Hydrothermal Treatment of Kitchen Waste as a Strategy for Dark Fermentation Biohydrogen Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum
2.2. Kitchen Waste
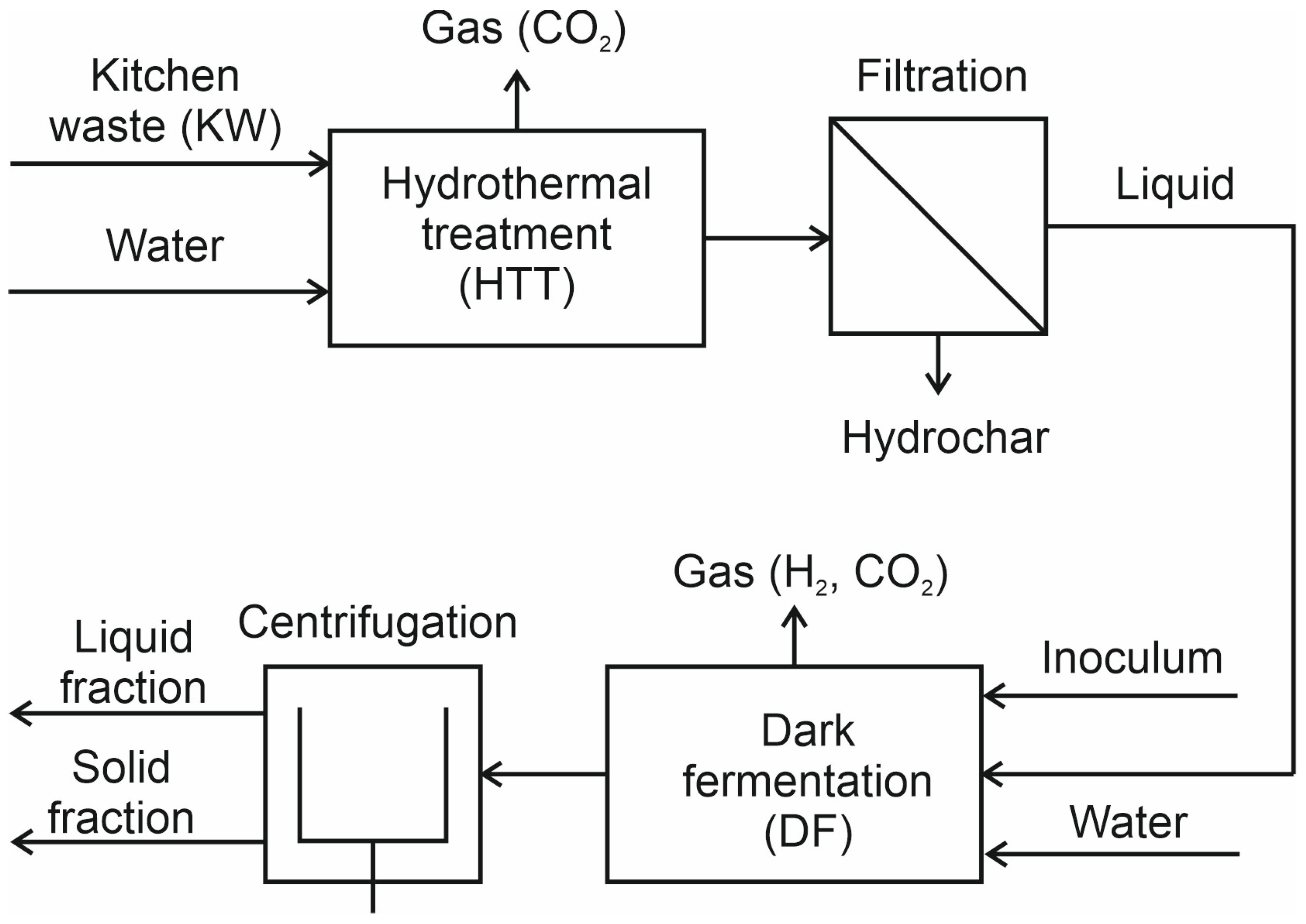
2.3. Experimental Setup
2.4. Analysis Procedures
3. Results and Discussion
3.1. Volatile Organic Compounds in the Liquid Fraction After Thermal Pretreatment
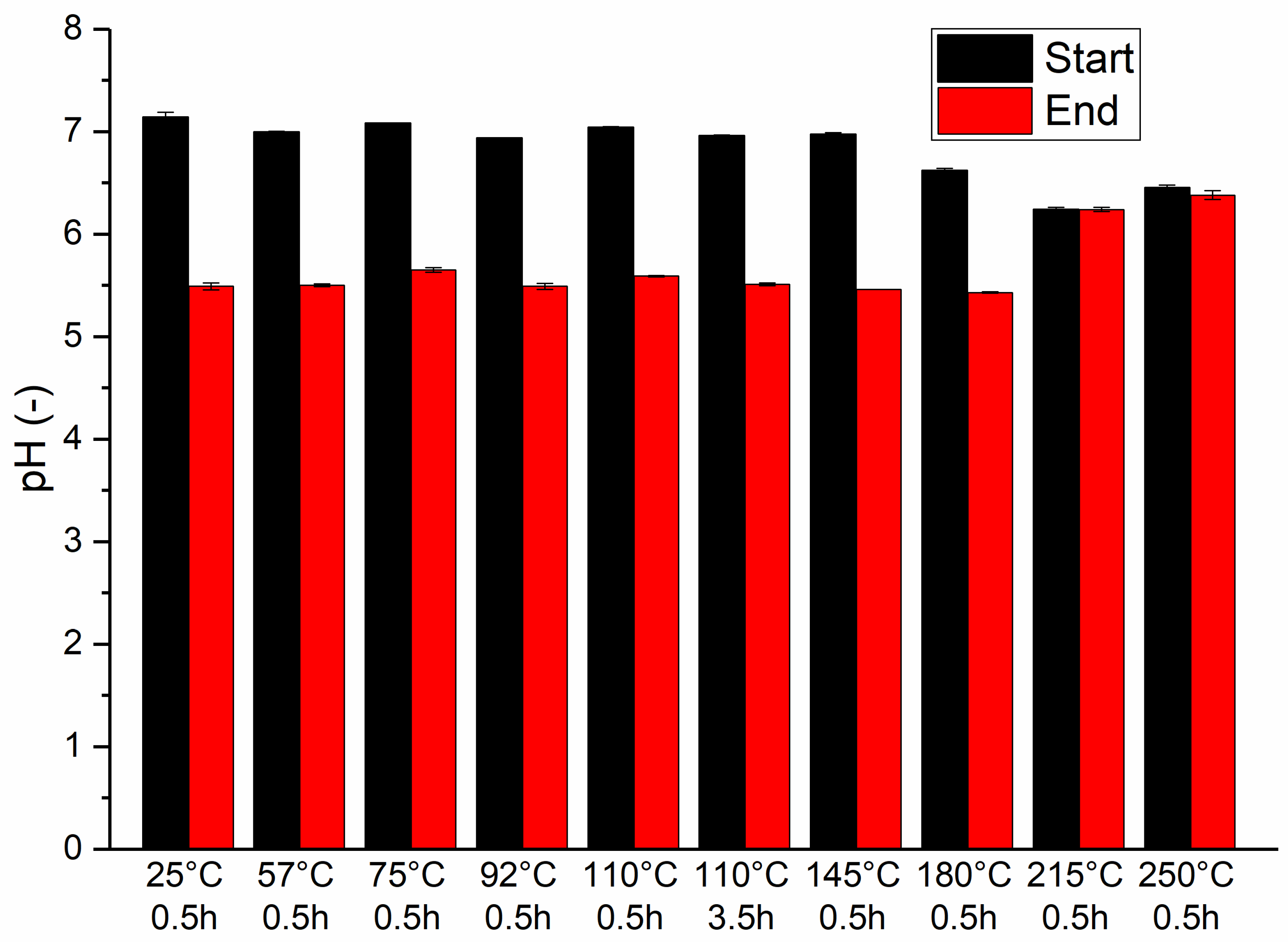
3.2. pH Changes
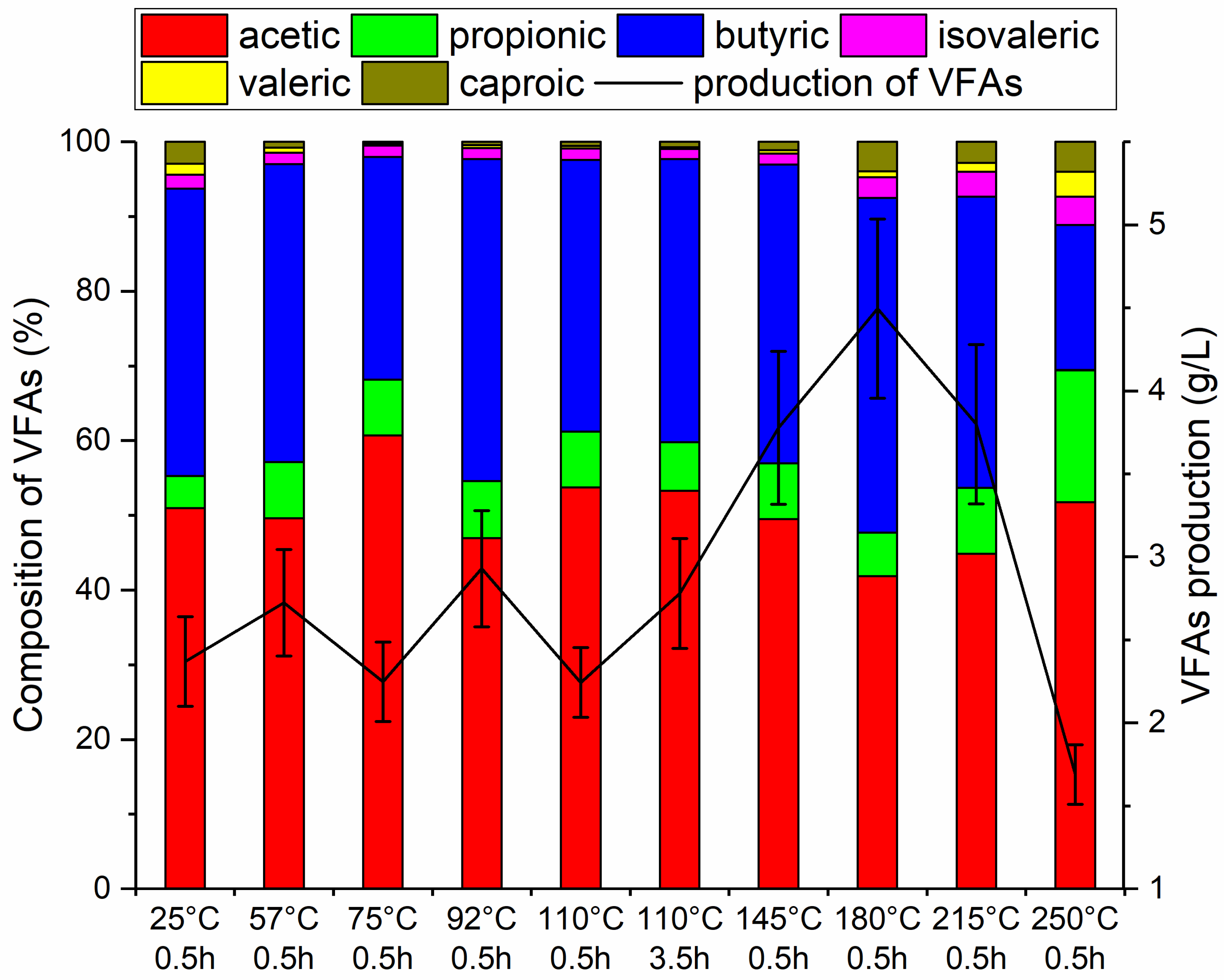
3.3. The Amount and Composition of VFAs
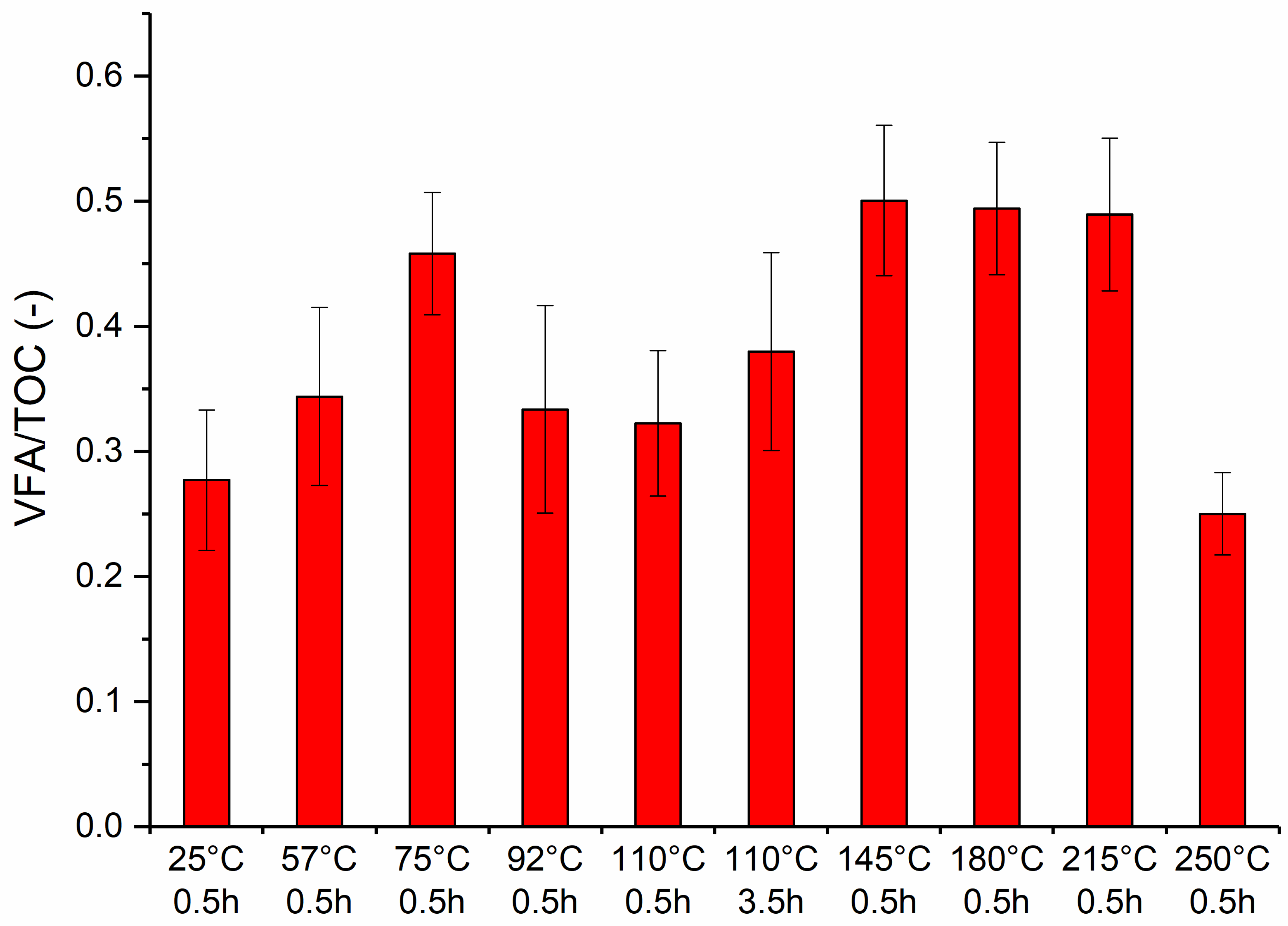
3.4. VFAs:TOC Ratio
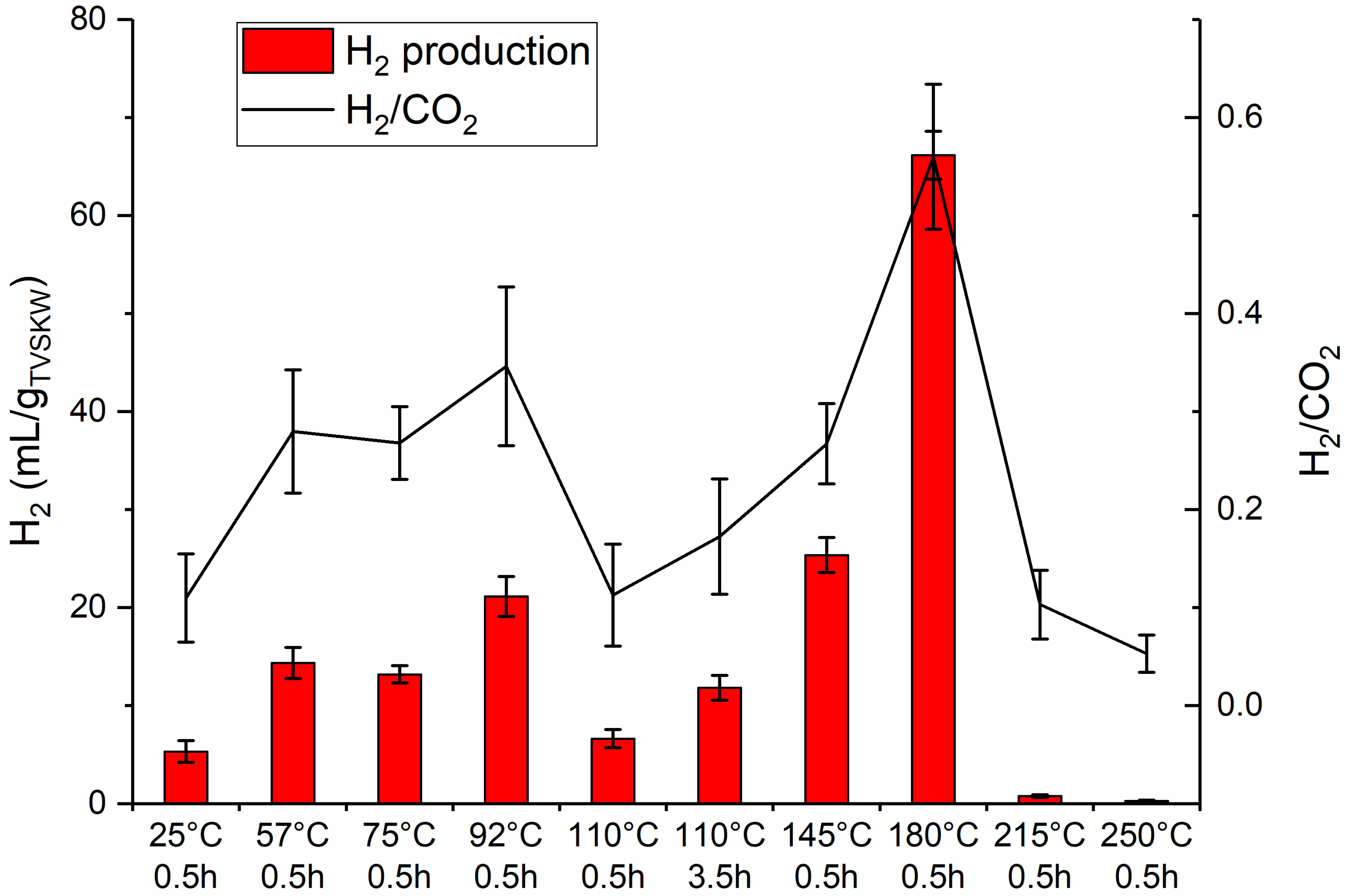
3.5. Hydrogen and Carbon Dioxide in Fermentation Gas
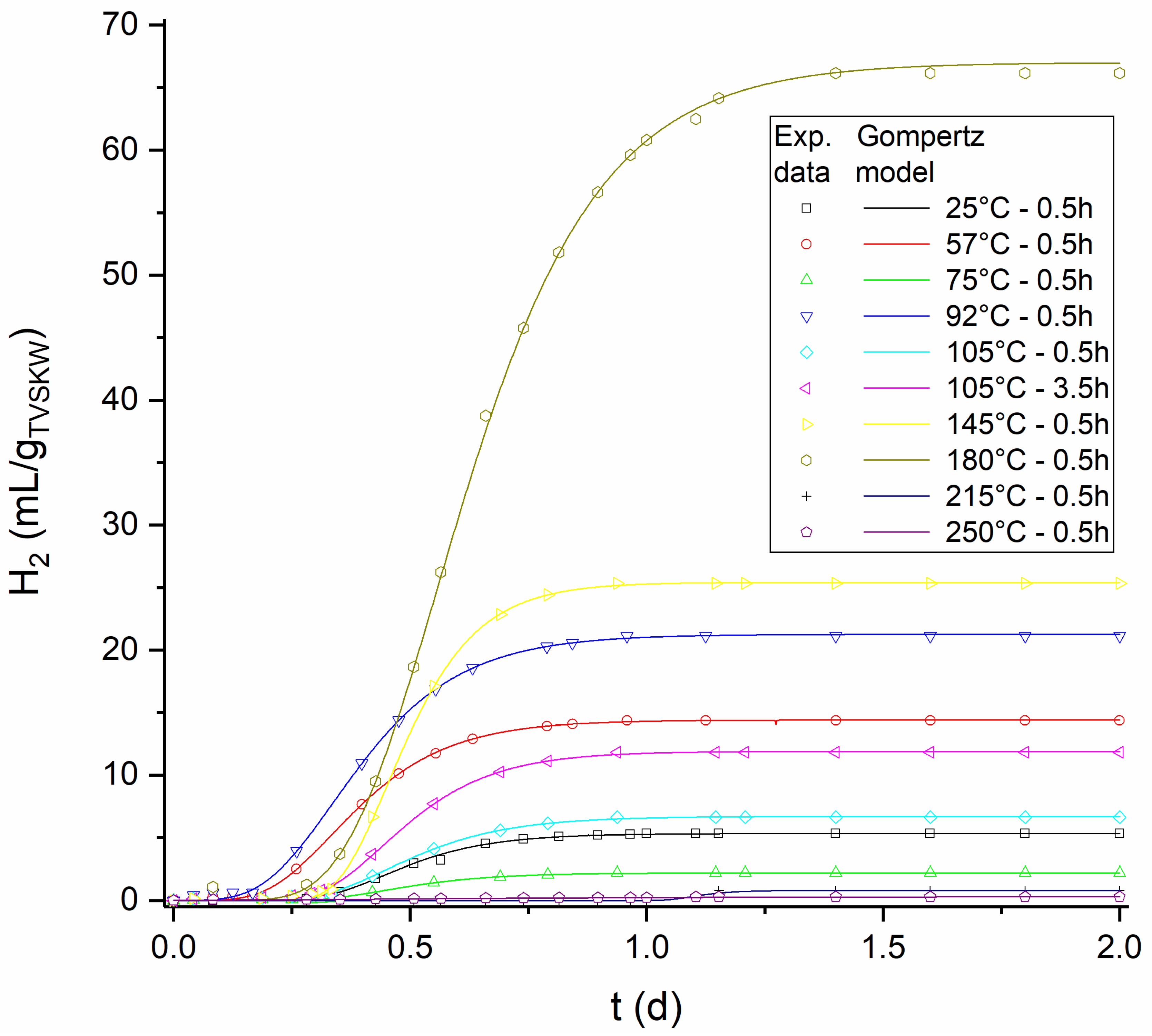
3.6. Gompertz Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, D. Hydrogen Production by Biological Processes: A Survey of Literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative Hydrogen Production: Principles, Progress, and Prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Szablowski, L.; Wojcik, M.; Dybinski, O. Review of Steam Methane Reforming as a Method of Hydrogen Production. Energy 2025, 316, 134540. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.-H.; Kim, D.-H. Fermentative Hydrogen Production Using Lignocellulose Biomass: An Overview of Pre-Treatment Methods, Inhibitor Effects and Detoxification Experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of Catalytic Supercritical Water Gasification for Hydrogen Production from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal Carbonization of Wet Biomass from Nitrogen and Phosphorus Approach: A Review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Claassen, P.A.M.; van Lier, J.B.; Lopez Contreras, A.M.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilisation of Biomass for the Supply of Energy Carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Kumarkhanal, S.; Sung, S. Kinetic Study of Biological Hydrogen Production by Anaerobic Fermentation. Int. J. Hydrogen Energy 2006, 31, 2170–2178. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological Hydrogen Production Measured in Batch Anaerobic Respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef]
- Mokhtarani, B.; Zanganeh, J.; Moghtaderi, B. A Review on Biohydrogen Production Through Dark Fermentation, Process Parameters and Simulation. Energies 2025, 18, 1092. [Google Scholar] [CrossRef]
- Taggar, M.S.; Kaur, A.; Jain, C.; Kalia, A.; Sooch, S.S. Hydrogen Production Via Dark Fermentation: A Review of Influential Factors. Cellul. Chem. Technol. 2024, 58, 1051–1063. [Google Scholar] [CrossRef]
- Albuquerque, M.M.; Sartor, G.D.; Martinez-Burgos, W.J.; Scapini, T.; Edwiges, T.; Soccol, C.R.; Medeiros, A.B. Biohydrogen Produced via Dark Fermentation: A Review. Methane 2024, 3, 500–532. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the Biological Hydrogen Production Pathway of Dark Fermentation: Insight into the Impact of Strain Improvement. Microb. Cell Fact. 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Panwar, N.L.; Jain, S.K.; Gupta, T.; Agarwal, C.; Meena, S.S. Bio-Hydrogen Production through Dark Fermentation: An Overview. Biomass Convers. Biorefinery 2024, 14, 12699–12724. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Yan, W.; Vásquez, V.R.; Coronella, C.J. Hydrothermal Carbonization of Various Lignocellulosic Biomass. Biomass Convers. Biorefinery 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Kravanja, G.; Knez, Ž. Hydrothermal Treatment of Biomass for Energy and Chemicals. Energy 2016, 116, 1312–1322. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Fang, M.; Wu, D.; Cui, D.; Pan, S.; Bai, J.; Xu, F.; Wang, Z. Hydrothermal Carbonization of Food Waste for Sustainable Biofuel Production: Advancements, Challenges, and Future Prospects. Sci. Total Environ. 2023, 897, 165327. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal Treatment of Biomass Feedstocks for Sustainable Production of Chemicals, Fuels, and Materials: Progress and Perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Monlau, F.; Vaneeckhaute, C. Valorisation of Anaerobic Digestate: Towards Value-Added Products. In Renewable Energy Technologies for Energy Efficient Sustainable Development; Springer Nature: Cham, Switzerland, 2022; pp. 227–262. [Google Scholar]
- Kumar, S. Hydrothermal Processing of Biomass for Biofuels. Biofuel Res. J. 2014, 1, 43. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating Strategies for Sustainable Conversion of Waste Biomass into Dark-Fermentative Hydrogen and Value-Added Products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Chalima, A.; Oliver, L.; Fernández de Castro, L.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Gopalakrishnan, B.; Khanna, N.; Das, D. Dark-Fermentative Biohydrogen Production. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444642035. [Google Scholar]
- Grossule, V.; Lavagnolo, M.C. Optimised Management of Semi-Aerobic Landfilling under Tropical Wet-Dry Conditions. Detritus 2020, 10, 160–169. [Google Scholar] [CrossRef]
- Moeller, L.; Zehnsdorf, A. Process Upsets in a Full-Scale Anaerobic Digestion Bioreactor: Over-Acidification and Foam Formation during Biogas Production. Energy. Sustain. Soc. 2016, 6, 30. [Google Scholar] [CrossRef]
- Slezak, R.; Grzelak, J.; Krzystek, L.; Ledakowicz, S. Influence of Initial PH on the Production of Volatile Fatty Acids and Hydrogen during Dark Fermentation of Kitchen Waste. Environ. Technol. 2021, 42, 4269–4278. [Google Scholar] [CrossRef]
- Md, M.R.; Mohamad, A.M.S.; Nasrin, S.; Kim, M.J.; Chang, S.R. Estimation of Total Volatile Fatty Acid (VFA) from Total Organic Carbons (TOCs) Assessment through in Vitro Fermentation of Livestock Feeds. African J. Microbiol. Res. 2013, 7, 1378–1384. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. The Gompertz Model and Its Applications in Microbial Growth and Bioproduction Kinetics: Past, Present and Future. Biotechnol. Adv. 2024, 72, 108335. [Google Scholar] [CrossRef]
- Boshagh, F.; Rostami, K.; van Niel, E.W.J. Application of Kinetic Models in Dark Fermentative Hydrogen Production–A Critical Review. Int. J. Hydrogen Energy 2022, 47, 21952–21968. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, J.; Kim, J.; Hee Yoon, H.; Khanh Thinh Nguyen, P.; Kim, J. Mathematical Modeling of Dark Fermentation of Macroalgae for Hydrogen and Volatile Fatty Acids Production. Bioresour. Technol. 2022, 354, 127193. [Google Scholar] [CrossRef]
- González-Figueredo, C.; Alejandro Flores-Estrella, R.A.; Rojas-Rejón, O. Fermentation: Metabolism, Kinetic Models, and Bioprocessing. In Current Topics in Biochemical Engineering; IntechOpen: London, UK, 2019. [Google Scholar]
- Mu, Y.; Wang, G.; Yu, H.-Q. Kinetic Modeling of Batch Hydrogen Production Process by Mixed Anaerobic Cultures. Bioresour. Technol. 2006, 97, 1302–1307. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; D’Ippolito, G.; Fontana, A.; Panico, A.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Model Development and Experimental Validation of Capnophilic Lactic Fermentation and Hydrogen Synthesis by Thermotoga neapolitana. Water Res. 2016, 99, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zou, Y.; Yang, H.; Fu, X.; Xiang, S.; Li, Z. Two-Stage Stochastic Energy Scheduling for Multi-Energy Rural Microgrids With Irrigation Systems and Biomass Fermentation. IEEE Trans. Smart Grid 2025, 16, 1075–1087. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Liu, C.-G.; Lin, Y.-H. A Novel Explainable Kinetic Model for Two-Stage Fermentation Profile. Chem. Eng. J. 2024, 493, 152745. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Kinetic Models for Fermentative Hydrogen Production: A Review. Int. J. Hydrogen Energy 2009, 34, 3313–3323. [Google Scholar] [CrossRef]
- Lim, S.W.; Nandong, J. Modeling of Biohydrogen Production Using Generalized Multi-Scale Kinetic Model: Impacts of Fermentation Conditions. Int. J. Hydrogen Energy 2022, 47, 17926–17945. [Google Scholar] [CrossRef]
- Domińska, M.; Ślęzak, R.; Świątkiewicz, J.; Paździor, K.; Ledakowicz, S. Influence of Inoculum Thermal Pretreatment Time on Hydrogen Production in Dark Fermentation. Energies 2024, 17, 974. [Google Scholar] [CrossRef]
- Domińska, M.; Paździor, K.; Ślęzak, R.; Ledakowicz, S. The Influence of Inoculum Source and Pretreatment on the Biohydrogen Production in the Dark Fermentation Process. Chem. Process Eng. New Front. 2024, 45, e63. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Broekaert, J.A.C.; Daniel, C. Harris: Quantitative Chemical Analysis, 9th ed. Anal. Bioanal. Chem. 2015, 407, 8943–8944. [Google Scholar] [CrossRef]
- Ikryannikova, L.N.; Kurbatov, L.K.; Gorokhovets, N.V.; Zamyatnin, A.A. Contact-Dependent Growth Inhibition in Bacteria: Do Not Get Too Close! Int. J. Mol. Sci. 2020, 21, 7990. [Google Scholar] [CrossRef]
- Bernier, S.P.; Surette, M.G. Concentration-Dependent Activity in Natural Environments. Front. Microbiol. 2013, 4, 20. [Google Scholar] [CrossRef]
- Tian, D.; Lin, Z.; Yu, J.; Yin, D. Influence Factors of Multicomponent Mixtures Containing Reactive Chemicals and Their Joint Effects. Chemosphere 2012, 88, 994–1000. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Melkania, U. Effect of Phenolic Compounds on Hydrogen Production from Municipal Solid Waste. Waste Manag. 2018, 78, 115–123. [Google Scholar] [CrossRef]
- Brandt, K. Final Report on the Safety Assessment of Hydroquinone and Pyrocatechol. J. Am. Coll. Toxicol. 1986, 5, 123–165. [Google Scholar] [CrossRef]
- Chen, H.; Yao, J.; Wang, F.; Zhou, Y.; Chen, K.; Zhuang, R.; Choi, M.M.F.; Zaray, G. Toxicity of Three Phenolic Compounds and Their Mixtures on the Gram-Positive Bacteria Bacillus Subtilis in the Aquatic Environment. Sci. Total Environ. 2010, 408, 1043–1049. [Google Scholar] [CrossRef]
- Torres-Vargas, J.A.; Cheng-Sánchez, I.; Martínez-Poveda, B.; Medina, M.Á.; Sarabia, F.; García-Caballero, M.; Quesada, A.R. Characterization of the Activity and the Mechanism of Action of a New Toluquinol Derivative with Improved Potential as an Antiangiogenic Drug. Biomed. Pharmacother. 2022, 155, 113759. [Google Scholar] [CrossRef]
- Nweke, C.O.; Okpokwasili, G.C. Influence of Exposure Time on Phenol Toxicity to Refinery Wastewater Bacteria. J. Environ. Chem. Ecotoxicol. 2010, 2, 20–27. [Google Scholar]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Cancellieri, M.A.; Chon, H.; Dagli, M.L.; Dekant, W.; Deodhar, C.; et al. RIFM Fragrance Ingredient Safety Assessment, 4-Hydroxybenzaldehyde, CAS Registry Number 123-08-0. Food Chem. Toxicol. 2023, 176, 113794. [Google Scholar] [CrossRef]
- Dzulkarnain, E.L.N.; Audu, J.O.; Wan Dagang, W.R.Z.; Abdul-Wahab, M.F. Microbiomes of Biohydrogen Production from Dark Fermentation of Industrial Wastes: Current Trends, Advanced Tools and Future Outlook. Bioresour. Bioprocess. 2022, 9, 16. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberan, A.; Chen, Y.; Yang, F.; Blaszczyk, M.K.; Sikora, A. Dynamics of Dark Fermentation Microbial Communities in the Light of Lactate and Butyrate Production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Laqa, K.F.; Razavi, A.S.; Hosseini Koupaie, E.; Hafez, H.; Elbeshbishy, E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids and Methane Production from Thickened Waste Activated Sludge. In Proceedings of the 93rd Water Environment Federation Technical Exhibition and Conference (WEFTEC 2020), New Orleans, LA, USA, 5–9 October 2020; pp. 1082–1087. [Google Scholar]
- Jilani, S.B.; Olson, D.G. Mechanism of Furfural Toxicity and Metabolic Strategies to Engineer Tolerance in Microbial Strains. Microb. Cell Fact. 2023, 22, 221. [Google Scholar] [CrossRef]
- Zeng, X.; Borole, A.P.; Pavlostathis, S.G. Biotransformation of Furanic and Phenolic Compounds with Hydrogen Gas Production in a Microbial Electrolysis Cell. Environ. Sci. Technol. 2015, 49, 13667–13675. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Sneha, N.P.; Rafi, S.M.; Sarkar, O. Dark Fermentative Hydrogen Production: Potential of Food Waste as Future Energy Needs. Sci. Total Environ. 2023, 888, 163801. [Google Scholar] [CrossRef]
| Parameter | Inoculum | Kitchen Waste |
|---|---|---|
| pH | 7.14 ± 0.04 | 4.86 ± 0.05 |
| TS (g/L) | 27.91 ± 0.31 | 0.158 ± 0.002 |
| TVS (g/L) | 17.49 ± 0.30 | 0.147 ± 0.002 |
| C (%TS) | 32.09 ± 0.13 | 45.48 ± 0.19 |
| N (%TS) | 3.96 ± 0.03 | 1.83 ± 0.01 |
| H (%TS) | 5.20 ± 0.04 | 6.13 ± 0.06 |
| C/N | 6.17 ± 0.17 | 24.85 ± 0.11 |
| Parameter | 25 °C | 57 °C | 75 °C | 92 °C | 110 °C 0.5 h | 110 °C 3.5 h | 145 °C | 180 °C | 215 °C | 250 °C | Unit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bmax | 5.32 | 14.41 | 2.2 | 21.26 | 6.7 | 11.9 | 25.43 | 67.02 | 0.8 | 0.297 | mL/gTVSKW |
| Rm | 15.64 | 39.28 | 6.33 | 53.03 | 17.58 | 33.97 | 88.98 | 128.47 | 5.89 | 0.267 | mL/gTVSKW/d |
| λ | 0.32 | 0.20 | 0.32 | 0.19 | 0.31 | 0.31 | 0.35 | 0.36 | 1.04 | 0.03 | d |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.936 | 0.921 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domińska, M.; Paździor, K.; Ślęzak, R.; Ledakowicz, S. Hydrothermal Treatment of Kitchen Waste as a Strategy for Dark Fermentation Biohydrogen Production. Energies 2025, 18, 5811. https://doi.org/10.3390/en18215811
Domińska M, Paździor K, Ślęzak R, Ledakowicz S. Hydrothermal Treatment of Kitchen Waste as a Strategy for Dark Fermentation Biohydrogen Production. Energies. 2025; 18(21):5811. https://doi.org/10.3390/en18215811
Chicago/Turabian StyleDomińska, Marlena, Katarzyna Paździor, Radosław Ślęzak, and Stanisław Ledakowicz. 2025. "Hydrothermal Treatment of Kitchen Waste as a Strategy for Dark Fermentation Biohydrogen Production" Energies 18, no. 21: 5811. https://doi.org/10.3390/en18215811
APA StyleDomińska, M., Paździor, K., Ślęzak, R., & Ledakowicz, S. (2025). Hydrothermal Treatment of Kitchen Waste as a Strategy for Dark Fermentation Biohydrogen Production. Energies, 18(21), 5811. https://doi.org/10.3390/en18215811







