies 18, no. 23: 6165. https://doi.org/10.3390/en18236165
APA StyleWang, Z., Xia, S., Ren, X., & Dong, Y. (2025). Phosphoric Acid Addition: Insight into the Mechanism Governing Biochar Structural Evolution. Energies, 18(23), 6165. https://doi.org/10.3390/en18236165






