Numerical Modelling of Biomethanation in UHS/UMR: The Role of Gas Solubility in Long-Term Dynamics
Abstract
1. Introduction
2. Object and Methods
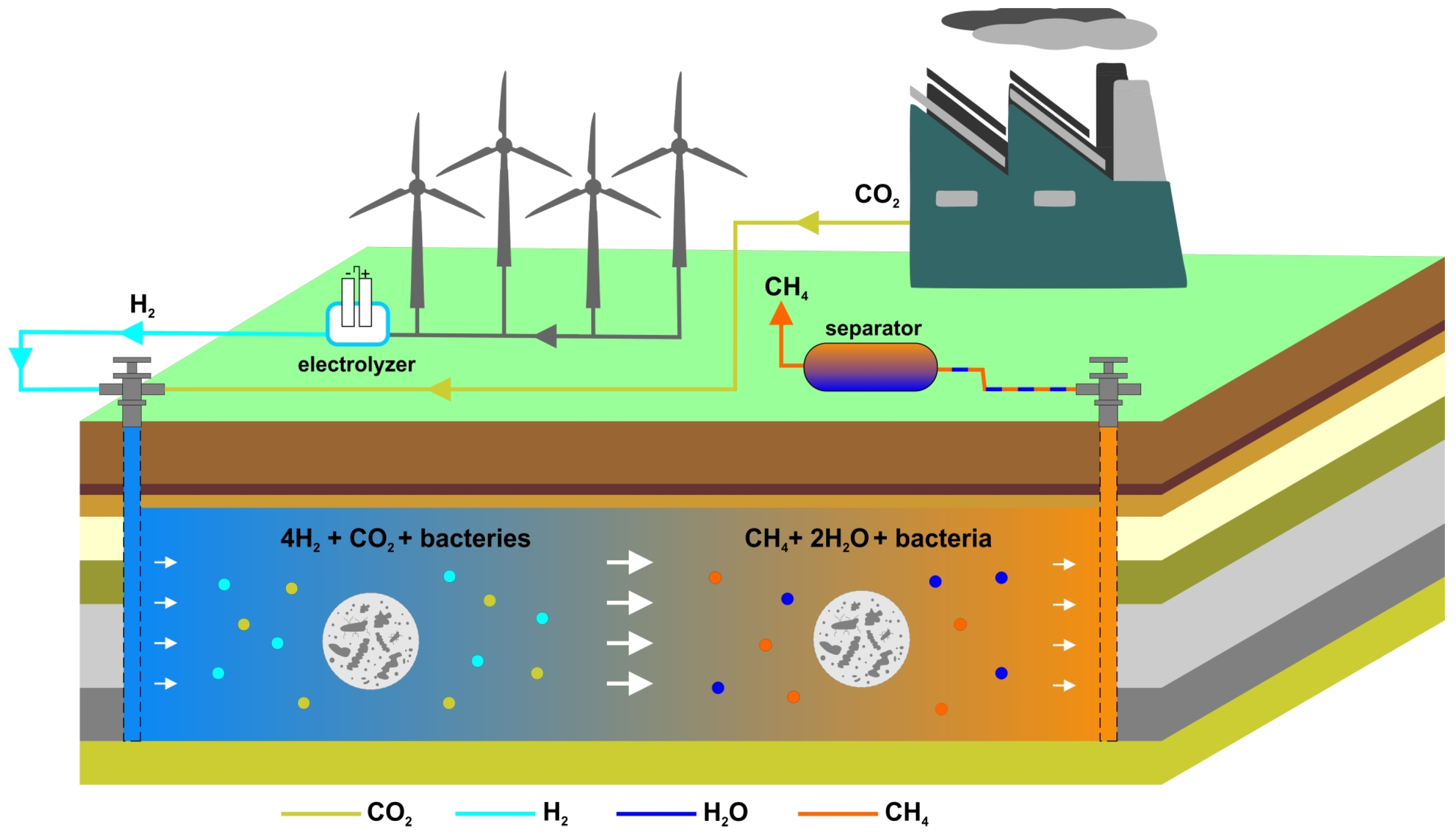
2.1. Underground Biomethanation Reactor (UMR) Model
2.2. Methanation and Biomethanation
- Abiotic methanation (Sabatier reaction): requires, using current technological conditions, nickel catalysis with temperature about 300° C [27].
- Injection of H2 into structures previously used for CCUS.
- Injection of green H2 (from surplus energy) together with CO2 (from industry) to generate CH4.
- Enhancement of CH4 content in synthetic gas mixtures or in aquifers during underground storage operations.
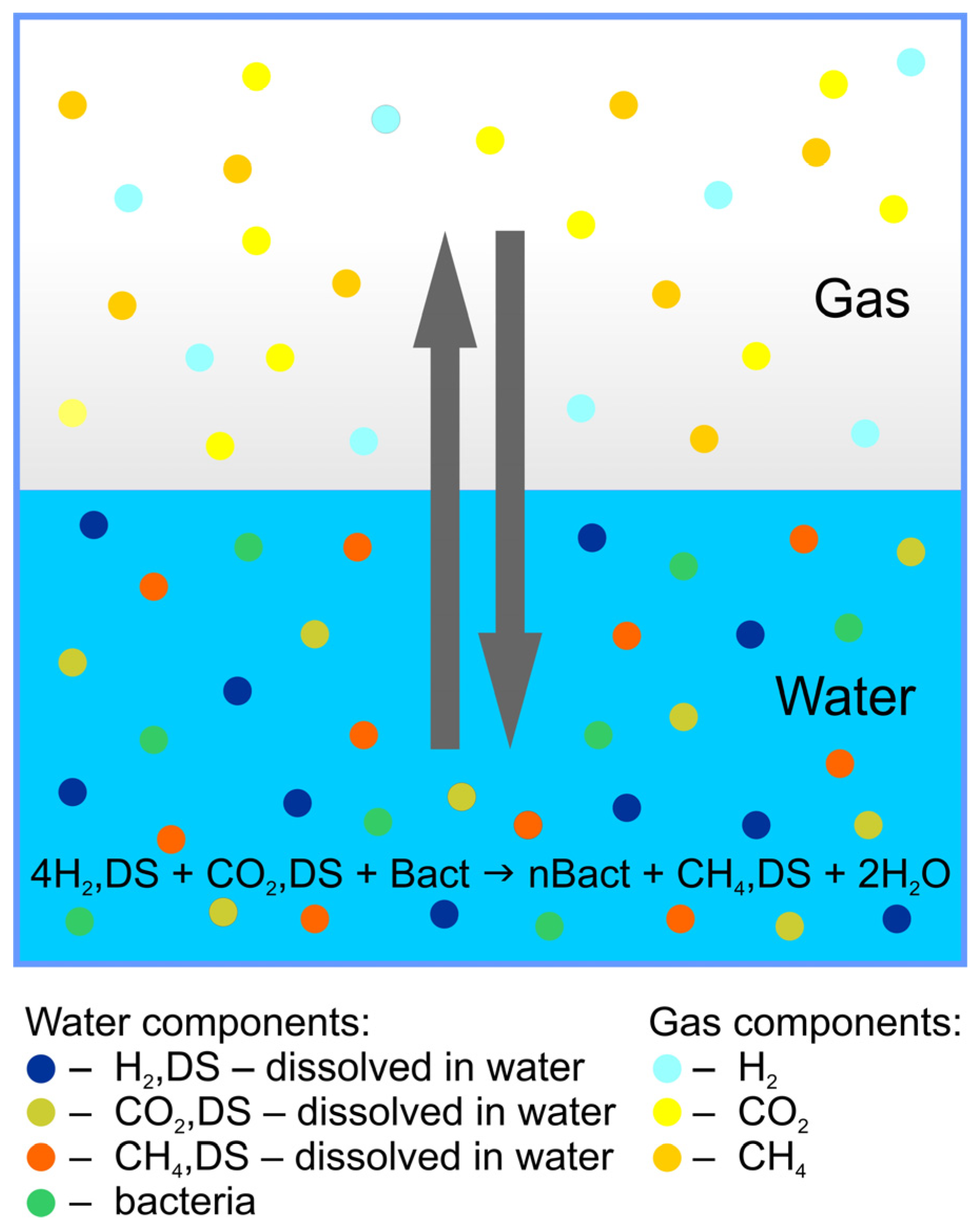
2.3. Physicochemical Model of Biomethanation in the Gas–Water System
- Two-phase flow of water and gas;
- Multicomponent model of the reservoir fluid;
- Multicomponent formation water model;
- Solubility of each component in formation water described by individual equilibrium constants, dependent on pressure, temperature;
- Methanogenic microorganisms treated as an aqueous component catalysing methanation according to the Sabatier reactions [27] (Götz et al., 2016):
- To implement biomethanation in the reservoir simulator, above assumption was expressed as [51]:where
- −
- —H2 dissolved in water;
- −
- —CO2 dissolved in water;
- −
- —H2 dissolved in water;
- −
- —bacteria;
- −
- —coefficient related to bacterial growth, n = 1.
- 2.
- −
- —dissolution of H2 in water along with the release of dissolved H2 into the gas phase;
- −
- —dissolution of CO2 in water along with the release of dissolved CO2 into the gas phase;
- −
- —dissolution of CH4 in water along with the release of dissolved CH4 into the gas phase.
2.4. Reservoir Fluid
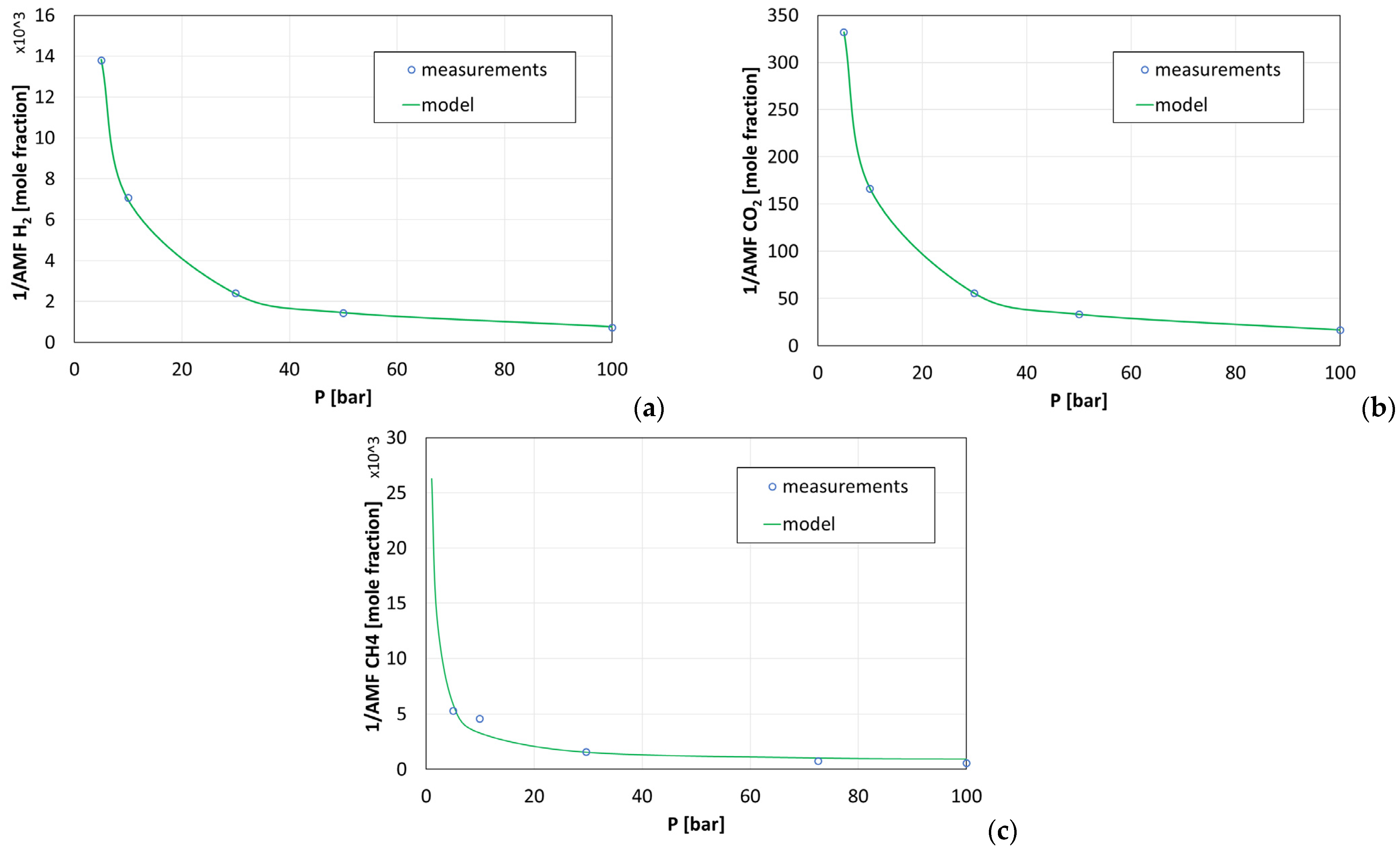
2.5. Solubility of Gases in Water
- −
- —concentration of k component in the gas phase;
- −
- —concentration of k component in the liquid phase.
- −
- —fitted constants;
- −
- —pressure.
2.6. Biomethanation Efficiency
- —bulk volume of the block (rock skeleton volume + pore volume);
- —Arrhenius constant;
- —molar activation energy of reaction;
- —universal gas constant;
- —temperature;
- —exponent of component i;
- —concentration of the i-th component in the phase where the reaction occurs, where for aqueous, the concentration of component I was expressed as follows:
- —porosity of the rock;
- —water saturation;
- —molar fraction of the i-th component in the aqueous phase;
- —molar density of water.
- —the specific volume given by
- —water component fraction in the aqueous phase;
- —reference density of component c;
- —molar mass of the water component c.
2.7. Phase Permeability
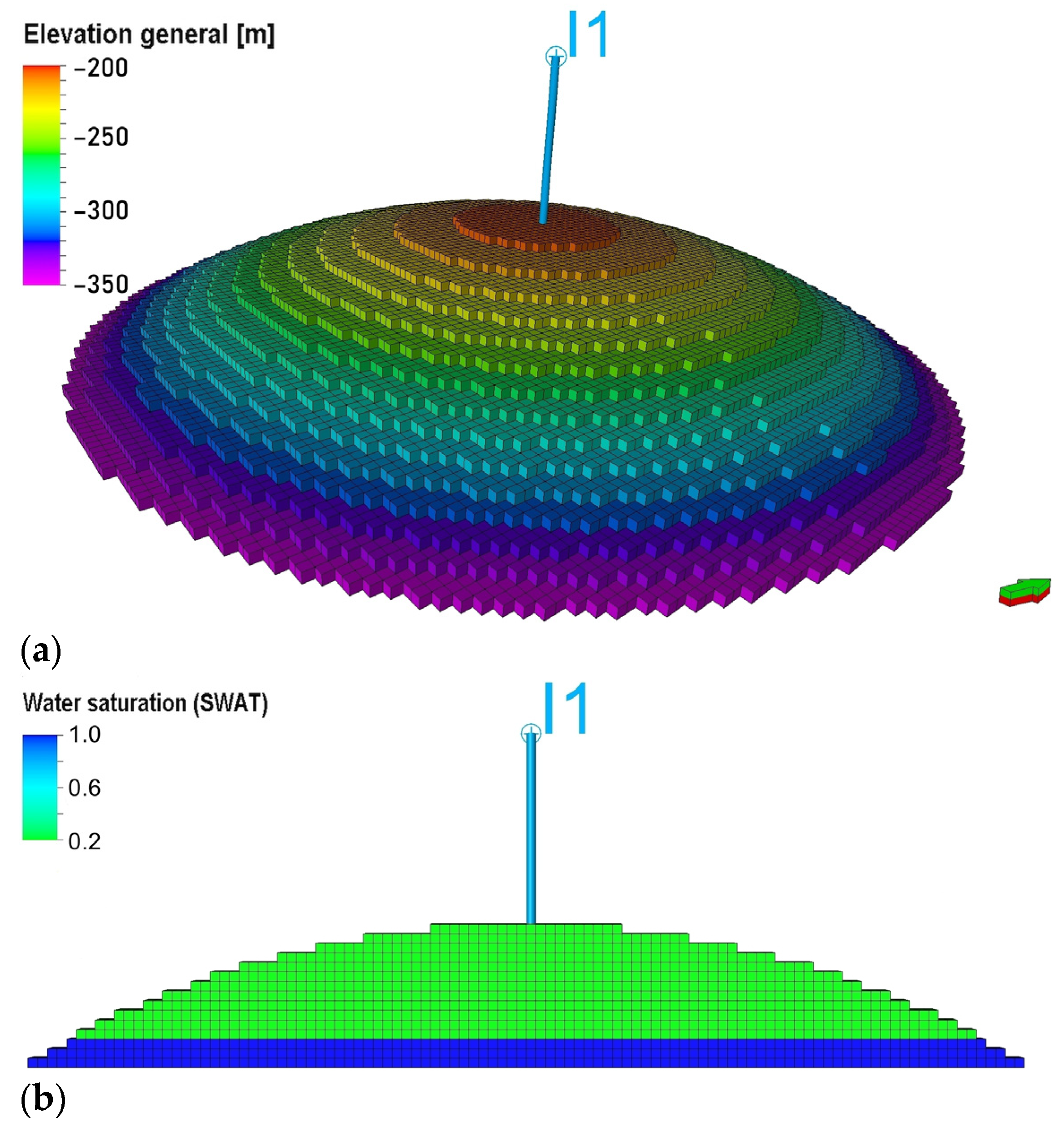
2.8. Model Initialization
- Initial reservoir pressure:The reference pressure at the gas–water contact depth (Hgwc = 320 m below sea level), Pini = 30 bar, based on values reported for storage operations in porous structure [49].
- Initial saturation distribution:The model was initialized with irreducible water saturation of Swcr = 20% across the gas-bearing zone, while the remaining pore space was filled by gas. Below the gas–water contact, it was assumed that the formation was fully water-saturated.
- Fluid composition:The initial gas phase consisted predominantly of CH4, as would be expected in a depleted natural gas reservoir. Trace amounts of dissolved components were calculated according to Henry’s law and the solubility correlations presented in Section 2.5.
- Temperature and salinity:Reservoir temperature was fixed at the reference value adopted for the base case, Tres = 40 °C, and the salinity was incorporated implicitly through solubility constants (Section 2.5).
3. Results
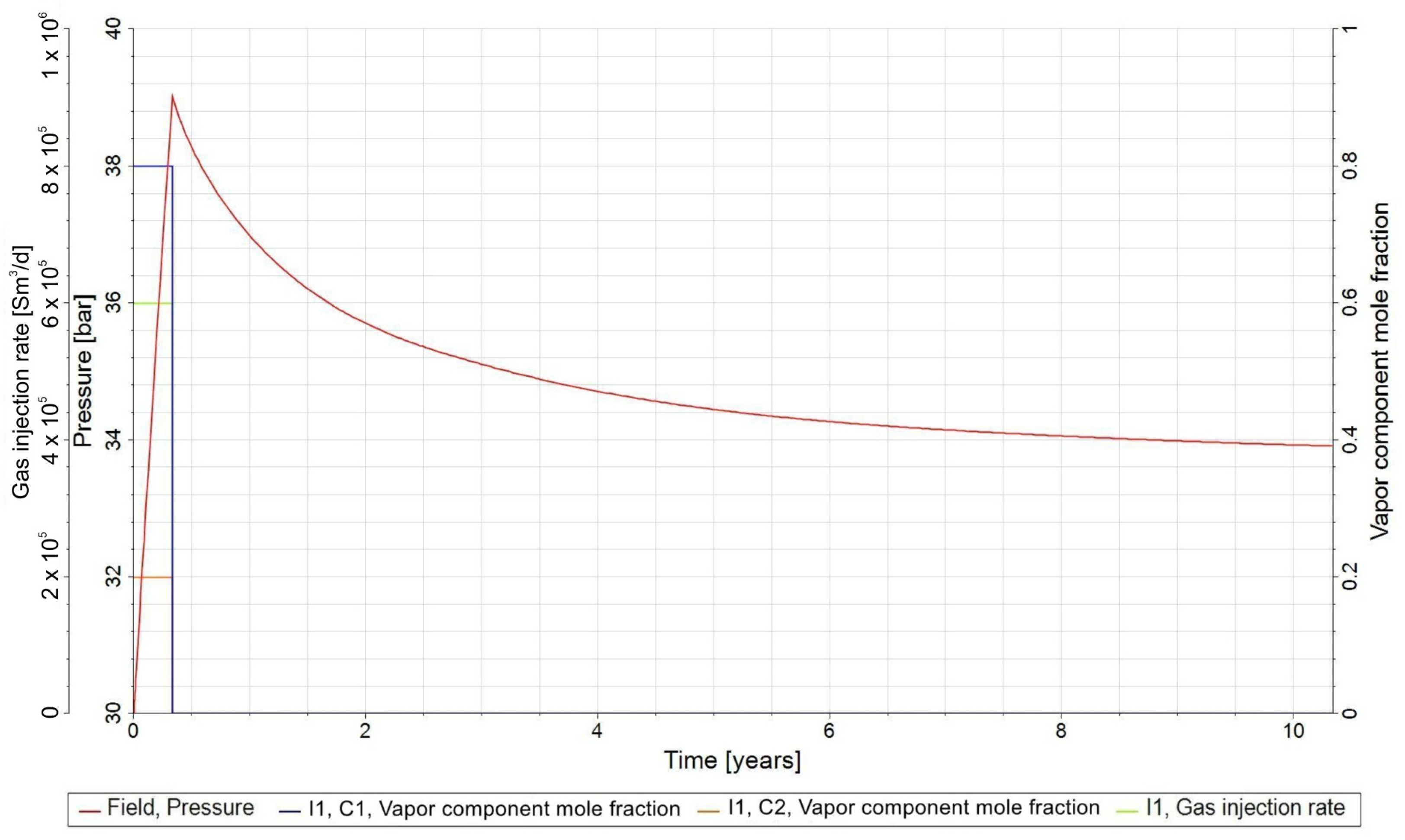
3.1. General Assumptions for the Forecast
- −
- Stage I—injection of a mixture of CO2 and H2 into the natural gas reservoir (injection period, tinj = 4 months;
- −
- Stage II—relaxation phase, trelax = 10 years.
- −
- composition of the injected gas resulting from the stoichiometric equation, cH2 = 80%, cCO2 = 20%;
- −
- injection well I1 completed in the lower half of the gas-bearing zone;
- −
- gas injection rate (CO2 and H2 mixtures), qg,inj = 600,000 Sm3/d;
- −
- initial pressure in UMR, Pini = 30 bar;
- −
- maximum bottom hole pressure in the injection phase, Pbhp,inj,max = 100 bar.
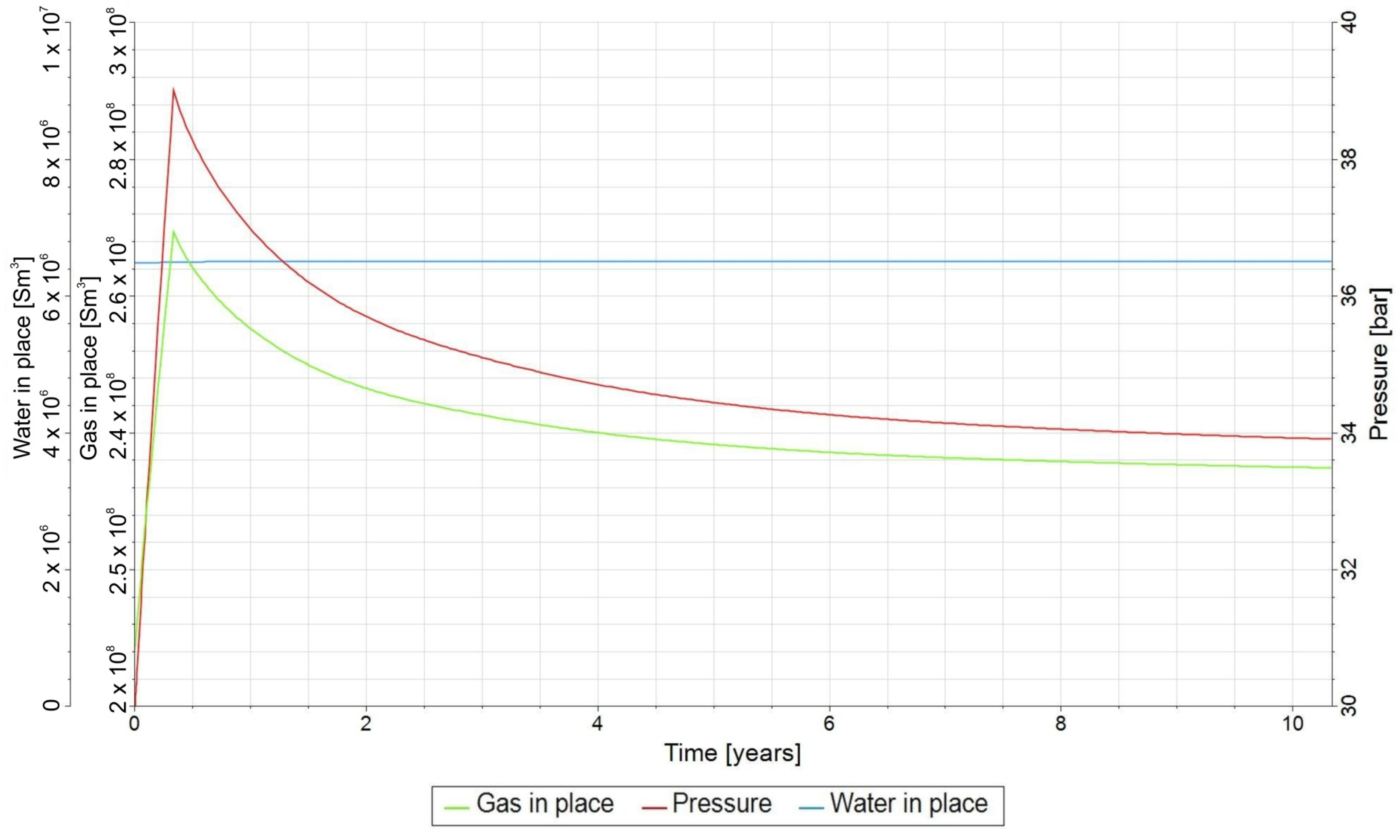
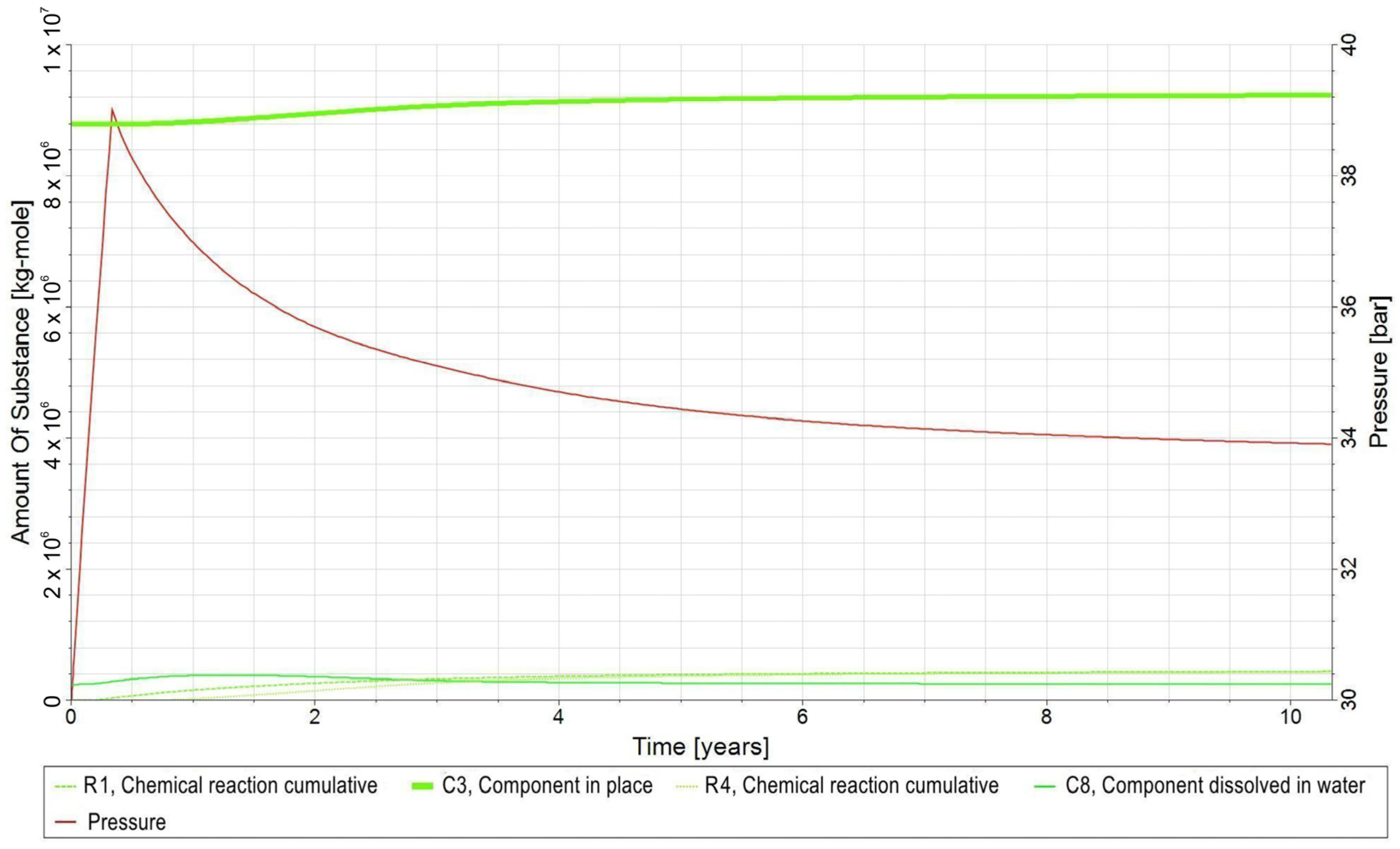
3.2. Forecasts and Simulation Results of the UMR
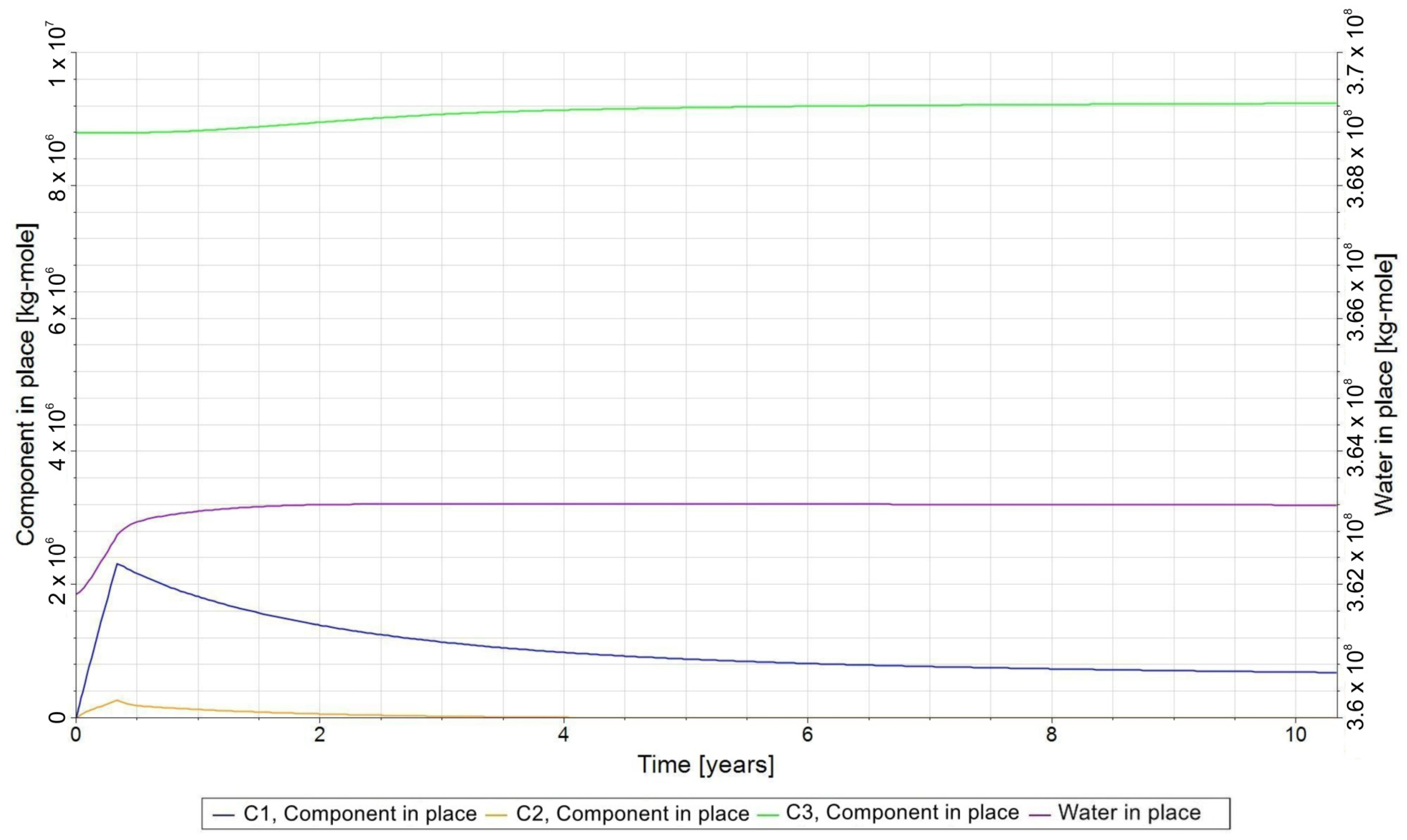
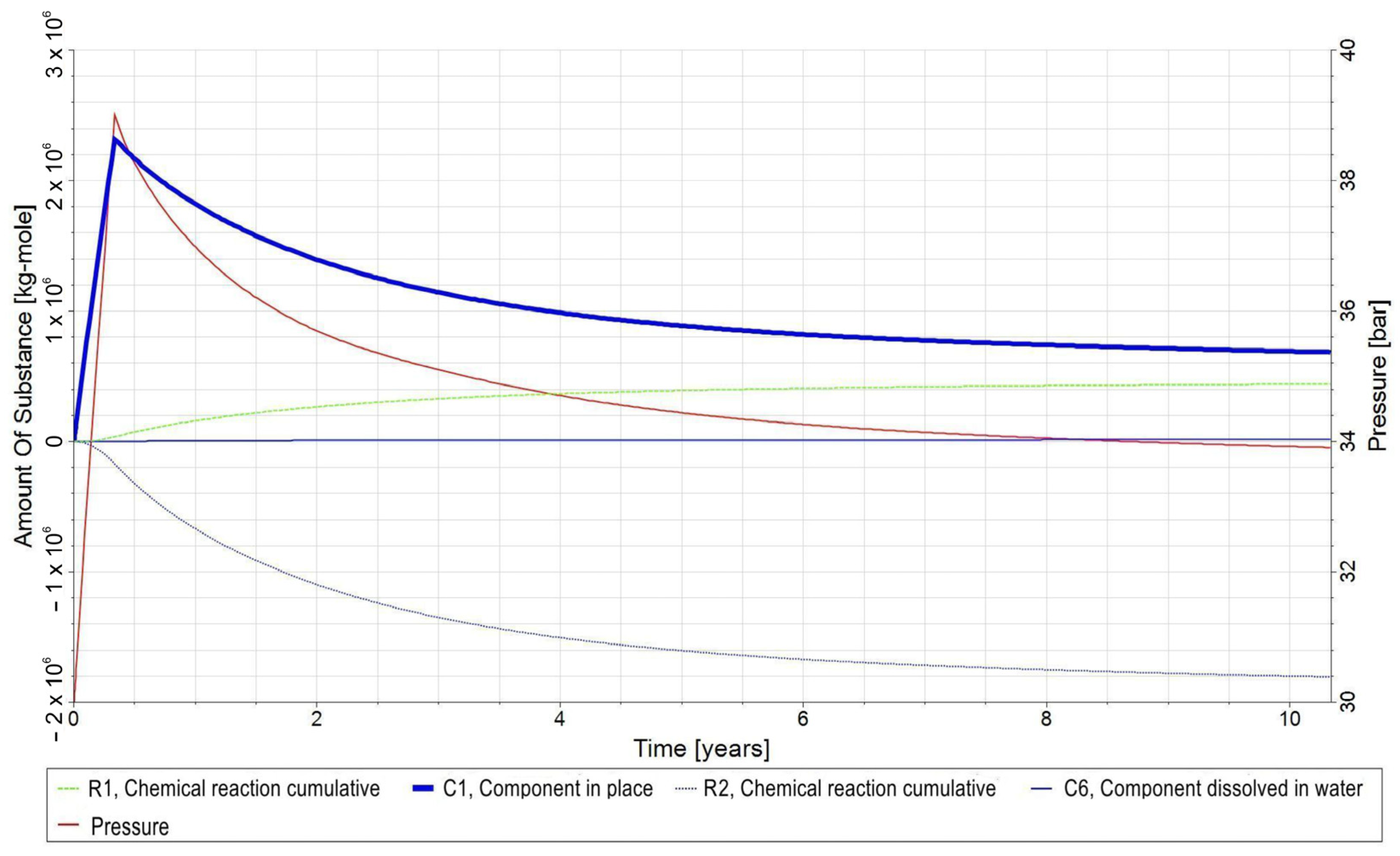
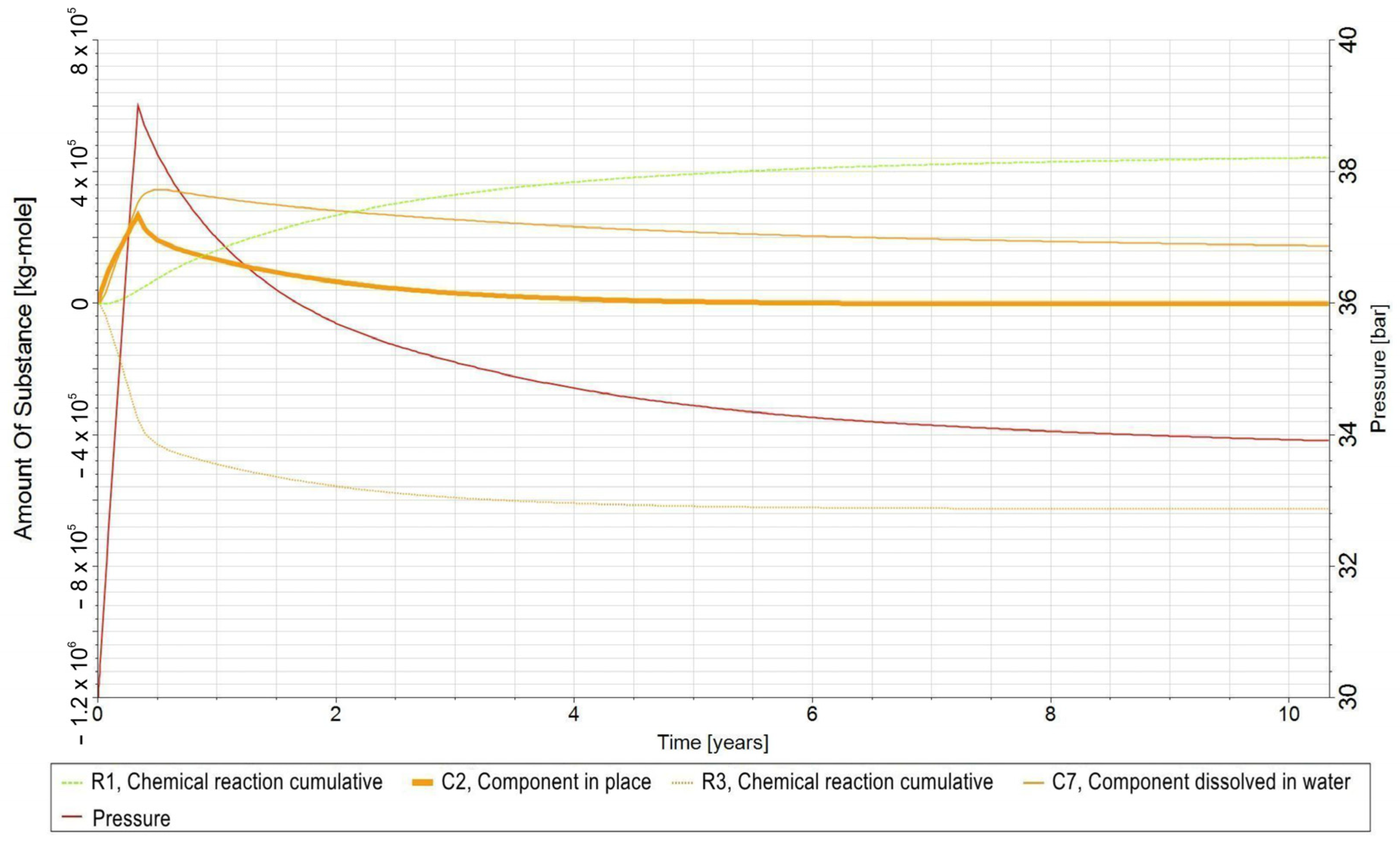
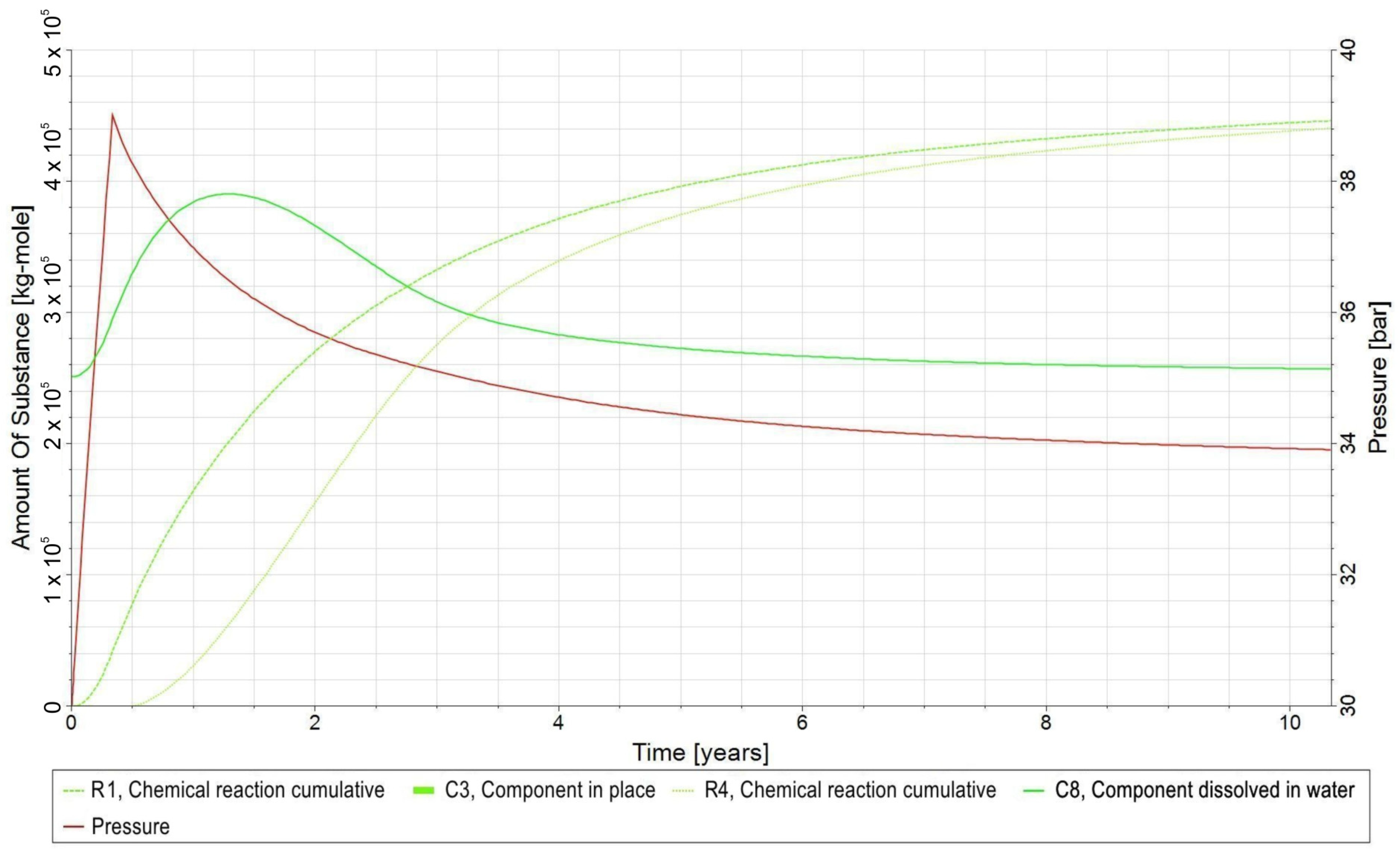
- −
- C1—H2 in gas phase;
- −
- C2—CO2 in the gas phase;
- −
- C3—CH4 in gas phase;
- −
- C4—H2O in the aqueous phase;
- −
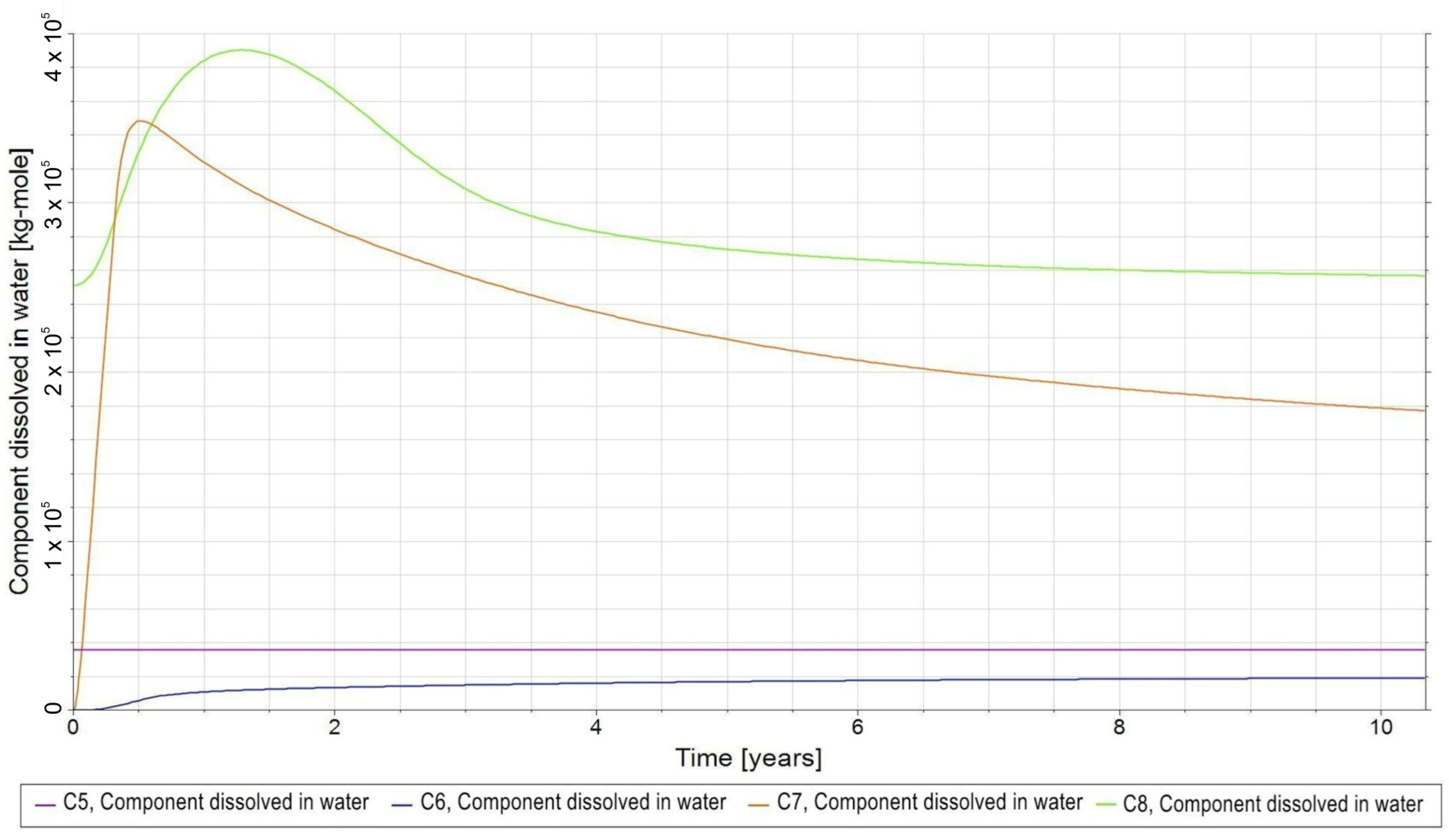
- C5—bacteria in the aqueous phase;
- −
- C6—H2,DS in the aqueous phase;
- −
- C7—CO2,DS in the aqueous phase;
- −
- C8—CH4,DS in the aqueous phase;
- −
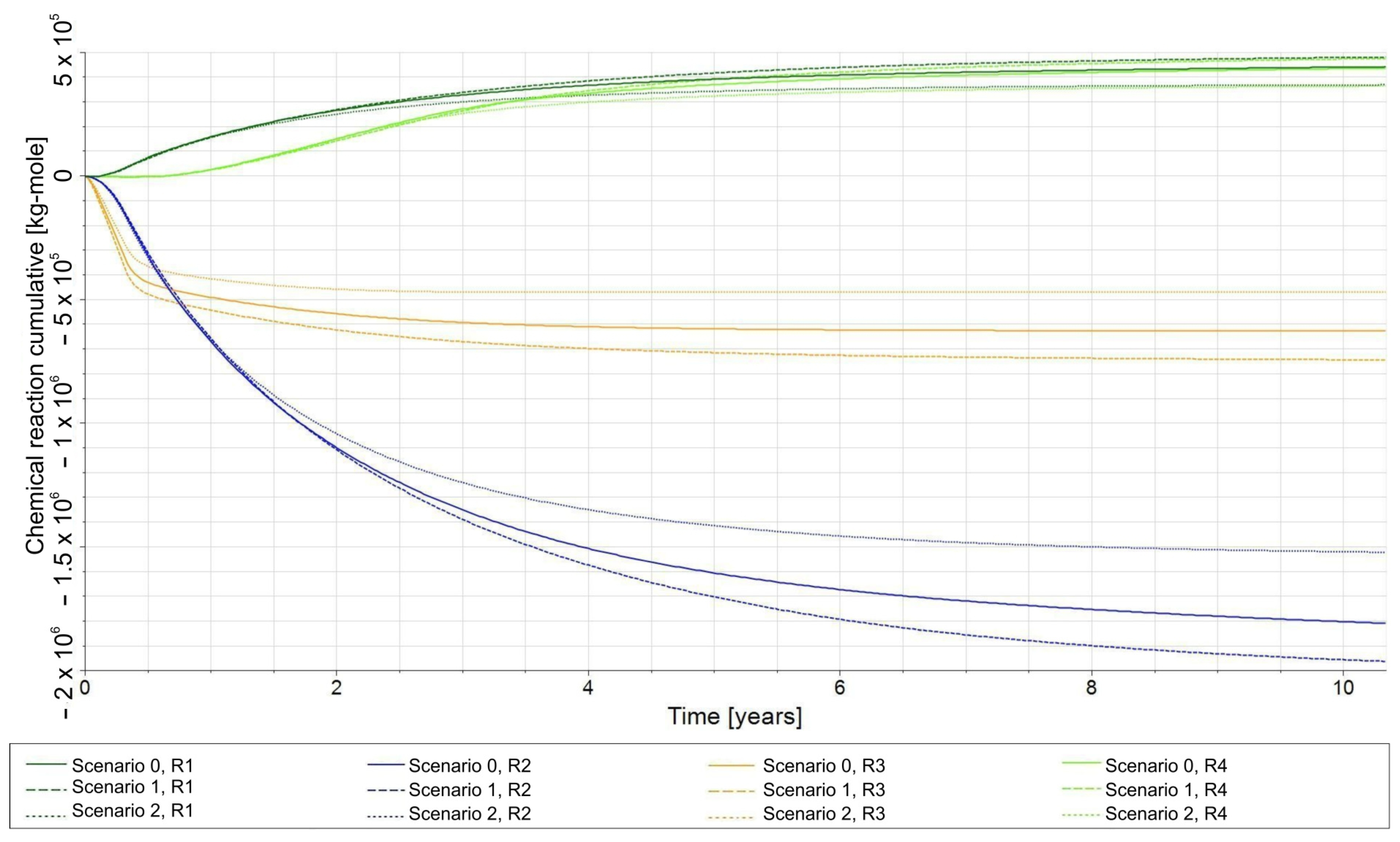
- R1—biomethanation reaction;
- −
- R2—the reaction of H2 dissolving in water and its release from water into the gas phase;
- −
- R3—the reaction of CO2 dissolving in water and its release from water into the gas phase;
- −
- R4—the reaction of dissolution of CH4 in water and its release from water into the gas phase.
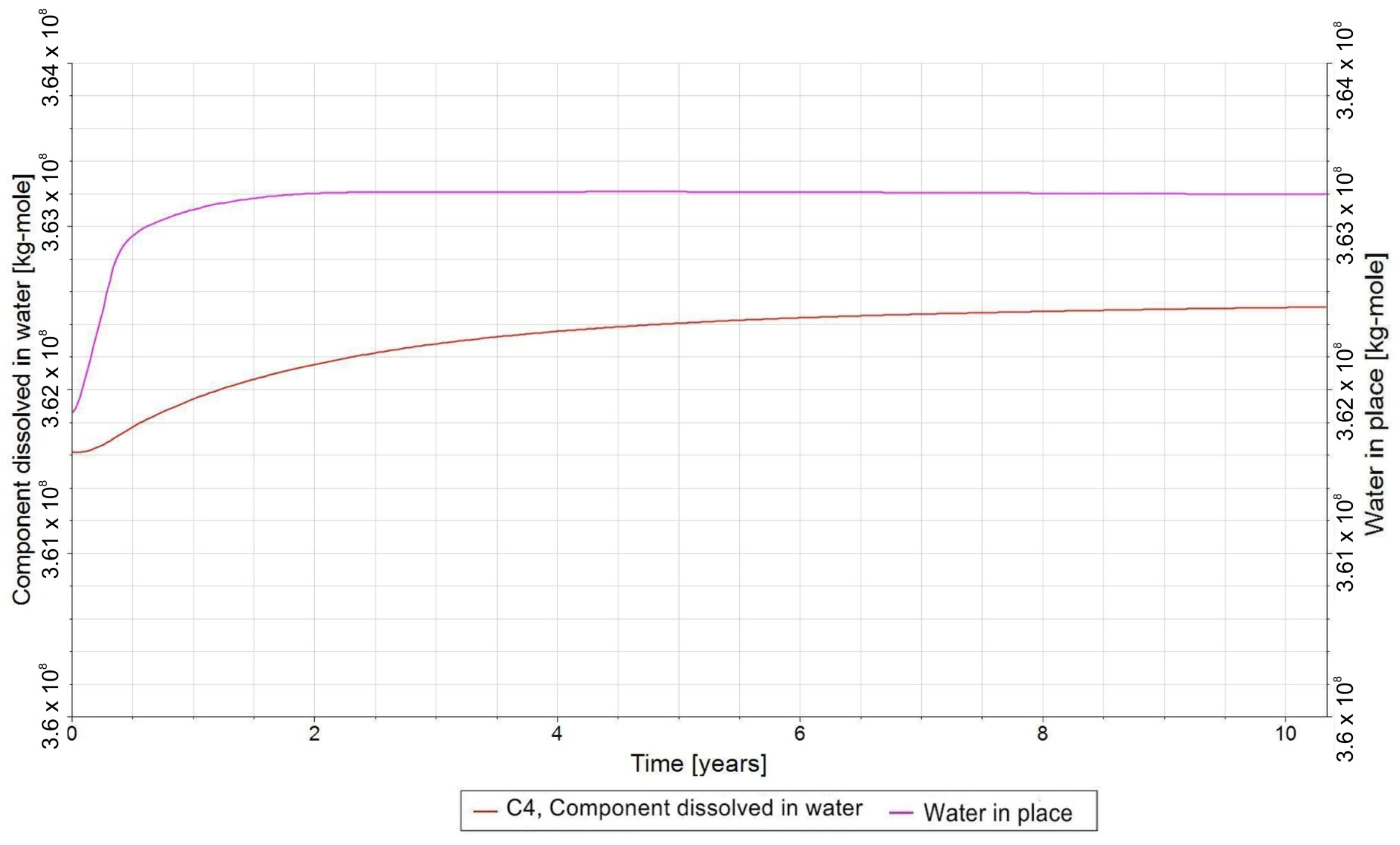
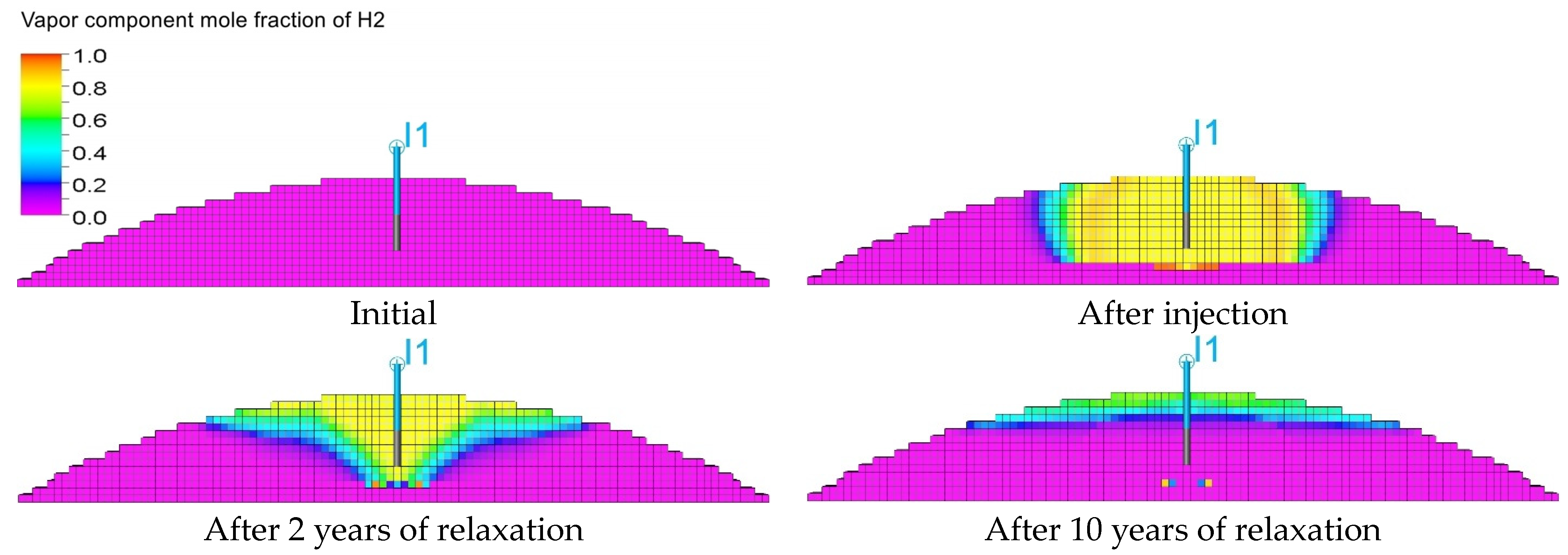
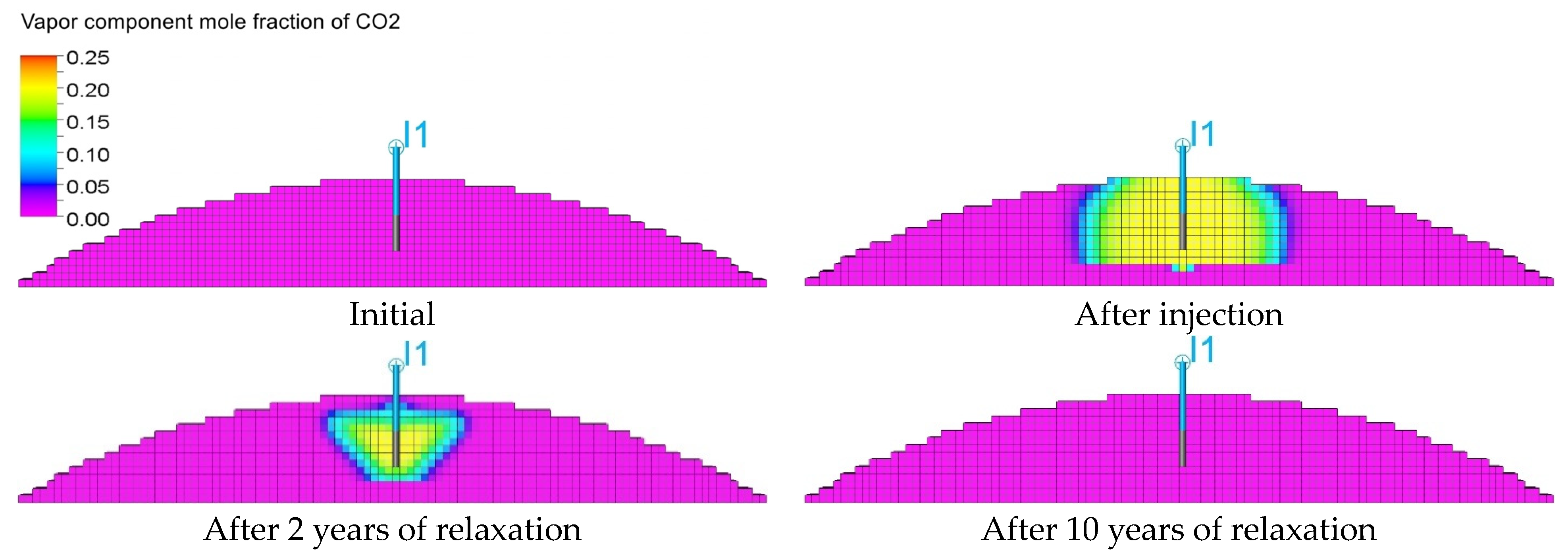
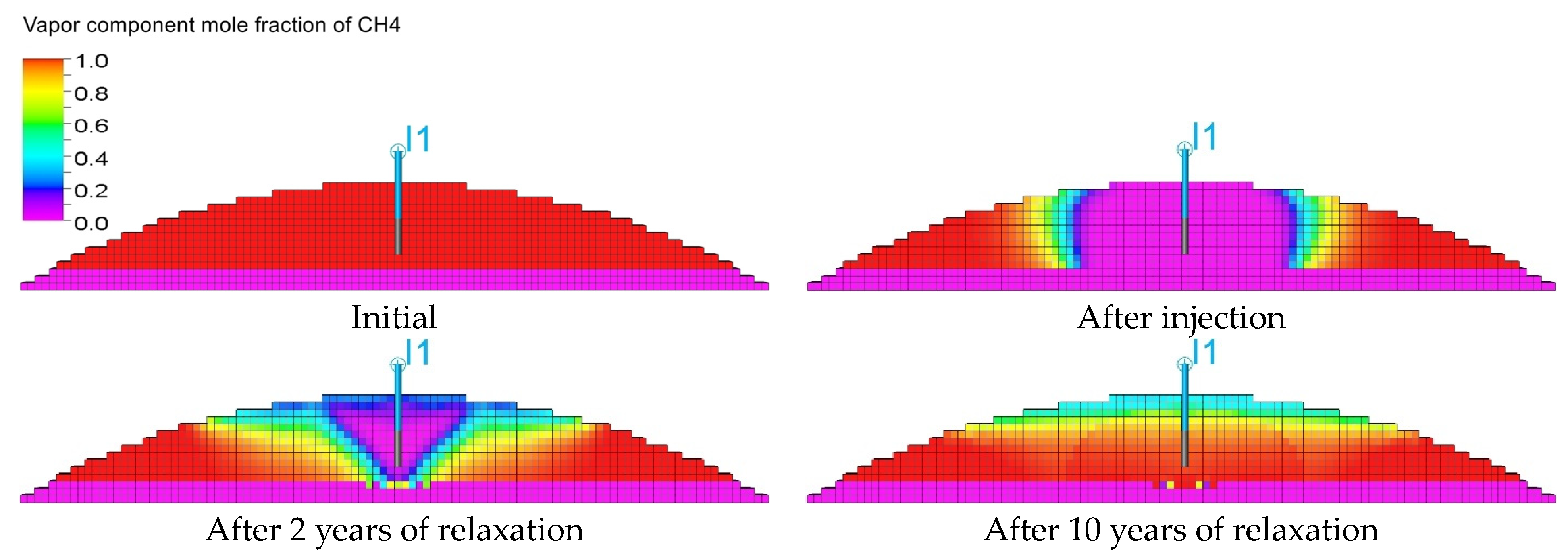
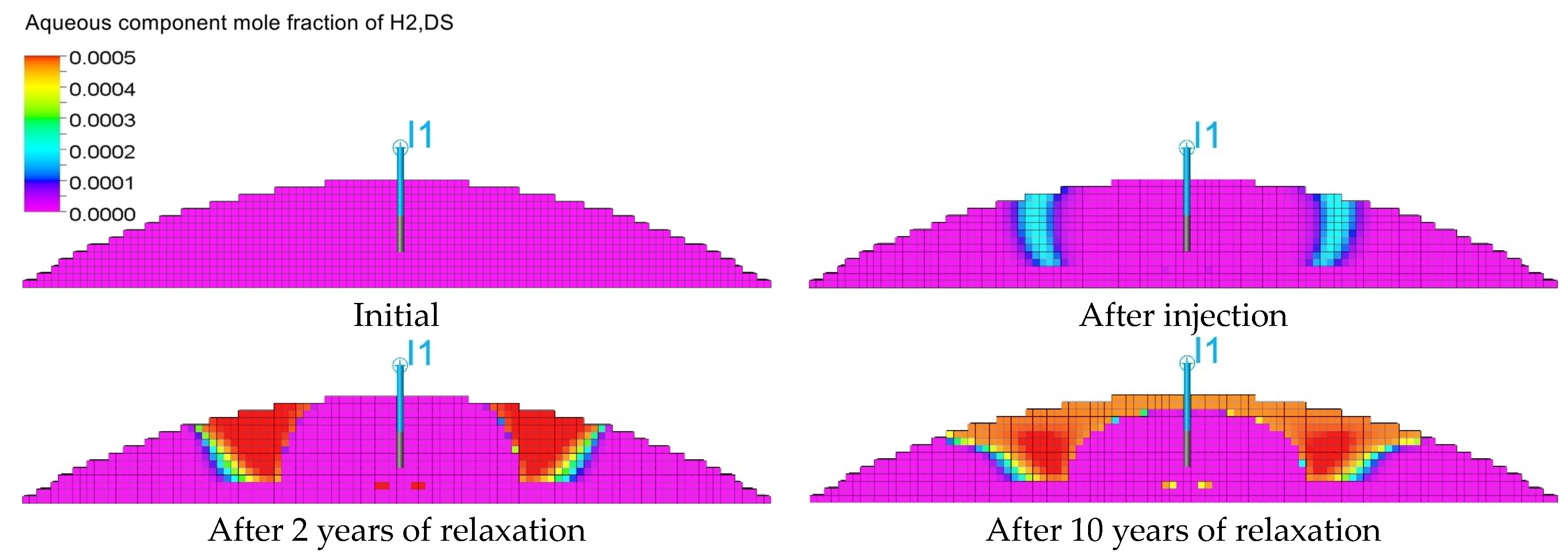
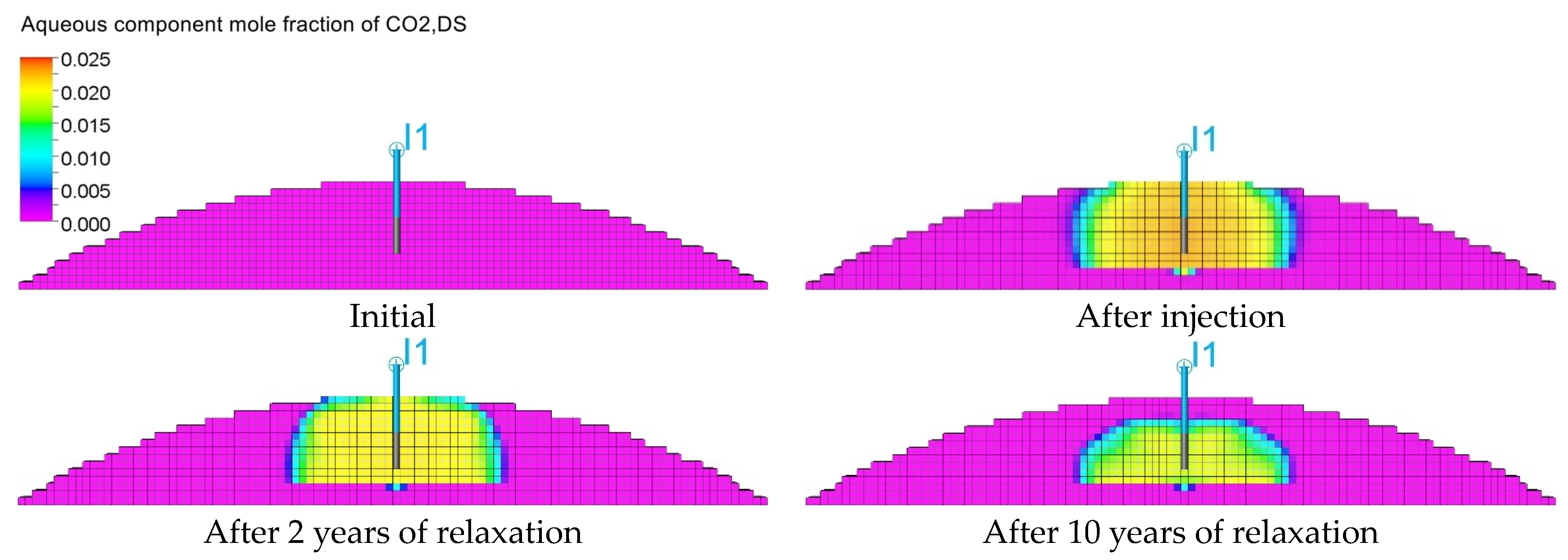
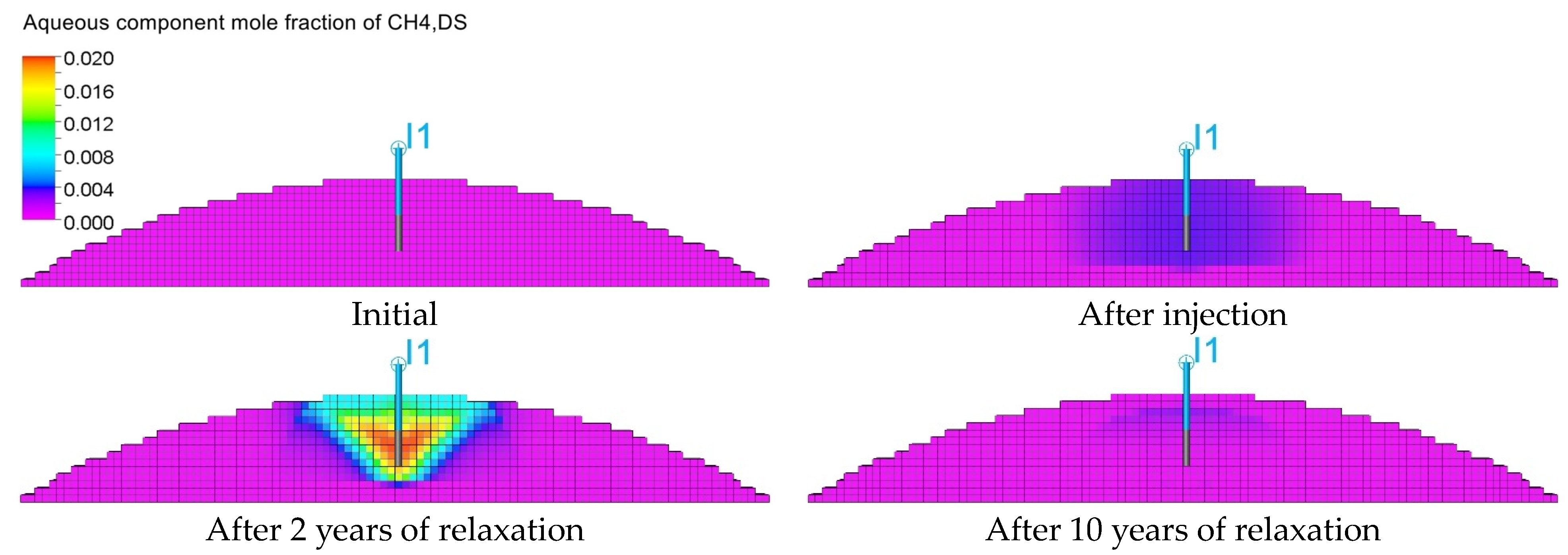
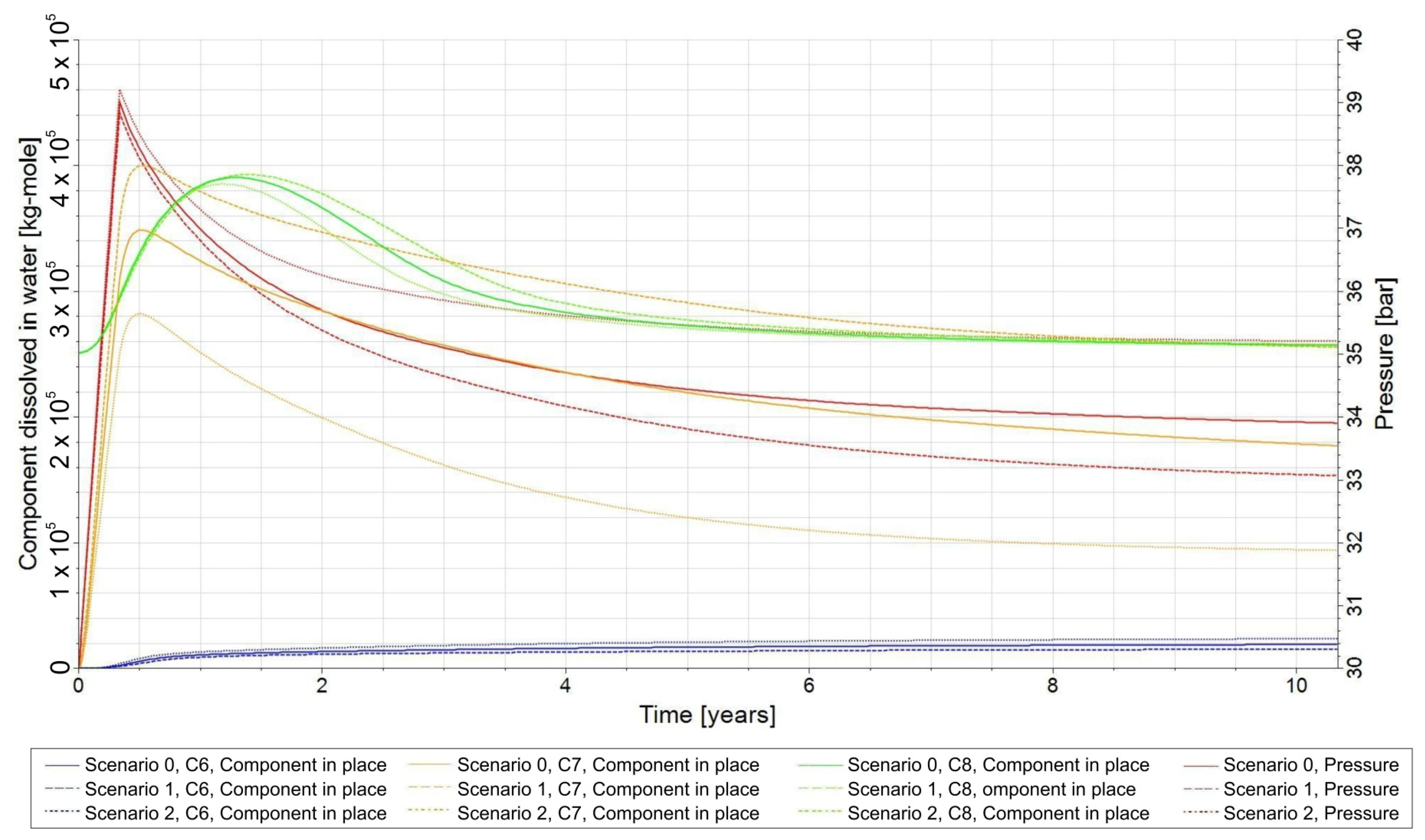
3.3. Qualitative Analysis of the Content Distributions of Selected Fluid Components
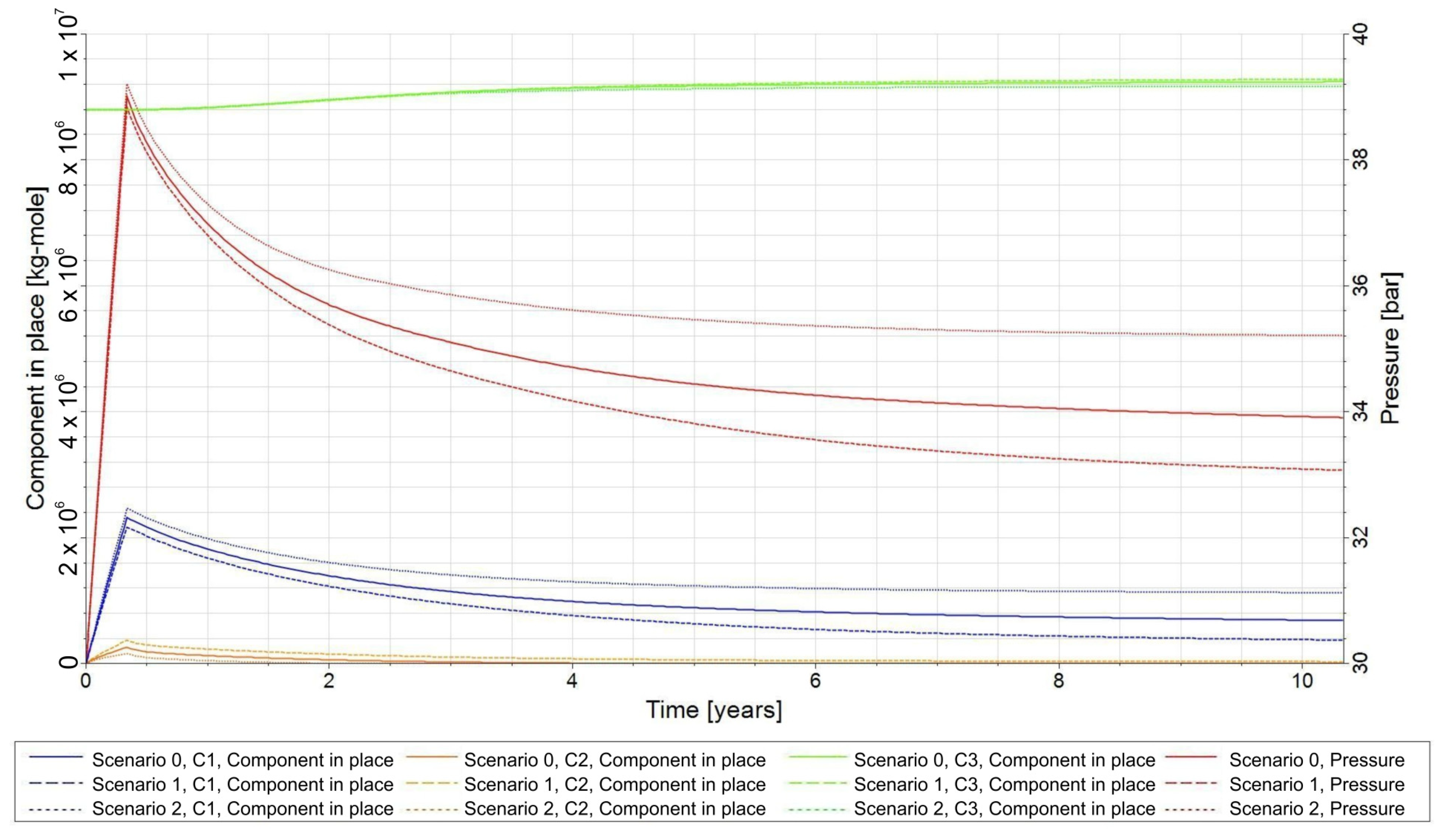
3.4. Influence of the Composition of the Injected Gas on the Phenomenon of Biomethanation
- −
- Scenario 0—CO2/H2 = 20:80;
- −
- Scenario 1—CO2/H2 = 25:75;
- −
- Scenario 2—CO2/H2 = 15:85.
4. Discussion
5. Conclusions
- This study confirmed the practical applicability of the commercial Eclipse reservoir simulator for modelling underground biomethanation (UMR).
- The solubility of gas components was identified as a key factor influencing the biomethanation process, particularly the low solubility of H2, which limits the efficiency of this process. Additionally, the biomethanation processes significantly affect the reservoir pressure, which in turn impacts the solubility of gases in formation water.
- For long-term relaxation, gravitational segregation plays a crucial role in the distribution of selected gas components.
- The analysis of CO2, H2, and CH4 distributions in both gaseous and aqueous phases indicates that process optimization could be achieved not only through the composition of injected gas but also by optimizing well placement and injection strategies.
- The composition of injected gas significantly affects CH4 production and H2 conversion efficiency, which reached up to 83% for the analysed scenarios. However, after a single injection cycle, the CH4 yield remained low, underscoring the need for process optimization and multi-cycle studies.
- The proposed workflow represents a methodological framework rather than a field-specific case study. However, it can be extended in future work for a real reservoir and coupled with a more advanced microbial growth model (e.g., Monod model).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| C1 | H2 in gas phase |
| C2 | CO2 in the gas phase |
| C3 | CH4 in gas phase |
| C4 | H2O in the aqueous phase |
| C5 | bacteria in the aqueous phase |
| C6 | H2,DS in the aqueous phase |
| C7 | CO2,DS in the aqueous phase |
| C8 | CH4,DS in the aqueous phase |
| R1 | biomethanation reaction |
| R2 | the reaction of H2 dissolving in water and its release from water into the gas phase |
| R3 | the reaction of CO2 dissolving in water and its release from water into the gas phase |
| R4 | the reaction of dissolution of CH4 in water and its release from water into the gas phase |
References
- Dorigoni, S.; Anzalone, G.A. Production of Energy from Renewable Sources and Financial Performance of European Utilities: A Panel-Data Analysis. Energy Policy 2024, 194, 114323. [Google Scholar] [CrossRef]
- Miłek, D.; Nowak, P.; Latosińska, J. The Development of Renewable Energy Sources in the European Union in the Light of the European Green Deal. Energies 2022, 15, 5576. [Google Scholar] [CrossRef]
- Miziołek, M.; Filar, B.; Kwilosz, T. Hydrogen Storage in Depleted Natural Gas Fields. Nafta-Gaz 2022, 2022, 219–239. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2024; IEA: Paris, France, 2024. [Google Scholar]
- Filar, B.; Miziołek, M.; Kwilosz, T. Assessment of Hydrogen Production Costs with the Use of Energy from a Photovoltaic Installation Build in Poland. Nafta-Gaz 2022, 2022, 451–459. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Miocic, J.M.; Hangx, S.J.T.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.J.; Schmidt-Hattenberger, C.; et al. Enabling Large-Scale Hydrogen Storage in Porous Media-the Scientific Challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Mouli-Castillo, J.; Heinemann, N.; Edlmann, K. Mapping Geological Hydrogen Storage Capacity and Regional Heating Demands: An Applied UK Case Study. Appl. Energy 2021, 283, 116348. [Google Scholar] [CrossRef]
- Tarkowski, R. Wybrane Aspekty Podziemnego Magazynowania Wodoru. Przegląd Geol. 2017, 65, 282–291. [Google Scholar]
- Such, P. Magazynowanie Wodoru w Obiektach Geologicznych. Nafta-Gaz 2020, 76, 794–798. [Google Scholar] [CrossRef]
- Jaworski, J.; Kułaga, P.; Kukulska-Zając, E. Wybrane Zagadnienia Dotyczące Wpływu Dodatku Wodoru Do Gazu Ziemnego Na Elementy Systemu Gazowniczego. Nafta-Gaz 2019, 75, 625–632. [Google Scholar] [CrossRef]
- Ciechanowska, M. Hydrogen Strategy for a Climate-Neutral Europe. Nafta-Gaz 2020, 2020, 951–954. [Google Scholar] [CrossRef]
- Mielich, T.; Dobler, R.; Hollnagel, J.; Akca, O.; Müller-Kirchenbauer, J. Europe’s Way from Natural Gas to Green Hydrogen: Modeling and Simulation of the Transforming European Gas Transport Infrastructure. Int. J. Hydrogen Energy 2025, 135, 156–171. [Google Scholar] [CrossRef]
- Siekierski, M.; Majewska, K.; Mroczkowska-Szerszeń, M. Metody Efektywnego i Bezpiecznego Magazynowania Wodoru Jako Warunek Powszechnego Jego Wykorzystania w Transporcie i Energetyce. Nafta-Gaz 2023, 79, 114–130. [Google Scholar] [CrossRef]
- Stopa, J.; Stanislaw, R.; Kosowski, P. The Role of Salt Caverns in Underground Gas Storage. Miner. Resour. Manag. 2008, 24, 11–23. [Google Scholar]
- Filar, B.; Miziołek, M.; Cicha-Szot, R.; Moska, A.; Kwilosz, T.; Szpunar, T. Analysis of the Possibility of Increasing the Working Capacity of Underground Gas Storage Facilities in Poland. Nafta-Gaz 2024, 80, 571–580. [Google Scholar] [CrossRef]
- Kaliski, M.; Janusz, P.; Szurlej, A. NAFTA-GAZ Maj 2010 ROK LXVI. Nafta-Gaz 2010, 66, 325–332. [Google Scholar]
- Ahmadi, P.; Chapoy, A. CO2 Solubility in Formation Water under Sequestration Conditions. Fluid. Phase Equilib. 2018, 463, 80–90. [Google Scholar] [CrossRef]
- Londe, L. Underground Storage of Hydrocarbons: Advantages, Lessons Learnt and Way Forward. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar]
- Such, P. Sekwestracja CO2 w Polsce Nie Ma Sensu?! Nafta-Gaz 2020, 76, 913–918. [Google Scholar] [CrossRef]
- Szott, W.; Miłek, K. Numerical Procedure to Effectively Assess Sequestration Capacity of Geological Structures. Nafta-Gaz 2021, 77, 783–794. [Google Scholar] [CrossRef]
- Terrien, P.; Lockwood, F.; Granados, L.; Morel, T. CO2 Capture from H2 Plants: Implementation for EOR. Energy Procedia 2014, 63, 7861–7866. [Google Scholar] [CrossRef]
- Pérez, A.; Pérez, E.; Dupraz, S.; Bolcich, J. Patagonia Wind-Hydrogen Project: Underground Storage and Methanation. In Proceedings of the 21st World Hydrogen Energy Conference 2016, Zaragoza, Spain, 13–16 June 2016. [Google Scholar]
- Cwicklinski, G.; Miras, R.; Pérard, J.; Rinaldi, C.; Darrouzet, E.; Cavazza, C. Development of a CO2-Biomethanation Reactor for Producing Methane from Green H2. Sustain. Energy Fuels 2024, 8, 1068–1076. [Google Scholar] [CrossRef]
- Heinemann, N.; Bünger, U.; Götz, R.; Hagemann, B.; Heinemann, T.; Henkel, S.; Kühn, M.; Schuppers, M. Hydrogen Underground Storage—Petrophysical and Geochemical Characteristics of Potential Storage Formations; Karlsruhe Institute of Technology (KIT): Karlsruhe, Germany, 2018; Available online: https://publikationen.bibliothek.kit.edu/1000094302 (accessed on 6 October 2025).
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground Bio-Methanation: Concept and Potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Hellerschmied, C.; Schritter, J.; Waldmann, N.; Zaduryan, A.B.; Rachbauer, L.; Scherr, K.E.; Andiappan, A.; Bauer, S.; Pichler, M.; Loibner, A.P. Hydrogen Storage and Geo-Methanation in a Depleted Underground Hydrocarbon Reservoir. Nat. Energy 2024, 9, 333–344. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A Technological and Economic Review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Espatolero, S.; Romeo, L.M. Power to Gas Technology under Spanish Future Energy Scenario. Energy Procedia 2017, 114, 6880–6885. [Google Scholar] [CrossRef]
- Bauer, S. Underground Sun Conversion; RAG Austria AG: Vienna, Austria, January 2021; Available online: https://www.underground-sun-conversion.at/en/ (accessed on 8 October 2025).
- Efenberger, M.; Brzezińska-Blaszczyk, E.; Wódz, K. Archaeons—Still Unknown Microorganisms. Postep. Hig. Med. Dosw. 2014, 68, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Wolicka, D.; Borkowski, A. Introduction to Enhanced Oil Recovery (EOR) Processes and Bioremediation of Oil-Contaminated Sites. In Microorganisms and Crude Oil; InTech: London, UK, 2012. [Google Scholar]
- Smigan, P.; Greksak, M.; Kozankova, J.; Buzek, F.; Onderka, V.; Wolf, I. Methanogenic Bacteria as a Key Factor Involved in Changes of Town Gas Stored in an Underground Reservoir. FEMS Microbiol. Ecol. 1990, 73, 221–224. [Google Scholar] [CrossRef]
- Moeininia, N.; Bueltemeier, H.; Krasper, L.; Nikolaev, D. Development of a New Workflow to Underground Biomethanation Simulation Using CMG STARS. In Proceedings of the World Gas Conference 2022 (WGC2022), Dageu, Republic of Korea, 23 May 2022. [Google Scholar]
- Panfilov, M.; Reitenbach, V.; Ganzer, L. Self-Organization and Shock Waves in Underground Methanation Reactors and Hydrogen Storages. Environ. Earth Sci. 2016, 75, 313. [Google Scholar] [CrossRef]
- Pudlo, D.; Ganzer, L.; Henkel, S.; Kühn, M.; Liebscher, A.; De Lucia, M.; Panfilov, M.; Pilz, P.; Reitenbach, V.; Albrecht, D.; et al. The H2STORE Project: Hydrogen Underground Storage—A Feasible Way in Storing Electrical Power in Geological Media? In Clean Energy Systems in the Subsurface: Production, Storage and Conversion; Springer Series in Geomechanics and Geoengineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 395–412. [Google Scholar]
- Bauer, S. Undeground Sun Storage—Final Report; RAG Austria AG: Vienna, Austria, 2020; Available online: https://www.underground-sun-storage.at/en/ (accessed on 8 October 2025).
- Konegger, H.; Loibner, A.; Waldmann, N.; Zaduryan, A.; Zeiser, M.; Rüdisüli, M.; Mutschler, R.; Sidler, D.; Boggiano, M.; Kunz, A.; et al. Underground Sun Conversion-Flexible Storage (USC-Flex Store); RAG Austria: Vienna, Austria, 2023; Available online: https://www.underground-sun-conversion.at/flexstore/presse/publikationen/endbericht.html (accessed on 8 October 2025).
- Panfilov, M. Underground Storage of Hydrogen: In Situ Self-Organisation and Methane Generation. Transp. Porous Media 2010, 85, 841–865. [Google Scholar] [CrossRef]
- Carroll, J.J.; Slupsky, J.D.; Mather, A.E. The Solubility of Carbon Dioxide in Water at Low Pressure. J. Phys. Chem. Ref. Data 1991, 20, 1201–1209. [Google Scholar] [CrossRef]
- Rezania, P.; Ranjbar, V.; Nasrifar, K. High Pressure Solubility of Light Gases (CH4, C2H6, C3H8, H2S, CO2, N2, Xe, Ar and Kr) and Certain Gas Mixtures in Water from Cubic Equations of State. J. Solut. Chem. 2021, 50, 1169–1188. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, Y.; Zheng, Z.; Chen, D. An Accurate Model for Estimating H2 Solubility in Pure Water and Aqueous NaCl Solutions. Energies 2022, 15, 5021. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; He, Z.; Fan, Q. Solubility Models of CH4, CO2, and Noble Gases and Their Geological Applications. J. Nat. Gas Geosci. 2023, 8, 337–347. [Google Scholar] [CrossRef]
- Hogeweg, S.; Strobel, G.; Hagemann, B. Benchmark Study for the Simulation of Underground Hydrogen Storage Operations. Comput. Geosci. 2022, 26, 1367–1378. [Google Scholar] [CrossRef]
- Wu, L.; Hou, Z.M.; Luo, Z.F.; Fang, Y.L.; Huang, L.C.; Wu, X.N.; Chen, Q.J.; Wang, Q.C. Impacts of Microbial Interactions on Underground Hydrogen Storage in Porous Media: A Comprehensive Review of Experimental, Numerical, and Field Studies. Pet. Sci. 2024, 21, 4067–4099. [Google Scholar] [CrossRef]
- Shabani, B.; Pashin, J.; Vilcáez, J. TOUGHREACT-CO2Bio—A New Module to Simulate Geological Carbon Storage under Biotic Conditions (Part 2): The Bio-Geochemical Reactive Transport of CO2-CH4-H2-H2S Gas Mixtures. J. Nat. Gas. Sci. Eng. 2020, 76, 103190. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. The Gompertz Model and Its Applications in Microbial Growth and Bioproduction Kinetics: Past, Present and Future. Biotechnol. Adv. 2024, 72, 108335. [Google Scholar] [CrossRef]
- Safari, Z.; Fatehi, R.; Azin, R. Developing a Numerical Model for Microbial Methanation in a Depleted Hydrocarbon Reservoir. Renew. Energy 2024, 227, 120426. [Google Scholar] [CrossRef]
- Wu, L.; Hou, Z.; Luo, Z.; Huang, L.; Xiong, Y.; Mehmood, F.; Liu, J.; Sun, W.; Xie, Y. Efficiency Assessment of Underground Biomethanation with Hydrogen and Carbon Dioxide in Depleted Gas Reservoirs: A Biogeochemical Simulation. Energy 2023, 283, 128539. [Google Scholar] [CrossRef]
- Szott, W.; Miłek, K. Numerical Simulations of Hydrogen Storage in a Partially Depleted Gas Reservoir. Nafta-Gaz 2022, 78, 41–55. [Google Scholar] [CrossRef]
- Feldmann, F.; Hagemann, B.; Ganzer, L.; Panfilov, M. Numerical Simulation of Hydrodynamic and Gas Mixing Processes in Underground Hydrogen Storages. Environ. Earth Sci. 2016, 75, 1165. [Google Scholar] [CrossRef]
- Schlumberger. Internal Technical Note: Simulation of Methanisation Process Using Multi-Component Water (Isothermal); Schlumberger: Houston, TX, USA, 2024; Unpublished Material. [Google Scholar]
- Wu, J.; Ansari, U. From CO2 Sequestration to Hydrogen Storage: Further Utilization of Depleted Gas Reservoirs. Reserv. Sci. 2025, 1, 19–35. [Google Scholar] [CrossRef]
- Wang, F.; Kobina, F. The Influence of Geological Factors and Transmission Fluids on the Exploitation of Reservoir Geothermal Resources: Factor Discussion and Mechanism Analysis. Reserv. Sci. 2025, 1, 3–18. [Google Scholar] [CrossRef]
- Panfilov, M. Physicochemical Fluid Dynamics in Porous Media; Wiley: Hoboken, NJ, USA, 2018; ISBN 9783527342358. [Google Scholar]
- Miłek, K.; Szott, W.; Tyburcy, J.; Lew, A. Reservoir Simulations of Hydrogen Generation from Natural Gas with CO2 EOR: A Case Study. Energies 2024, 17, 2321. [Google Scholar] [CrossRef]
- Yao, C.; Meng, X.; Qu, X.; Cheng, T.; Da, Q.; Zhang, K.; Lei, G. Kinetic Model and Numerical Simulation of Microbial Growth, Migration, and Oil Displacement in Reservoir Porous Media. ACS Omega 2022, 7, 32549–32561. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger. ECLIPSE Technical Description, version 2019.1; Schlumberger Ltd.: Houston, TX, USA, 2019. [Google Scholar]
| Component | Molecular Weight | Tcrit | Pcrit | Vcrit | Zcrit | Vol Shift | Acentric Factor | Parachor | Omega A | Omega B |
|---|---|---|---|---|---|---|---|---|---|---|
| [kg/kmole] | [K] | [Bar] | [m3/kg-Moles] | [-] | [-] | [-] | [Dyne/cm] | [-] | [-] | |
| H2 | 2.02 | 33.20 | 13.00 | 0.065 | 0.3061 | 0 | −0.218 | 34 | 0.4275 | 0.0866 |
| CO2 | 44.01 | 304.70 | 73.87 | 0.094 | 0.2741 | 0 | 0.225 | 78 | 0.4275 | 0.0866 |
| CH4 | 16.04 | 190.60 | 46.04 | 0.098 | 0.2847 | 0 | 0.013 | 77 | 0.4275 | 0.0866 |
| Component | Molecular Weight | Compressibility | Density |
|---|---|---|---|
| [kg/kmole] | [1/Bar] | [kg/m3] | |
| H2O | 18.02 | 4.74 × 10−7 | 1004.8 |
| Bact | 10 | 4.74 × 10−7 | 1004.8 |
| H2,DS | 2.02 | 4.74 × 10−7 | 1004.8 |
| CO2,DS | 44.01 | 4.74 × 10−7 | 1004.8 |
| CH4,DS | 16.04 | 4.74 × 10−7 | 1004.8 |
| Component | Molar Composition [%] |
|---|---|
| pure H2O | 99.9205 |
| bacteria | 0.01 |
| H2,DS | <0.01 |
| CO2,DS | <0.01 |
| CH4,DS | 0.0695 |
| Size | Scenario | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Initial amount of CH4 in gas | 8,805,000 | 8,805,000 | 8,805,000 |
| Amount of injected H2 [kmol] | 2,497,001 | 2,340,938 | 2,653,063 |
| Amount of CO2 injected [kmol] | 624,250 | 780,313 | 468,188 |
| Amount of dissolved H2,DS in water [kmol] | 1,806,232 | 1,959,229 | 1,519,725 |
| Amount of CO2,DS dissolved in water [kmol] | 624,290 | 741,657 | 468,277 |
| Amount of CH4,DS produced [kmol] | 446,643 | 485,839 | 373,867 |
| Conversion efficiency of H2 to CH4 [%] | 71.55 | 83.02 | 56.37 |
| Amount of CH4,DS released from water [kmol] | 440,836 | 479,814 | 368,743 |
| Relative rise of CH4 in gas phase [%] | 5.01 | 5.45 | 4.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, K.; Szott, W.; Stopa, J. Numerical Modelling of Biomethanation in UHS/UMR: The Role of Gas Solubility in Long-Term Dynamics. Energies 2025, 18, 5661. https://doi.org/10.3390/en18215661
Miłek K, Szott W, Stopa J. Numerical Modelling of Biomethanation in UHS/UMR: The Role of Gas Solubility in Long-Term Dynamics. Energies. 2025; 18(21):5661. https://doi.org/10.3390/en18215661
Chicago/Turabian StyleMiłek, Krzysztof, Wiesław Szott, and Jerzy Stopa. 2025. "Numerical Modelling of Biomethanation in UHS/UMR: The Role of Gas Solubility in Long-Term Dynamics" Energies 18, no. 21: 5661. https://doi.org/10.3390/en18215661
APA StyleMiłek, K., Szott, W., & Stopa, J. (2025). Numerical Modelling of Biomethanation in UHS/UMR: The Role of Gas Solubility in Long-Term Dynamics. Energies, 18(21), 5661. https://doi.org/10.3390/en18215661







