Abstract
This work introduces a novel approach to enhancing the performance of zinc anodes in zinc–air batteries through a photopolymerizable organic–inorganic hybrid coating. Electrochemical tests were conducted in a neutral NaCl electrolyte, selected to minimize electrolyte carbonation, anode corrosion, and zinc dendrite formation. The behavior of bare and coated zinc electrodes was investigated using linear sweep voltammetry, electrochemical impedance spectroscopy (EIS), potentiostatic measurements, galvanostatic discharge tests, and charge-discharge tests, while morphological and structural characterizations were carried out by Atomic Force Microscopy (AFM), Raman spectroscopy, and X-ray Diffraction (XRD). The results confirmed that the hybrid coating acts as a corrosion-resistant barrier, enhancing the reversibility and stability of zinc electrodes through a barrier mechanism. Charge–discharge tests further confirmed the improved performance of the coated electrode, obtaining at a current density of 1 mA/cm2, a coulombic efficiency of 92.61% and a capacity retention of 90.18%, respectively, after 16 cycles. These findings highlight the effectiveness of the photopolymerizable hybrid coating in improving the durability and rechargeability of zinc–air batteries.
1. Introduction
Rechargeable batteries operate as electrochemical energy storage systems by transforming chemical energy into electrical energy through oxidation-reduction reactions occurring between the anode and the cathode [1,2]. Among various rechargeable batteries, metal–air batteries (MABs) have gained attention as promising systems because of their cost-effective anode materials, such as aluminum, zinc, and iron, and atmospheric oxygen as the cathodic reactant. This configuration reduces the overall cost and significantly decreases battery weight [3,4,5,6]. Zinc-air batteries (ZABs), in particular, have gained widespread attention. Zinc offers a high theoretical specific capacity (820 mAh g−1 and 5855 Ah L−1) and a low electrochemical potential (−0.762 V vs. SHE), making ZABs well-suited for large-scale applications. Their further advantages include high energy density, natural abundance, low toxicity, affordability, excellent safety, long shelf life (under dry conditions), environmental friendliness, and stable performance across a range of temperatures and loads [7,8,9,10,11,12,13,14].
Despite these benefits, ZABs face several challenges: limited power output, irreversibility of redox reactions at the air electrode, dendrite formation, hydrogen evolution due to anode corrosion, sluggish oxygen reduction kinetics, narrow temperature operating ranges, carbonation of alkaline electrolytes, and high overpotentials at the air cathode [10,11,12,15].
Some of these issues are mainly related to alkaline Zn-air batteries, where the high concentration of OH− ions promotes dendrite growth, air cathode corrosion, and electrolyte carbonation. Neutral electrolytes have been proposed as an alternative to overcome these limitations. Lower OH− concentrations help suppress carbonation, reduce electrode corrosion, and inhibit dendrite formation, thereby extending battery cycle life. Neutral or near-neutral electrolytes also offer enhanced safety and non-toxicity, making them attractive for practical ZAB applications [16,17,18].
The use of chloride-based neutral electrolytes was first introduced in 1973 for primary ZABs [19], and later extended to rechargeable systems in 2012 [17,20]. Since then, Cl−-based neutral electrolytes have been widely studied [16,20,21,22,23]. However, they can induce chlorine evolution reactions, leading to a pH drop in the electrolyte. To mitigate this, NaCl-based solutions have been recommended for better pH control, improved ionic conductivity, and minimized chlorine evolution [18].
Several studies have investigated NaCl-based systems for Zn-air batteries. The performance of zinc and Zn-Al alloy anodes has been examined in NaCl electrolytes [16]; Zinc electrode behavior has been analyzed in pure and EDTA-modified NaCl solutions [17]. Seawater-based electrolytes have been assessed to evaluate the impact of chloride ions on cathode electrocatalytic activity and overall ZAB performance [24]. Despite these advances, challenges remain, and further research is essential to fully realize the potential of NaCl-based neutral electrolytes in Zn-air battery systems [25,26,27].
In addition to electrolyte optimization, considerable research has been devoted to developing interfacial strategies aimed at mitigating the intrinsic challenges of zinc anodes. Among these, surface coatings have emerged as one of the most effective approaches, as they can promote uniform zinc deposition while suppressing undesirable side reactions. Because organic coatings alone are susceptible to fracture after several charge/discharge cycles, organic–inorganic hybrid coatings have shown significant promise. These systems combine the advantageous properties of both organic and inorganic components: the organic phase provides corrosion resistance, flexibility, and excellent barrier properties, whereas the inorganic phase enhances zinc-ion desolvation and facilitates faster ion transport kinetics. Moreover, the use of photopolymerization enables controlled, light-induced curing, resulting in the rapid formation of dense, homogeneous, and defect-free protective layers on the zinc surface. Furthermore, photopolymerization helps address the environmental issues associated with polymers in solutions, as it significantly reduces volatile solvent emissions. This synergistic design not only stabilizes the anode/electrolyte interface but also prolongs the electrochemical stability and cycle life of zinc-based batteries [28,29,30].
This study focused on assessing the role of a photopolymerizable organic-inorganic coating, labelled as “Hybrid”, on the performance of zinc anode material within a Zinc-air battery configuration, all set within a 1 M NaCl solution, to evaluate the potential advantages for use in a rechargeable battery. Its specially formulated, light-curing, solvent-free coating is known for its nanostructured composition and exceptional protective properties. The coating was designed to protect porous substrates, such as natural stone, and due to the proven effectiveness of Hybrid coating in reducing the corrosion rate, its potential as an anti-corrosive top-coating for non-porous substrates, including metals such as pure zinc, is explored, which was the focus of our investigation. Hybrid is characterized by its super-hydrophobic nature, making it an ideal candidate for providing long-lasting protection to the surfaces [31,32]. The choice of a photopolymerizable system is related to the benefits of the photopolymerization process. For several years, it has been well known that light-curing is a valid alternative to traditional thermal curing, as it is a solvent-free technique that is usually carried out at room temperature in a very short time (within a few hours). Consequently, photo-cured polymeric films demonstrate enhanced performance in terms of hydrophobicity, thermal-mechanical properties, chemical resistance and durability compared to coatings produced from conventional polymeric solutions or emulsions [33,34].
In this study, the efficacy of this innovative coating has been evaluated through electrochemical and spectroelectrochemical experiments. Finally, the general discharge performance of the zinc-air battery is carefully assessed through long-term discharge tests and a series of charge/discharge tests at various current densities.
2. Materials and Methods
2.1. Materials
For the electrochemical characterization tests and morphological measurements, the electrodes were plates of 99% pure zinc (E-Nettech, Mumbai, India). For all tests, the samples had an exposed surface area of 1.44 cm2 in contact with the electrolyte, except for the charge and discharge test, where it was 3.30 cm2. To prepare the nearly neutral solution (1 M NaCl, pH 7), analytical grade NaCl (Sigma-Aldrich, Darmstadt, Germany) and distilled water were used. The volume of electrolyte for the electrochemical measurements was 200 mL, and for the charge/discharge test, it was approximately 5 mL.
The coating used in this study is a photopolymerizable product developed by some of the authors as a protective coating for several surfaces. The synthesis procedure and the composition of the hybrid coating are covered by a European patent [35]. The formulation is crosslinkable by solar radiations, according to the invention, contains at least 4–15% by weight of an alkoxy- silane compound, 22–90% by weight of a trimethoxypropyl silane methacrylate and functionalized poly(dimethylsiloxane)-terminated vinyl, 5–60% by weight of a methacrylic resin and 1–5% by weight of a photoinitiator mixture containing at least one UV photoinitiator and at least one photoinitiator that can be activated by visible light. The amounts given in % by weight are referred to the total weight of the organic-inorganic hybrid formulation [35].
The application of the hybrid coating involved the following steps:
(i) Immersion: the bare samples were immersed in the hybrid coating solution. The immersion was total or partial, depending on the specific test to be conducted. The samples were immersed for 6 min, with each face being immersed for approximately 3 min. This step ensured that the hybrid coating covered the entire surface of the samples.
(ii) Exposure to light radiation: after the immersion step, the samples were exposed to light radiation. This exposure was achieved by placing the samples under natural sunlight or using a sunlamp for 10 h. This step was crucial for the light-curing process of the hybrid coating, which enhances its stability and durability.
(iii) Dual-curing: following the light exposure, the samples underwent a dual-curing process. The samples were subjected to heating in an oven at a temperature of 120 °C for 1 h. This step enabled the complete curing and bonding of the hybrid coating to the surface of the samples. The dual-curing process ensured optimal adhesion.
2.2. Electrochemical, Morphological and Spectroscopic Tests
To assess the effectiveness of the hybrid coating on the corrosion resistance of zinc samples, various electrochemical tests were performed at room temperature employing a Parstat 2273 potentiostat/galvanostat (Ametek SI, Oak Ridge, TN, USA) with an integrated Impedance Analyzer linked directly to the cell.
The electrochemical cell used for the electrochemical tests was composed of a conventional three-electrode configuration. The investigated working electrodes were bare and Hybrid-coated zinc samples. A platinized titanium mesh functioned as the counter electrode, with Ag/AgCl (KCl 3 M) as the reference electrode. All measured potentials are referenced to Ag/AgCl. The electrochemical characterization was done by performing open circuit potential (OCP), linear sweep voltammetry (LSV), potentiostatic (PS), electrochemical impedance spectroscopy (EIS), galvanostatic discharge and charge/discharge tests.
OCP was recorded for 30 min, verifying that the voltage fluctuations were less than 5 mV. LSV tests were performed at a scan rate of 1 mV/s. Corrosion current density (icorr) and corrosion potential (Ecorr) were determined from LSV curves by Origin software (version 8.5). The potentiostatic corrosion tests were conducted at a constant potential of −0.8 V for 60 min. The EIS measurements were carried out on bare and Hybrid-coated samples, in the frequency range from 1 MHz to 10 mHz, at OCP with an AC amplitude of 10 mV. To obtain the related corrosion parameters, an equivalent circuit model was applied. Each experiment was conducted in triplicate to guarantee repeatability.
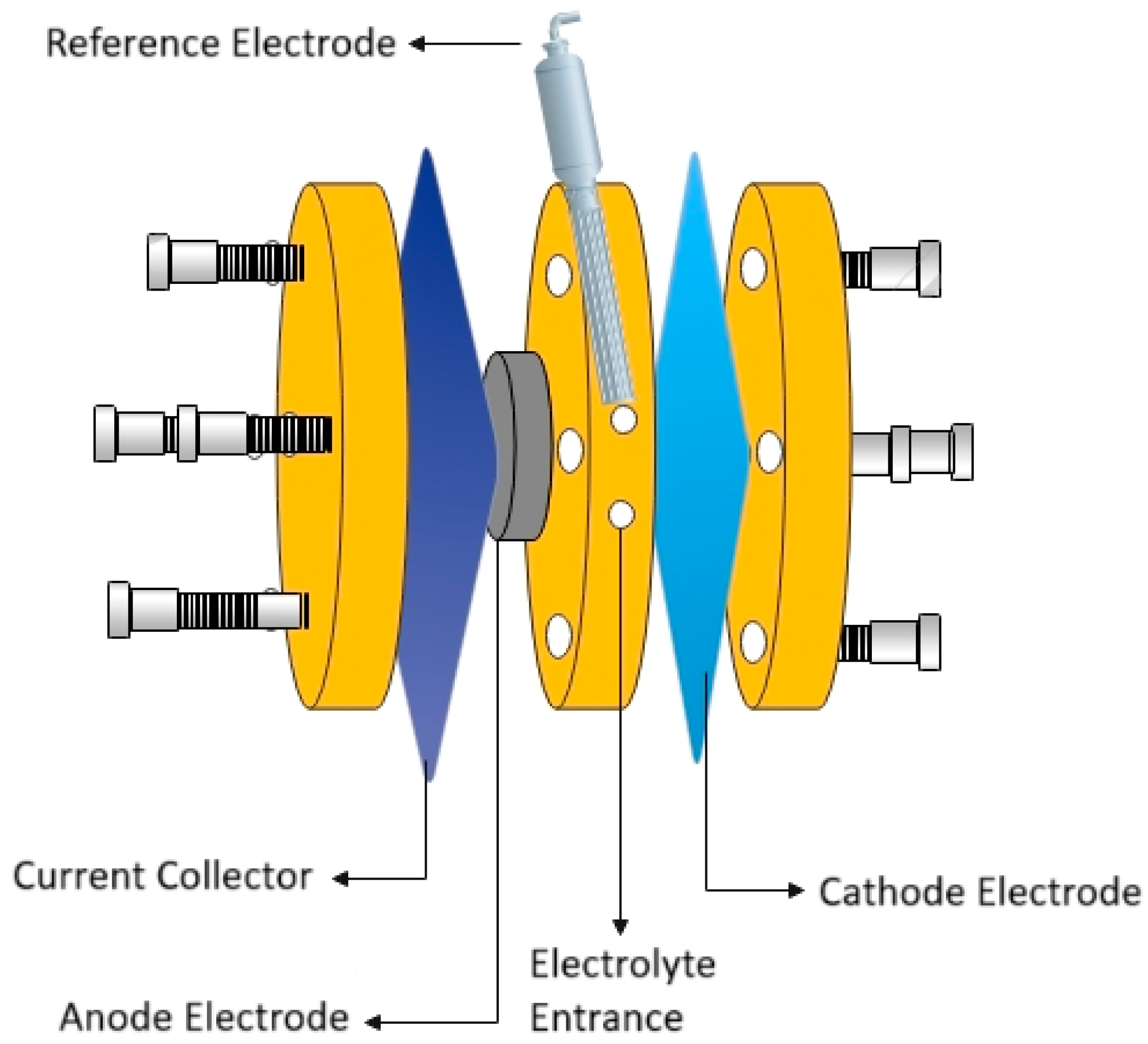
Galvanostatic discharge and charge/discharge tests were performed in a self-fabricated laboratory plane-parallel primary battery [13,36] composed of a cathode (a MnO2-carbon black (Vulcan) air cathode on a Ni grid), a bare zinc disc, and the Hybrid-coated zinc serving as the anode electrode and a Celgard 3510 separator. This setup also featured a Ni plate functioning as the anode current collector. A sketch of the Zn-air battery’s components is presented in Figure 1. The discharge capacitance of the Zn-air battery with bare zinc and Hybrid-coated zinc as anode material was evaluated at a fixed current of 1 mA/cm2 to a cut-off voltage of 0.5 V and the charge/and discharge tests were conducted at the current densities of 1 mA/cm2 and 10 mA/cm2 for 24 h.
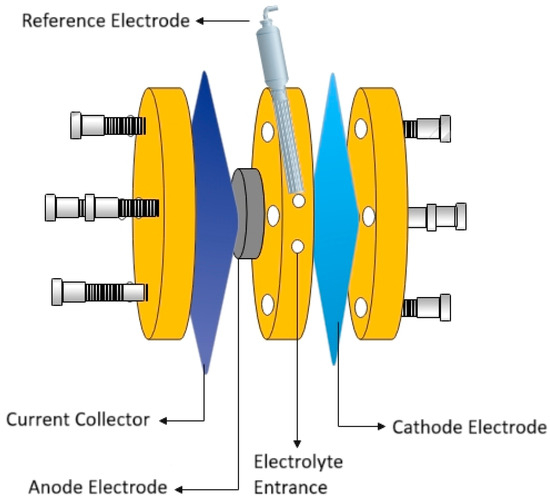
Figure 1.
Graphical sketch of the zinc-air battery’s components, including the zinc anode, air cathode, electrolyte and separator.
Notably, for each test iteration, a fresh cathode material was employed. Charge/discharge tests were performed based on a half-cell assembly with a zinc-base sample, with and without coating, as an anode and cathode and a zinc wire as a reference electrode.
Surface roughness and topography of the samples were measured in air employing a Bruker Multimode 8 AFM operating in quantitative nanomechanical mode, providing valuable height and peak force error data. The scan size of the images was 3 × 3 µm2 and the scan rate was 0.5 Hz. The cantilever was RTESPA-300 (Bruker, Dresden, Germany), having a resonance frequency of about 300 kHz and a spring constant of 40 N/m. For the data processing, Nanoscope Analysis software (version 1.5) was applied.
The chemical nature of the samples, with and without coating, was studied before and after corrosion experiments via Raman spectroscopy using a LabRam confocal microscope (Palaiseau, France), a 10× objective, and 633 nm He–Ne laser excitation at 7 mW.
XRD analyses were carried out using a Rigaku Ultima+ diffractometer equipped with a Bragg–Brentano goniometer (Rigaku, Neu-Isenburg, Germany) and Cu Kα radiation (λ = 1.5406 Å), at 40 kV and 20 mA, with a 0.02° step size.
3. Results
3.1. Electrochemical Tests
The effectiveness of the hybrid coating has been evaluated by subjecting both bare and Hybrid-coated zinc samples to a series of electrochemical experiments within a 1M NaCl electrolyte. The outcomes of each of these experiments are discussed in the subsequent sections. The average coating thickness was measured by a digital micrometer (Mcbazel, Wenzhou, Zhejiang, China) and was 130 ± 6 µm, confirming a homogeneous layer formation.
OCP values of the samples were recorded by immersing them in the electrolyte for 30 min and monitoring the potential change until its fluctuations reached less than 5 mV. For the uncoated zinc sample, immediately after immersion, the potential showed a slight decrease, and approximately 15 min after immersion, it reached an almost constant value. Contrarily, the potential profiles of the Hybrid-coated zinc remained notably stable throughout the immersion duration, sustaining this stability during the whole test. At the end of the test, the open-circuit potentials for the zinc and Hybrid-coated zinc samples were recorded as −1.03 and −1.01 V, respectively. The lower activity of the Hybrid-coated sample compared to the bare sample suggests the formation of a barrier film between the substrate and the electrolyte.
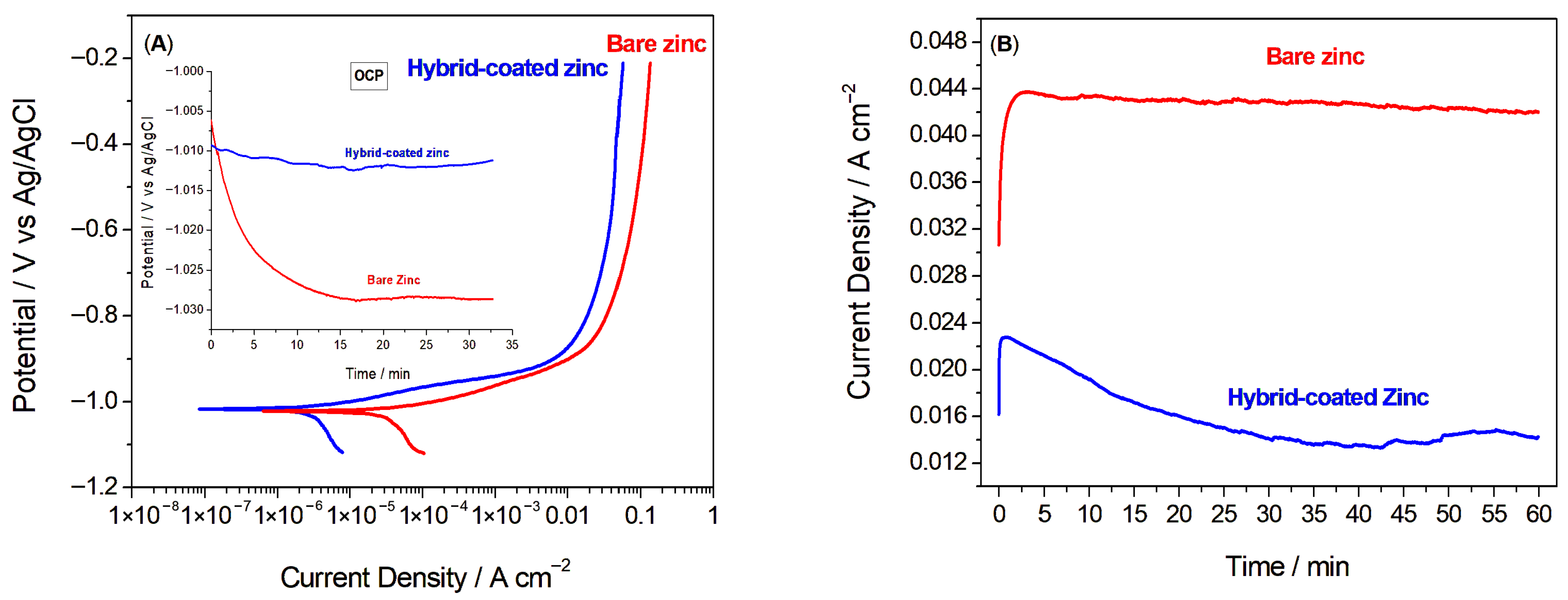
The linear sweep voltammetry (LSV) curves for both bare and Hybrid-coated zinc samples are presented in Figure 2A. The LSV results show that, beyond the activation region, the current density remained nearly constant with increasing potential, suggesting the formation of a surface oxide layer [23,37]. As anticipated, the bare zinc exhibited a more negative Ecorr and a significantly higher icorr than the coated sample, highlighting the enhanced corrosion resistance of the Hybrid-coated sample. Quantitative values of the polarization resistance (Rp) of the samples were calculated from corrosion potential and current and also the Tafel slopes, which were found to be 0.119 and 0.681 Ω.cm2 for bare and Hybrid-coated zinc, respectively. The results imply that the hybrid protective coating can substantially reduce the corrosion rate of zinc [38].
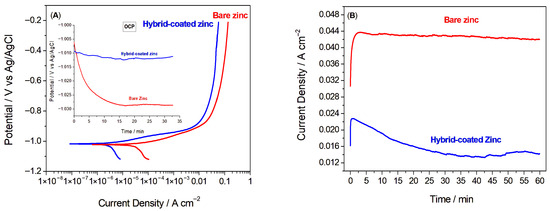
Figure 2.
(A) Linear sweep voltammetry curves of bare and Hybrid-coated zinc samples (Inset: Open circuit potential of the bare and Hybrid-coated zinc vs. time), (B) at −0.8 V on bare versus Hybrid-coated zinc samples in 1 M NaCl electrolyte.
The effect of the applied coating was also evaluated by potentiostatic tests carried out for 60 min at a selected potential of −0.8 V, a value corresponding to an almost constant current density in the LSVs. The graphs of the measured current density over time are shown in Figure 2B. The samples demonstrated a rise in current density initially, which subsequently stabilized, reaching nearly constant values of 42 and 14 mA/cm2 after 60 min for zinc without and with hybrid coating, respectively. The reduced current density observed for the coated zinc revealed the satisfying corrosion behaviour of Hybrid as a protective coating against corrosion.
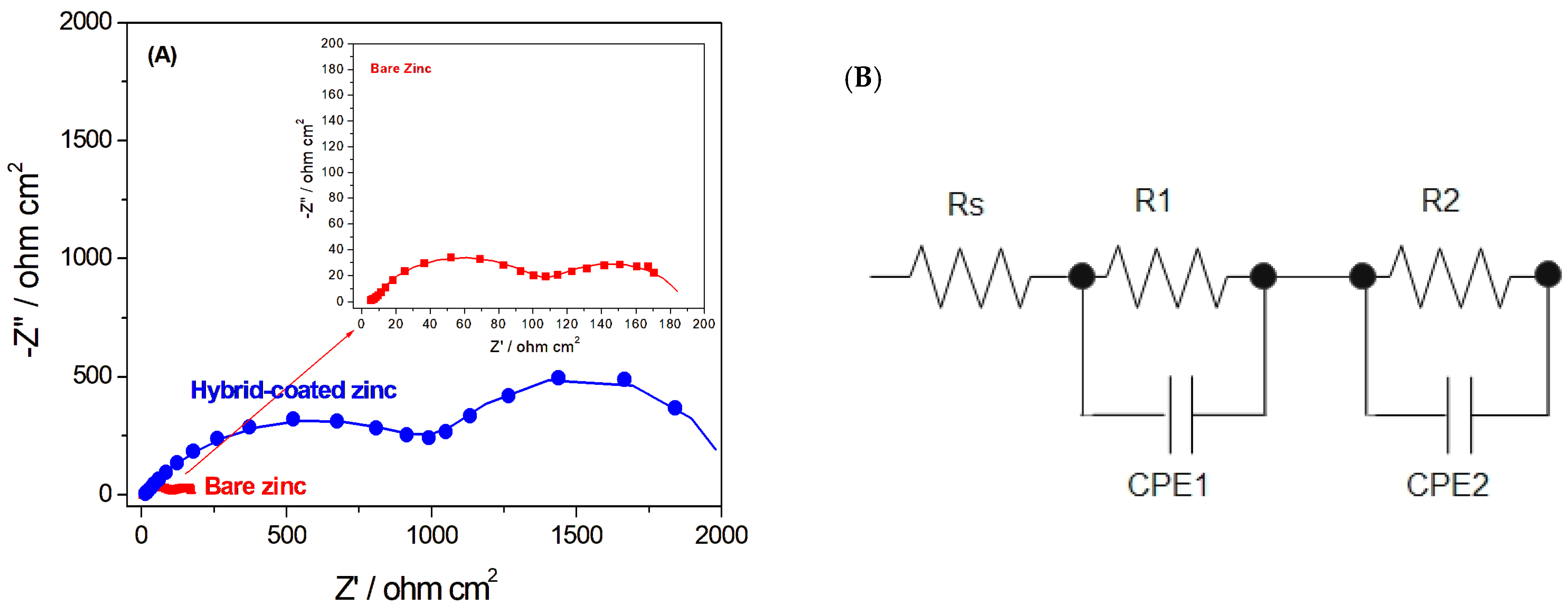
The electrochemical behavior and corrosion resistance of the Hybrid-coated samples were further investigated by electrochemical impedance spectroscopy (EIS). As shown in the Nyquist plots (Figure 3A), the capacitive loop exhibited a noticeably larger diameter, indicating enhanced corrosion resistance of the coated sample, which demonstrates the effectiveness of the coating [21,39].
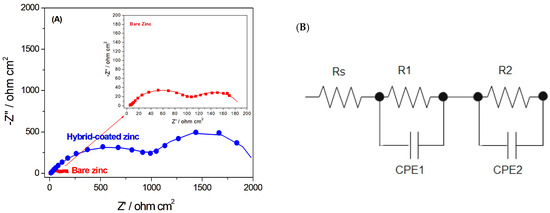
Figure 3.
(A) Nyquist plots for bare and Hybrid-coated zinc, showing the experimental EIS data (symbols) and the corresponding fit results (lines), (B) schematic of the equivalent circuit used to fit the EIS data.
Two capacitive time constants were evident in the Nyquist plots of the coated and bare zinc samples. For bare zinc, the presence of two capacitive time constants indicated that the corrosion products formed on its surface were composed of two relatively dense and porous layers. [39] The equivalent circuit used to analyse the EIS data and fit the plots is shown in Figure 3B. Rs represents the electrolyte resistance. The high-frequency capacitive time constant corresponds to the interfacial film resistance (R1), reflecting the resistance of corrosion products created on the surface of the sample. The low-frequency capacitive time constant is associated with the charge transfer resistance (R2) at the electrode/electrolyte interface, which depends on surface conditions and indicates the resistance to charge transfer during electrochemical reactions [39,40]. CPE1 represents the coating capacitor, which is associated with the dielectric character of the coating material. It reflects the capacitive behaviour of the protective layer. CPE2 is the double-layer capacitor, which is attributed to the capacitance at the electrode/electrolyte interface. Y represents the magnitude of the constant phase element (CPE), and n is the exponent that reflects the deviation from ideal capacitive behavior. When n = 1, the CPE functions as an ideal capacitor, whereas n = 0 corresponds to a pure resistor; and when n = 0.5, it corresponds to a Warburg element, reflecting diffusion-controlled processes [39,41,42,43].
The resulting values of the fits are shown in Table 1. In particular, the R1 value, representing the coating resistance, was significantly higher for the Hybrid-coated zinc compared to the bare sample. This outcome confirms the effectiveness of the hybrid coating in enhancing the corrosion protection of zinc.

Table 1.
Equivalent electrical circuit parameters of the zinc material, before and after corrosion.
The hybrid coating provides corrosion protection mainly by functioning as a compact and hydrophobic barrier. Alkoxy-silane and silanes undergo hydrolysis and subsequent condensation to form a highly crosslinked siloxane (Si–O–Si) network, which exhibits strong interfacial adhesion to the metallic substrate. In parallel, a methacrylic resin reinforces the organic phase of the coating, imparting additional toughness and flexibility that help mitigate cracking or mechanical failure. The synergistic contribution of silane groups and organic segments increases the hydrophobic character of the film, effectively reducing water uptake and restricting the permeation of aggressive species such as chloride ions and dissolved oxygen. These combined effects result in a robust and long-lasting protective layer that physically separates the metal surface from the surrounding corrosive environment [44,45,46].
The ionic conductivity of the coating was determined using impedance measurements. The ionic conductivity (δ) was calculated according to the following Equation:
where L is the coating thickness, A is the exposed area, and Rb represents the coating resistance [47]. The ionic conductivity of the Hybrid-coated zinc in a 1 M NaCl solution was 0.013 mS/cm.
3.2. Charge/Discharge Tests
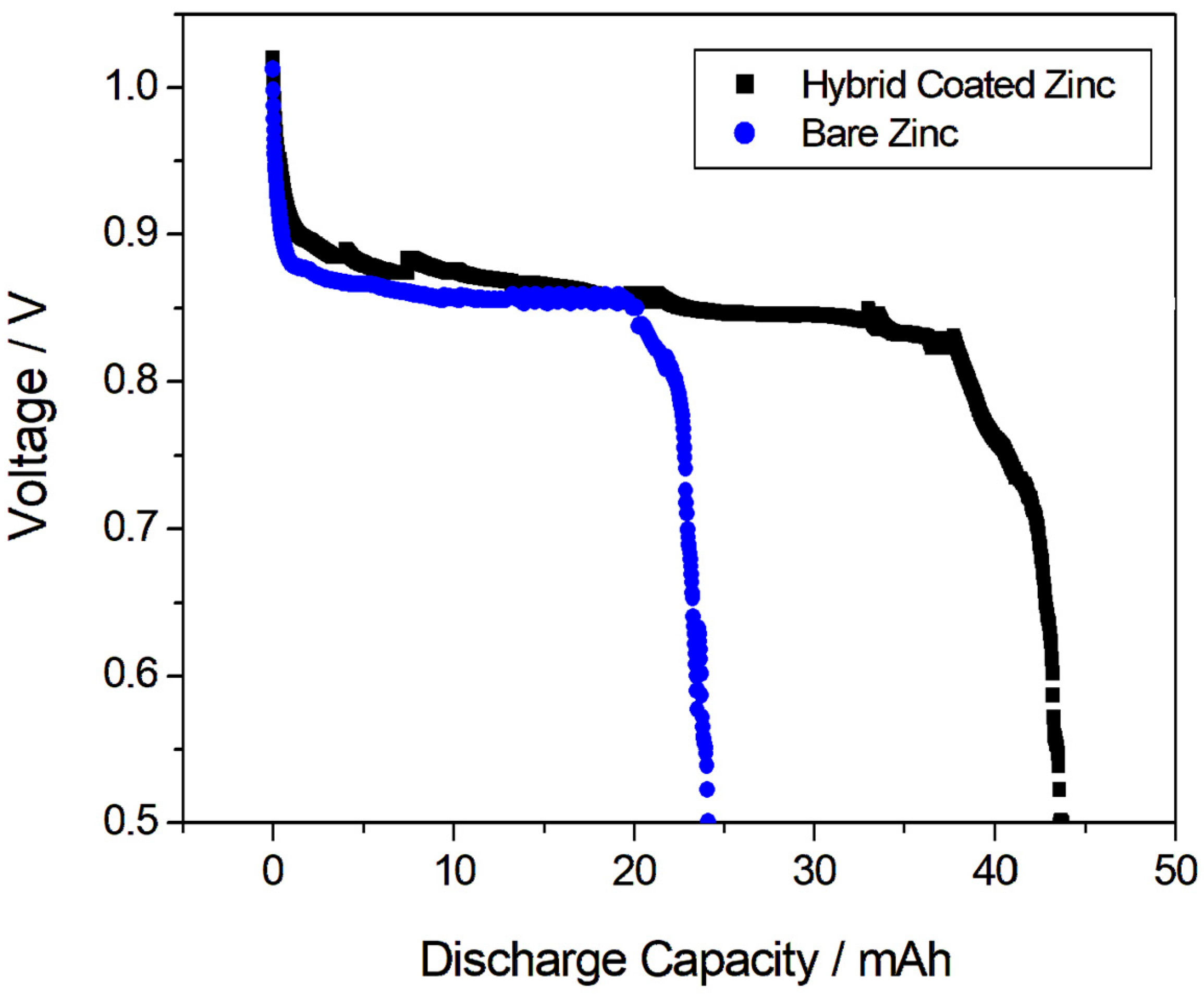
The effectiveness of the coating was then evaluated by long-term discharge tests performed in galvanostatic mode for a zinc-air battery. The zinc-air battery cell was composed of an air cathode, bare zinc or Hybrid-coated zinc as the anode and a separator, in NaCl solution. Figure 4 presents the discharge characteristics obtained with a Hybrid-coated and bare zinc electrode, at a discharge current density of 1 mA/cm2, down to a threshold voltage of 0.5 V in a full-cell assembly. The obtained higher discharge capacity of the coated zinc electrode (43.7 mAh) compared to the bare zinc electrode (24.1 mAh) indicated that the coating successfully increased the corrosion resistance of the zinc anode, delaying its passivation process. By effectively slowing down passivation, the coating allows the zinc anode to maintain its active state for a longer period during discharge, resulting in higher capacity utilization. This is a pivotal factor in enhancing the performance and efficiency of zinc-air batteries, particularly for long-duration applications.
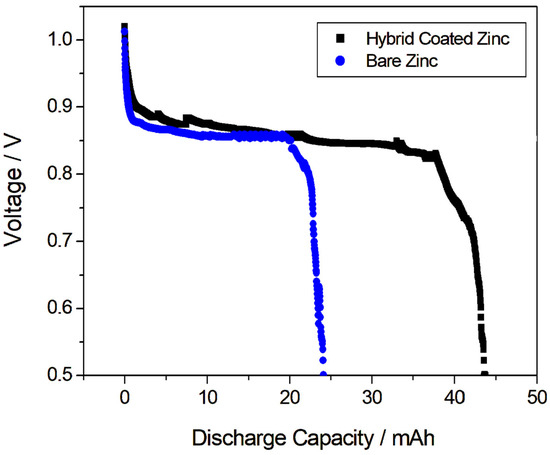
Figure 4.
Discharge characteristics obtained with a Hybrid-coated and bare zinc electrode, at a discharge current density of 1 mA/cm2.
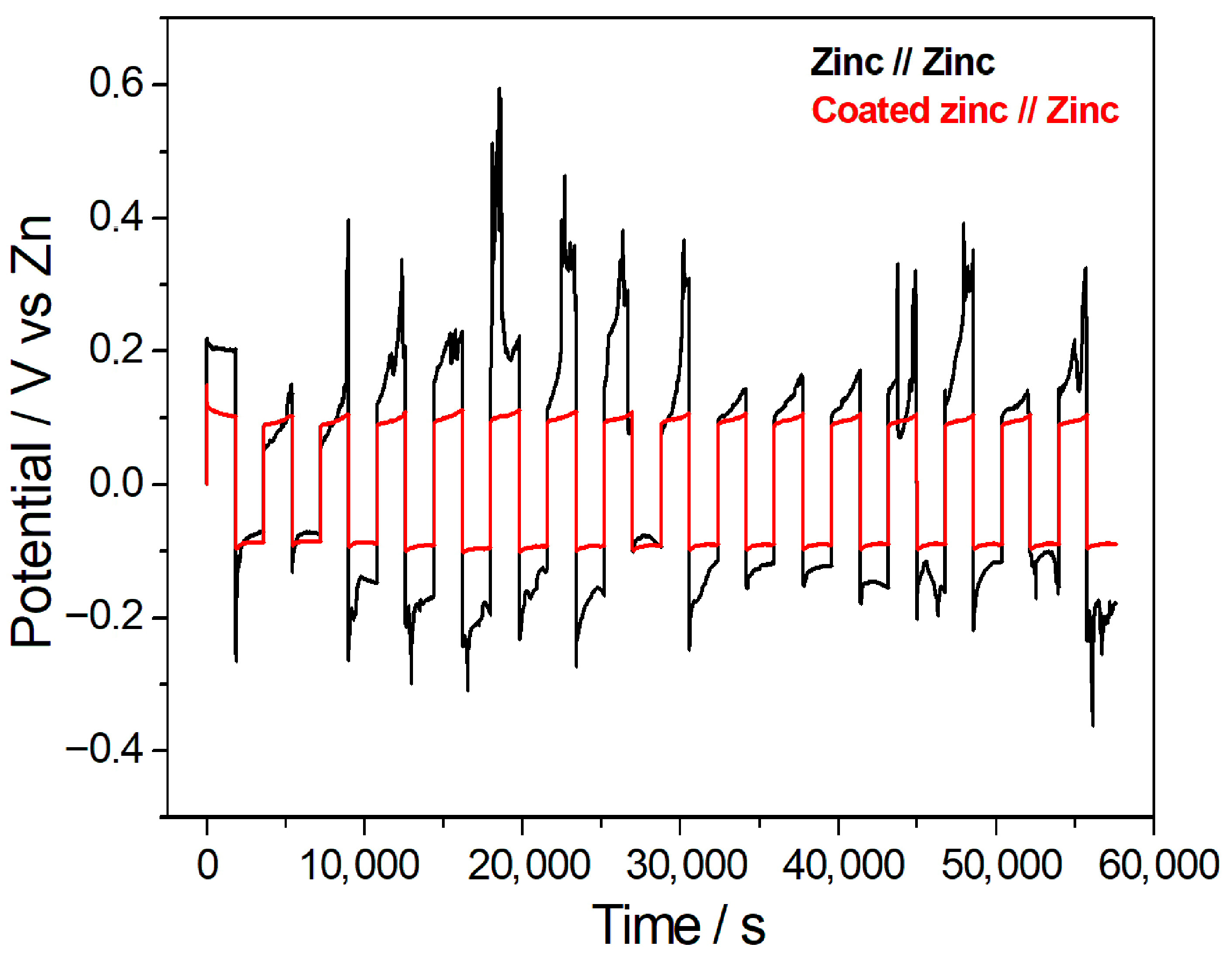
The same self-fabricated laboratory cell was used to conduct several charge/discharge tests in 1 M NaCl electrolyte, in a symmetric cell configuration for assessing anode stability [48]. The charge/discharge behaviour of a cell consisting of Hybrid-coated or bare zinc vs. bare zinc was investigated at two current densities, 1 mA/cm2 and 10 mA/cm2.
The charge/discharge performances of zinc//zinc and coated zinc//zinc at the current density of 1 mA/cm2 were examined and are presented in Figure 5. With the zinc//zinc cell the charge/discharge plots were very unstable with an overpotential, whereas Hybrid-coated Zn electrodes showed a stable cyclability. At a current density of 1 mA/cm2, the symmetric cell with bare zinc electrodes exhibited a coulombic efficiency of 83.25% and a capacity retention of 88.68%, after 16 cycles, whereas the Hybrid-coated zinc versus bare zinc cell achieved values of 92.61% and 90.18%, respectively. These results indicate that the hybrid coating markedly improves the coulombic efficiency, also slightly improving capacity retention compared to bare zinc.
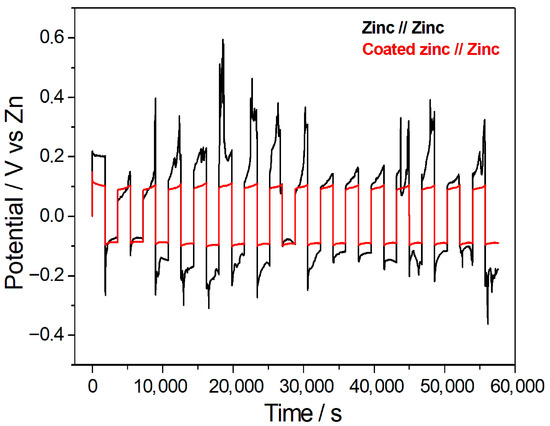
Figure 5.
Charge/discharge behaviour of a cell composed of Hybrid-coated zinc//zinc and bare zinc//zinc measured at the current density of 1 mA/cm2.
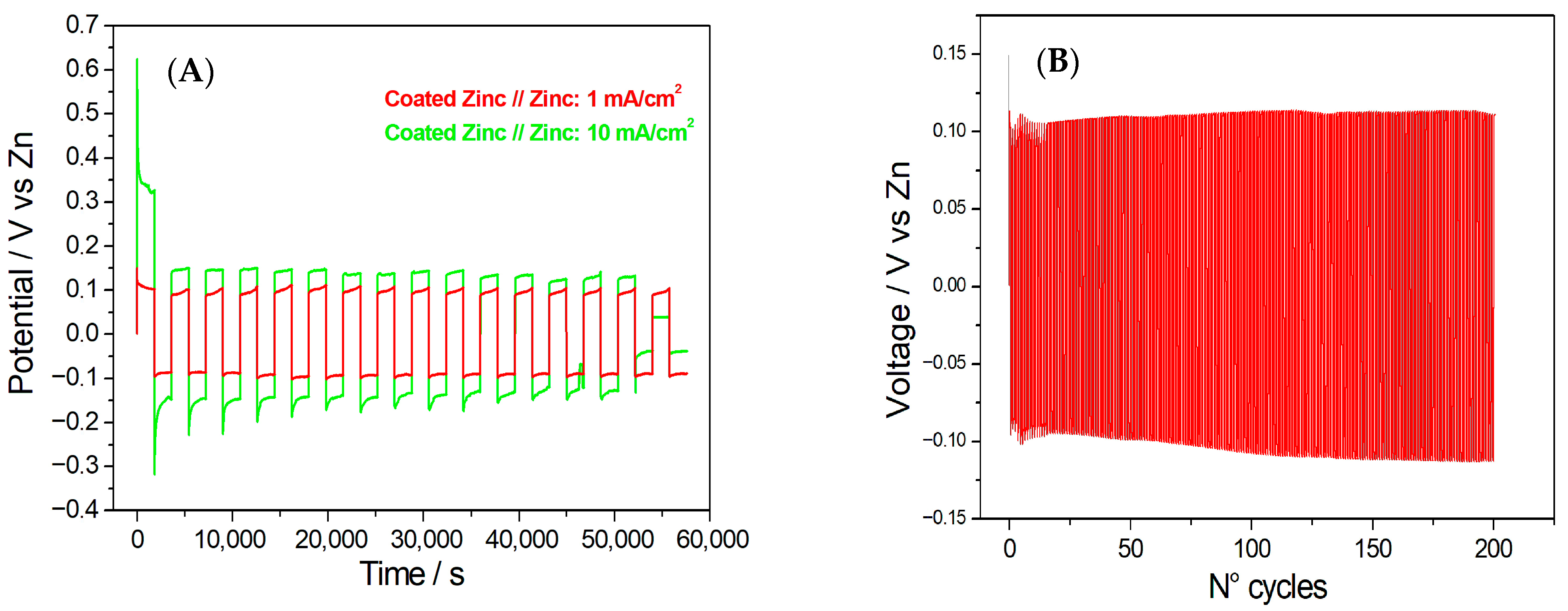
In Figure 6A, the charge/discharge behaviour of a cell composed of coated zinc vs. bare zinc at 1 mA/cm2 and 10 mA/cm2 is reported. The cell exhibited very stable anodic behaviour at 1 mA/cm2. Stability in the anodic processes indicated that the electrode maintains its electrochemical activity without significant passivation or degradation [49,50,51]. At a current density of 10 mA/cm2, the cell demonstrated a tendency towards passivation. In this case, the passivation phenomenon might hinder the desired electrochemical processes during the charge and discharge cycles. The coated zinc electrode appears to have limited effectiveness in preventing passivation or suppressing the formation of non-reactive layers on the electrode’s surface. This suggests that the coating used on the zinc electrode may not be optimally designed to mitigate passivation effects at this particular current density. Passivation of Zn in neutral solutions proceeds via the gradual formation of ZnO/Zn(OH)2 layers, which increases interfacial resistance [49,50,51]. In addition, the battery cell demonstrates very stable cathodic behaviour at both 1 and 10 mA/cm2. Cathodic processes involve reduction reactions at the electrode. The cell exhibited nucleation and growth with a very limited tendency for outgrowth, which indicates highly stable cathodic behaviour. The coulombic efficiency and capacity retention of symmetric cells assembled with coated zinc vs. bare zinc electrodes were determined from galvanostatic charge–discharge measurements. At a current density of 10 mA/cm2, the cell exhibited a coulombic efficiency of 91.07% and a capacity retention of 27.10%. When the current density was decreased to 1 mA/cm2, the coulombic efficiency slightly improved to 92.61%, while the capacity retention markedly increased to 88.68%. This trend suggests that the coated zinc electrode exhibits enhanced stability and reversibility at lower current densities.
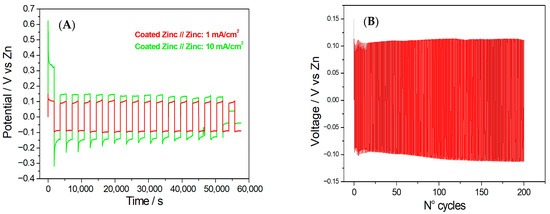
Figure 6.
(A) Charge/discharge behaviour of a cell composed of Hybrid-coated zinc vs. bare zinc at 1 mA/cm2 and 10 mA/cm2; (B) Long-term galvanostatic charge/discharge cycles of Hybrid-coated zinc vs. bare zinc at 1 mA/cm2 (1 h per cycle).
A long-term cyclability test with the coated zinc//zinc cell was also conducted at 1 mA/cm2 (Figure 6B). A remarkable cycling stability is observed, sustaining 90.79% energy efficiency even after 200 cycles (1 h per cycle).
All in all, while the coating may not eliminate corrosion and passivation of the zinc electrode in secondary zinc-air batteries, its presence can significantly reduce their impact.
3.3. Morphological Studies
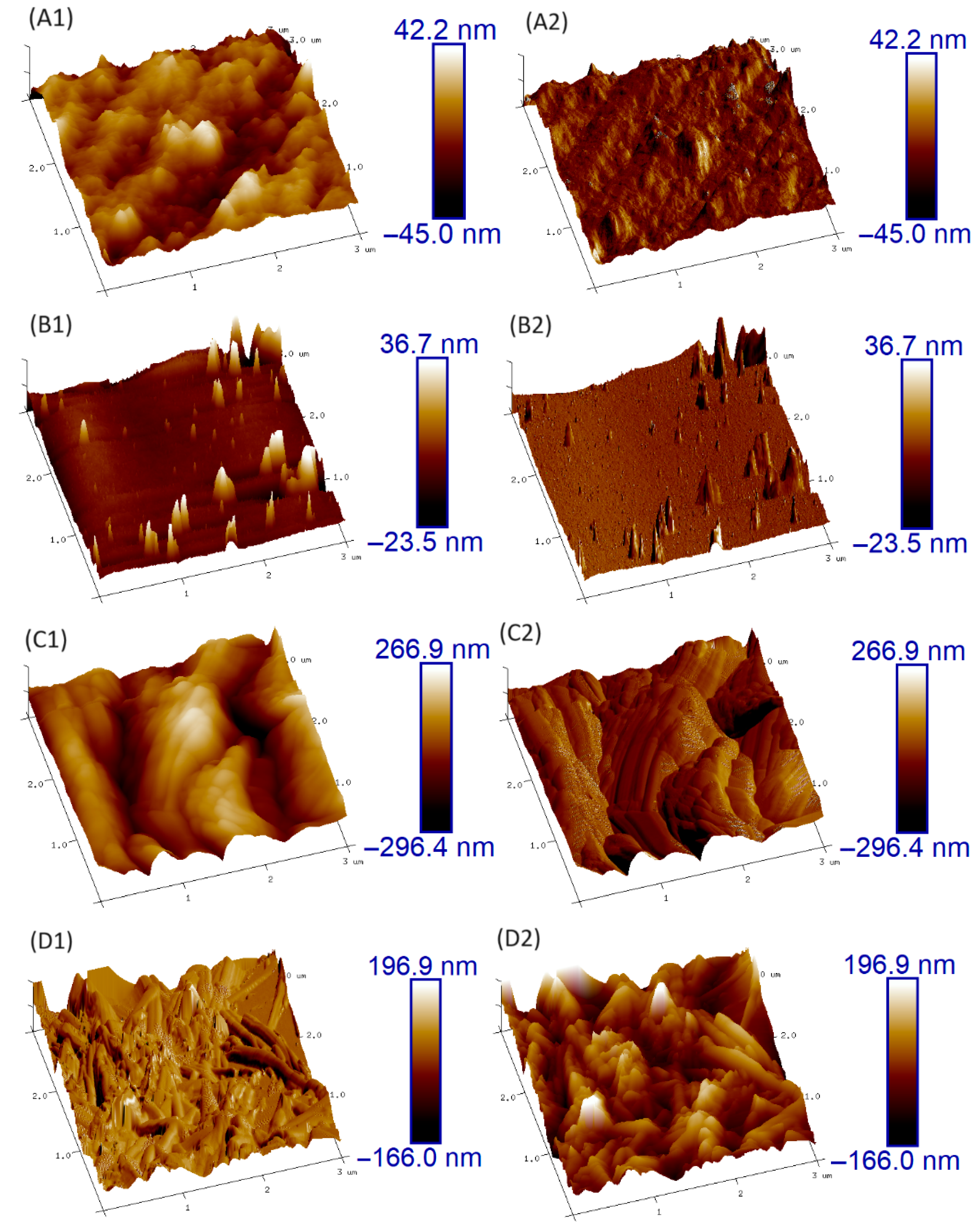
Figure 7 presents the 3D topographic images of bare and Hybrid-coated zinc samples obtained by AFM, before and after corrosion. The corroded surfaces correspond to those exposed to potentiostatic testing at –0.8 V for 60 min in 1 M NaCl solution. Before corrosion, both samples exhibited relatively smooth surfaces, with the coated sample displaying a noticeably smoother and more homogeneous morphology than the uncoated one.
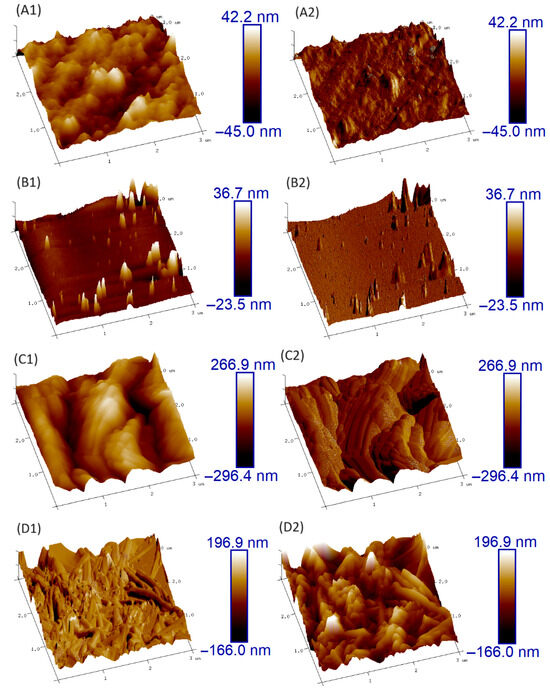
Figure 7.
Height and Peak force Error AFM images of (A1,A2) Bare Zinc, (B1,B2) Hybrid-coated zinc, (C1,C2) Bare zinc after corrosion, and (D1,D2) Hybrid-Coated zinc after corrosion.
After the corrosion test, the differences in the surface condition between the coated and uncoated samples became more evident. The bare zinc surface was severely damaged due to corrosion, while the coated sample showed much less corrosion and some areas remained unaffected. This demonstrated the protective effect of the coating in mitigating corrosion and preserving the integrity of the underlying material by forming a barrier film on the surface.
The surface roughness (Rq) was evaluated with Nanoscope Analysis 1.5 software, calculated as the mean of ten distinct 500 nm × 500 nm regions within the imaged area. The results, along with the corresponding standard deviations, are summarized in Table 2. The obtained Rq values revealed the effectiveness of the coating in reducing surface roughness and preserving the smoothness of the coated sample, even after the corrosion test. The lower Rq values for the coated sample, relative to the uncoated one, further validate the protective and anticorrosive effectiveness of the applied coating.

Table 2.
Rq values of zinc specimens without and with coating, before and after corrosion, extracted from AFM images reported in Figure 7.
3.4. Spectroscopic Studies
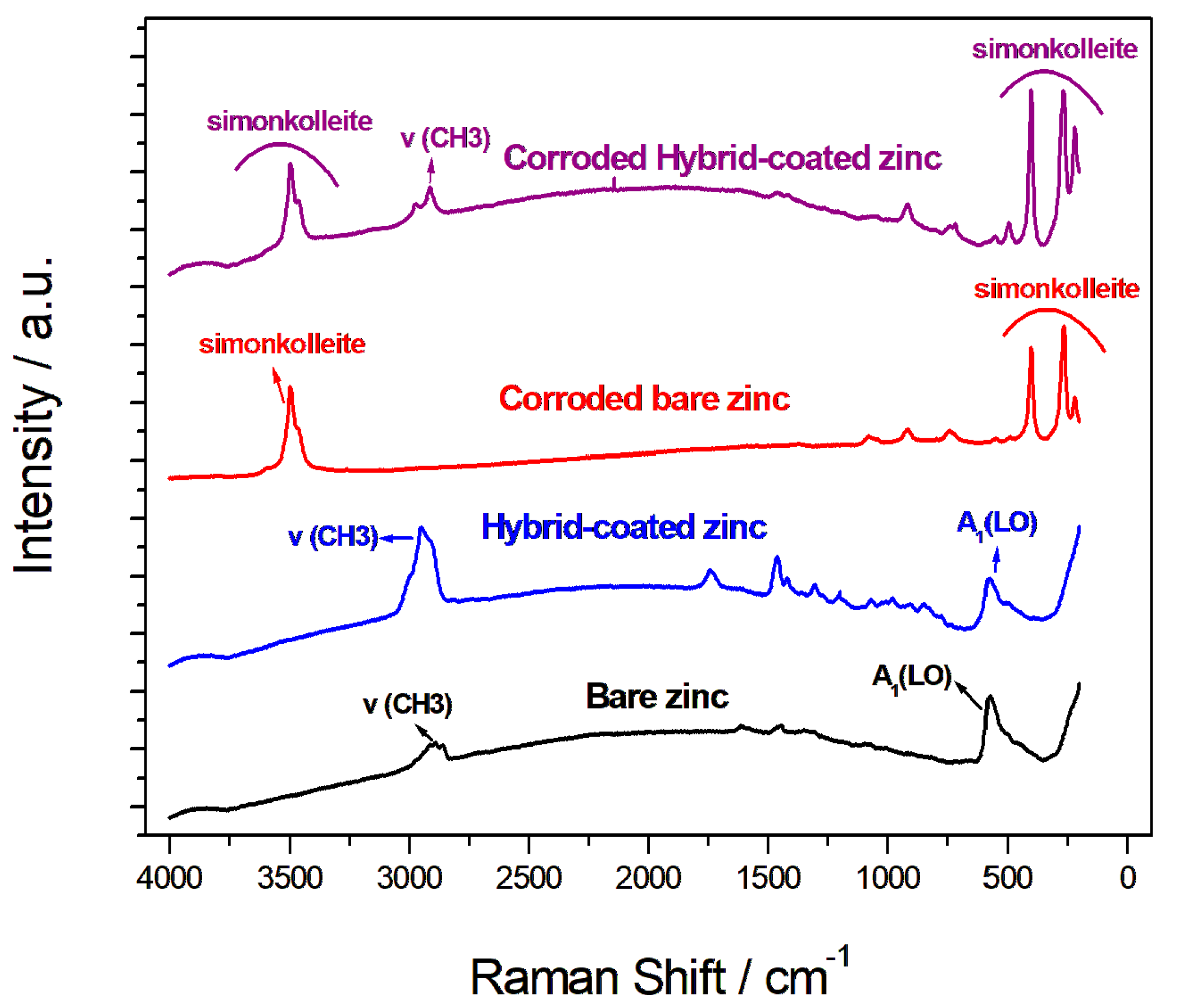
The Raman spectroscopy analysis of the zinc samples, both coated and uncoated, before and after controlled corrosion obtained by potentiostatic tests carried out for 60 min at −0.8 V in 1 M NaCl solution, is shown in Figure 8. The Raman spectra provide valuable information on the chemical and structural changes that take place during the corrosive attack. The Raman spectra of the zinc sample before corrosion showed characteristic peaks associated with the formation of zinc oxide (ZnO) due to atmospheric exposure. The main observed band at 575 cm−1 could be assigned to the A1(LO) mode, which is characteristic of zinc oxide. Additionally, there were peaks between 2850 cm−1 and 3000 cm−1 corresponding to symmetrical and asymmetrical ν(CH3) vibrations. The spectrum of the coated zinc sample showed the same peaks related to zinc oxide formation, as well as additional peaks between 700 cm−1 and 1700 cm−1, which are attributable to the organic polymer present in the coating [52]. Following the corrosive attack, both the coated and bare zinc samples showed clear peaks at 265 cm−1 and 400 cm−1, as well as others at about 730 cm−1 and 900 cm−1, which are indicative of the formation of Zn(Cl)2(Zn[OH]2)4 (simonkolleite), a corrosion product [53,54]. The presence of simonkolleite was further confirmed by the peaks at 3460 cm−1 and 3495 cm−1, which correspond to OH vibration modes. Additionally, the peak at 1078 cm−1 in the corroded zinc spectrum could be assigned to zinc carbonate [53]. Interestingly, after corrosion, the Raman spectrum of the coated zinc still displayed the characteristic bands of zinc oxide (ZnO), indicating that the coating provided some level of protection and slowed down the passivation process. However, in the uncoated zinc sample after corrosion, the oxide peaks were no longer present, indicating that the corrosion had progressed more extensively in the absence of protection.
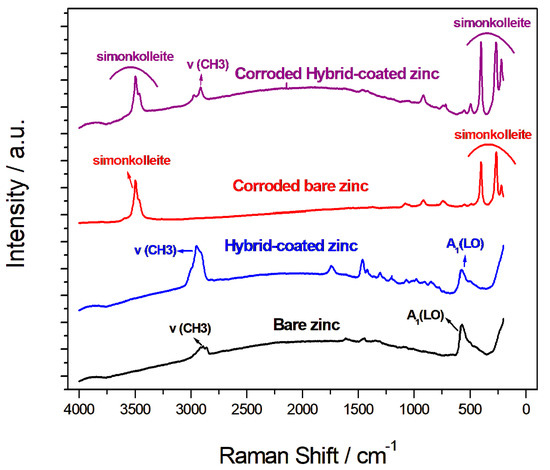
Figure 8.
Raman spectra of the bare and coated samples before and after corrosion.
Furthermore, the peaks associated with the organic polymer coating were still present in the coated zinc sample after corrosion, although they were less evident. These results suggested that the protective hybrid coating on the zinc electrode provides some level of resistance against corrosive attack, slowing down passivation without blocking corrosion through a barrier mechanism [44,45,46]. This is a positive aspect of recharging the zinc electrode in the zinc-air battery, as it allows for a more controlled and stable electrochemical performance.
3.5. XRD Studies
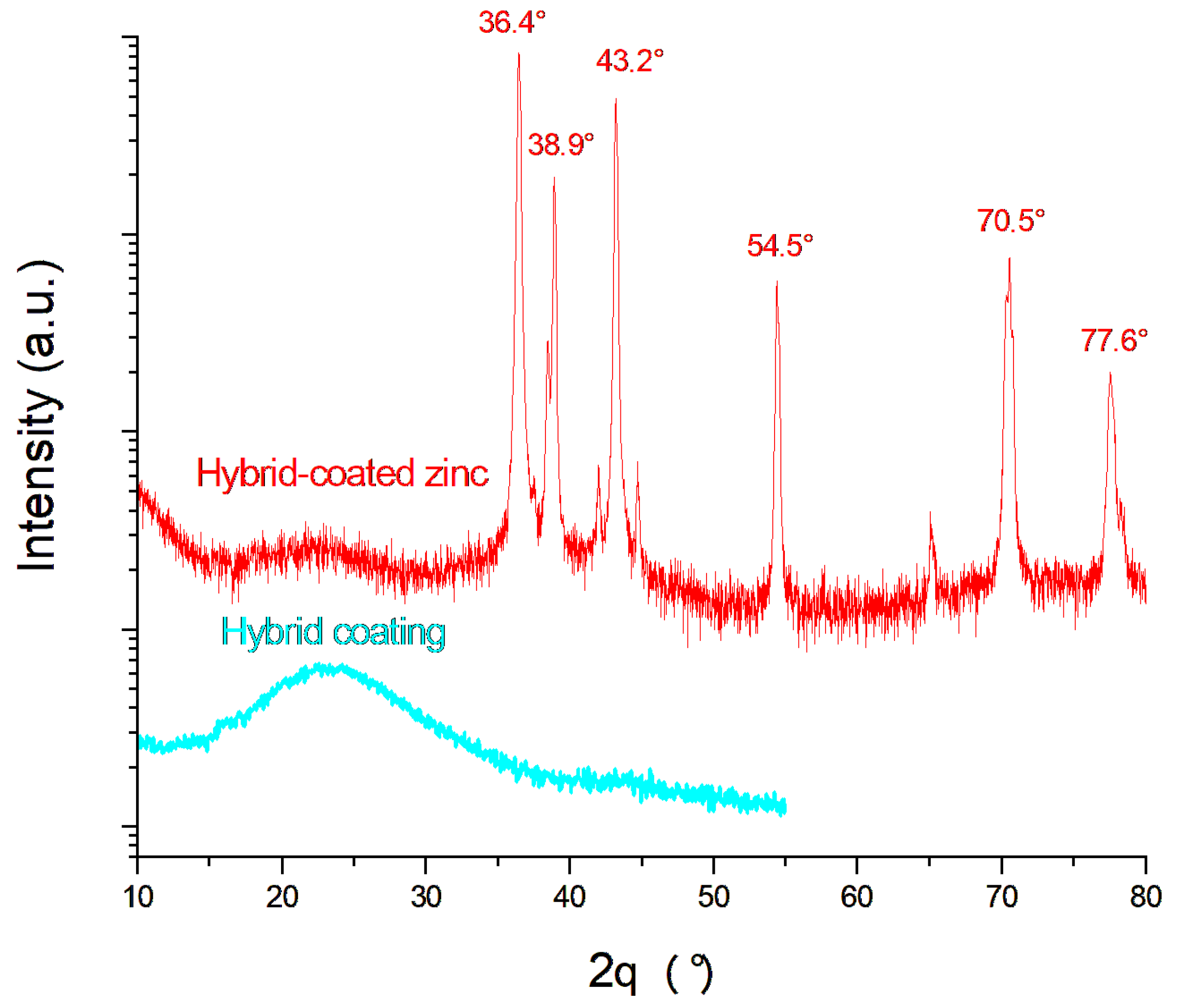
An XRD analysis was performed on the Hybrid-coated zinc sample after the potentiostatic corrosion test at −0.8 V for 1 h, and the corresponding diffraction pattern is shown in Figure 9, together with that of the coating alone. The characteristic zinc diffraction peaks at 36.4°, 38.9°, 43.2°, 54.5°, 70.5°, and 77.6° correspond to the International Centre for Diffraction Data (JCPDS card No. 03-065-5973) and remain visible even after the corrosion test. The broad amorphous signal centered around 23° is attributed to the coating. Its persistence on the Hybrid-coated zinc after corrosion confirms the protective effectiveness of the Hybrid coating.
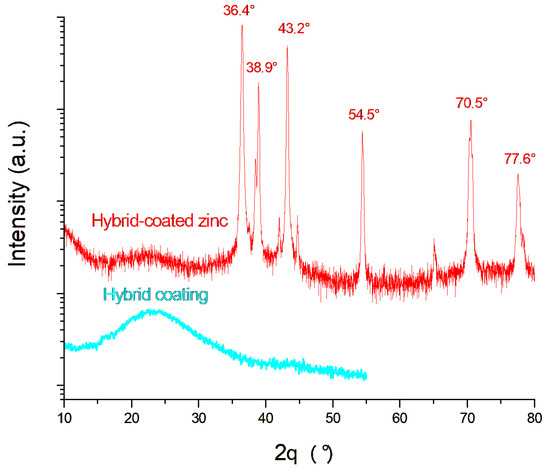
Figure 9.
XRD spectra of the bare and coated samples after corrosion.
4. Conclusions
In this study, a hybrid coating was applied on the surface of a zinc electrode to enhance the corrosion resistance in a neutral electrolyte. The effectiveness of this approach was investigated through electrochemical and spectroelectrochemical techniques.
The electrochemical results indicated that the applied coating was successful in reducing the corrosion current density, thus enhancing the corrosion resistance of the zinc electrode. Morphological studies and roughness measurements using Atomic Force Microscopy further supported the reduced corrosion susceptibility of the coated zinc sample. Raman spectroscopy combined with XRD studies demonstrated that the coating slowed down the passivation process without blocking corrosion, and the hybrid coating provides corrosion protection mainly by functioning as a compact and hydrophobic barrier.
Long-term charge/discharge tests of the coated zinc vs. zinc at the current density of 1 mA/cm2 showed a remarkable cycle stability even after 200 cycles with a coulombic efficiency of 90.79%. This allowed for a greater discharge capacity, making the coating advantageous for the recharging of the zinc electrode in the zinc-air battery.
Author Contributions
Conceptualization, S.B., B.B., C.E.C., R.S. and C.M.; methodology, S.B., B.B., C.E.C., R.S. and C.M.; validation, S.B. and C.M.; investigation, S.B., B.B., C.E.C., R.S. and C.M.; data curation, S.B., C.E.C., R.S. and C.M.; writing—original draft preparation, S.B. and C.M.; writing—review and editing, S.B. and C.M.; supervision, B.B., C.E.C. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by PRIN2022 PNRR “BAT-MEN” (BATtery Modeling, Experiments & Numerics) Enhancing battery lifetime, Project code: P20228C2PP 001, CUP: F53D23010020001, funded by MIUR (Italian Ministry of University and Research) and European Union–NextGenerationEU.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yuan, Z.; Yin, Y.; Xie, C.; Zhang, H.; Yao, Y.; Li, X. Advanced Materials for Zinc-Based Flow Battery: Development and Challenge. Adv. Mater. 2019, 31, 1902025. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Zinc dendrites Inhibition for Zinc-based Battery. ChemSusChem 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent Progress of Metal—Air Batteries—A Mini Review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Mele, C.; Bozzini, B. Spectroelectrochemical investigation of the anodic and cathodic behaviour of zinc in 5.3 M KOH. J. Appl. Electrochem. 2015, 45, 43–50. [Google Scholar] [CrossRef]
- Mele, C.; Bilotta, A.; Bocchetta, P.; Bozzini, B. Characterization of the particulate anode of a laboratory flow Zn–air fuel cell. J. Appl. Electrochem. 2017, 47, 877–888. [Google Scholar] [CrossRef]
- Bozzini, B.; Kazemian, M.; Kiskinova, M.; Kourousias, G.; Mele, C.; Gianoncelli, A. Operando soft X-ray microscope study of rechargeable Zn–air battery anodes in deep eutectic solvent electrolyte. X-Ray Spectrom. 2019, 48, 527–535. [Google Scholar] [CrossRef]
- Mu, T.; Lu, H.; Wang, H.; Wang, Y.; Lou, S.; Zhang, Y.; Gao, J.; Ma, Y.; Zuo, P.; Zhu, X.; et al. Artificial organic-inorganic hybrid interface enables reversible Zn anodes. Nano Energy 2025, 138, 110835. [Google Scholar] [CrossRef]
- Suppanucroa, N.; Yoopensuk, W.; Pimoei, J.; Thanapong-a-morn, W.; Kao-Ian, W.; Pakawanit, P.; Mahlendorf, F.; Kheawhom, S.; Somwangthanaroj, A. Enhanced long-term stability of zinc-air batteries using a quaternized PVA-chitosan composite separator with thin-layered MoS2. Electrochim. Acta 2025, 510, 145361. [Google Scholar] [CrossRef]
- Shinde, S.S.; Kim, S.H.; Wagh, N.K.; Lee, J.H. Design Strategies for Practical Zinc-Air Batteries Toward Electric Vehicles and beyond. Adv. Energy Mater. 2025, 15, 2405326. [Google Scholar] [CrossRef]
- Wang, Y.; Kwok, H.; Pan, W.; Zhang, H.; Leung, D.Y.C. Innovative paper-based Al-air batteries as a low-cost and green energy technology for the miniwatt market. J. Power Sources 2019, 414, 278–282. [Google Scholar] [CrossRef]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Blázquez, J.A. Alkaline aqueous electrolytes for secondary zinc–air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; TRD McGraw-Hill: Singapore, 2002. [Google Scholar]
- Bozzini, B.; Altissimo, M.; Amati, M.; Bocchetta, P.; Gianoncelli, A.; Gregoratti, L.; Kourousias, G.; Mancini, L.; Mele, C.; Kiskinova, M. In situ and ex situ X-ray microspectroelectrochemical methods for the study of zinc-air batteries. Encycl. Interfacial Chem. Surf. Sci. Electrochem. 2018, 174–194. [Google Scholar] [CrossRef]
- Bozzini, B.; Mele, C.; Veneziano, A.; Sodini, N.; Lanzafame, G.; Taurino, A.; Mancini, L. Morphological Evolution of Zn-Sponge Electrodes Monitored by in Situ X-ray Computed Microtomography. ACS Appl. Energy Mater. 2020, 3, 4931–4940. [Google Scholar] [CrossRef]
- Zhao, H. Recent Advances in Rechargeable Zn-Air Batteries. Molecules 2024, 29, 5313. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Guerrero, S.S.M.; Tempel, H.; Hausen, F.; Kungl, H.; Eichel, R.A. Influence of Al Alloying on the Electrochemical Behavior of Zn Electrodes for Zn–Air Batteries with Neutral Sodium Chloride Electrolyte. Front. Chem. 2019, 7, 800. [Google Scholar] [CrossRef]
- Guerrero, S.S.M.; Durmus, Y.E.; Dzieciol, K.; Basak, S.; Tempel, H.; van Waasen, S.; Kungl, H.; Eichel, R.A. Improved Electrochemical Performance of Zinc Anodes by EDTA in Near-Neutral Zinc−Air Batteries. Batter. Supercaps 2021, 4, 1830–1842. [Google Scholar] [CrossRef]
- Wei, W.; Xu, J.; Chen, W.; Mi, L.; Zhang, J. A review of sodium chloride-based electrolytes and materials for electrochemical energy technology. J. Mater. Chem. A 2022, 10, 2637–2671. [Google Scholar] [CrossRef]
- Jindra, J.; Mrha, J.; Musilová, M. Zinc-air cell with neutral electrolyte. J. Appl. Electrochem. 1973, 3, 297–301. [Google Scholar] [CrossRef]
- Amendola, S.; Binder, M.; Black, P.J.; Sharp-Goldman, S.; Johnson, L.; Kunz, M.; Oster, M.; Chciuk, T.; Johnson, R. Electrically Rechargeable, Metal-Air Battery Systems and Methods. US Patent US20120021303A1, 26 January 2012. [Google Scholar]
- Goh, F.W.T.; Liu, Z.; Hor, T.S.A.; Zhang, J.; Ge, X.; Zong, Y.; Yu, A.; Khoo, W. A Near-Neutral Chloride Electrolyte for Electrically Rechargeable Zinc-Air Batteries. J. Electrochem. Soc. 2014, 161, A2080–A2086. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Zheng, G.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Durable rechargeable zinc-air batteries with neutral electrolyte and manganese oxide catalyst. J. Power Sources 2016, 332, 330–336. [Google Scholar] [CrossRef]
- Clark, S.; Latz, A.; Horstmann, B. Rational Development of Neutral Aqueous Electrolytes for Zinc–Air Batteries. ChemSusChem 2017, 10, 4735–4747. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, C.-X.; Liu, J.-N.; Li, B.-Q.; Tang, C.; Zhang, Q. Seawater-based electrolyte for zinc–air batteries. Green. Chem. Eng. 2020, 1, 117–123. [Google Scholar] [CrossRef]
- Neburchilov, V.; Wang, H.; Martin, J.J.; Qu, W. A review on air cathodes for zinc-air fuel cells. J. Power Sources 2010, 195, 1271–1291. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc-air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Cao, R.; Lee, J.S.; Liu, M.; Cho, J. Recent progress in non-precious catalysts for metal-air batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Xue, G.; Bian, H.; Wang, B.; Wu, H.; Li, F.; Wang, C.; Zhou, Q.; Jia, S.; Hu, Z.; Ma, Y.; et al. An organic-inorganic composite coating with excellent protection and ion regulation effects for highly stable zinc anodes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135272. [Google Scholar] [CrossRef]
- Li, B.; Yang, H.; He, J.; Yu, S.; Xiao, R.; Luo, H.; Wen, Y.; Peng, S.; Liao, X.; Yang, D. Photopolymerization of Coating Materials for Protection against Carbon Steel Corrosion. Materials 2023, 16, 2015. [Google Scholar] [CrossRef]
- Corcione, C.E.; Striani, R.; Frigione, M. Organic-inorganic UV-cured methacrylic-based hybrids as protective coatings for different substrates. Prog. Org. Coat. 2014, 77, 1117–1125. [Google Scholar] [CrossRef]
- Striani, R.; Cappai, M.; Casnedi, L.; Corcione, C.E.; Pia, G. Coating’s influence on wind erosion of porous stones used in the Cultural Heritage of Southern Italy: Surface characterisation and resistance. Case Stud. Constr. Mater. 2022, 17, e01501. [Google Scholar] [CrossRef]
- Pia, G.; Corcione, C.E.; Striani, R.; Casnedi, L.; Sanna, U. Coating’s influence on water vapour permeability of porous stones typically used in cultural heritage of Mediterranean area: Experimental tests and model controlling procedure. Prog. Org. Coat. 2017, 102, 239–246. [Google Scholar] [CrossRef]
- Striani, R.; Corcione, C.E.; Muia, G.D.; Frigione, M. Durability of a sunlight-curable organic–inorganic hybrid protective coating for porous stones in natural and artificial weathering conditions. Prog. Org. Coat. 2016, 101, 1–14. [Google Scholar] [CrossRef]
- Fouassier, J.P. An introduction to the basic principles in UV-curing. Radiat. Curing Polym. Sci. Technol. 1993, 1, 49–113. [Google Scholar]
- Corcione, C.E.; Frigione, M.; Striani, R. Hybrid Organic-Inorganic Nanostructured UV-Curable Formulation and Method for Preparation Thereof. European Patent 13001868.2, 11 April 2013. [Google Scholar]
- Bozzini, B.; Bagheri, S.; Boniardi, M.; Mancini, L.; Marini, E.; Sgura, I.; Mele, C. Quantifying and rationalizing polarization curves of Zn-air fuel-cells: A simple enabling contribution to device-scale analysis and monitoring. Electrochim. Acta 2022, 425, 140712. [Google Scholar] [CrossRef]
- Mele, C.; Bozzini, B. Corrosion performance of austenitic stainless steel bipolar plates for Nafion- and room-temperature ionic-liquid-based PEMFCs. Open Fuels Energy Sci. J. 2012, 5, 47–52. [Google Scholar] [CrossRef][Green Version]
- Pakshir, M.; Bagheri, T.; Kazemi, M.R. In vitro evaluation of the electrochemical behaviour of stainless steel and Ni-Ti orthodontic archwires at different temperatures. Eur. J. Orthod. 2013, 35, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, L.; Zhang, D.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.N. Initial formation of corrosion products on pure zinc in saline solution. Bioact. Mater. 2019, 4, 87–96. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Miikkulainen, V.; Mäntymäki, M.; Mousavihashemi, S.; Lahtinen, J.; Lide, Y.; Jiang, H.; Mizohata, K.; Kankaanpää, T.; Kallio, T. Understanding the Stabilizing Effects of Nanoscale Metal Oxide and Li-Metal Oxide Coatings on Lithium-Ion Battery Positive Electrode Materials. ACS Appl. Mater. Interfaces 2021, 13, 42773–42790. [Google Scholar] [CrossRef]
- Duchoslav, J.; Steinberger, R.; Arndt, M.; Keppert, T.; Luckeneder, G.; Stellnberger, K.H.; Hagler, J.; Angeli, G.; Riener, C.K.; Stifter, D. Evolution of the surface chemistry of hot dip galvanized Zn-Mg-Al and Zn coatings on steel during short term exposure to sodium chloride containing environments. Corros. Sci. 2015, 91, 311–320. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.; Zou, Y.; Luo, K.; Zhang, F.; Zeng, R.C.; Li, S. In vitro corrosion and antibacterial performance of polysiloxane and poly(acrylic acid)/gentamicin sulfate composite coatings on AZ31 alloy. Surf. Coat. Technol. 2016, 291, 7–14. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.W.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J.S.; Zhang, G.; Suganuma, K. The role of Zn precipitates and Cl- anions in pitting corrosion of Sn-Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Longhi, M.; Kunsta, S.R.; Beltrami, L.V.R.; Kerstner, E.K.; Filho, C.I.S.; Sarmento, V.H.V.; Malfatti, C. Effect of tetraethoxy-silane (TEOS) amounts on the corrosion prevention properties of siloxane-pmma hybrid coatings on galvanized steel substrates. Mater. Res. 2015, 18, 1140–1155. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Torrico, R.F.O.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Organic-Inorganic Hybrid Coatings for Corrosion Protection of Metallic Surfaces. New Technol. Prot. Coat. 2017, 290, 153–162. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Li, Q.; Chen, A.; Wang, D.; Pei, Z.; Zhi, C. “Soft Shorts” Hidden in Zinc Metal Anode Research. Joule 2022, 6, 273–279. [Google Scholar] [CrossRef]
- Fuchs, D.; Müller, C.; Schaffeld, M.; Mahlendorf, F.; Hoster, H.E. New Insights Into Zinc Passivation Through Operando Measured Zincate Concentrations. Batter. Supercaps 2024, 7, e202400298. [Google Scholar] [CrossRef]
- Bockelmann, M.; Reining, L.; Kunz, U.; Turek, T. Electrochemical characterization and mathematical modeling of zinc passivation in alkaline solutions: A review. Electrochim. Acta 2017, 237, 276–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Wang, N.; Lai, W.H.; Liu, Y.; Chou, S.L.; Liu, H.K.; Dou, S.X.; Wang, Y.X. Anode optimization strategies for aqueous zinc-ion batteries. Chem. Sci. 2022, 13, 14246–14263. [Google Scholar] [CrossRef]
- Nikitenko, V.A.; Plekhanov, V.G.; Mukhin, S.V. Raman Spectra of Oxide zinc powder and single crystals. J. Appl. Spectrosc. 1996, 63, 350–352. [Google Scholar] [CrossRef]
- Gu, R.A.; Shen, X.Y.; Liu, G.K.; Ren, B.; Tian, Z.Q. Surface-enhanced raman scattering from bare Zn electrode. J. Phys. Chem. B 2004, 108, 17519–17522. [Google Scholar] [CrossRef]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, mid-infrared, near-infrared and ultraviolet-visible spectroscopy of PDMS silicone rubber for characterization of polymer optical waveguide materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).