Quantitative Assessment of Free and Adsorbed Shale Oil in Kerogen Pores Using Molecular Dynamics Simulations and Experiment Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Experimental Methods
2.1.1. Pyrolysis-GC-MS
2.1.2. Solid-State 13C NMR
2.1.3. FTIR
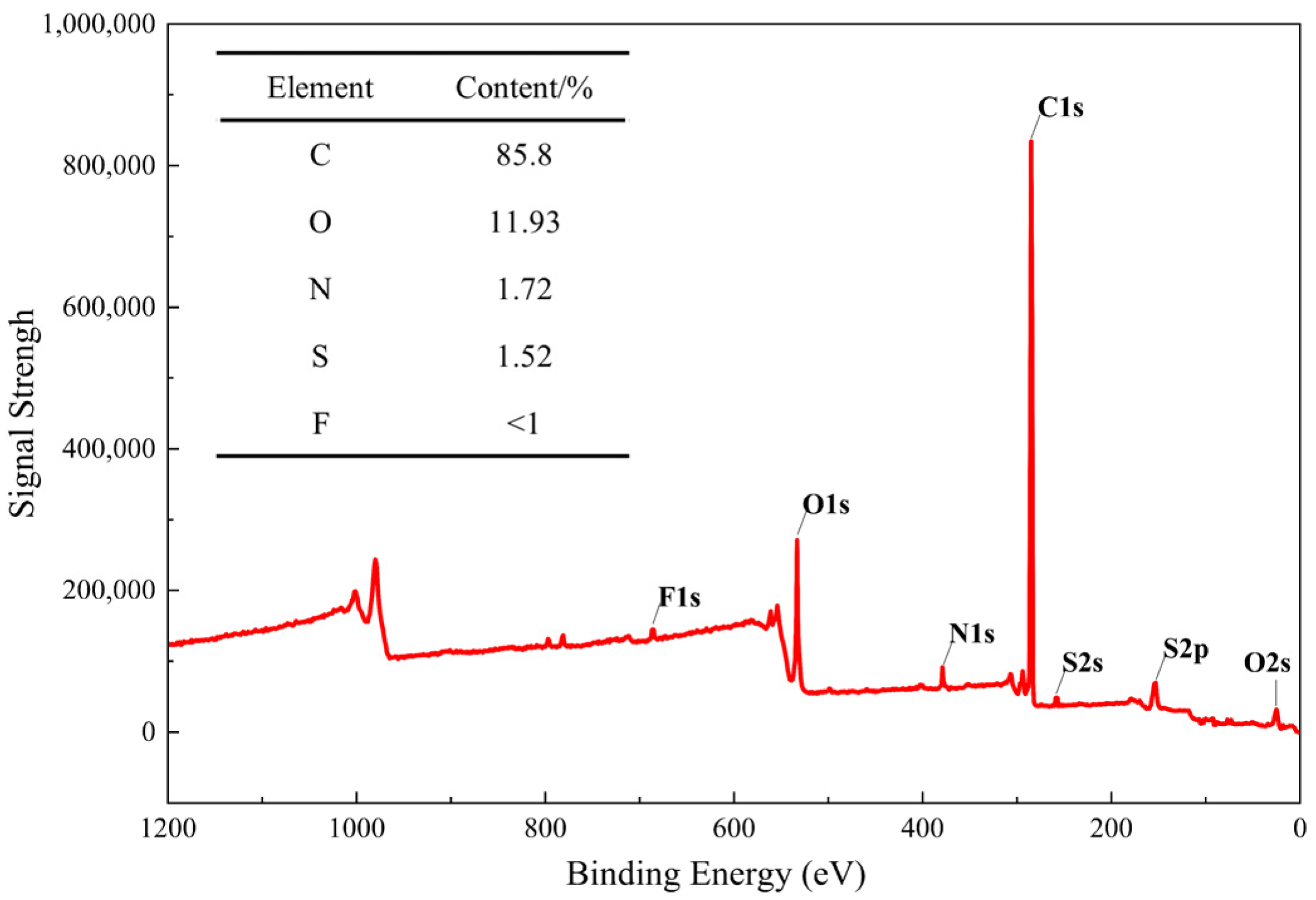
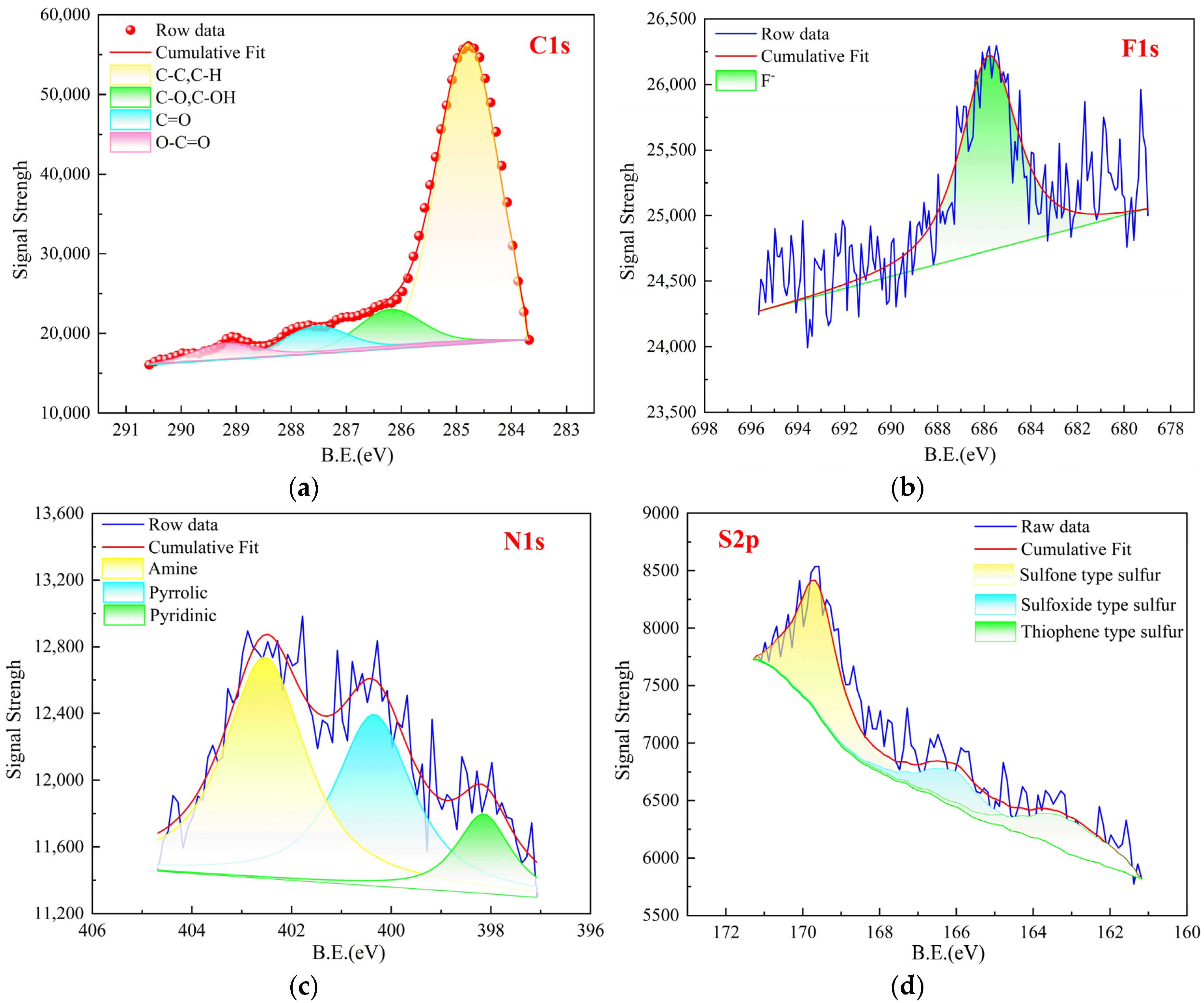
2.1.4. XPS
2.2. Molecular Simulation Methods
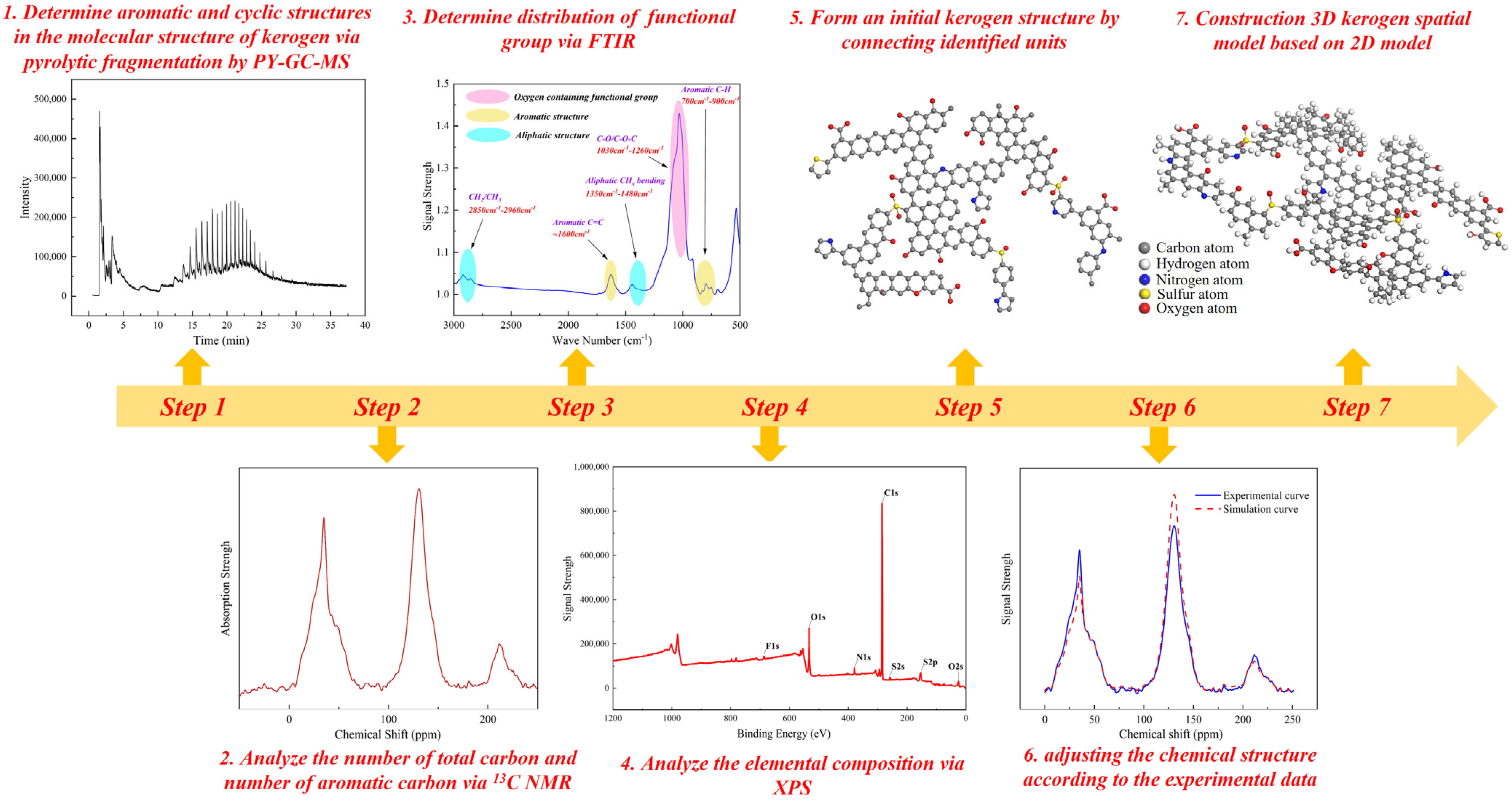
2.2.1. Construction of Kerogen Molecular Structure
- (1)
- Determining the aromatic and cyclic structures within the kerogen molecular framework based on pyrolysis fragment analysis from PY-GC-MS.
- (2)
- Extracting molecular skeletal parameters from 13C NMR data, including protonated aromatic carbon (faH), esterification degree (far), aromaticity (fal), aromatic substitution degree (δ), and branched aromatic carbon (farC).
- (3)
- Identifying the distribution of characteristic functional groups through FTIR analysis.
- (4)
- Analyzing the occurrence states of O, N, S, and F elements using XPS data.
- (5)
- Connecting the identified molecular units to construct an initial kerogen structure.
- (6)
- Refining the chemical structure to match experimental observations, yielding a final two-dimensional kerogen model.
- (7)
- Constructing the three-dimensional spatial kerogen structure based on the established two-dimensional chemical and physical framework (Figure 2).
2.2.2. Construction of Kerogen Slit Structure
2.2.3. GCMC Simulation
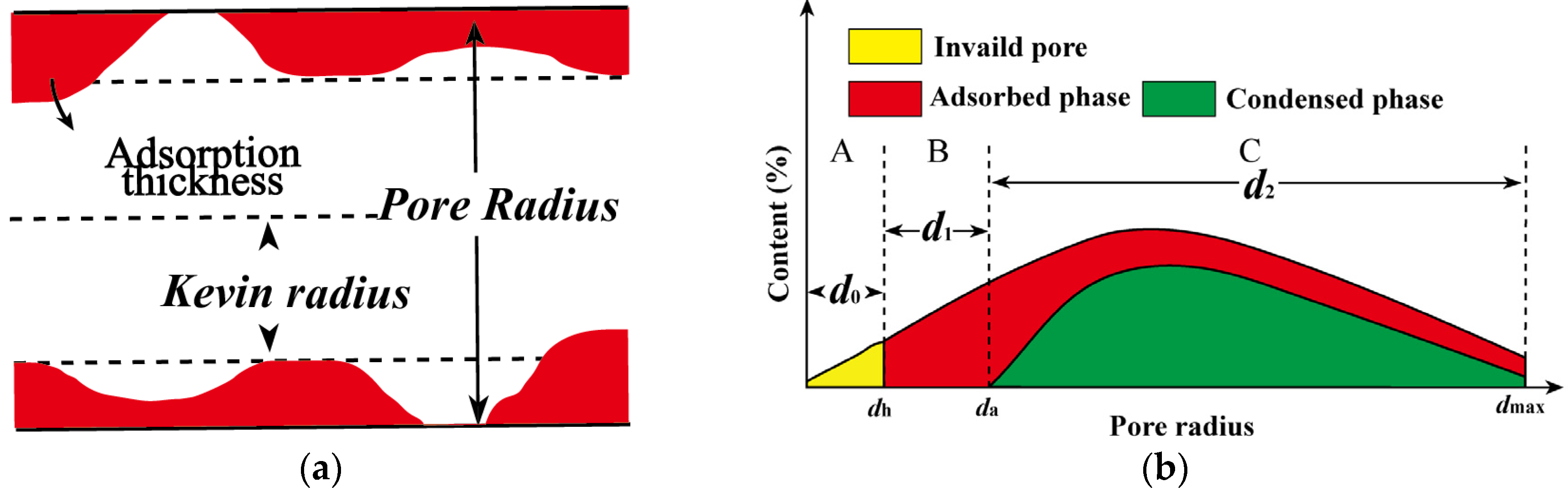
2.3. Theoretical Model of Adsorbed and Free Oil
3. Results and Discussions
3.1. Characterization of Kerogen Chemical Structure
3.1.1. PY-GC-MS Data Analysis
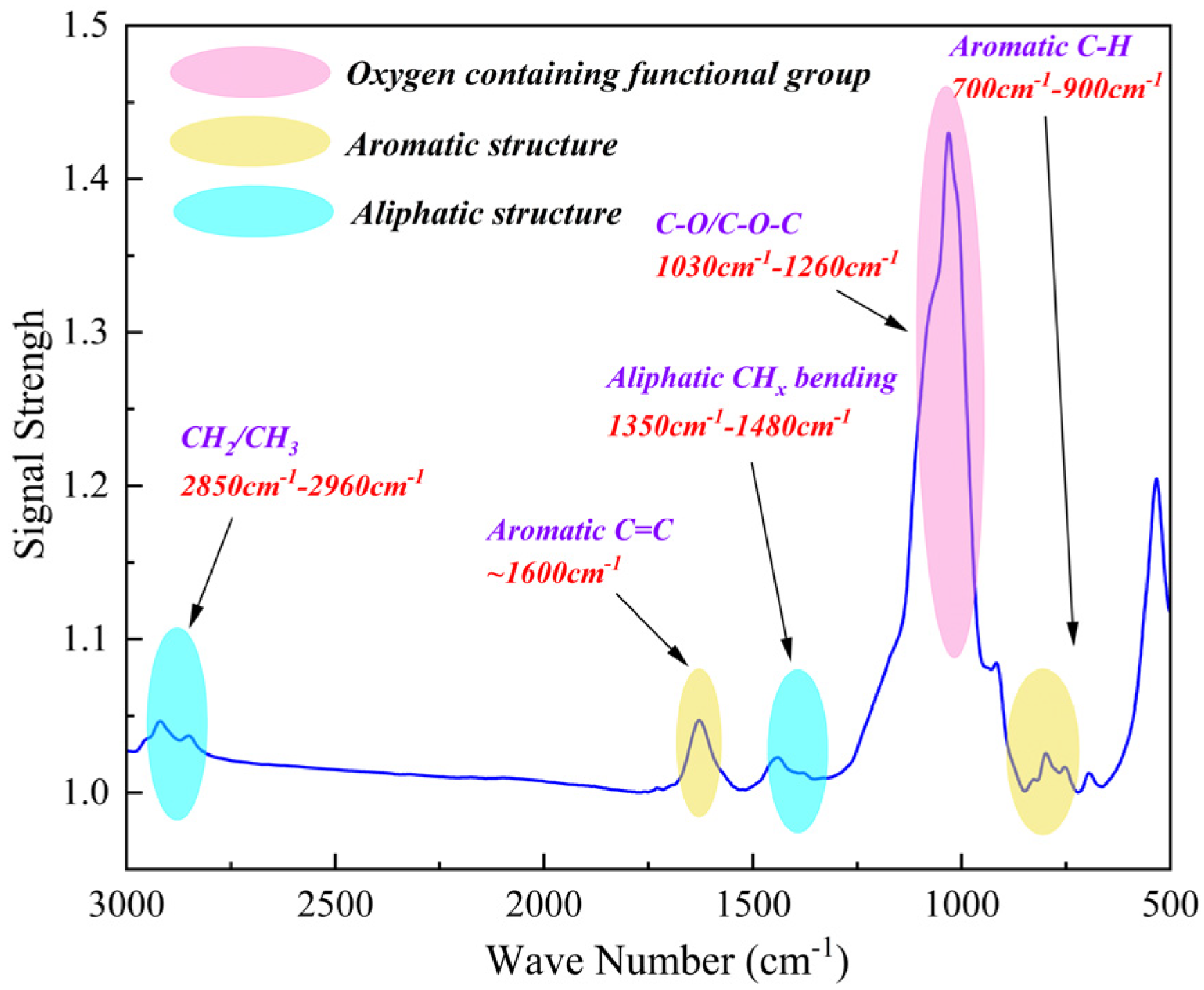
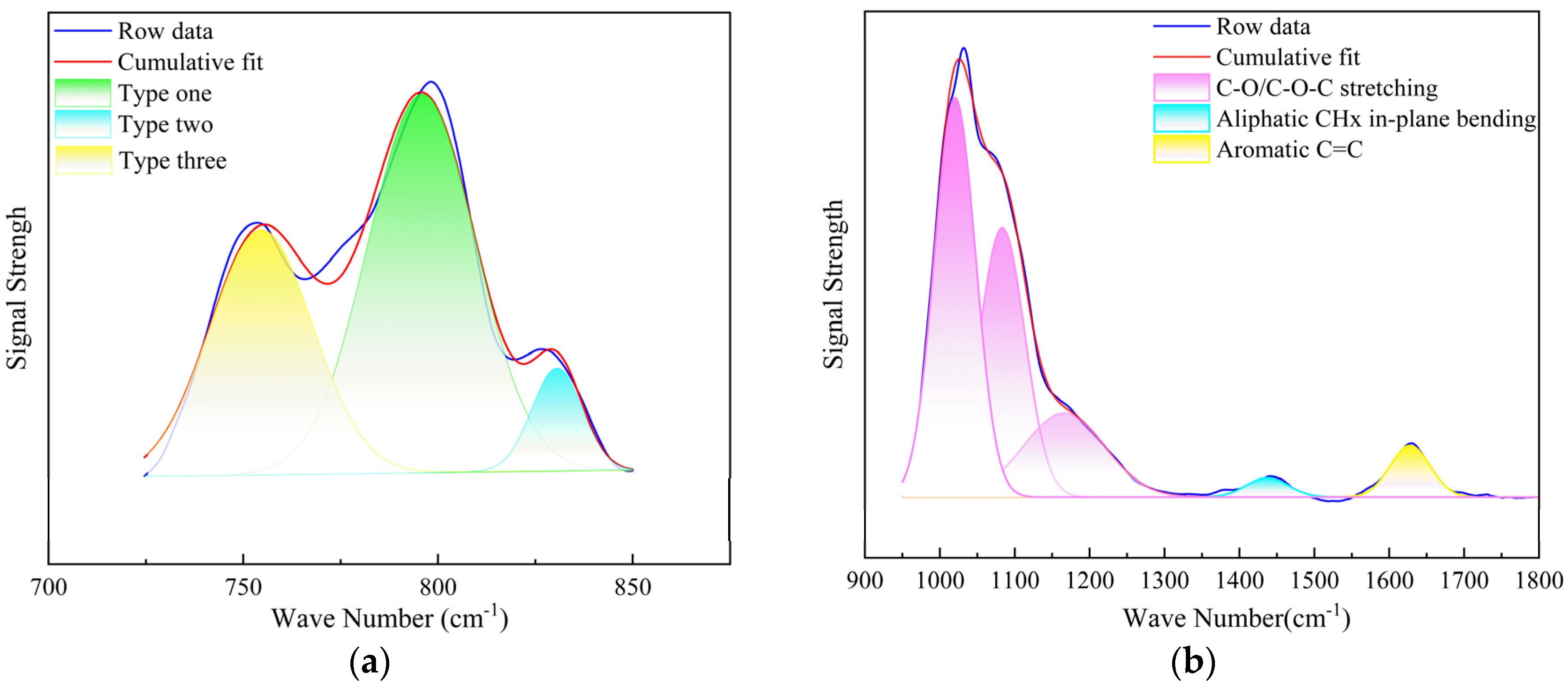
3.1.2. FTIR Data Analysis
3.1.3. Solid-State 13C NMR Data Analysis
3.1.4. XPS Data Analysis
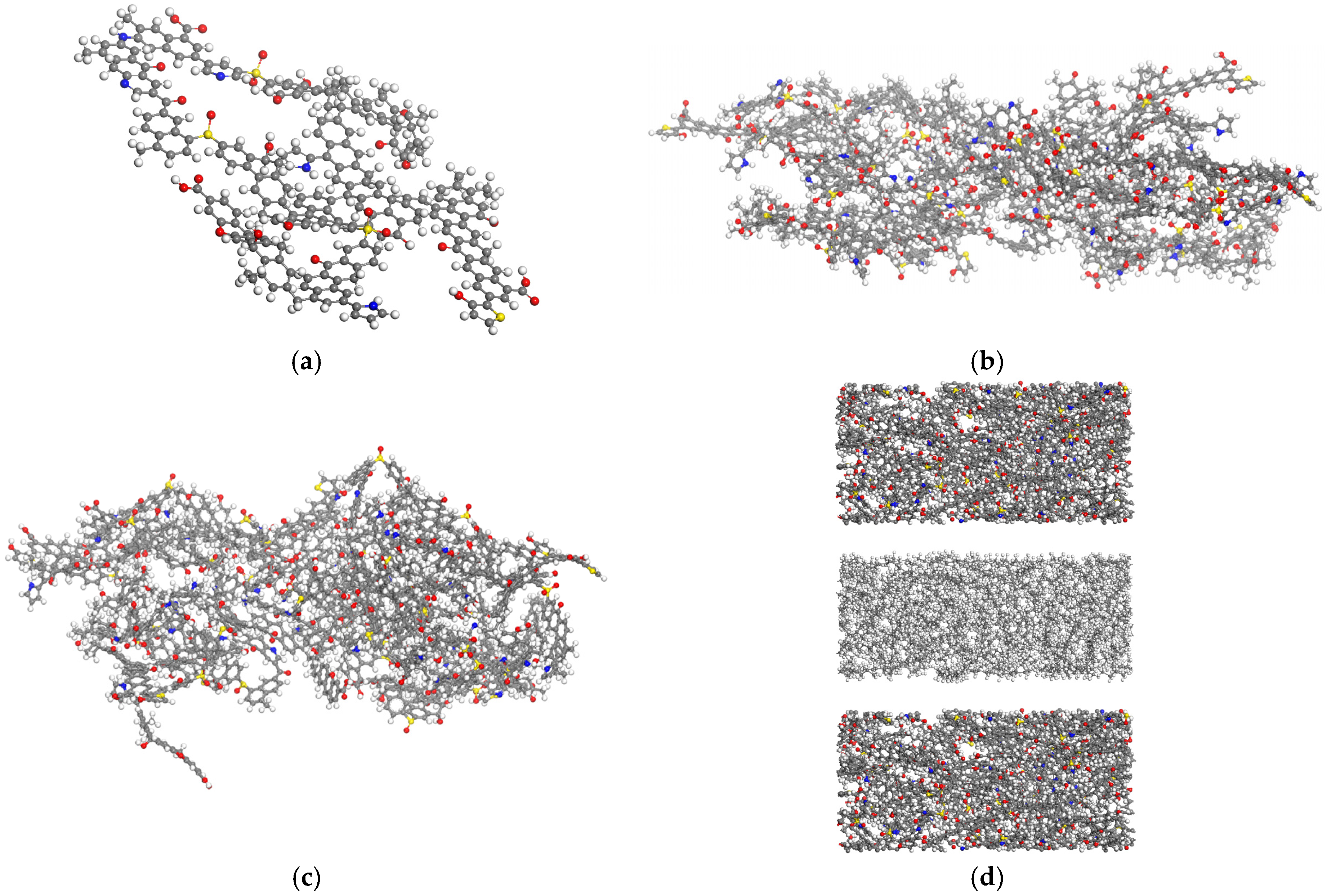
3.2. Molecular Modeling of Kerogen Structure
3.2.1. Molecular Structure of Kerogen Unit and Validation
3.2.2. Construction of Kerogen Slit Nanopore
3.3. The Effect on n-Dodecane Adsorption
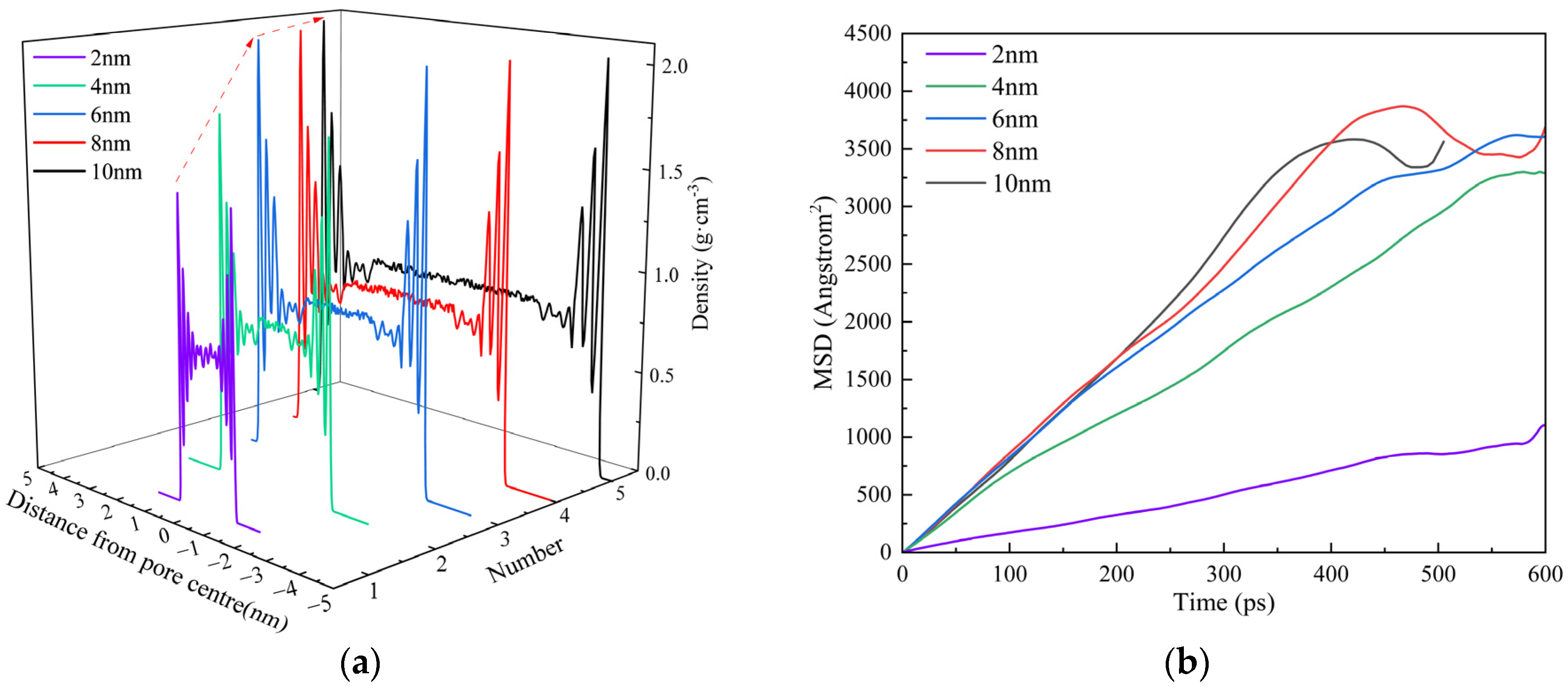
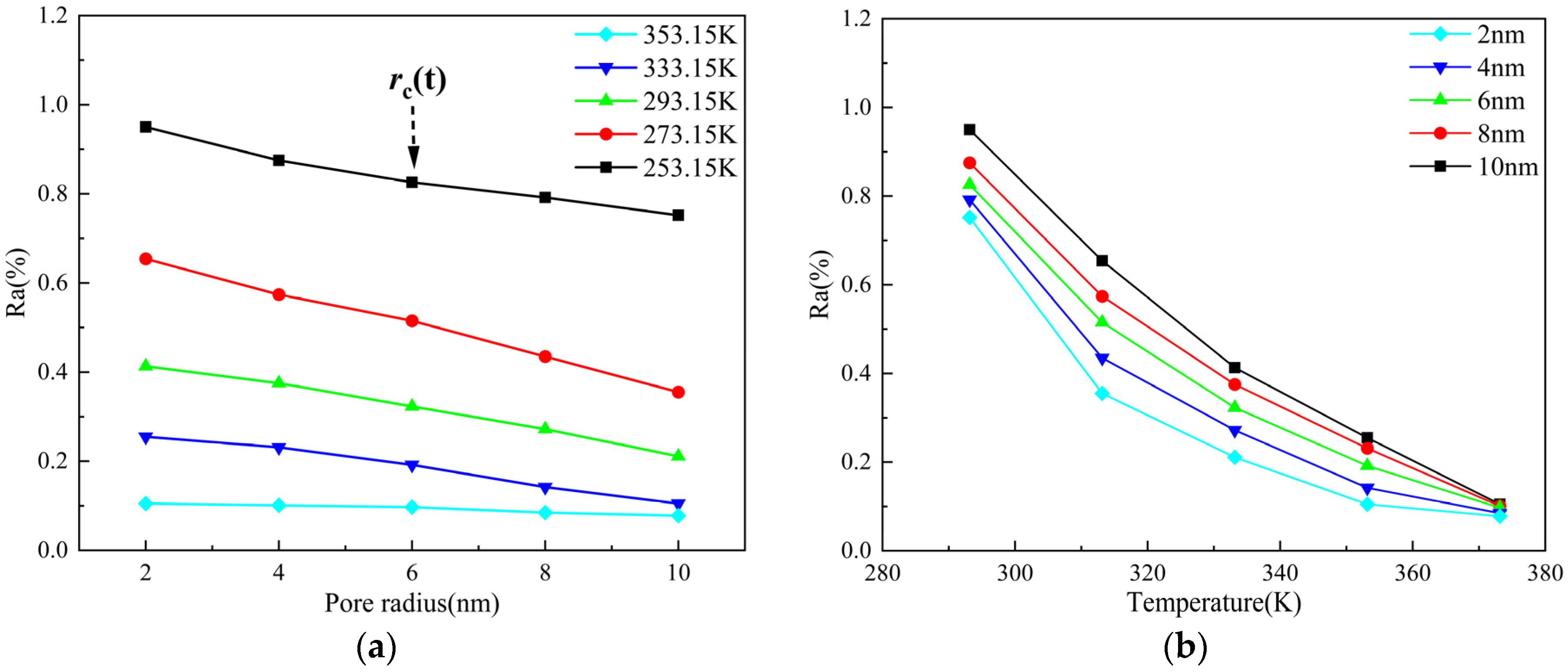
3.3.1. Pore Radius
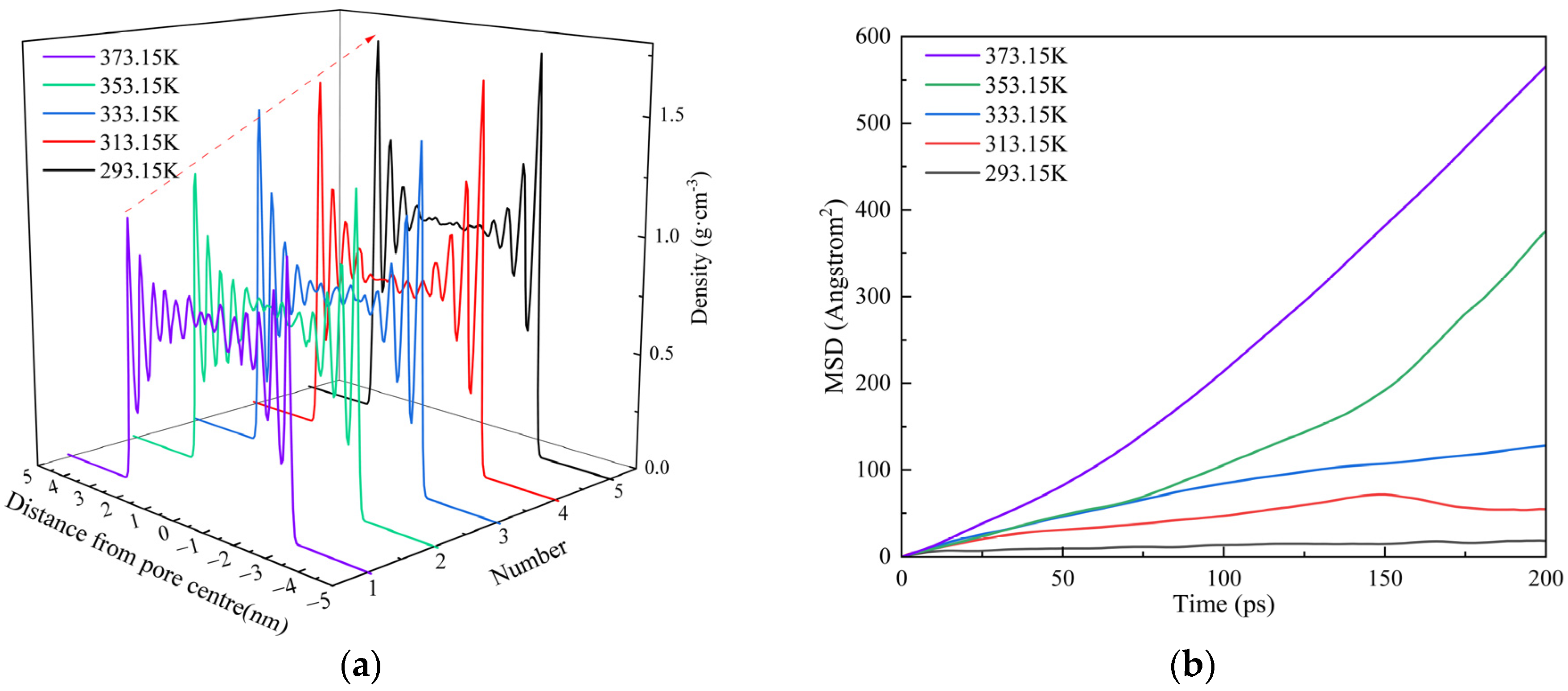
3.3.2. Temperature
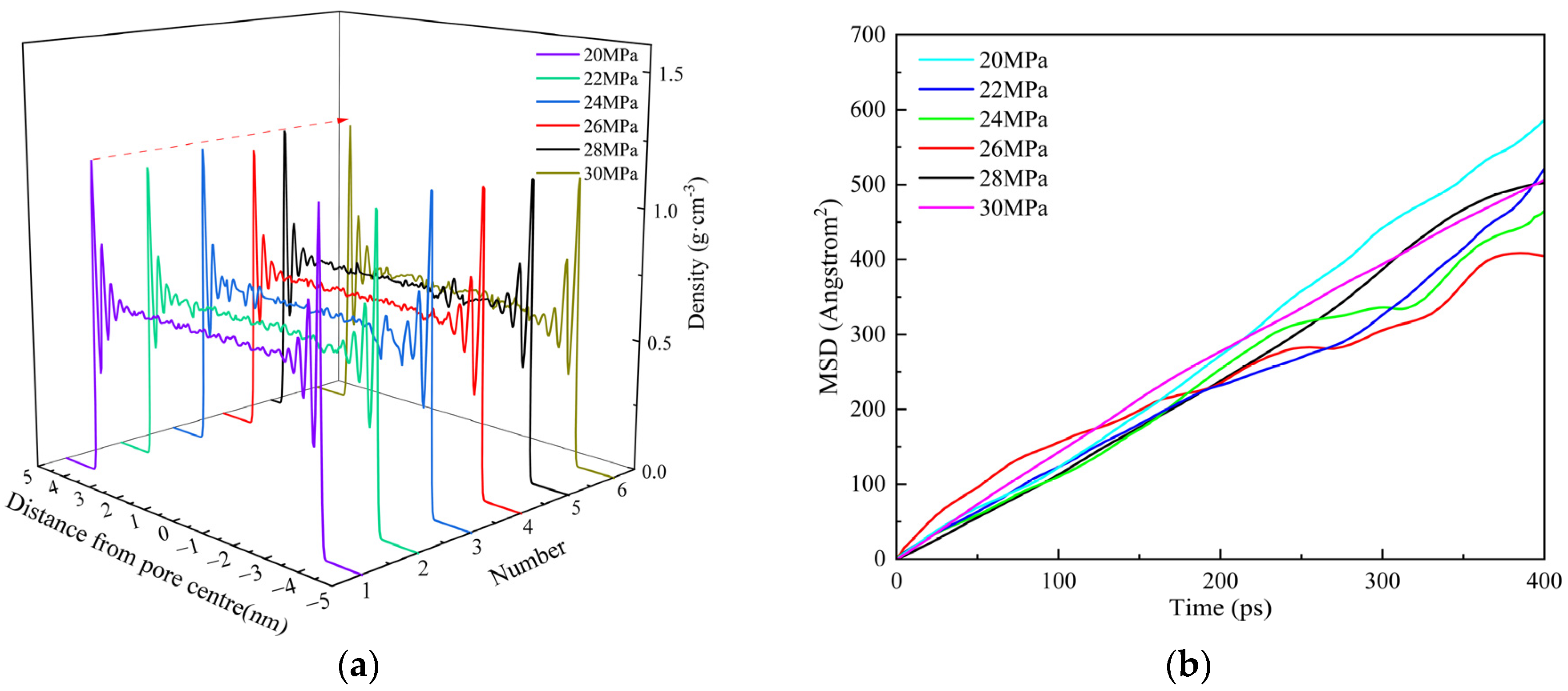
3.3.3. Pressure
3.3.4. Sensitivity Analysis
3.3.5. Microscopic Mechanism of Shale Oil Occurrence
4. Conclusions
- (1)
- The pyrolysis products of kerogen samples primarily consist of hydrocarbons, nitrogen-containing heteroatom compounds, aromatic compounds with benzene ring structures, and oxygen-containing alcohol compounds. FTIR results indicate that the chemical structure of kerogen is mainly composed of aromatic groups, aliphatic groups, and oxygen-containing groups. 13C NMR results suggest that aromatic carbon forms the main framework of the kerogen structure. XPS experimental results show that fluorine exists in the form of ions in kerogen, while nitrogen exists in the forms of pyridine, pyrrole, amines, and nitrogen oxides, and sulfur exists in three forms: thiophene-type, sulfone-type, and sulfoxide-type sulfur.
- (2)
- Shale oil molecules preferentially form an adsorption layer in kerogen pores. As pore size increases, the number of adsorption layers gradually increases. When the pore diameter exceeds 8 nm, the density profile of the adsorption layer no longer changes. As the temperature increases, the peak of the adsorption layer near the wall decreases with temperature, while the peak of the adsorption layer farther from the wall shows minimal reduction. Pressure has a limiting effect on the adsorption amount of dodecane molecules.
- (3)
- With an increase in pore size, the adsorbed oil content gradually decreases, but the overall reduction is less than 20%. After the pore diameter exceeds a certain threshold, the change in shale oil adsorption becomes negligible. Meanwhile, the amount of shale oil adsorbed decreases significantly with increasing temperature, with a total reduction exceeding 50%. This suggests that in comparison with pore size, temperature has a stronger effect on shale oil adsorption.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PY-GC-MS | Pyrolysis–gas chromatography–mass spectrometry |
| 13C NMR | Solid-state 13C nuclear magnetic resonance |
| FTIR | Fourier transform infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| GCMC | Grand Canonical Monte Carlo |
References
- Du, J.H.; Hu, S.Y.; Pang, Z.L.; Du, S.H.; Hou, L.H.; Zhu, R.K. The types, potentials and prospects of continental shale oil in China. China Pet. Explor. 2019, 24, 560–568. [Google Scholar]
- Mănesu, C.B.; Nuño, G. Quantitative effects of the shale oil revolution. Energy Policy 2015, 86, 855–866. [Google Scholar] [CrossRef]
- Douglas, B.; Umekwe, M.P. Shale-oil development prospects: The role of shale-gas in developing shale-oil. Energies 2019, 12, 3331. [Google Scholar]
- Zhou, C.N.; Pan, S.Q.; Jing, Z.H.; Gao, J.L.; Yang, Z.; Wu, S.T. Shale oil and gas revolution and its impact. Acta Pet. Sin. 2020, 41, 1–12. [Google Scholar]
- Temoor, M.; Ahmed, H.; Fahad, S.; Hassan, A.; Amaar, S. Unconventional hydrocarbon resources: Geological statistics, petrophysical characterization, and field development strategies. Pet. Explor. Prod. Technol. 2022, 12, 1463–1488. [Google Scholar]
- Katz, B.; Lin, F. Lacustrine basin unconventional resource plays: Key differences. Mar. Pet. Geol. 2014, 56, 255–265. [Google Scholar] [CrossRef]
- Zhou, C.N.; Yang, Z.; Cui, J.W.; Zhu, R.K.; Hou, L.H.; Tao, S.Z. Formation mechanism, geological characteristics and development strategy of nonmarine shale oil in China. Pet. Explor. Dev. 2013, 40, 14–26. [Google Scholar] [CrossRef]
- Zhang, J.C.; Lin, L.M.; Li, Y.X.; Tang, X.; Zhu, L.L. Classification and evaluation of shale oil. Earth Sci. Front. 2012, 19, 322–331. [Google Scholar] [CrossRef]
- Hu, D.F.; Wei, Z.H.; Wei, X.F.; Shi, W.B.; Wang, D.J.; Liu, X.J. Breakthrough in the exploration of continental shale oil/gas of Jurassic Lianggaoshan Formation in the Fuxing area of the Sichuan Basin and its inspiration. Nat. Gas Ind. 2025, 45, 1–13. [Google Scholar]
- Clementz, D.M. Effect of oil and bitumen saturation on source-rock pyrolysis. AAPG Bull. 1978, 63, 2227–2232. [Google Scholar]
- Xu, Y.; Lun, Z.M.; Pan, Z.J.; Wang, H.T.; Xia, Z.; Zhao, C.P. Occurrence space and state of shale oil: A Review. J. Pet. Sci. Eng. 2022, 211, 110183. [Google Scholar] [CrossRef]
- Kuang, L.C.; Hou, L.H.; Wu, S.T.; Cui, J.W.; Tian, H.; Zhang, L.J. Organic matter occurrence and pore-forming mechanisms in lacustrine shales in China. Pet. Sci. 2022, 19, 1460–1472. [Google Scholar] [CrossRef]
- Dordzie, G.; Balhoff, M. A review of chemical methods and testing techniques for enhanced oil recovery in shale reservoirs. Fuel 2025, 394, 135060. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Gasparik, M.; Amann-Hildenbrand, A.; Gensterblum, Y.; Krooss, B.M. Experimental study of fluid transport processes in the matrix system of the European organic-rich shales: I. Scandinavian Alum Shale. Mar. Pet. Geol. 2014, 51, 79–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; He, Z.; Li, Y.; Xiao, D.; Chen, G. Coupling between Source Rock and Reservoir of Shale Gas in Wufeng-Longmaxi Formation in Sichuan Basin, South China. Energies 2021, 14, 2679. [Google Scholar] [CrossRef]
- Fathi, E.; Akkutlu, I.Y. Multi-component gas transport and adsorption effects during CO injection and enhanced shale gas recovery. Int. J. Coal. Geol. 2014, 123, 52–61. [Google Scholar] [CrossRef]
- Akkutlu, I.Y.; Fathi, E. Multiscale gas transport in shales with local kerogen heterogeneities. SPE J. 2012, 17, 1002–1011. [Google Scholar] [CrossRef]
- Nikolaev, M.Y.; Kazak, A.V. Liquid saturation evaluation in organic-rich unconventional reservoirs: A Comprehensive Review. Earth Sci. Rev. 2019, 194, 327–349. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Y.; Wang, Q.; Bo, J.R.; Xu, X.M.; Wang, Q. DFT and thermodynamic analysis of pyrolysis mechanism of sulfur-containing compounds in shale oil. J. China Coal Soc. 2024, 42, 572–586. [Google Scholar]
- Félix, G.; Djimasbe, R.; Tirado, A.; Varfolomeev, M.A. Detailed kinetic modeling for the organic-rich oil shale upgrading using supercritical water. Int. J. Hydrogen Energy 2025, 136, 126–138. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Li, M.K.; Qian, M.H.; Li, Z.M.; Li, Z. Quantitative characterization of shale oil in different occurrence states and its application. Pet. Geol. Exp. 2016, 38, 843–849. [Google Scholar]
- Qian, M.H.; Jiang, Q.G.; Li, M.W.; Li, Z.M.; Liu, P. Quantitative characterization of extractable organic matter in lacustrine shale with different occurrence. Pet. Geol. Exp. 2017, 39, 278–286. [Google Scholar]
- Li, J.Q.; Lu, S.F.; Xie, L.J.; Zhang, J.; Xue, H.T. Modeling of hydrocarbon adsorption on continental oil shale: A case study on n-alkane. Fuel 2017, 206, 603–613. [Google Scholar] [CrossRef]
- Qian, M.H.; Wang, X.L.; Li, M.K.; Li, Z.M.; Leng, J.Y.; Kong, Z.L. Oil-bearing properties and hydrocarbon occurrence states of Fengcheng formation shale in Well Maye-1, Mahu Sag. Xinjiang Pet. Geol. 2022, 43, 693–703. [Google Scholar]
- Jarvie, D.M. Unconventional oil petroleum systems: Shales and shale hybrids. In Proceedings of the AAPG International Conference and Exhibition, Calgary, AB, Canada, 12–15 September 2010; pp. 12–15. [Google Scholar]
- Jarvie, D.M. Shale resource systems for oil and gas: Part 2−shale-oil resource systems. AAPG Mem. 2012, 97, 89–119. [Google Scholar]
- Jarvie, D.M. Components and processes affecting producibility and commerciality of shale resource systems. Geol. Acta 2014, 12, 307–325. [Google Scholar]
- Ertas, D.; Kelemen, S.R.; Halsey, T.C. Petroleum expulsion part 1. Theory of kerogen swelling in multicomponent solvents. Energy Fuels 2006, 20, 295–300. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y. Atomic mechanisms and equation of state of methane adsorption in carbon nanopores. J. Phys. Chem. C 2014, 118, 17737–17744. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhu, X.; Lin, K.; Zhao, Y. Molecular dynamics simulations of the enhanced recovery of confined methane with carbon dioxide. Phys. Chem. Chem. Phys. 2015, 17, 31887–31893. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, Y.; Bhatia, S.K.; Rudolph, V. Simulation of binary mixture adsorption of methane and CO2 at supercritical conditions in carbons. AIChE J. 2006, 52, 957–967. [Google Scholar] [CrossRef]
- Czerwinski, F. Current trends in automotive lightweighting strategies and materials. Materials 2021, 14, 6631. [Google Scholar] [CrossRef]
- Qing, W.; Xinmin, W.; Shuo, P. The three-dimensional molecular structure model of Fushun oil shale kerogen, China. J. Mol. Struct. 2022, 1255, 132380. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, W.; Zhang, N. Molecular insights into recovery of shale gas by CO2 injection in kerogen slit nanopores. J. Nat. Gas Sci. Eng. 2021, 90, 103903. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z. Hydrocarbon mixture and CO2 adsorptions in a nanopore-bulk multiscale system in relation to CO2 enhanced shale gas recovery. Chem. Eng. J. 2021, 415, 128398. [Google Scholar] [CrossRef]
- Wang, S.; Yao, X.; Feng, Q.; Javadpour, F.; Yang, Y.; Xue, Q. Molecular insights into carbon dioxide enhanced multi-component shale gas recovery and its sequestration in realistic kerogen. Chem. Eng. J. 2021, 425, 130292. [Google Scholar] [CrossRef]
- Adewumi, K.; Babatunde, B.; Mamo, N.; Rashik, M.; Ahmed, T.Y.; Regassa, J.S. Molecular simulation study of CO2/CH4 adsorption on realistic heterogeneous shale surfaces. Appl. Surf. Sci. 2021, 543, 148789. [Google Scholar] [CrossRef]
- Li, J.Q.; Lu, S.F.; Cai, J.C.; Zhang, P.F.; Xue, H.T.; Zhao, X.B. Adsorbed and Free Oil in Lacustrine Nanoporous Shale: A Theoretical Model and a Case Study. Energy Fuels 2018, 32, 12247–12258. [Google Scholar] [CrossRef]
- Kirsanov, A.M.; Komkov, I.K.; Paizanskaya, I.L. Geochemical methods for prediction and assessment of shale oil resources: Case study of the bazhenov formation. Russ. Geol. Geophys. 2017, 58, 403–409. [Google Scholar] [CrossRef]
- Isaksen, G.H. Central North Sea hydrocarbon systems: Generation, migration, entrapment, and thermal degradation of oil and gas. AAPG Bull. 2004, 88, 1545–1572. [Google Scholar] [CrossRef]
- Cao, H.; Zou, Y.; Lei, Y.; Xi, D.; Wan, X. Shale oil assessment for the Songliao basin, northeastern China, using oil generation-sorption method. Energy Fuels 2017, 31, 4826–4842. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, Y.Q.; Yang, C.C.; Wang, Z.L.; Sun, S.S. Geological characteristics of shale oil in the Jurassic Lianggaoshan Formation in Sichuan Basin. China Pet. Explor. 2023, 28, 66–78. [Google Scholar]
- Huang, S.; Ma, X.; Yang, H.; Wu, J.; Zhang, J. Experimental characterization and molecular modeling of kerogen in Silurian deep gas shale from southern Sichuan Basin, China. Energy Rep. 2022, 8, 1497–1507. [Google Scholar] [CrossRef]
- Wang, X.H.; Huang, X.F.; Lin, K.; Zhao, Y.P. The constructions and pyrolysis of 3D kerogen macromolecular models: Experiments and simulations. Glob. Chall. 2019, 3, 1900006. [Google Scholar] [CrossRef]
- Wang, X.H.; Huang, X.F.; Gao, M.N.; Zhao, Y.P. Mechanical response of kerogen at high strain rates. Int. J. Impact Eng. 2021, 155, 103905. [Google Scholar] [CrossRef]
- Hou, D.L.; Qiu, X.D.; Gong, F.M.; Dejam, M.; Nasrabadi, H. Characterization of kerogen molecular structure and its effect on methane adsorption behavior: A comparative study on outcrop and core samples from Longmaxi shale. Chem. Eng. J. 2023, 466, 143293. [Google Scholar] [CrossRef]
- Meunier, M.; Robertson, S. Materials Studio 20th anniversary. Mol. Simul. 2021, 47, 537–539. [Google Scholar] [CrossRef]
- Dou, X.J.; Dai, J.J.; Peng, M.G.; He, Y.F.; Zhu, P.F. Study on the adsorption and deformation laws of multi-components in shale oil with nanopores—Insights from the molecular simulation. J. Pet. Explor. Prod. Technol. 2024, 14, 3091–3109. [Google Scholar] [CrossRef]
- Cui, S.T.; McCabe, C.; Cummings, P.T.; Cochran, H.D. Molecular dynamics study of the nano-rheology of n-dodecane confined between planar surfaces. J. Chem. Phys. 2003, 118, 8941–8944. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Li, R.C.; Yu, S.C.; Guo, M.Z.; Yan, Y.G. Screening and verification of molecular force field based on physical property simulation of deep oil. J. China Univ. Pet. 2020, 44, 163–169. [Google Scholar]
- Curtis, M.E.; Sondergeld, C.H.; Rai, C.S. Relationship between organic shale microstructure and hydrocarbon generation. In Proceedings of the SPE Unconventional Resources Conference/Gas Technology Symposium, The Woodlands, TX, USA, 10–12 April 2013; p. SPE-164540-MS. [Google Scholar]
- Liu, W.D.; Liu, J.; Sun, L.H.; Li, Y.; Lan, X.Y. Influence of fluid boundary layer on fluid flow in low permeability oil fields. Sci. Technol. 2011, 29, 42–44. [Google Scholar]
- Ling, H.C.; Yang, Z.M.; Xiao, Q.H.; Xu, Q.Y. Study on new porous flow model in tight oil formation. Sci. Technol. Eng. 2013, 13, 7624–7628. [Google Scholar]
- Philippe, U.; Julien, C.; Marianna, Y. Molecular Modeling of the Volumetric and Thermodynamic Properties of Kerogen: Influence of Organic Type and Maturity. Energy Fuels 2015, 29, 91–105. [Google Scholar]
- Katritzky, A.R.; Akhmedov, N.G.; Güven, A. gNMR simulated 1H and proton-coupled 13C NMR spectra of substituted 3-nitropyridines. GIAO/DFT calculated values of proton and carbon chemical shifts and coupling constants. J. Mol. Struct. 2006, 787, 131–147. [Google Scholar] [CrossRef]

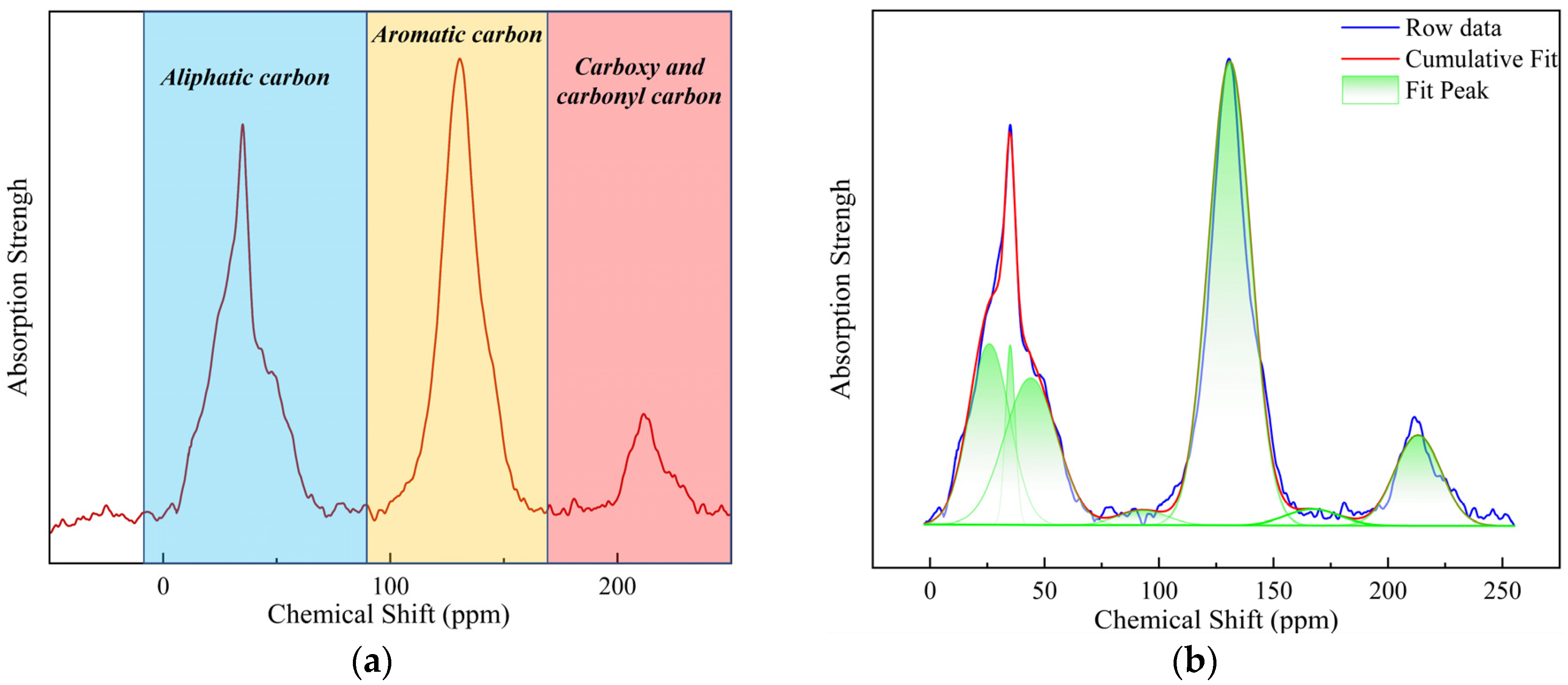
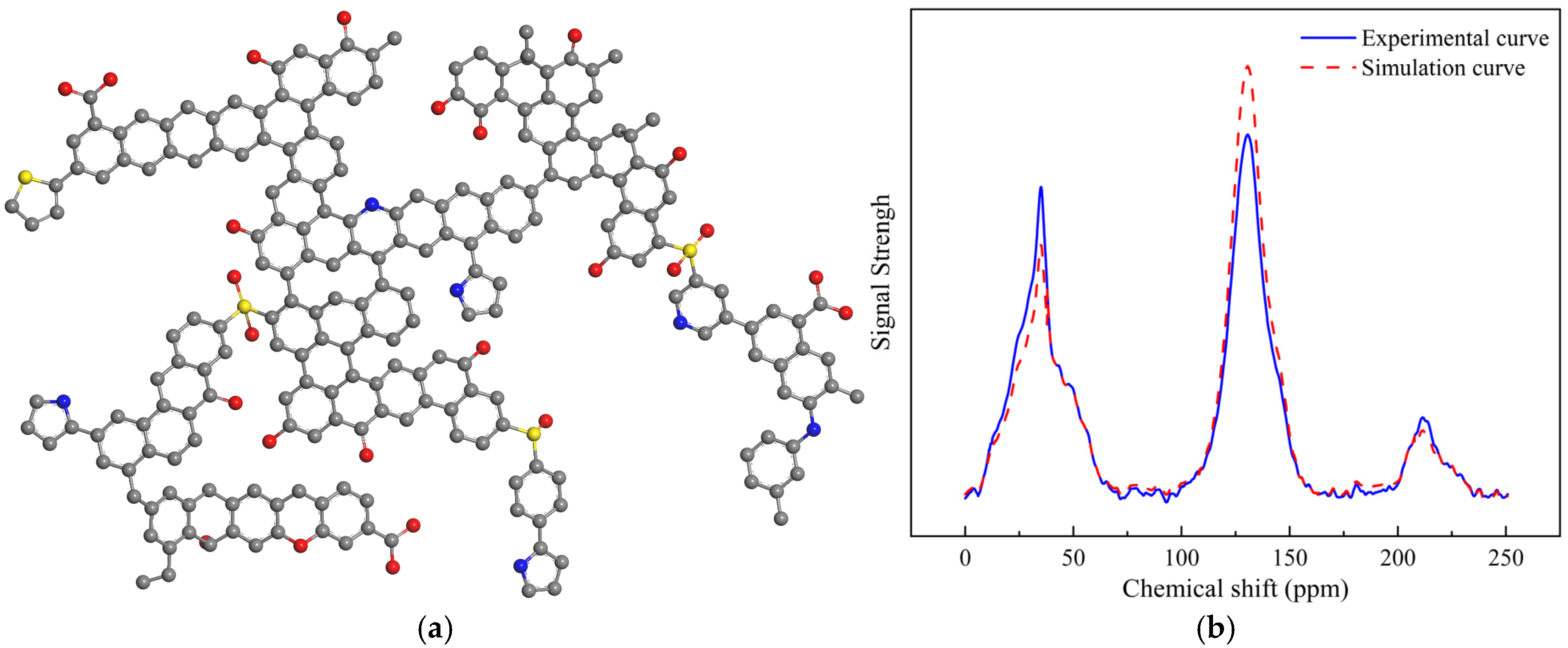
| Structural Parameter | Carbon Functionality | Position | Chemical Shift/ppm | Area Fraction (%) |
|---|---|---|---|---|
| falm | Aliphatic methyl | Cal-C*H3 | 16 | \ |
| falA | Aromatic methyl | Car-C*H3 | 20 | \ |
| falB | Aliphatic C(2) carbon | C*H2-CH3 | 25 | 17.34 |
| falH | Methylene | C*H2 | 33 | \ |
| falD | Methine, Quaternary sp3 carbon | C*, C*H | 35–50 | 4.14 |
| Oxygen-methylene | C*H2-O | 55 | 18.26 | |
| falO | Oxygen-methine | C*H-O | 62,69 | 1.69 |
| Oxygen-aliphatic ring | Cal*-O | 83,89 | \ | |
| farA | Oxygen-aromatic ring | Car*-Car-O | 99,105 | 2.10 |
| farH | Protonated aromatic carbon | Car-H | 100–129 | \ |
| farB | Bridged aromatic carbon | Car*-Car | 129–139 | 46.51 |
| farC | Branched aromatic carbon | Car*-Cal | 139–152 | \ |
| farO | Oxygen-aromatic carbon | Car*-O | 152–165 | \ |
| faO | Carboxyl, carbonyl | RC*=OR | 165–220 | 9.94 |
| Lattice Parameters | Description | Definition | Area Fraction (%) |
|---|---|---|---|
| fal | Aliphaticity | falm + falA + falB + falH + falD + falO | 19.46 |
| far | Aromaticity | farA + farH + farB + farC + farO | 70.61 |
| XBP | Degree of condensation | farB/(farA + farH + farC + farO) | 22.11 |
| BI | Aliphatic branch | (falD + falO)/fal | 14.10 |
| δ | Aromatic substitution | 1 − farH/far | 4.32 |
| Parameters | Perturbation | ∆Ra (3 nm Pore) | ∆Ra (5 nm Pore) |
|---|---|---|---|
| ρ1 | +10% | +18% | +12% |
| ρ1 | −10% | −15% | −10% |
| ρ2 | +10% | −5% | −2% |
| ρ2 | −10% | +4% | +1% |
| dm | +10% | −14% | −8% |
| dm | −10% | +16% | +10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Sima, L.; Wang, L.; Tang, S.; Li, J.; Jin, W.; Liu, B.; Li, B. Quantitative Assessment of Free and Adsorbed Shale Oil in Kerogen Pores Using Molecular Dynamics Simulations and Experiment Characterization. Energies 2025, 18, 5695. https://doi.org/10.3390/en18215695
Guo Y, Sima L, Wang L, Tang S, Li J, Jin W, Liu B, Li B. Quantitative Assessment of Free and Adsorbed Shale Oil in Kerogen Pores Using Molecular Dynamics Simulations and Experiment Characterization. Energies. 2025; 18(21):5695. https://doi.org/10.3390/en18215695
Chicago/Turabian StyleGuo, Yuhao, Liqiang Sima, Liang Wang, Song Tang, Jun Li, Wujun Jin, Bowen Liu, and Bojie Li. 2025. "Quantitative Assessment of Free and Adsorbed Shale Oil in Kerogen Pores Using Molecular Dynamics Simulations and Experiment Characterization" Energies 18, no. 21: 5695. https://doi.org/10.3390/en18215695
APA StyleGuo, Y., Sima, L., Wang, L., Tang, S., Li, J., Jin, W., Liu, B., & Li, B. (2025). Quantitative Assessment of Free and Adsorbed Shale Oil in Kerogen Pores Using Molecular Dynamics Simulations and Experiment Characterization. Energies, 18(21), 5695. https://doi.org/10.3390/en18215695






