Study on Electrochemical Performance and Magnesium Storage Mechanism of Na3V2(PO4)3@C Cathode in Mg(TFSI)2/DME Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Assembly of Coin Cells
2.3. Characterization and Testing
2.3.1. Structural and Morphological Characterization
2.3.2. Electrochemical Performance Testing
3. Results and Discussions
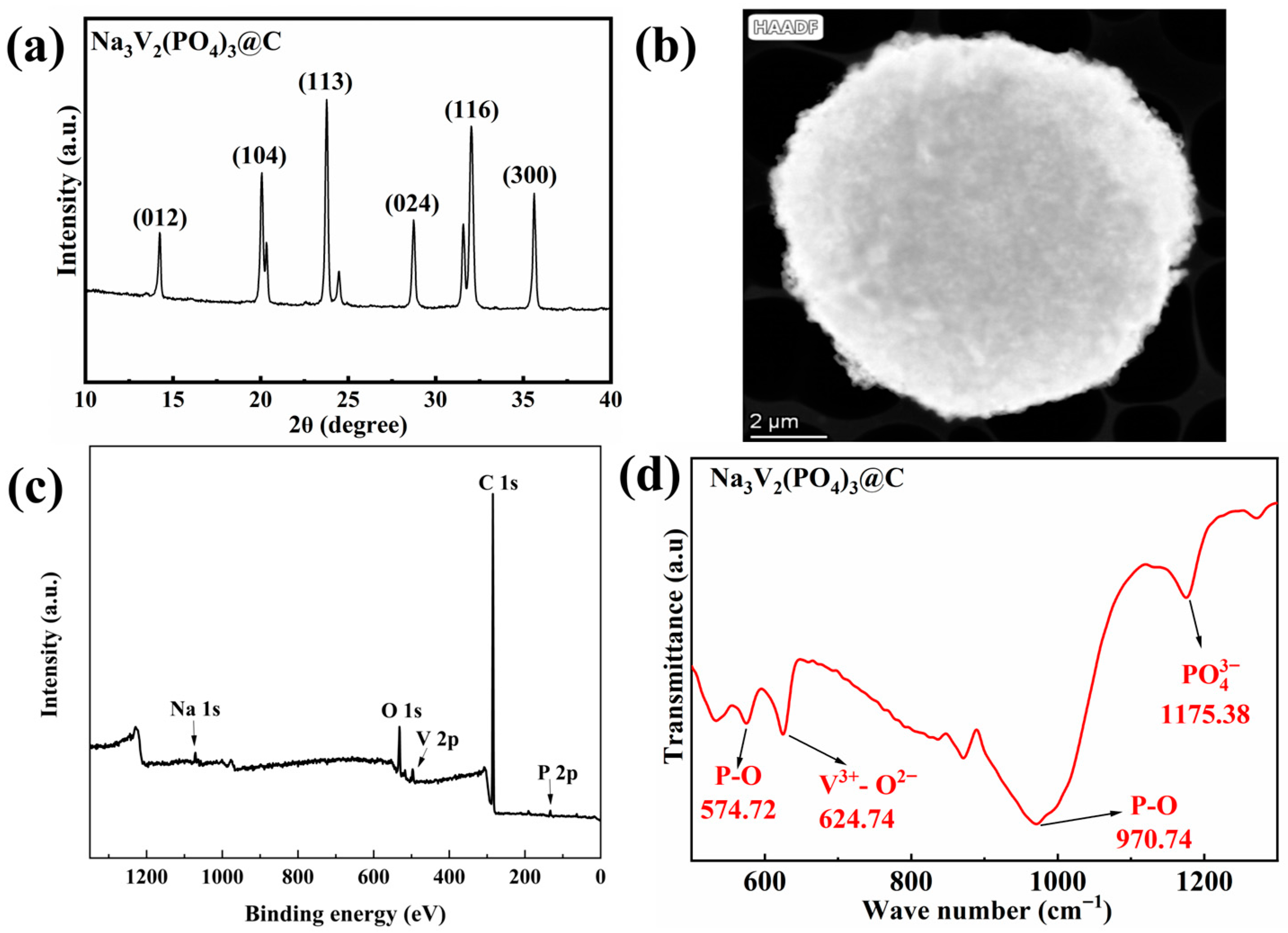
3.1. Structural and Morphological Characteristics of NVP@C
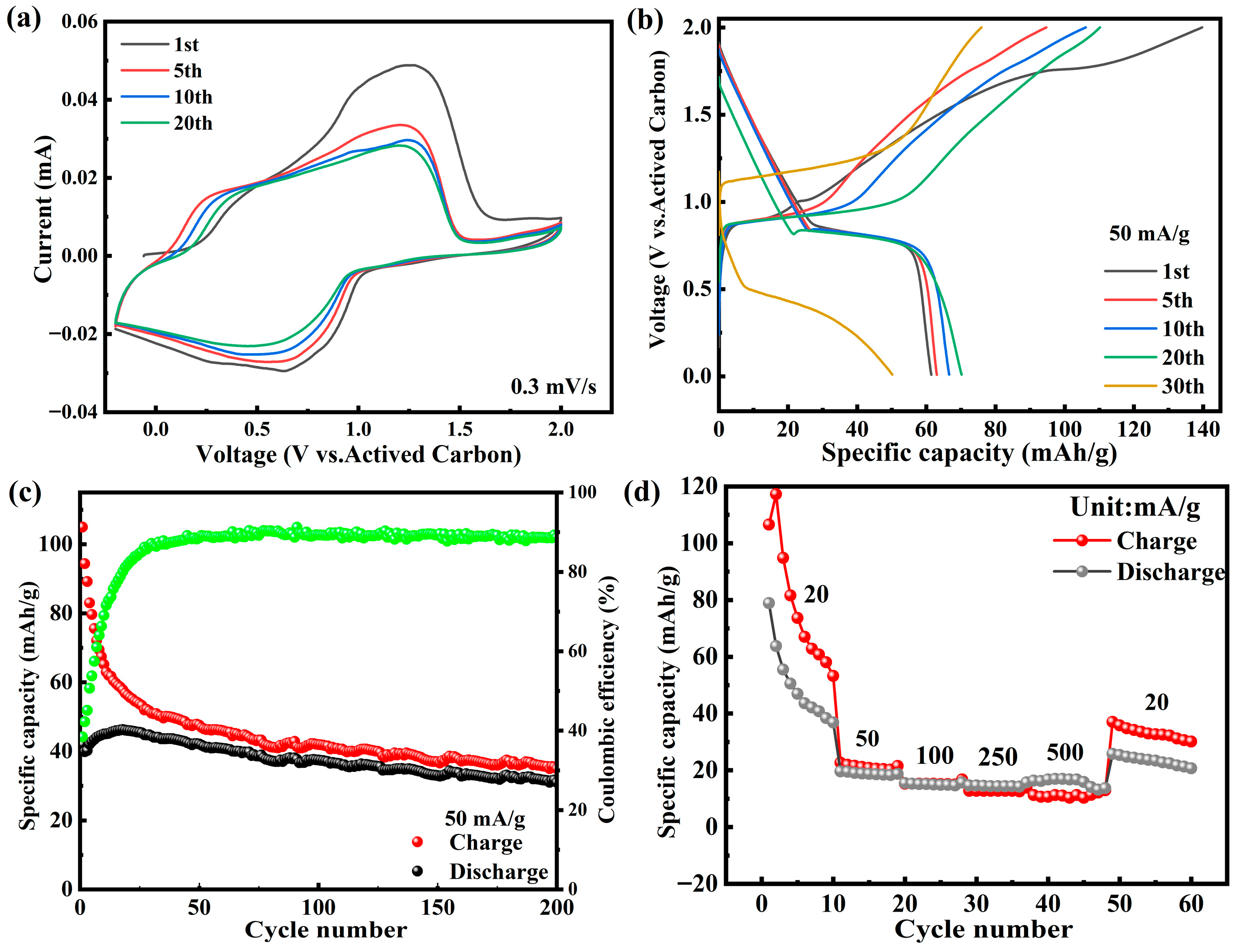
3.2. Electrochemical Performance of NVP@C
3.3. Magnesium Storage Reaction Process of NVP@C in Mg(TFSI)2/DME Electrolyte
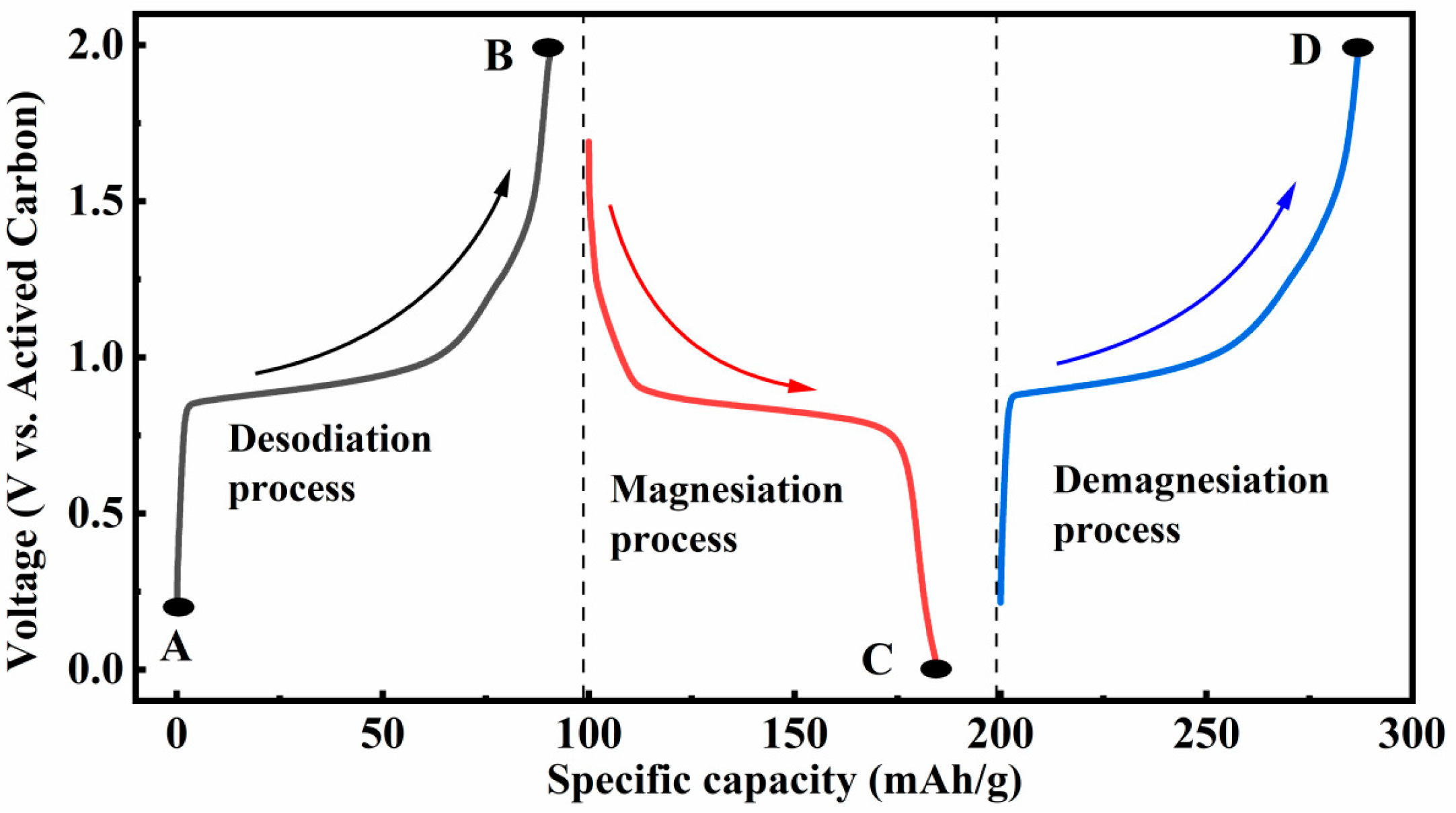
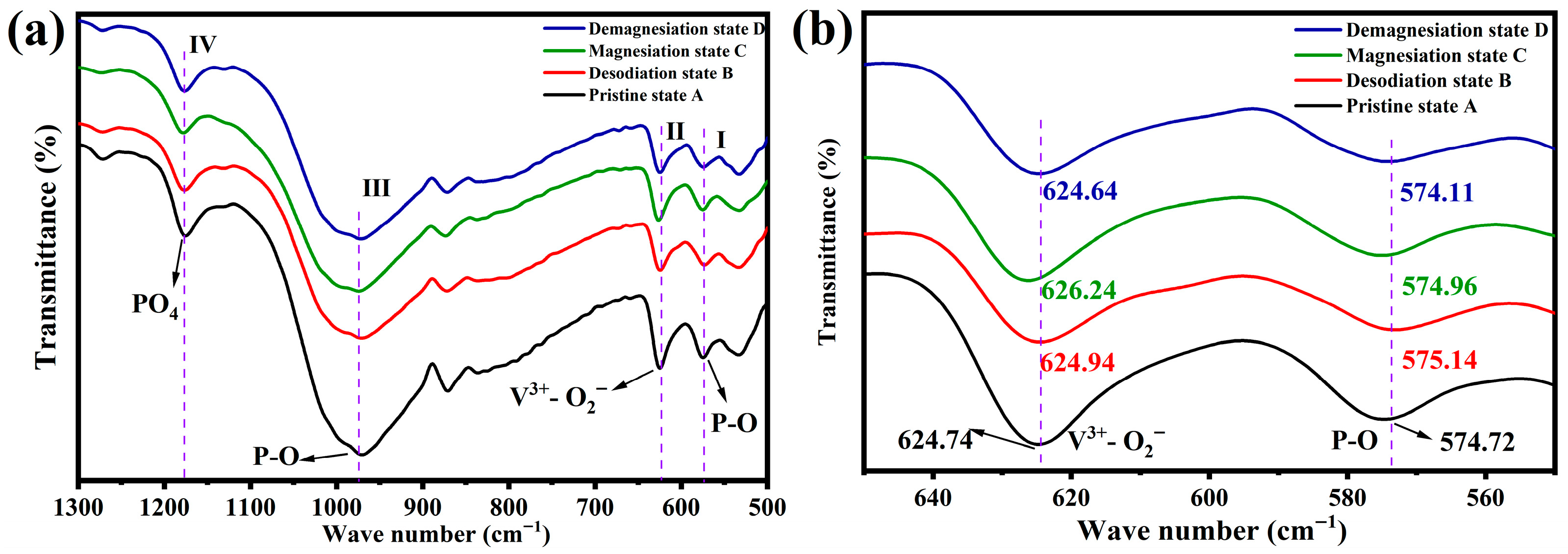
3.3.1. Reaction Stages of NVP@C in Mg(TFSI)2/DME Electrolyte
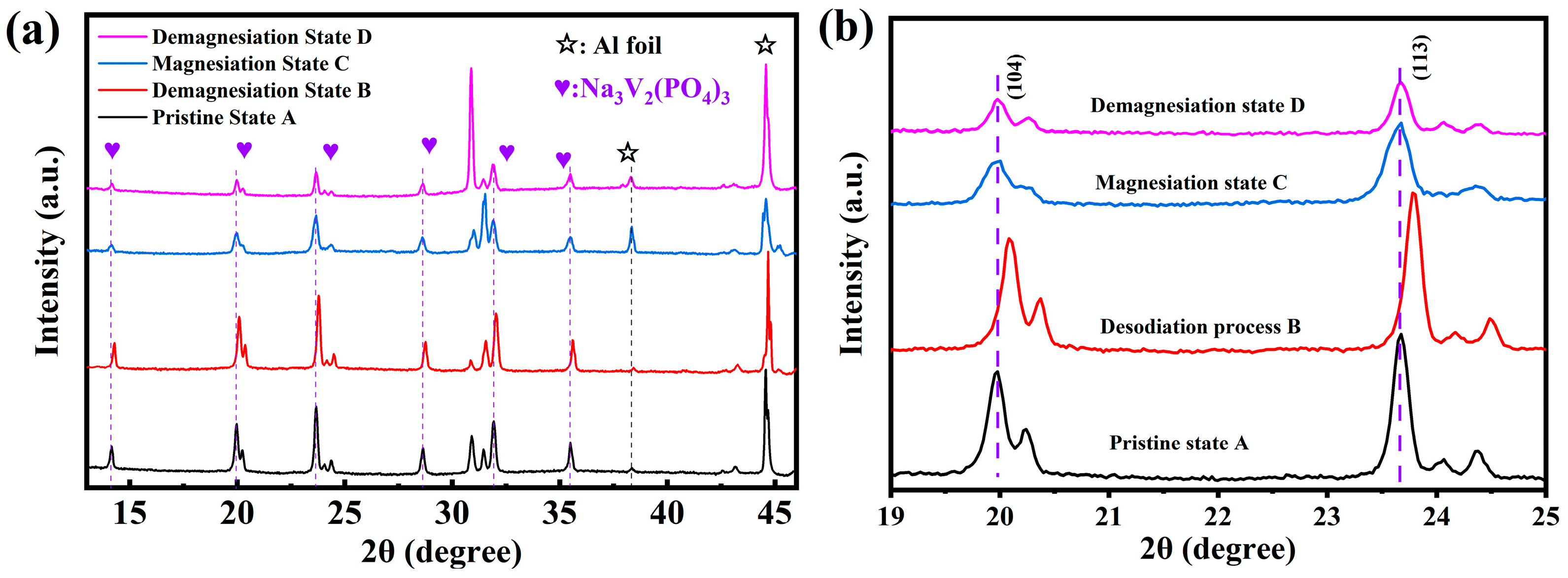
3.3.2. Reaction Mechanism of NVP@C in Mg(TFSI)2/DME Electrolyte
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.L.; Liu, Y.; Dou, S.X. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: An on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. [Google Scholar] [CrossRef]
- Gregory, T.D.; Hoffman, R.J.; Winterton, R.C. Nonaqueous Electrochemistry of Magnesium—Applications to Energy-Storage. J. Electrochem. Soc. 1990, 137, 775–780. [Google Scholar] [CrossRef]
- Lu, Z.; Schechter, A.; Moshkovich, M.; Aurbach, D. On the electrochemical behavior of magnesium electrodes in polar aprotic electrolyte solutions. J. Electroanal. Chem. 1999, 466, 203–217. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, H.; Wei, W.; Ma, J.; Chen, L.; Li, C.C. Challenges and recent progress in the design of advanced electrode materials for rechargeable Mg batteries. Energy Storage Mater. 2019, 20, 118–138. [Google Scholar] [CrossRef]
- Sun, C.; Wang, H.; Yang, F.; Tang, A.; Huang, G.; Li, L.; Wang, Z.; Qu, B.; Xu, C.; Tan, S.; et al. Layered buserite Mg-Mn oxide cathode for aqueous rechargeable Mg-ion battery. J. Magnes. Alloys 2023, 11, 840–850. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.Z.; Xu, T.; Li, H.J.; Chen, Y.G.; Yan, Y.G. High cycling stability of MnO2 cathode for aqueous Mg-ion batteries enabled by Fe doping. J. Power Sources 2024, 611, 9. [Google Scholar] [CrossRef]
- Choyal, V.; Dey, D.; Sai Gautam, G. Exploration of Amorphous V2O5 as Cathode for Magnesium Batteries. Small 2025, e05851. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kempaiah Devaraju, M.; Nguyen, D.N.; Gambe, Y.; Nayuki, K.; Sasaki, Y.; Tran, P.D.; Honma, I. Disulfide-Bridged (Mo3S11) Cluster Polymer: Molecular Dynamics and Application as Electrode Material for a Rechargeable Magnesium Battery. Nano Lett. 2016, 16, 5829–5835. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xiong, F.; Fan, Y.; Xiong, Y.; Jian, Z. Hierarchical Copper Sulfide Porous Nanocages for Rechargeable Multivalent-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 10471–10478. [Google Scholar] [CrossRef]
- Fei, Y.; Wang, H.; Xu, Y.; Song, L.; Man, Y.; Du, Y.; Bao, J.; Zhou, X. Bimetallic sulfide CoNi2S4 hollow nanospheres as a high-performance cathode material for Mg-ion batteries. Chem. Eng. J. 2024, 480, 148255. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Ghosh, T.; Bai, Y.; Lim, C.Y.J.; Zhang, L.; Seng, D.H.L.; Goh, W.P.; Xing, Z.; Liu, Z.; et al. Hierarchical FeS2 cathode with suppressed shuttle effect for high performance magnesium-ion batteries. Nano Energy 2024, 119, 109082. [Google Scholar] [CrossRef]
- Truong, Q.D.; Devaraju, M.K.; Honma, I. Nanocrystalline MgMnSiO4 and MgCoSiO4 particles for rechargeable Mg-ion batteries. J. Power Sources 2017, 361, 195–202. [Google Scholar] [CrossRef]
- Perez-Vicente, C.; Rubio, S.; Ruiz, R.; Zuo, W.; Liang, Z.; Yang, Y.; Ortiz, G.F. Olivine-Type MgMn0.5 Zn0.5 SiO4 Cathode for Mg-Batteries: Experimental Studies and First Principles Calculations. Small 2023, 19, e2206010. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hong, Y.; Dou, S.; Wu, J.; Zhang, J.; Wang, Q.; Wen, T.; Song, Y.; Liu, W.D.; Zeng, J.; et al. Ultrafast Synthesis of Oxygen Vacancy-Rich MgFeSiO4 Cathode to Boost Diffusion Kinetics for Rechargeable Magnesium-Ion Batteries. Nano Lett. 2025, 25, 730–739. [Google Scholar] [CrossRef]
- Canepa, P.; Bo, S.H.; Sai Gautam, G.; Key, B.; Richards, W.D.; Shi, T.; Tian, Y.; Wang, Y.; Li, J.; Ceder, G. High magnesium mobility in ternary spinel chalcogenides. Nat. Commun. 2017, 8, 1759. [Google Scholar] [CrossRef]
- Medina, A.; Pérez-Vicente, C.; Alcántara, R. Spinel-type MgxMn2-yFeyO4 as a new electrode for sodium ion batteries. Electrochim. Acta 2022, 421, 140492. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, T.; Miao, J.; Ning, W.; Sun, Y.; Qiu, F.; Shi, X.; Miao, S. Mg(Al, Fe, Mn, REE)2O4 Spinel Prepared from Pelagic REE-Rich Clays and Application as Magnesium-Ion-Battery Cathodes. ACS Appl. Mater. Interfaces 2024, 16, 60122–60131. [Google Scholar] [CrossRef]
- Mei, L.; Xu, J.; Wei, Z.; Liu, H.; Li, Y.; Ma, J.; Dou, S. Chevrel Phase Mo6 T8 (T = S, Se) as Electrodes for Advanced Energy Storage. Small 2017, 13, 1701441. [Google Scholar] [CrossRef]
- Sun, X.; Bonnick, P.; Nazar, L.F. Layered TiS2 Positive Electrode for Mg Batteries. ACS Energy Lett. 2016, 1, 297–301. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Z.; Li, Q.; Wang, X.; Xu, X.; Meng, J.; Ren, W.; Zhang, X.; Huang, Y.; Mai, L. 3.0 V High Energy Density Symmetric Sodium-Ion Battery: Na4V2(PO4)3||Na3V2(PO4)3. ACS Appl. Mater. Interfaces 2018, 10, 10022–10028. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, G.; Sun, J.; Xie, L.; Jiang, Y.; Huang, Y.; Chen, S. High performance cathode material based on Na3V2(PO4)2F3 and Na3V2(PO4)3 for sodium-ion batteries. Energy Storage Mater. 2020, 25, 724–730. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, J.; Guo, Y.; Zhu, H.; Huang, X. Research Progress on Na3V2(PO4)3 Cathode Material of Sodium Ion Battery. Front. Chem. 2020, 8, 635. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, K.; Lv, X.; Shi, K.; Wang, Y.; Nian, Z.; Li, Y.; Wang, L.; Dai, L.; He, Z. Recent advances of NASICON-Na3V2(PO4)3 as cathode for sodium-ion batteries: Synthesis, modifications, and perspectives. J. Alloys Compd. 2021, 867, 159060. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Ma, L.; Liu, Y.; Hou, B.; Shang, N.; Zhang, S.; Song, J.; Chen, S.; Zhao, X. Surface Crystal Modification of Na3V2(PO4)3 to Cast Intermediate Na2V2(PO4)3 Phase toward High-Rate Sodium Storage. Adv. Sci. 2024, 11, e2306168. [Google Scholar] [CrossRef]
- Jian, Z.; Han, W.; Lu, X.; Yang, H.; Hu, Y.S.; Zhou, J.; Zhou, Z.; Li, J.; Chen, W.; Chen, D.; et al. Superior Electrochemical Performance and Storage Mechanism of Na3V2(PO4)3 Cathode for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2012, 3, 156–160. [Google Scholar] [CrossRef]
- Jian, Z.; Yuan, C.; Han, W.; Lu, X.; Gu, L.; Xi, X.; Hu, Y.S.; Li, H.; Chen, W.; Chen, D.; et al. Atomic Structure and Kinetics of NASICON NaxV2(PO4)3 Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 4265–4272. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liang, Q.; Xu, L.; Ding, C.; Liu, Y.; Gao, Y. Optimization Strategies of Na3V2(PO4)3 Cathode Materials for Sodium-Ion Batteries. Nano-Micro Lett. 2024, 17, 33. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, X.; Wang, Z.; Zhang, L.; Zhou, L.; Lai, L.; Liu, X. Selection of graphene dopants for Na3V2(PO4)3 graphene composite as high rate, ultra long-life sodium-ion battery cathodes. Electrochim. Acta 2019, 306, 558–567. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Li, W.; Zeng, L.; Pan, F.; Wang, M.; Wei, X.; Hu, G.; Gu, L.; Yu, Y. Nanoconfined Carbon-Coated Na3V2(PO4)3 Particles in Mesoporous Carbon Enabling Ultralong Cycle Life for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1402104. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Wei, Z.; Wang, D.; Gao, Y.; Wei, Y.; Du, F.; Chen, G. Core/Double-Shell Structured Na3V2(PO4)2F3@C Nanocomposite as the High Power and Long Lifespan Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 31709–31715. [Google Scholar] [CrossRef]
- Rangasamy, V.S.; Thayumanasundaram, S.; Locquet, J.-P. Ionic liquid electrolytes based on sulfonium cation for lithium rechargeable batteries. Electrochim. Acta 2019, 328, 135133. [Google Scholar] [CrossRef]
- Novák, Z.; Kozma, G.; Kukovecz, Á. Electrolyte effect on the electroactuation behavior of multilayer polypyrrole films intercalated with TFSi−, ClO4−, NO3− anions in lithium and potassium based electrolyte solutions. J. Mol. Struct. 2022, 1262, 133057. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Zeng, Z.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. A Nonflammable Na+-Based Dual-Carbon Battery with Low-Cost, High Voltage, and Long Cycle Life. Adv. Energy Mater. 2018, 8, 1802176. [Google Scholar] [CrossRef]
- Serra Moreno, J.; Armand, M.; Berman, M.B.; Greenbaum, S.G.; Scrosati, B.; Panero, S. Composite PEOn:NaTFSI polymer electrolyte: Preparation, thermal and electrochemical characterization. J. Power Sources 2014, 248, 695–702. [Google Scholar] [CrossRef]
- Wan, S.; Song, K.; Chen, J.; Zhao, S.; Ma, W.; Chen, W.; Chen, S. Reductive Competition Effect-Derived Solid Electrolyte Interphase with Evenly Scattered Inorganics Enabling Ultrahigh Rate and Long-Life Span Sodium Metal Batteries. J. Am. Chem. Soc. 2023, 145, 21661–21671. [Google Scholar] [CrossRef]
- Sun, J.; O’Dell, L.A.; Armand, M.; Howlett, P.C.; Forsyth, M. Anion-Derived Solid-Electrolyte Interphase Enables Long Life Na-Ion Batteries Using Superconcentrated Ionic Liquid Electrolytes. ACS Energy Lett. 2021, 6, 2481–2490. [Google Scholar] [CrossRef]
- Manohar, C.V.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. Role of N-Propyl-N-Methyl Pyrrolidinium bis(trifluoromethanesulfonyl)imide as an Electrolyte Additive in Sodium Battery Electrochemistry. Energy Technol. 2018, 6, 2232–2237. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, Y.; Lai, S.; Huang, J.; Zhang, Y.; Wang, J.; Zhao, J. A Promising High-Voltage Cathode Material Based on Mesoporous Na3V2(PO4)3/C for Rechargeable Magnesium Batteries. Chemistry 2017, 23, 16898–16905. [Google Scholar] [CrossRef]
- Ha, S.Y.; Lee, Y.W.; Woo, S.W.; Koo, B.; Kim, J.S.; Cho, J.; Lee, K.T.; Choi, N.S. Magnesium(II) bis(trifluoromethane sulfonyl) imide-based electrolytes with wide electrochemical windows for rechargeable magnesium batteries. ACS Appl. Mater. Interfaces 2014, 6, 4063–4073. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Ye, J.; Wen, J.; Wang, J.; Huang, G.; Wang, J. A High Voltage Magnesium Ion Battery Based on Na3V2(PO4)3@C Cathode and Bi Nanowire Anode. Langmuir 2025, 41, 18919–18928. [Google Scholar] [CrossRef]
- Deng, Y.J.; Li, G.Y.; Deng, R.R.; Zhang, B.W.; Cao, L.Y.; Huang, G.S.; Wang, J.F.; Pan, F.S. Dual-Site Modulation of Mg2+ Desolvation Beyond the Primary Solvation Shell in Magnesium Battery Electrolytes. Angew. Chem.-Int. Edit. 2025, 64, 7. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.G.; Karuppiah, C.; Walle, K.Z.; Abdelaal, M.M.; Kotobuki, M.; Lu, L. Review on dendrite formation of Mg metal anode and its prevention. Nano Energy 2024, 131, 16. [Google Scholar] [CrossRef]
- Guan, Q.H.; Zhuang, Q.; Xu, W.L.; Zhang, Y.Z.; Cheng, S.; Zhang, J.; Liu, M.N.; Lin, H.Z.; Wang, J. Accelerated Kinetics of Desolvation and Redox Transformation Enabled by MOF Sieving for High-Loading Mg-S Battery. Adv. Funct. Mater. 2025, 35, 2506397. [Google Scholar] [CrossRef]
- Ihsan-Ul-Haq, M.; Cui, J.; Mubarak, N.; Xu, M.Y.; Shen, X.; Luo, Z.T.; Huang, B.L.; Kim, J.K. Revealing Cathode-Electrolyte Interface on Flower-Shaped Na3V2(PO4)3/C Cathode through Cryogenic Electron Microscopy. Adv. Energy Sustain. Res. 2021, 2, 8. [Google Scholar] [CrossRef]


| State of Na3V2(PO4)3 | Experimental Composition of Na3V2(PO4)3 |
|---|---|
| initial state A | Na3.03±0.05V2(PO4)3 |
| sodium-extracted state B | Mg0.03±0.01Na0.99±0.04V2(PO4)3 |
| magnesium-intercalated state C | Mg0.66±0.01Na0.91±0.02V2(PO4)3 |
| magnesium-deintercalated state D | Mg0.04Na0.97±0.03V2(PO4)3 |
| Impedance | 50 Circles | 100 Circles | 500 Circles | 1000 Circles |
|---|---|---|---|---|
| Rs (Ω) | 5.517 | 5.831 | 6.206 | 6.338 |
| Rct (Ω) | 179.3 | 51.07 | 80.57 | 47.55 |
| W1 (Ω) | 216.2 | 98.2 | 131.04 | 75.13 |
| Parameters | 50 Circles | 100 Circles | 500 Circles | 1000 Circles |
|---|---|---|---|---|
| D Mg2+ (cm2/s) | 4.2 × 10−15 | 1.5 × 10−14 | 9.3 × 10−15 | 8.6 × 10−15 |
| σ | 117.50 | 62.61 | 79.21 | 81.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, P.; Mou, X.; Yang, J.; Wang, J.; Huang, G.; Wang, J. Study on Electrochemical Performance and Magnesium Storage Mechanism of Na3V2(PO4)3@C Cathode in Mg(TFSI)2/DME Electrolyte. Energies 2025, 18, 5975. https://doi.org/10.3390/en18225975
Wang J, Zhang P, Mou X, Yang J, Wang J, Huang G, Wang J. Study on Electrochemical Performance and Magnesium Storage Mechanism of Na3V2(PO4)3@C Cathode in Mg(TFSI)2/DME Electrolyte. Energies. 2025; 18(22):5975. https://doi.org/10.3390/en18225975
Chicago/Turabian StyleWang, Jinxing, Peiyang Zhang, Xuan Mou, Jingdong Yang, Jiaxu Wang, Guangsheng Huang, and Jingfeng Wang. 2025. "Study on Electrochemical Performance and Magnesium Storage Mechanism of Na3V2(PO4)3@C Cathode in Mg(TFSI)2/DME Electrolyte" Energies 18, no. 22: 5975. https://doi.org/10.3390/en18225975
APA StyleWang, J., Zhang, P., Mou, X., Yang, J., Wang, J., Huang, G., & Wang, J. (2025). Study on Electrochemical Performance and Magnesium Storage Mechanism of Na3V2(PO4)3@C Cathode in Mg(TFSI)2/DME Electrolyte. Energies, 18(22), 5975. https://doi.org/10.3390/en18225975







